Intensification of Waste Valorization Techniques for Biogas Production on the Example of Clarias gariepinus Droppings
Abstract
:1. Introduction
- -
- To achieve the aim of the study, the following tasks were performed:
- -
- An investigation of the dynamics of hydrochemical parameters in a series of experiments conducted on the intensification of the anaerobic digestion of clariid catfish droppings;
- -
- A comparative analysis of the effect of different types of treatment on biogas yield and quality in the digestion of clariid catfish droppings;
- -
- The modeling of the species composition of a consortium of methanogenic microorganisms;
- -
- The formalization of the electro-fermentation system in aquaculture technology.
2. Materials and Methods
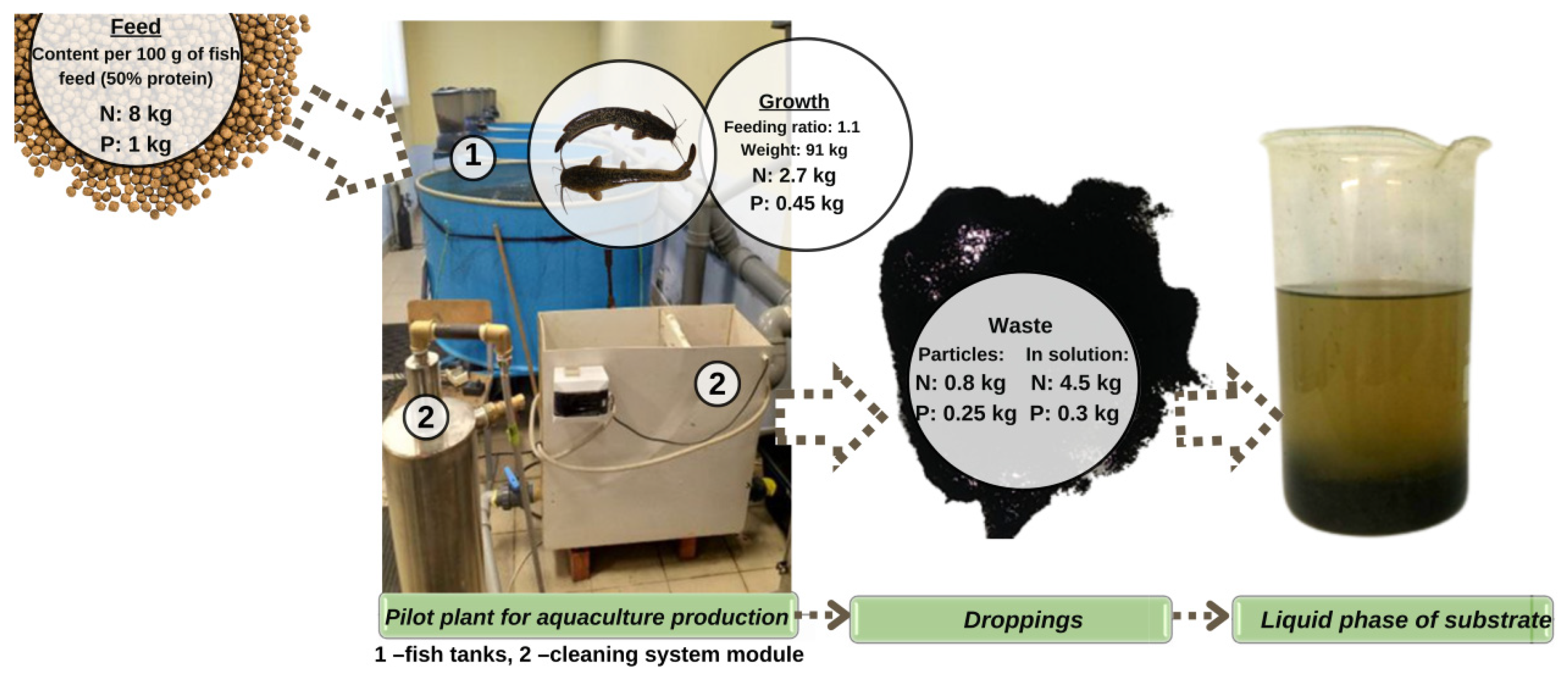
2.1. Characteristics of the Substrate Used—Excreta of the Clariid Catfish
2.2. Characteristics of Stimulant Supplements
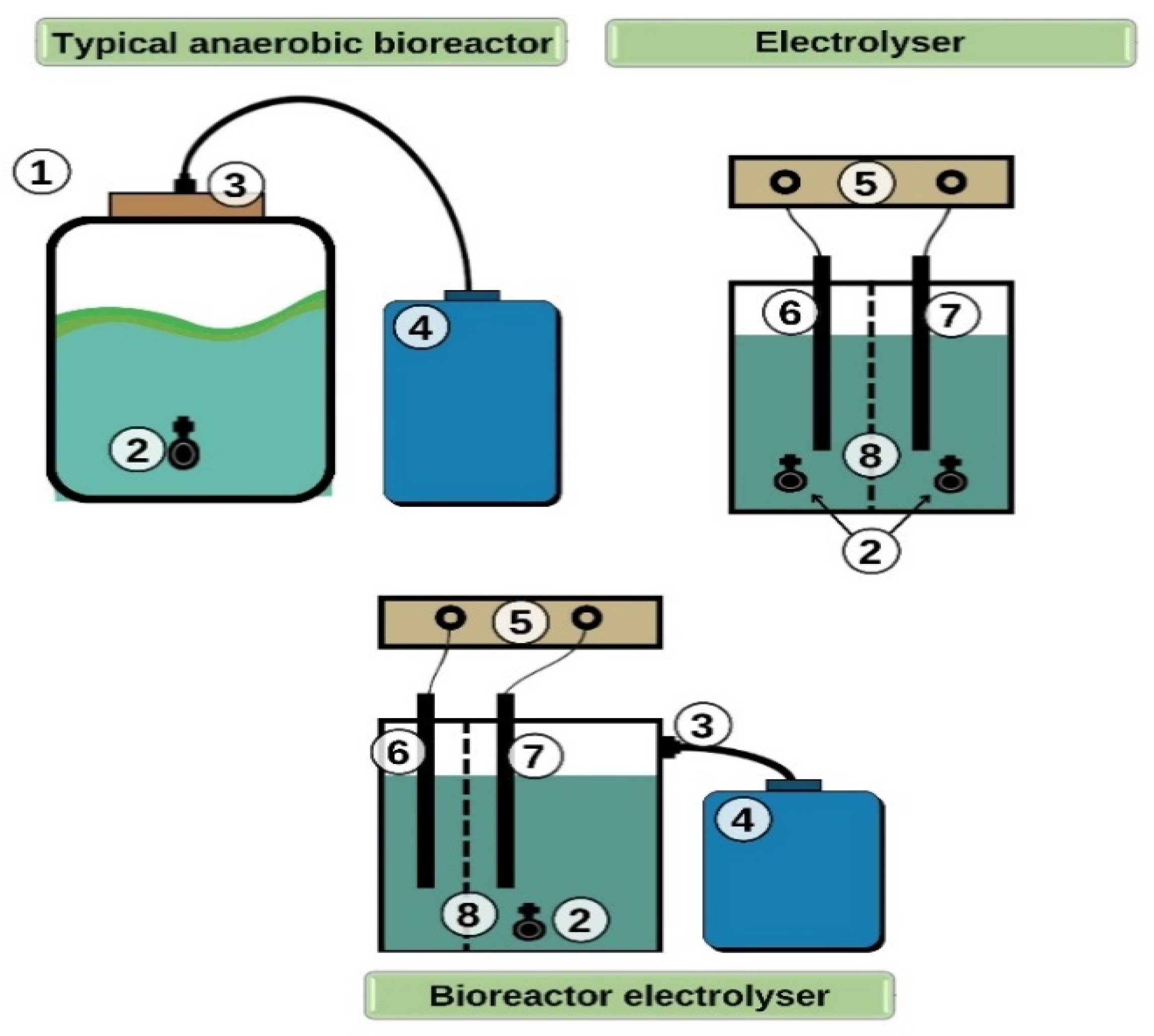
2.3. Lab Benches Used for Biogas Production Stimulation
- Substrate digestion with stimulating additives: zeolite and humic fertilizer;
- Pretreatment of the substrate by electrolysis;
- Treatment of the substrate by electric current during anaerobic digestion every day (electro-fermentation);
- Anaerobic digestion of substrate without treatment and stimulating additives (control).
