Influence of Organic Loading Rate on Methane Production from Brewery Wastewater in Bioelectrochemical Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Sludge and Brewery Wastewater
2.2. Electrode Fabrication
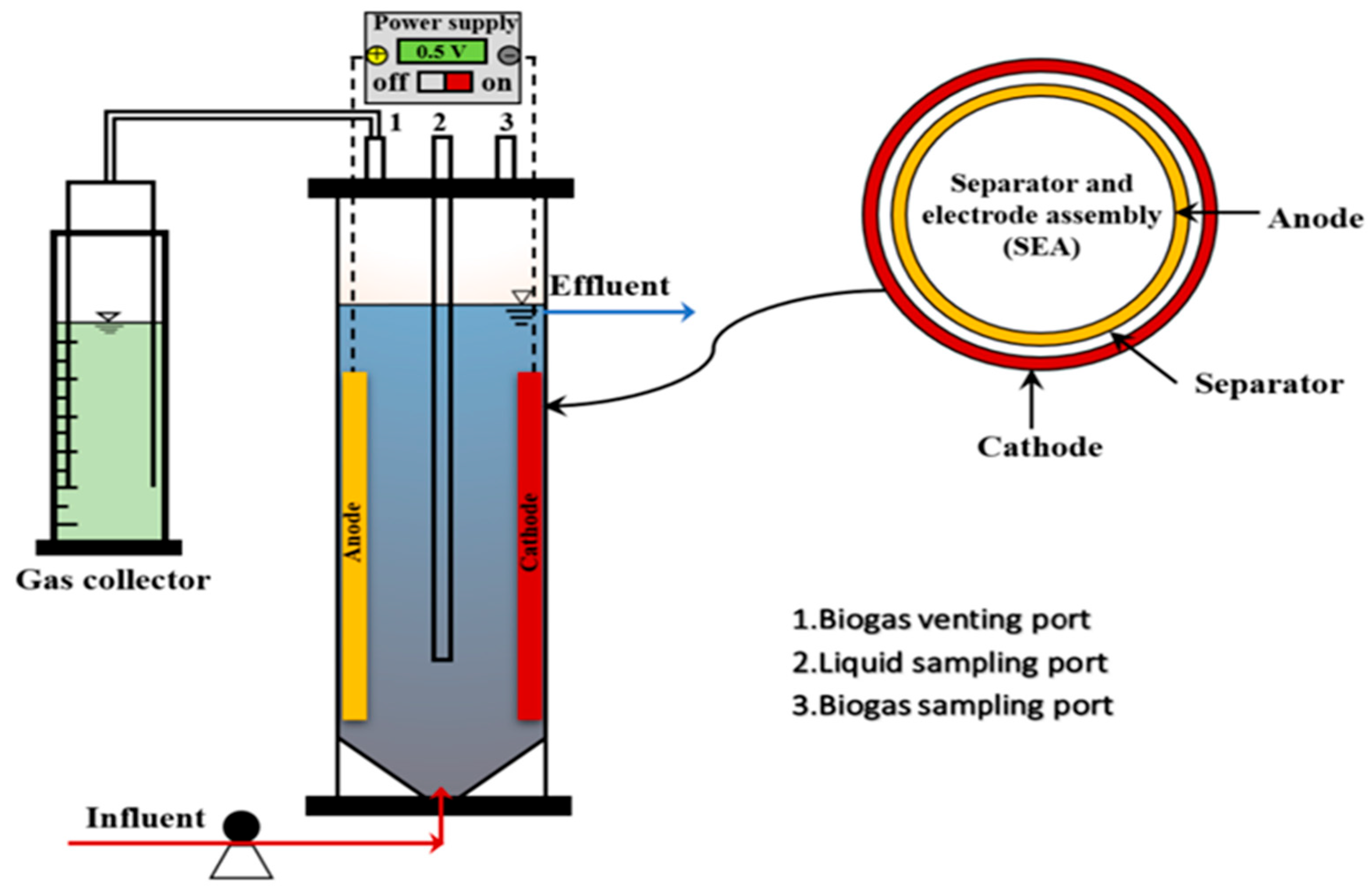
2.3. Reactor Design and Operation
2.4. Measurements and Calculations
3. Result and Discussion
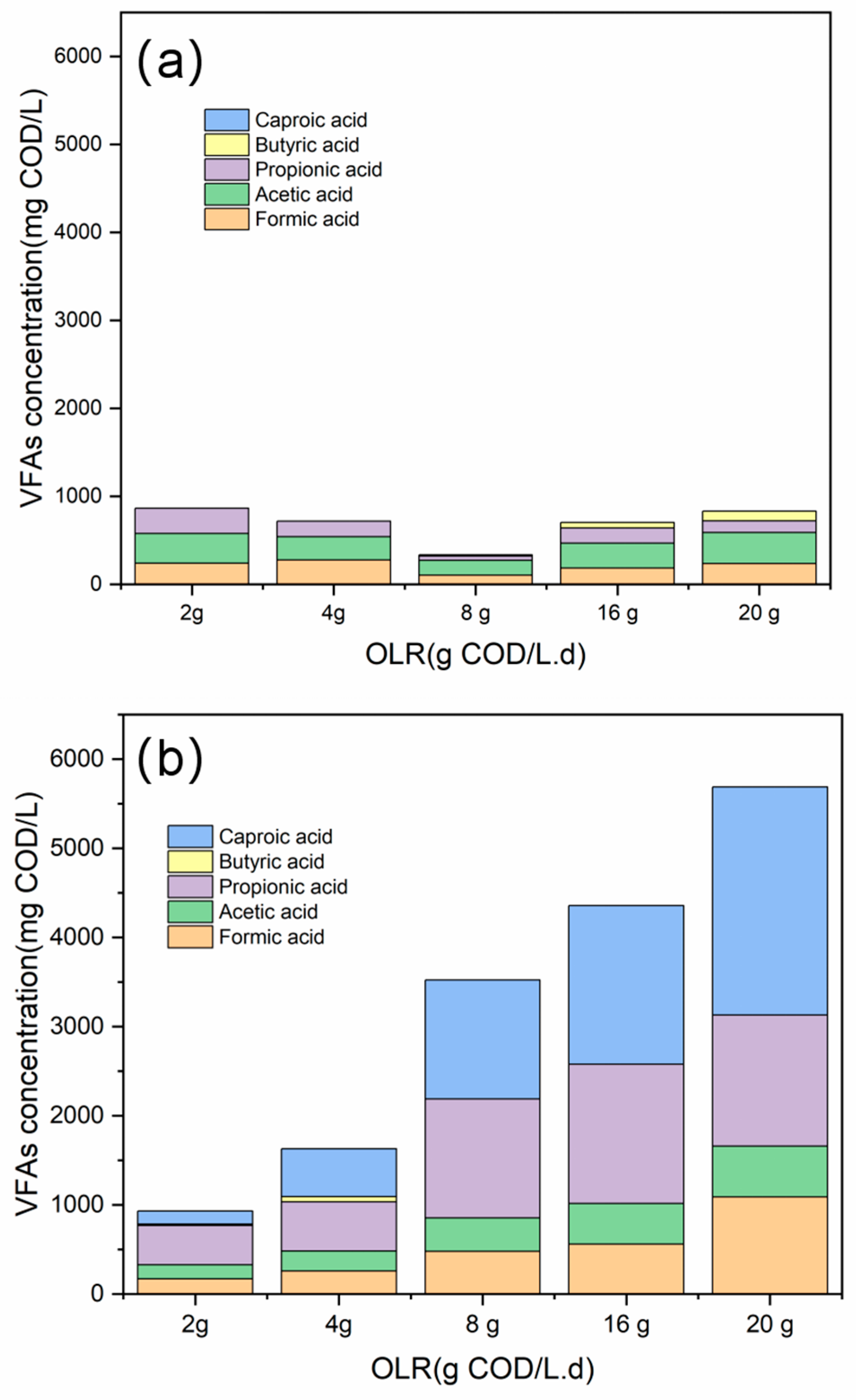
3.1. State Variables (pH, Alkalinity, and VFA) under Different OLRs
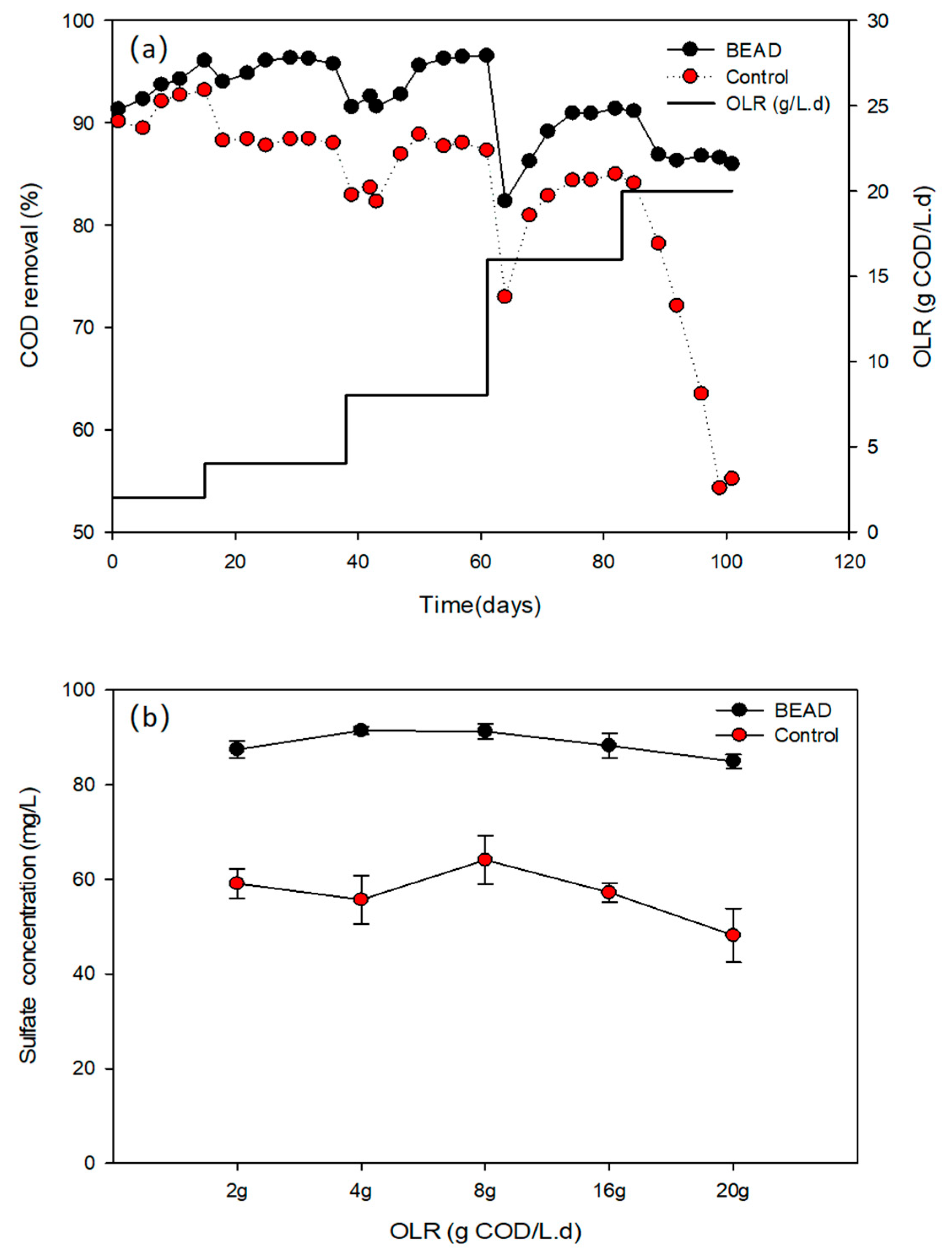
3.2. COD and Sulphate Removal under Different OLRs
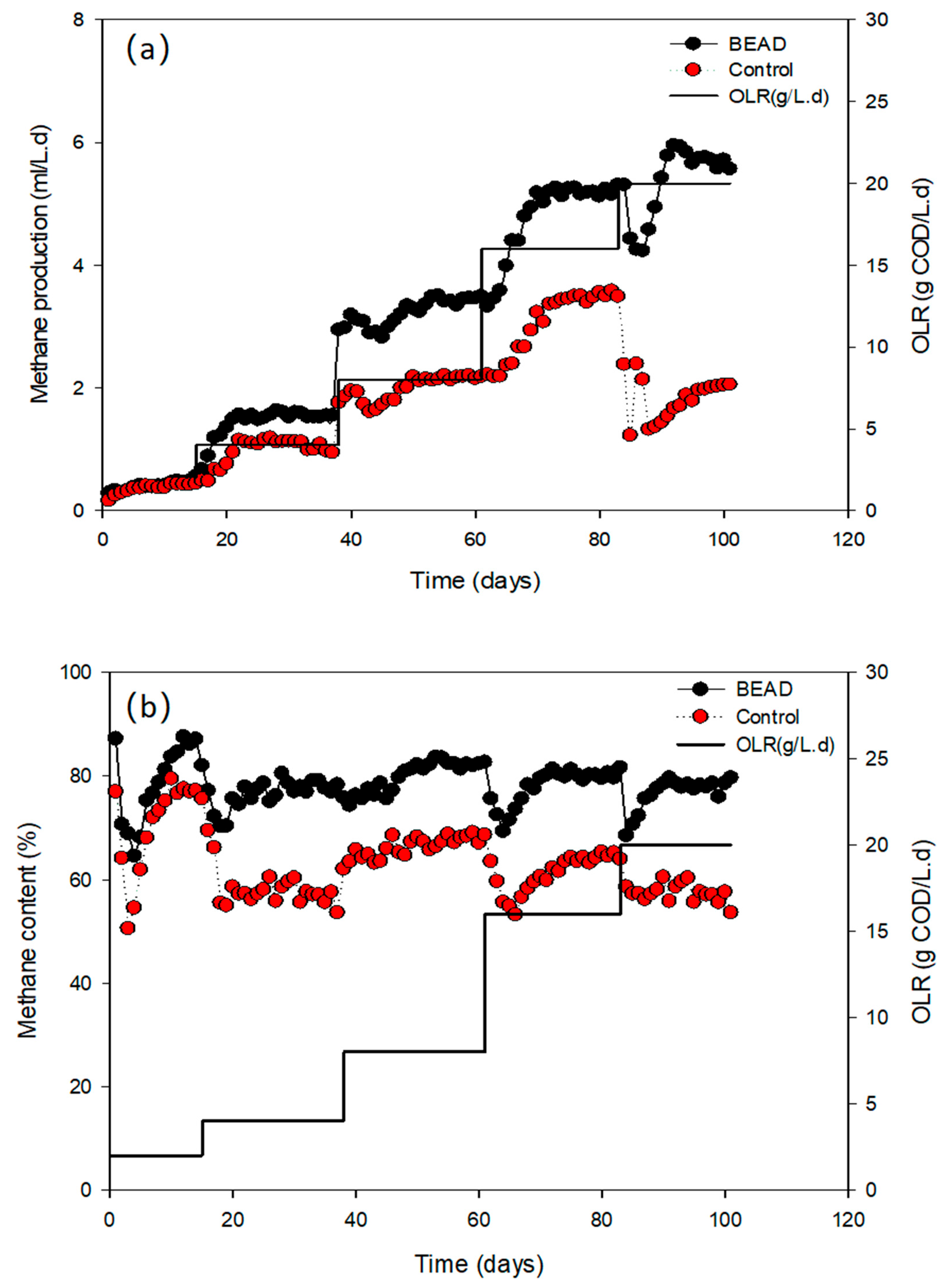
3.3. Methane Production under Different OLRs
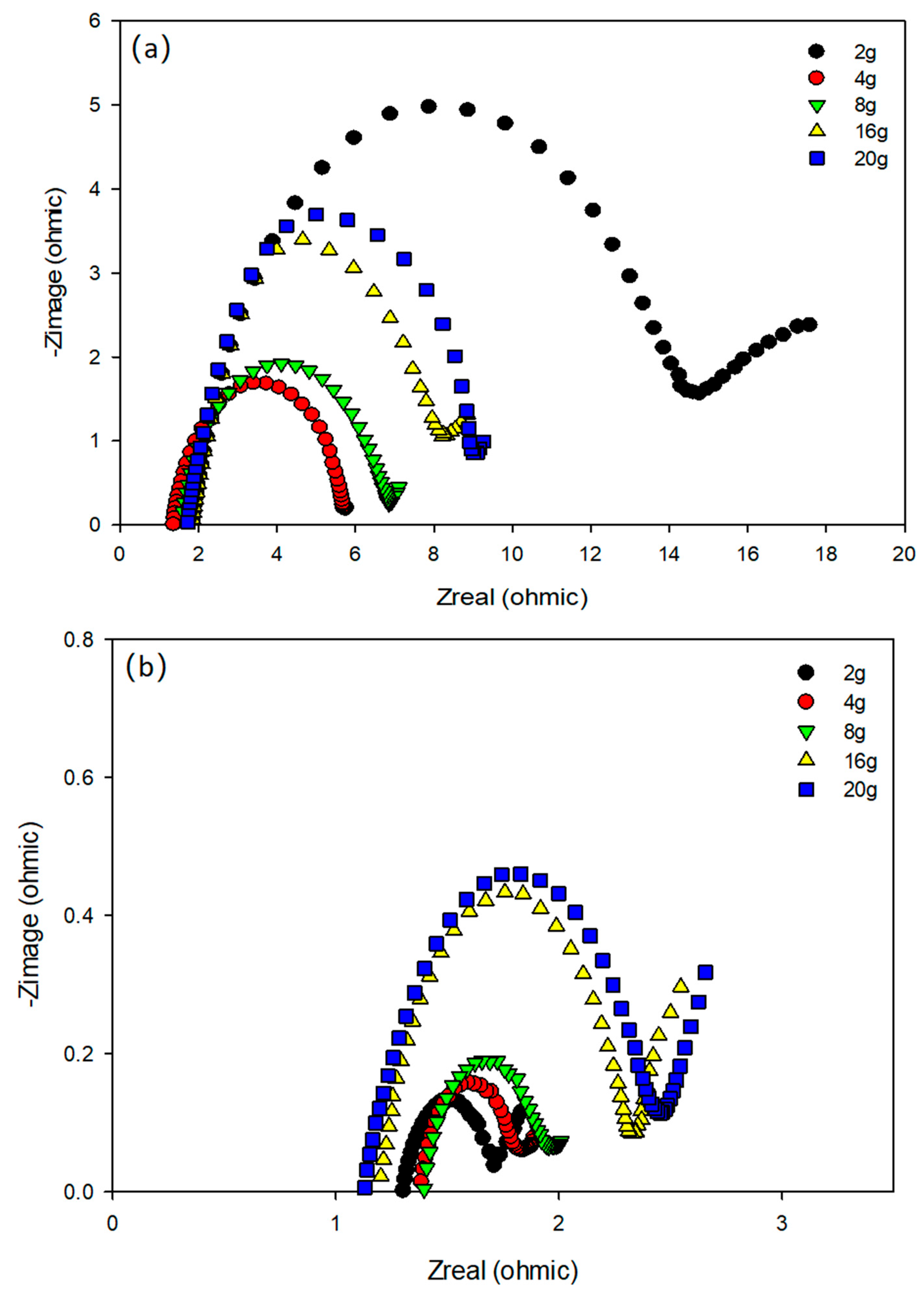
3.4. Electrochemical Characteristics of Electrodes under Different OLRs
3.5. Implications of Bioelectrochemistry for Brewery Wastewater Methanation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cieslik, M.; Dach, J.; Lewicki, A.; Smurzynska, A.; Janczak, D.; Pawlicka-Kaczorowska, J.; Boniecki, P.; Cyplik, P.; Czekala, W.; Jozwiakowski, K. Methane fermentation of the maize straw silage under meso- and thermophilic conditions. Energy 2016, 115, 1495–1502. [Google Scholar] [CrossRef]
- Chen, J.F.; Liu, Y.Y.; Liu, K.; Hu, L.J.; Yang, J.Q.; Wang, X.M.; Song, Z.L.; Yang, Y.W.; Tang, M.Z.; Wang, R.J. Bacterial community composition of internal circulation reactor at different heights for large-scale brewery wastewater treatment. Bioresour. Technol. 2021, 331, 125027. [Google Scholar] [CrossRef] [PubMed]
