The Microbial Communities of Anaerobic Respiration and Fermentation Degrading Chitin Exist in the Anaerobic Sludge of Microbial Fuel Cell Anodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Suspended Chitin
2.2. Experimental Operation
2.3. Chemical Analyses
2.4. Scanning Electron Microscope Observation
2.5. High-Throughput Sequencing
2.6. Data Analysis
3. Results and Discussion
3.1. Chitin Degradation in the Anaerobic Respiration and Fermentation Systems
3.2. Electrical Properties of Chitin Degradation in the MFC
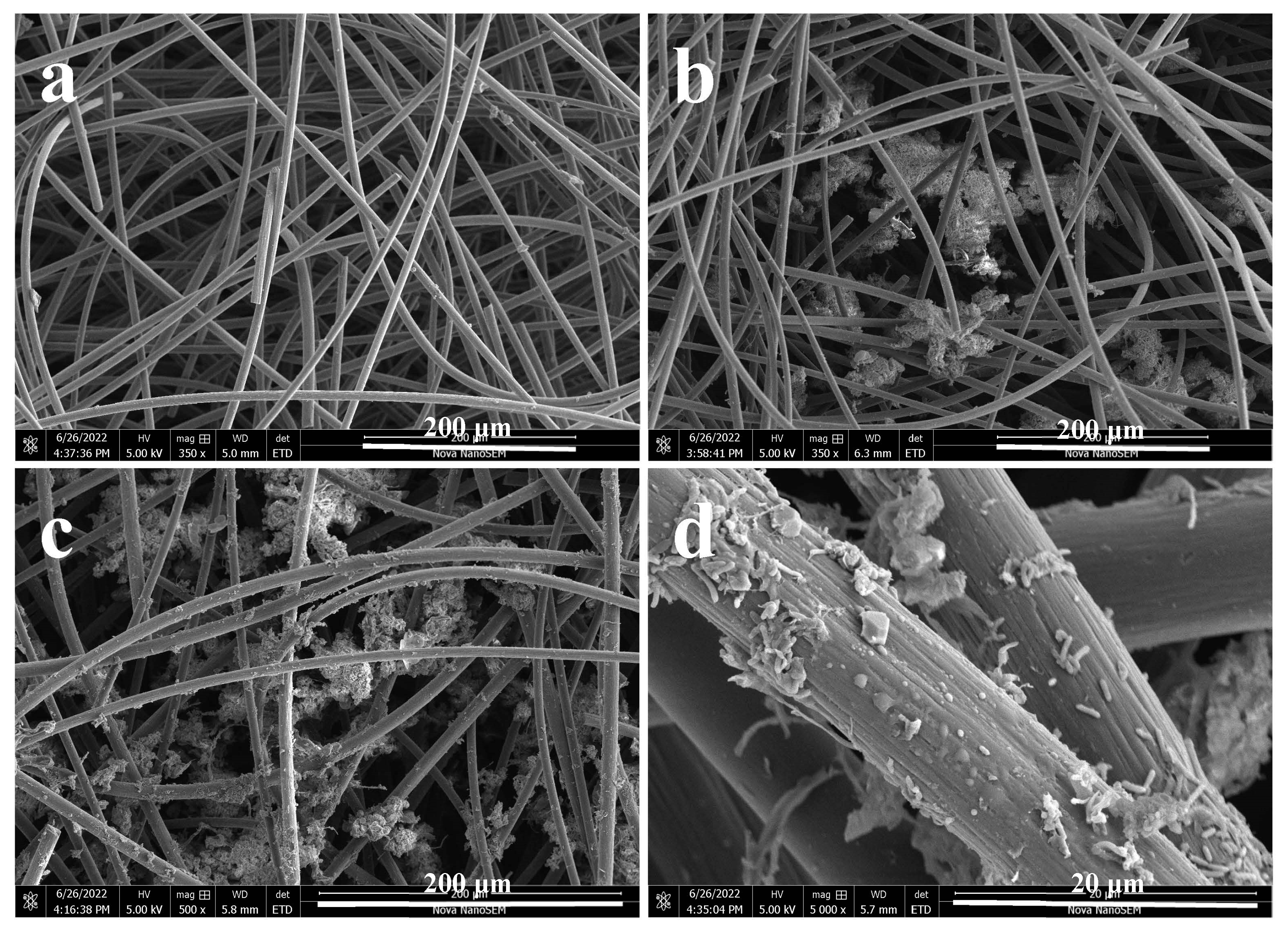
3.3. Microbial Morphology of the Anode-Attached Sludge
3.4. Microbial Community of Anode Sludge in the Anaerobic Respiration and Fermentation Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boulaiche, W.; Hamdi, B.; Trari, M. Removal of heavy metals by chitin: Equilibrium, kinetic and thermodynamic studies. Appl. Water Sci. 2019, 9, 39. [Google Scholar] [CrossRef]
- Sherin, P.; Nathalie, L.; Deepu, G.; Hanna, J.M.; Ange, N.; Sabu, T. Chitin and chitosan based composites for energy and environmental applications: A review. Waste Biomass Valori. 2020, 12, 4777–4804. [Google Scholar]
- Lv, J.; Lv, X.; Ma, M.; Oh, D.-H.; Jiang, Z.; Fu, X. Chitin and chitin-based biomaterials: A review of advances in processing and food applications. Carbohyd. Polym. 2023, 299, 120142. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.N. Aerobic and anaerobic degradation and mineralization of 14C-chitin by water column and sediment inocula of the York River estuary, Virginia. Appl. Environ. Microb. 1994, 60, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Biologically extracting energy from wastewater biohydrogen production and microbial fuel cells. Environ. Sci. Technol. 2004, 38, 159–167. [Google Scholar]
- Zhang, E.R.; Xu, W.; Diao, G.W.; Shuang, C.D. Electricity generation from acetate and glucose by sedimentary bacterium attached to electrode in microbial-anode fuel cells. J. Power Sources 2006, 161, 820–825. [Google Scholar] [CrossRef]
- Herrero-Hernandez, E.; Smith, T.J.; Akid, R. Electricity generation from wastewaters with starch as carbon source using a mediatorless microbial fuel cell. Biosens. Bioelectron. 2013, 39, 194–198. [Google Scholar] [CrossRef]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef]
- Rezaei, F.; Richard, T.L.; Logan, B.E. Analysis of chitin particle size on maximum power generation, power longevity, and coulombic efficiency in solid-substrate microbial fuel cells. J. Power Sources 2009, 192, 304–309. [Google Scholar] [CrossRef]
- Li, S.W.; Zeng, R.J.; Sheng, G.P. An excellent anaerobic respiration mode for chitin degradation by Shewanella oneidensis MR-1 in microbial fuel cells. Biochem. Eng. J. 2017, 118, 20–24. [Google Scholar] [CrossRef]