2.4. Methods
2.4.1. Microbiological Studies
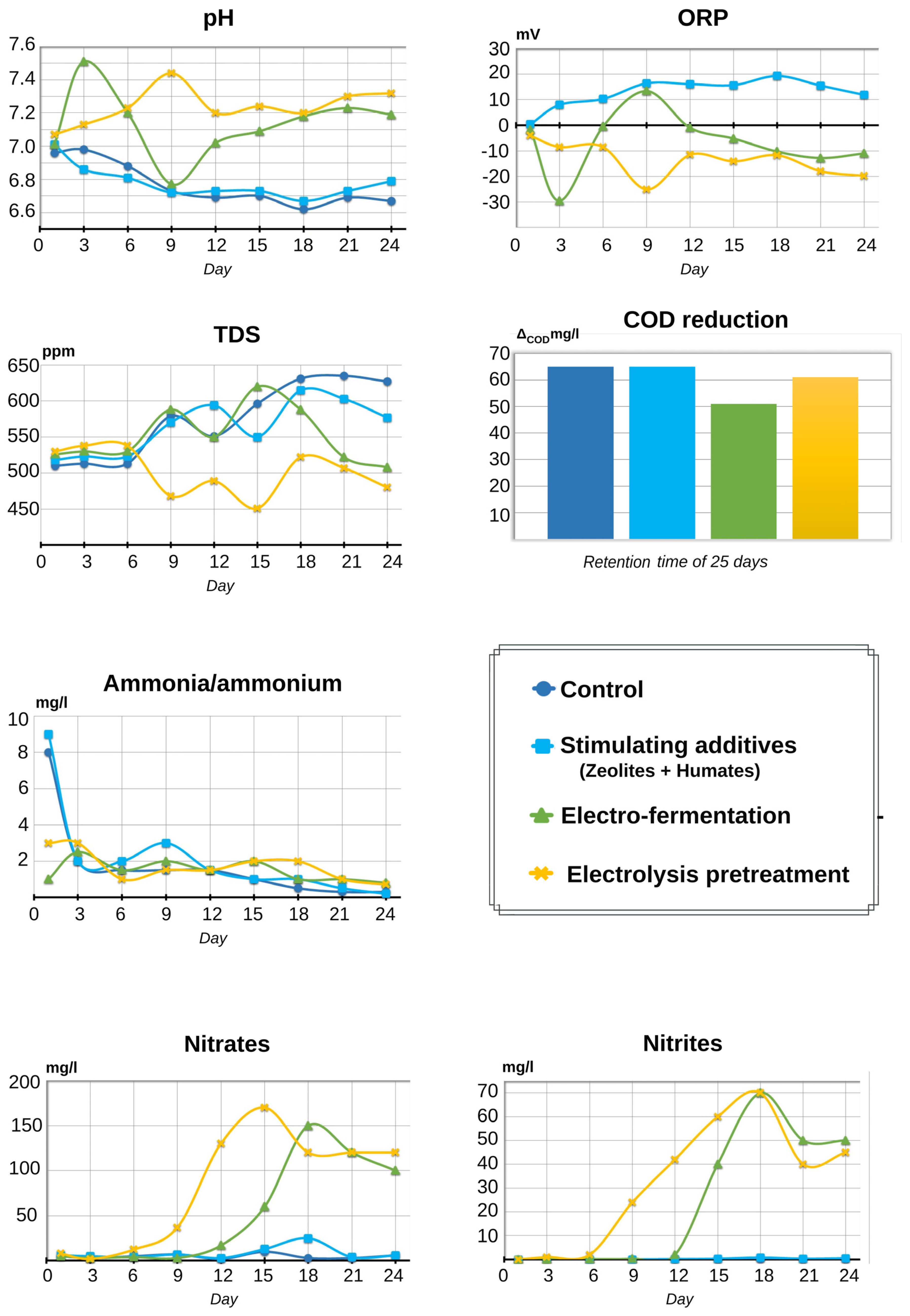
2.4.2. Control of Parameters during the Anaerobic Digestion Process
- -
- Nitrate level (NO3–);
- -
- Ammonia–ammonium level (NH3/NH4+);
- -
- Nitrite level (NO2–);
- -
- Solution salinity (TDS);
- -
- Redox potential (ORP);
- -
- Hydrogen potential (pH).