- Satyawali, Y.; Balakrishnan, M. Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: A review. J. Environ. Manag. 2008, 86, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chang, S.; Guo, Q.B.; Hong, Y.S.; Wu, P. Brewery wastewater treatment using an anaerobic membrane bioreactor. Biochem. Eng. J. 2016, 105, 321–331. [Google Scholar] [CrossRef]
- Wang, D.X.; Han, H.J.; Han, Y.X.; Li, K.; Zhu, H. Enhanced treatment of Fischer-Tropsch (F-T) wastewater using the up-flow anaerobic sludge blanket coupled with bioelectrochemical system: Effect of electric field. Bioresour. Technol. 2017, 232, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Clauwaert, P.; Aelterman, P.; Pham, T.H.; De Schamphelaire, L.; Carballa, M.; Rabaey, K.; Verstraete, W. Minimizing losses in bio-electrochemical systems: The road to applications. Appl. Microbiol. Biotechnol. 2008, 79, 901–913. [Google Scholar] [CrossRef]
- Wang, H.M.; Ren, Z.Y.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; van Mechelen, W.; Pratt, M.; Phys Activity Series, E. The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C.; Bae, B.U. Influence of applied voltage on the performance of bioelectrochemical anaerobic digestion of sewage sludge and planktonic microbial communities at ambient temperature. Bioresour. Technol. 2016, 220, 500–508. [Google Scholar] [CrossRef]