- Li, S.W.; He, H.; Zeng, R.J.; Sheng, G.P. Chitin degradation and electricity generation by Aeromonas hydrophila in microbial fuel cells. Chemosphere 2017, 168, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Bhatia, S.K.; Moon, Y.M.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Song, H.S.; Jeon, J.M.; Yoon, J.J.; Kim, Y.G.; et al. One-pot exploitation of chitin biomass for simultaneous production of electricity, n-acetylglucosamine and polyhydroxyalkanoates in microbial fuel cell using novel marine bacterium Arenibacter palladensis YHY2. J. Clean. Prod. 2019, 209, 324–332. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.-L.; Yang, S.-F.; Tay, J.-H. Mechanisms and models for anaerobic granulation in upflow anaerobic sludge blanket reactor. Water Res. 2003, 37, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, J.-T.; Yuan, X.-Z.; Guo, R.-B.; Qiu, Y.-L. Hydrogenispora ethanolica gen. nov., sp. nov., an anaerobic carbohydrate-fermenting bacterium from anaerobic sludge. Int. J. Syst. Evol. Micr 2014, 64 Pt 5, 1756–1762. [Google Scholar] [CrossRef]
- Kotay, S.M.; Das, D. Microbial hydrogen production with Bacillus coagulans IIT-BT S1 isolated from anaerobic sewage sludge. Bioresource Technol. 2007, 98, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Bond, D.R.; O’Neil, R.A.; Reimers, C.E.; Tender, L.R.; Lovley, D.R. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol. 2004, 48, 178–190. [Google Scholar] [CrossRef]
- Logan, B.E.; Murano, C.; Scott, K.; Gray, N.D.; Head, I.M. Electricity generation from cysteine in a microbial fuel cell. Water Res. 2005, 39, 942–952. [Google Scholar] [CrossRef]
- Veeresh, G.S.; Kumar, P.; Mehrotra, I. Treatment of phenol and cresols in upflow anaerobic sludge blanket (UASB) process: A review. Water Res. 2005, 39, 154–170. [Google Scholar] [CrossRef]
- Lu, Y.; Lai, Q.; Zhang, C.; Zhao, H.; Ma, K.; Zhao, X.; Chen, H.; Liu, D.; Xing, X.H. Characteristics of hydrogen and methane production from cornstalks by an augmented two- or three-stage anaerobic fermentation process. Bioresour. Technol. 2009, 100, 2889–2895. [Google Scholar] [CrossRef]
- Gao, Q.; Li, S.W.; Xie, Y.J.; Zheng, M.X.; Wei, J.; Luo, Z.J.; Zhou, X.T.; Liu, Z.G.; Li, Y.; Wu, Z.R. Rapid cultivation of anammox sludge based on Ca-alginate cell beads. Water Sci. Technol. 2022, 85, 2899–2911. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, L.; Wang, X.; Yang, Y.; Wang, Z.; Li, J. Macroscale and microscale analysis of anammox in anaerobic rotating biological contactor. J. Environ. Sci. 2011, 23, 1679–1683. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R.; Law, Y.; Cheng, K.Y. Ammonium as a sustainable proton shuttle in bioelectrochemical systems. Bioresour. Technol. 2011, 102, 9691–9696. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.S.; Abd-Alla, M.H.; Abdul-Raouf, U.M. Characterization of anodic biofilm bacterial communities and performance evaluation of a mediator-free microbial fuel cell. Environ. Eng. Res. 2020, 25, 862–870. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Richard, T.L.; Brennan, R.A.; Logan, B.E. Substrate-enhanced microbial fuel cells for improved remote power generation from sediment-based systems. Environ. Sci. Technol. 2007, 41, 4053–4058. [Google Scholar] [CrossRef]