3. Results and Discussions
3.1. Comparison of Changes Occurring in Control Parameters in a Series of Experiments Conducted on the Intensification of the Anaerobic Digestion of Clarionic Catfish Excreta
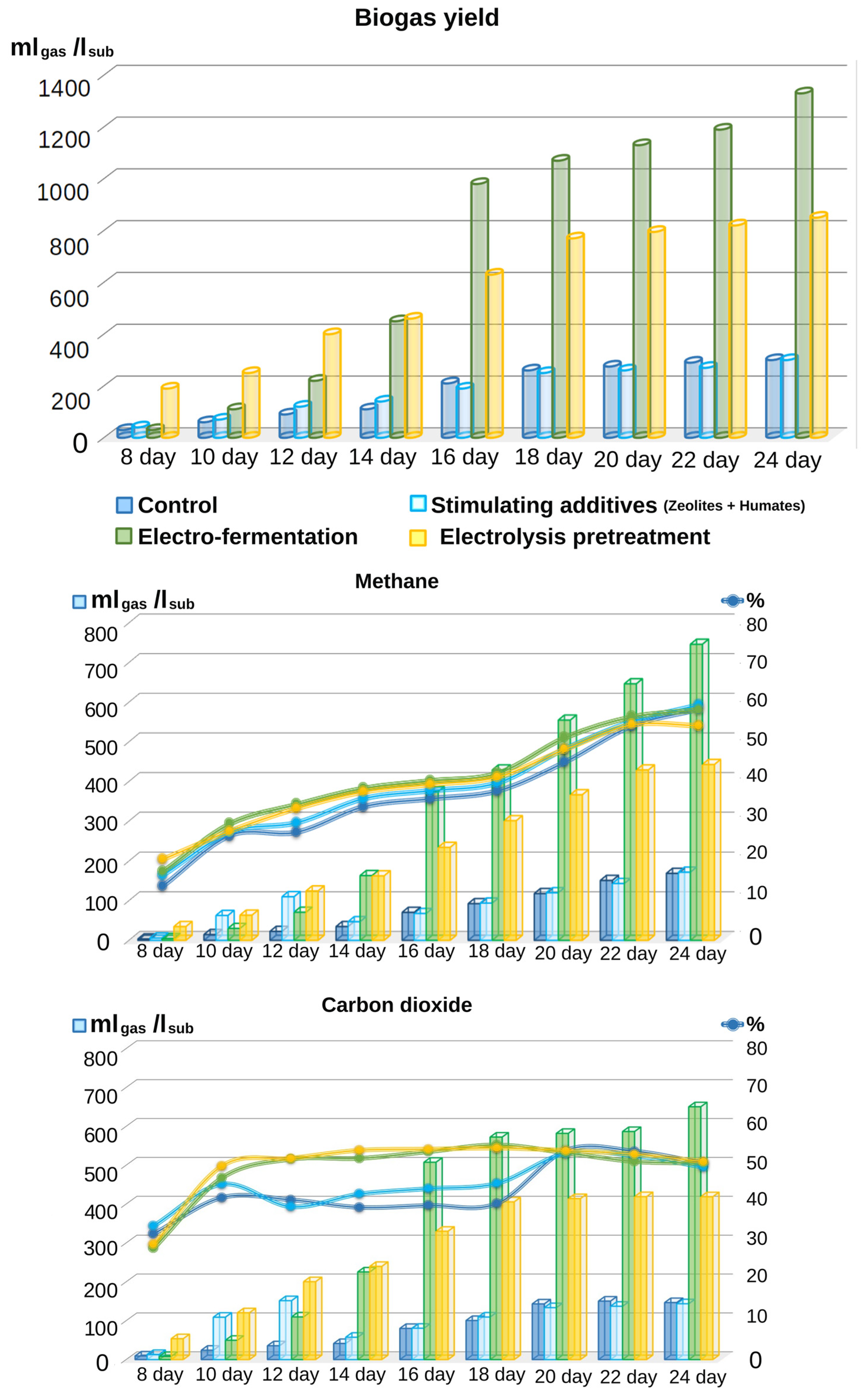
3.2. Comparative Analysis of the Effect of Different Types of Treatment on Biogas Yield and Its Quality
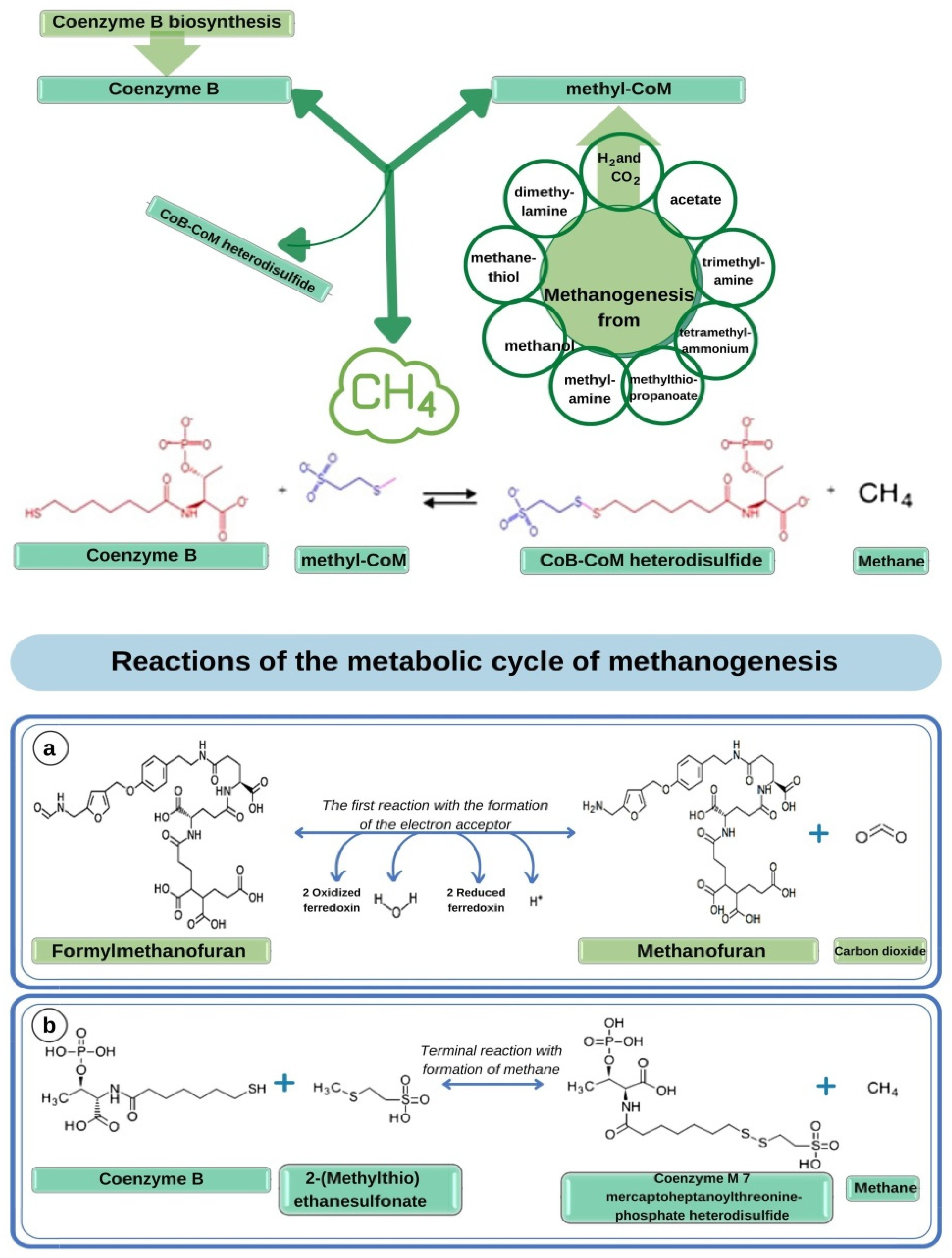
3.3. Microscopy and Modeling of the Species Composition of the Methanogenic Microorganisms Consortium
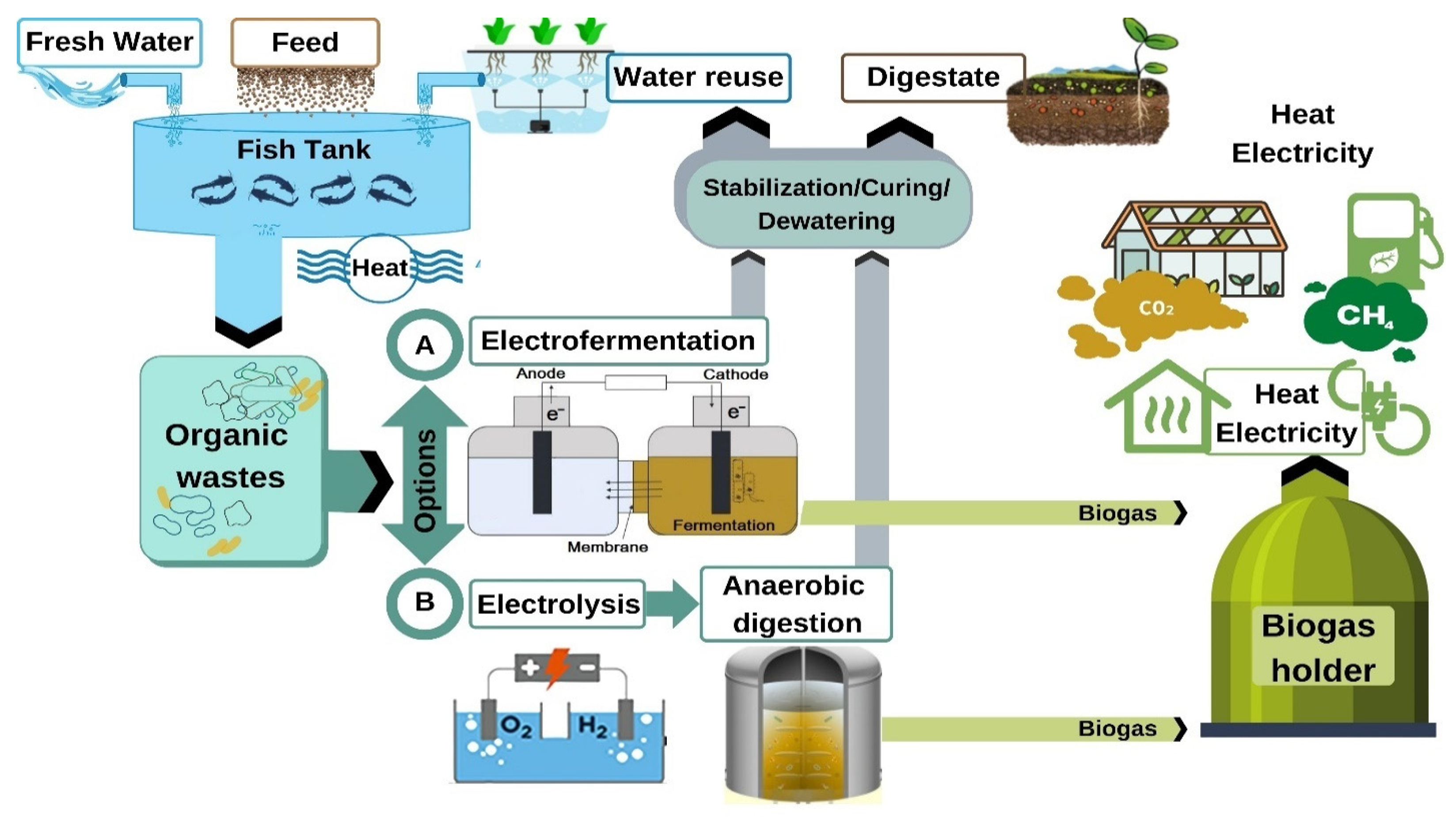
3.4. Formalization of the Electro-Fermentation System in Aquaculture Technology
- -
- In a closed water supply system designed for the cultivation of clariid catfish, the following materials are supplied: prepared water (feed), combined feed, and heat to maintain the required temperature of the water solution;
- -
- The catfish consume and digest food, and release metabolic waste products into the water. These include proteins, peptides, and nitrogen compounds harmful to the fish, for which protein molecules from the feces can be broken down;
- -
- Aquaculture waste is removed from the fish tank using filtering elements and extracted for further processing;
- -
- The separated droppings are sent to an anaerobic bioreactor, integrating a substrate electrolysis process into its structure. Furthermore, during the digestion cycle, the waste is treated daily with a constant electric current (electro-fermentation process);
- -
- During the process of anaerobic conversion, biogas is released as a by-product of methanogenic microorganisms. The gas is drained from the reactor into a gas holder. Methane contained in this gas mixture is an energy carrier and can be used to generate heat and electricity;
- -
- Carbon dioxide (CO2) can be accumulated in liquefied form, and used to feed plants in greenhouses as one of the possible applications;
- -
- Water obtained after dewatering the sludge can be sent back to the fish farm by passing through a hydroponic system, and the dry residue rich in mineral salts and available forms of nitrogen can be used as fertilizer.