- Estrada-Arriaga, E.B.; Reynoso-Deloya, M.G.; Guillén-Garcés, R.A.; Falcón-Rojas, A.; García-Sánchez, L. Enhanced methane production and organic matter removal from tequila vinasses by anaerobic digestion assisted via bioelec-trochemical power-to-gas. Bioresour. Technol. 2021, 320, 124344. [Google Scholar] [CrossRef]
- Ino, K.; Onodera, T.; Fukuda, M.T.; Nashimoto, Y.; Shiku, H. Electrochemical imaging using redox mediators for cell activity of three-dimensional cultured cells. In Proceedings of the Conference on Advanced Environmental, Chemical, and Biological Sensing Technologies XV held at SPIE Defense + Commercial Sensing Conference, Baltimore, MD, USA, 14–16 April 2019. [Google Scholar]
- Kondaveeti, S.; Lee, J.; Kakarla, R.; Kim, H.S.; Min, B. Low-cost separators for enhanced power production and field application of microbial fuel cells (MFCs). Electrochim. Acta 2014, 132, 434–440. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, N.; Kim, S.; Jung, S. Dynamic behaviors of redox mediators within the hydrophobic layers as an important factor for effective microbial fuel cell operation. Bull. Korean Chem. Soc. 2003, 24, 437–440. [Google Scholar]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Y.; Hu, X.Y.; Chen, H.B.; Zhou, Y.Y.; Zhou, Y.F.; Wang, D.B. Advances in enhanced volatile fatty acid production from anaerobic fermentation of waste activated sludge. Sci. Total Environ. 2019, 694, 133741. [Google Scholar] [CrossRef]