- Ramasamy, R.P.; Ren, Z.; Mench, M.M.; Regan, J.M. Impact of initial biofilm growth on the anode impedance of microbial fuel cells. Biotechnol. Bioeng. 2008, 101, 101–108. [Google Scholar] [CrossRef]
- Srivastava, P.; Abbassi, R.; Yadav, A.K.; Garaniya, V.; Lewis, T.; Zhao, Y.; Aminabhavi, T. Interrelation between sulphur and conductive materials and its impact on ammonium and organic pollutants removal in electroactive wetlands. J. Hazard. Mater. 2021, 419, 126417. [Google Scholar] [CrossRef]
- Ramanaiah, S.V.; Cordas, C.M.; Matias, S.C.; Reddy, M.V.; Leitão, J.H.; Fonseca, L.P. Bioelectricity generation using long-term operated biocathode: RFLP based microbial diversity analysis. Biotechnol. Rep. 2021, 32, e00693. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Wang, H.Y.; Dong, W.Y.; Chang, Y.; Yan, G.K.; Chu, Z.S.; Ling, Y.; Wang, Z.M.; Fan, T.F.; Li, C.Y. Nitrogen removal and microbial community for the treatment of rural domestic sewage with low C/N ratio by A/O biofilter with Arundo donax as carbon source and filter media. J. Water Process Eng. 2020, 37, 101509. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, R.; Du, C.; Dong, S.; Sun, J. Effects of continuous sulfamonomethoxine shock on the power generation performance and microbial community structure of MFCs under seasonal temperature variation. Biochem. Eng. J. 2021, 167, 107909. [Google Scholar] [CrossRef]
- Hug, L.A.; Castelle, C.J.; Wrighton, K.C.; Thomas, B.C.; Sharon, I.; Frischkorn, K.R.; Williams, K.H.; Tringe, S.G.; Banfield, J.F. Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 2013, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, J.A. The planctomycetes emerging models for microbial ecology, evolution and cell biology. Microbiology 1995, 141, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Jing, Z.; Tao, Z.; Luo, H.; Zuo, S.; Li, Y.-Y. Efficient nitrogen removal in microbial fuel cell—Constructed wetland with corncobs addition for secondary effluent treatment. J. Clean. Prod. 2022, 332, 130108. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Chen, P.; Sathishkumar, K.; Lu, Y.; Naraginti, S.; Wu, Y.; Wu, H. Biological mediated synthesis of reduced graphene oxide (rGO) as a potential electron shuttle for facilitated biological denitrification: Insight into the electron transfer process. J. Environ. Chem. Eng. 2022, 10, 108225. [Google Scholar] [CrossRef]