- -
- Until the organic substrate is obtained from the fish farm, the algorithm of action is similar to that described for the scheme presented in Figure 7 (option A);
- -
- After the droppings are removed from the filter, they are sent to the electrolyzer, where they are treated with a direct electric current;
- -
- Following the electrolysis treatment, the substrate is sent to the classical anaerobic bioreactor;
- -
- The subsequent algorithm obtained from the end of the digestion period is the same as in option A.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markovcev, V.G. Improvement of biotechnologies of nitrogen and phosphorus removal from municipal wastewater [Sovershenstvovanie biotekhnologij udaleniya azota i fosfora iz gorodskih stochnyh vod]. Izv. TINRO 2016, 152, 289–299. [Google Scholar]
- Yogev, U.; Vogler, M.; Nir, O.; Londong, J.; Gross, A. Phosphorous recovery from a novel recirculating aquaculture system followed by its sustainable reuse as a fertilizer. Sci. Total Environ. 2020, 722, 137949. [Google Scholar] [CrossRef]
- Adegbola, I.P.; Aborisade, B.A.; Adetutu, A. Health Risk Assessment and Heavy Metal Accumulation in Fish Species (Clarias Gariepinus and Sarotherodon Melanotheron) from Industrially Polluted Ogun and Eleyele Rivers, Nigeria. Toxicol. Rep. 2021, 8, 1445–1460. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, A.A.; Akegbejo-Samsons, Y.; Fawole, F.J.; Olatunji, P.O.; Muller, N.; Wan, A.H.L.; Davies, S.J. From Waste to Feed: Dietary Utilisation of Bacterial Protein from Fermentation of Agricultural Wastes in African Catfish (Clarias Gariepinus) Production and Health. Aquaculture 2021, 531, 735850. [Google Scholar] [CrossRef]
- Gicana, R.G.; Yeh, F.-I.; Hsiao, T.-H.; Chiang, Y.-R.; Yan, J.-S.; Wang, P.-H. Valorization of Fish Waste and Sugarcane Bagasse for Alcalase Production by Bacillus Megaterium via a Circular Bioeconomy Model. J. Taiwan Inst. Chem. Eng. 2022, 135, 104358. [Google Scholar] [CrossRef]
- Maksimov, A.S.; Ilarionov, S.A.; Dyogtev, M.I. Current state and prospects for the development of biogas technologies [Sovremennoe sostoyanie i perspektivy razvitiya biogazovyh tekhnologij]. Perm Univ. Her. 2012, 1, 76–85. [Google Scholar]
- Hisamova, A.I.; Yugina, N.A.; Mihajlova, E.O. Analysis of the effect of biologically active substances on the growth of anaerobic microorganisms of activated sludge [Analiz vliyaniya biologicheski aktivnyh veshchestv na rost anaerobnyh mikroorganizmov aktivnogo ila]. Bull. Kazan Univ. Technol. 2013, 10, 201–203. [Google Scholar]
- Mihajlenko, V.V.; Kapustin, A.E. Evaluating the effectiveness of wastewater treatment by anaerobic digestion [Ocenka effektivnosti ochistki stochnyh vod metodom anaerobnogo sbrazhivaniya]. Technol Audit. Prod Reserves 2016, 3, 72–76. [Google Scholar]
- Balintova, M.; Kovacova, Z.; Demcak, S.; Chernysh, Y.; Junakova, N. Comparison of Sorption Efficiency of Natural and MnO 2 Coated Zeolite for Copper Removal from Model Solutions. IOP Conf. Ser. Earth Environ. Sci. 2021, 900, 012003. [Google Scholar] [CrossRef]