- Tan, L.; Nishimura, H.; Wang, Y.F.; Sun, Z.Y.; Tang, Y.Q.; Kida, K.; Morimura, S. Effect of organic loading rate on thermophilic methane fermentation of stillage eluted from ethanol fermentation of waste paper and kitchen waste. J. Biosci. Bioeng. 2019, 127, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Gou, C.L.; Yang, Z.H.; Huang, J.; Wang, H.L.; Xu, H.Y.; Wang, L.K. Effects of temperature and organic loading rate on the performance and microbial community of anaerobic co-digestion of waste activated sludge and food waste. Chemosphere 2014, 105, 146–151. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Greses, S.; Gonzalez-Fernandez, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar] [CrossRef]
- Jiang, J.G.; Zhang, Y.J.; Li, K.M.; Wang, Q.; Gong, C.X.; Li, M.L. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, B.J.; Jeong, C.M.; Choi, J.D.R.; Ahn, Y.H.; Chang, H.N. Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour. Technol. 2008, 99, 7866–7874. [Google Scholar] [CrossRef]
- Dogan, E.; Demirer, G.N. Volatile Fatty Acid Production from Organic Fraction of Municipal Solid Waste Through Anaerobic Acidogenic Digestion. Environ. Eng. Sci. 2009, 26, 1443–1450. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Wu, H.R.; Weimer, P.J.; Dalke, R.; Granda, C.B.; Mai, J.S.; Urgun-Demirtas, M. Microbial communities for valorizing biomass using the carboxylate platform to produce volatile fatty acids: A review. Bioresour. Technol. 2022, 344, 126253. [Google Scholar] [CrossRef]