- Pan, J.-J.; Tan, L.-Y.; Fan, Q.-Q.; Cao, X.-Y.; Huang, J.; Gu, Y.-K.; Chen, T.-M. Effect of different carbon sources on sulfate reduction and microbial community structure in bioelectrochemical systems. Environ. Sci. Pollut. R. 2023, 30, 18312–18324. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, C.F.; Huang, J.X.; Wang, L.M.; Pang, Q.Q.; Peng, F.Q.; Hou, J.; Ni, L.X.; He, F.; Xu, B. The effect of sulfamethoxazole on nitrogen removal and electricity generation in a tidal flow constructed wetland coupled with a microbial fuel cell system: Microbial response. Chem. Eng. J. 2022, 431, 134070. [Google Scholar] [CrossRef]
- Yu, Y.; Ali, J.; Yang, Y.; Kuang, P.; Zhang, W.; Lu, Y.; Li, Y. Synchronous Cr(VI) remediation and energy production using microbial fuel cell from a subsurface environment: A review. Energies 2022, 15, 1989. [Google Scholar] [CrossRef]
- Franco, A.; Elbahnasy, M.; Rosenbaum, M.A. Screening of natural phenazine producers for electroactivity in bioelectrochemical systems. Microb. Biotechnol. 2023, 16, 579–594. [Google Scholar] [CrossRef]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 2006, 72, 7345–7348. [Google Scholar] [CrossRef]
- Yamada, T.; Sekiguchi, Y. Cultivation of uncultured Chloroflexi subphyla: Significance and ecophysiology of formerly uncultured Chloroflexi ‘subphylum i’ with natural and biotechnological relevance. Microbes Environ. 2009, 24, 205–216. [Google Scholar] [CrossRef]






| Metabolic Mode | Experimental Group | Generated TOC Concentration (mg/L) | vchitin (C-mg/L·d−1) |
|---|---|---|---|
| Anaerobic respiration | Closed-circuit MFC | 63.93 ± 8.60 | 7.10 ± 0.96 |
| Fermentation | Open-circuit MFC | 61.19 ± 4.24 | 6.96 ± 0.23 |
| Serum bottle | 62.60 ± 2.92 | 6.96 ± 0.32 | |
| Abiotic group | Sludge-free MFC | 0.47± 0.72 | 0.05 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhen, S.-H.; Yu, Y.-Y.; Xie, R.-R.; Xu, W.; Li, S.-W. The Microbial Communities of Anaerobic Respiration and Fermentation Degrading Chitin Exist in the Anaerobic Sludge of Microbial Fuel Cell Anodes. Fermentation 2023, 9, 983. https://doi.org/10.3390/fermentation9110983
Zhen S-H, Yu Y-Y, Xie R-R, Xu W, Li S-W. The Microbial Communities of Anaerobic Respiration and Fermentation Degrading Chitin Exist in the Anaerobic Sludge of Microbial Fuel Cell Anodes. Fermentation. 2023; 9(11):983. https://doi.org/10.3390/fermentation9110983
Chicago/Turabian StyleZhen, Sheng-Hu, Yang-Yang Yu, Rong-Rong Xie, Wei Xu, and Shan-Wei Li. 2023. "The Microbial Communities of Anaerobic Respiration and Fermentation Degrading Chitin Exist in the Anaerobic Sludge of Microbial Fuel Cell Anodes" Fermentation 9, no. 11: 983. https://doi.org/10.3390/fermentation9110983
APA StyleZhen, S.-H., Yu, Y.-Y., Xie, R.-R., Xu, W., & Li, S.-W. (2023). The Microbial Communities of Anaerobic Respiration and Fermentation Degrading Chitin Exist in the Anaerobic Sludge of Microbial Fuel Cell Anodes. Fermentation, 9(11), 983. https://doi.org/10.3390/fermentation9110983