- Ferguson, R.M.W.; Coulon, F.; Villa, R. Organic loading rate: A promising microbial management tool in anaerobic digestion. Water Res. 2016, 100, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Fradinho, J.; Oehmen, A.; Reis, M.A.M. Photosynthetic mixed culture polyhydroxyalkanoate (PHA) production from individual and mixed volatile fatty acids (VFAs): Substrate preferences and co-substrate uptake. J. Biotechnol. 2014, 185, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Y.C.; Yoo, K.; Kuppanan, N.; Subudhi, S.; Lal, B. Bioelectrochemical enhancement of direct interspecies electron transfer in upflow anaerobic reactor with effluent recirculation for acidic distillery wastewater. Bioresour. Technol. 2017, 241, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Chan, D.S.; Lin, K.H.; Jow, J.J. A simple route to electrophoretic deposition of transition metal-coated nickel oxide films for electrochemical capacitors. Mater. Chem. Phys. 2011, 130, 1239–1245. [Google Scholar] [CrossRef]
- Alavi, B.; Aghajani, H.; Rasooli, A. Electrophoretic deposition of electroless nickel coated YSZ core-shell nanoparticles on a nickel based superalloy. J. Eur. Ceram. Soc. 2019, 39, 2526–2534. [Google Scholar] [CrossRef]
- Kazazi, M. Facile preparation of nanoflake-structured nickel oxide/carbon nanotube composite films by electrophoretic deposition as binder-free electrodes for high-performance pseudocapacitors. Curr. Appl. Phys. 2017, 17, 240–248. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C. Surface Modification of a Graphite Fiber Fabric Anode for Enhanced Bioelectrochemical Methane Production. Energy Fuels 2016, 30, 6467–6474. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Zhang, Y.B.; Wang, L.Y.; Quan, X. Potential for direct interspecies electron transfer in an electric-anaerobic system to increase methane production from sludge digestion. Sci. Rep. 2015, 5, 11094. [Google Scholar] [CrossRef]
- Pant, D.; Adholeya, A. Nitrogen Removal from Biomethanated Spentwash Using Hydroponic Treatment Followed by Fungal Decolorization. Environ. Eng. Sci. 2009, 26, 559–565. [Google Scholar] [CrossRef]
- Song, Y.C.; Kwon, S.J.; Woo, J.H. Mesophilic and thermophilic temperature co-phase anaerobic digestion compared with single-stage mesophilic- and thermophilic digestion of sewage sludge. Water Res. 2004, 38, 1653–1662. [Google Scholar] [CrossRef]
- Kato, S. Biotechnological Aspects of Microbial Extracellular Electron Transfer. Microbes Environ. 2015, 30, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhao, Q.C.; Wu, X.E.; Li, X.Z.; Li, Q.B.; Wang, Y.P. Interspecies electron transfer in syntrophic methanogenic consortia: From cultures to bioreactors. Renew. Sustain. Energy Rev. 2016, 54, 1358–1367. [Google Scholar] [CrossRef]
- Zhao, Z.S.; Zhang, Y.B.; Quan, X.; Zhao, H.M. Evaluation on direct interspecies electron transfer in anaerobic sludge digestion of microbial electrolysis cell. Bioresour. Technol. 2016, 200, 235–244. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Brewery Wastewater | Anaerobic Sludge |
|---|---|---|
| pH | 3.6 ± 0.2 | 6.93 |
| Alkalinity (mg/L as CaCO3) | - | 1052 |
| COD (g/L) | 34.8 ± 1.6 | 18.2 |
| TS (g/L) | 28.3 ± 1.8 | 14.3 |
| VS (g/L) | 22.9 ± 1.6 | 7.6 |
| Sulphate (g/L) | 1.5 ± 0.5 | - |
| OLR (COD/L.d) | 2 | 4 | 8 | 16 | 20 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reactors | BEAD | Control | BEAD | Control | BEAD | Control | BEAD | Control | BEAD | Control |
| pH | 7.58 ± 0.03 | 7.55 ± 0.04 | 7.41 ± 0.02 | 7.11 ± 0.04 | 7.42 ± 0.05 | 7.25 ± 0.03 | 7.34 ± 0.01 | 7.07 ± 0.02 | 7.27 ± 0.04 | 6.29 ± 0.22 |
| Alkalinity (mg/L as CaCO3) | 6374 ± 131 | 6084 ± 202 | 5933 ± 79 | 5104 ± 88 | 5366 ± 57 | 4536 ± 66 | 5241 ± 63 | 4339 ± 69 | 4867 ± 66 | 3325 ± 24 |
| VFAs (mg/L as COD) | 864 ± 102 | 931 ± 152 | 719 ± 52 | 1627 ± 135 | 334 ± 57 | 3524 ± 256 | 703 ± 72 | 4357 ± 539 | 831 ± 64 | 5690 ± 340 |
| VFA/alkalinity | 0.14 | 0.15 | 0.12 | 0.32 | 0.06 | 0.78 | 0.13 | 1.00 | 0.17 | 1.71 |
| COD removal rate (%) | 94.0 ± 0.4 | 92.4 ± 0.4 | 96.0 ± 0.4 | 88.3 ± 0.3 | 96.5 ± 0.1 | 87.9 ± 0.2 | 91.3 ± 0.2 | 84.7 ± 0.4 | 86.3 ± 0.4 | 54.8 ± 0.6 |
| Sulphate removal rate (%) | 87.5 ± 1.8 | 59.1 ± 3.1 | 91.5 ± 0.8 | 55.7 ± 5.1 | 91.3 ± 1.6 | 64.1 ± 5.2 | 88.3 ± 2.6 | 57.2 ± 2.0 | 84.9 ± 1.5 | 48.2 ± 5.7 |
| Specific methane production rate (L/L.d) | 0.48 ± 0.01 | 0.43 ± 0.01 | 1.54 ± 0.02 | 1.00 ± 0.06 | 3.43 ± 0.06 | 2.18 ± 0.02 | 5.18 ± 0.05 | 3.53 ± 0.05 | 5.64 ± 0.09 | 2.04 ± 0.01 |
| Methane content (%) | 86.3 ± 1.3 | 77.1 ± 0.4 | 78.0 ± 0.9 | 56.0 ± 1.8 | 82.0 ± 0.5 | 68.2 ± 0.8 | 80.0 ± 0.5 | 64.8 ± 0.5 | 78.2 ± 1.6 | 55.9 ± 1.8 |
| Methane yield (mL CH4/g COD) | 240 ± 8 | 217 ± 4 | 385 ± 4 | 250 ± 15 | 400 ± 7 | 272 ± 3 | 343 ± 4 | 221 ± 3 | 352 ± 6 | 127 ± 10 |
| Anode | Cathode | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ORL (COD/L.d) | 2 | 4 | 8 | 16 | 20 | 2 | 4 | 8 | 16 | 20 |
| Rs (ohm) | 1.37 | 1.52 | 1.36 | 1.85 | 1.73 | 1.42 | 1.45 | 1.31 | 1.19 | 1.13 |
| Rct (ohm) | 12.54 | 4.33 | 5.88 | 7.43 | 7.98 | 2.23 | 1.54 | 1.65 | 2.24 | 2.35 |
| C (uF) | 548 | 205 | 258 | 198 | 342 | 544 | 853 | 2631 | 2115 | 1524 |
| W (1/ohm sqrt(Hz)) | 5.67 | 1.44 | 0.985 | 8.01 | 4.85 | 4.54 | 8.76 | 9.36 | 20.16 | 15.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Feng, Q.; Zhao, Y.; Li, X.; Zi, H. Influence of Organic Loading Rate on Methane Production from Brewery Wastewater in Bioelectrochemical Anaerobic Digestion. Fermentation 2023, 9, 932. https://doi.org/10.3390/fermentation9110932
Pan H, Feng Q, Zhao Y, Li X, Zi H. Influence of Organic Loading Rate on Methane Production from Brewery Wastewater in Bioelectrochemical Anaerobic Digestion. Fermentation. 2023; 9(11):932. https://doi.org/10.3390/fermentation9110932
Chicago/Turabian StylePan, Hongda, Qing Feng, Yong Zhao, Xiaoxiang Li, and Hao Zi. 2023. "Influence of Organic Loading Rate on Methane Production from Brewery Wastewater in Bioelectrochemical Anaerobic Digestion" Fermentation 9, no. 11: 932. https://doi.org/10.3390/fermentation9110932