- Foysal, M.J.; Nguyen, T.T.T.; Sialumano, M.; Phiri, S.; Chaklader, M.R.; Fotedar, R.; Gagnon, M.M.; Tay, A. Zeolite Mediated Processing of Nitrogenous Waste in the Rearing Environment Influences Gut and Sediment Microbial Community in Freshwater Crayfish (Cherax Cainii) Culture. Chemosphere 2022, 298, 134276. [Google Scholar] [CrossRef]
- Holub, M.; Balintova, M.; Pavlikova, P.; Palascakova, L. Study of Sorption Properties of Zeolite in Acidic Conditions in Dependence on Particle Size. Chem. Eng. Trans. 2013, 32, 559–564. [Google Scholar] [CrossRef]
- Montalvo, S.; Guerrero, L.; Borja, R.; Sánchez, E.; Milán, Z.; Cortés, I.; Angeles de la la Rubia, M. Application of Natural Zeolites in Anaerobic Digestion Processes: A Review. Appl. Clay Sci. 2012, 58, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Palominos, N.; Castillo, A.; Guerrero, L.; Borja, R.; Huiliñir, C. Coupling of Anaerobic Digestion and Struvite Precipitation in the Same Reactor: Effect of Zeolite and Bischofite as Mg2+ Source. Front. Environ. Sci. 2021, 9. [Google Scholar] [CrossRef]
- Lisova, Y.A.; Koroleva, E.A. Comparison of the effectiveness of loading materials used in filters in the final stages of water treatment [Sravnenie effektivnosti zagruzochnyh materialov, primenyaemyh v fil’trah na zaklyuchitel’nyh stadiyah ochistki vody]. J. Plumb. Heat. Cond. Energy Effic. 2020, 12, 32–35. [Google Scholar]
- Rush, E.A.; Obuzdina, M.V. Development and study of modified adsorbents based on zeolites from Eastern Transbaikalia for industrial wastewater treatment in locomotive depots [Sozdaniye i issledovaniye modifitsirovannykh adsorbentov na osnove tseolitov Vostochnogo Zabaykalʹya dlya ochistki promyshlennykh stochnykh vod v lokomotivnykh depo]. J. Transsib Railw. Stud. 2013, 1, 27–34. [Google Scholar]
- Paritosh, K.; Yadav, M.; Chawade, A.; Sahoo, D.; Kesharwani, N.; Pareek, N.; Vivekanand, V. Additives as a Support Structure for Specific Biochemical Activity Boosts in Anaerobic Digestion: A Review. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Schievano, A.; Goglio, A.; Erckert, C.; Marzorati, S.; Rago, L.; Cristiani, P. Organic Waste and Bioelectrochemical Systems: A Future Interface between Electricity and Methane Distribution Grids. Detritus 2018, 1, 57–63. [Google Scholar]
- Luo, Q.; Wang, H.; Zhang, X.; Qian, Y. Effect of direct electric current on the cell surface properties of phenol-degrading bacteria. Appl. Environ. Microbiol. 2005, 71, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.E. Influence of Electricity on Micro-Organisms. Bot. Gaz. 1909, 48, 359–379. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Kim, J.S.; Park, R.J. Effect of Electrical Stimulation on Bacterial Growth. J. Korean Soc. Phys. Ther. 1994, 6, 109–119. [Google Scholar]
- Chernysh, Y.; Balintova, M.; Shtepa, V.; Chubur, V.; Junakova, N. Effect of Electrolysis on Activated Sludge during the Hydrolysis and Acidogenesis Stages in the Anaerobic Digestion of Poultry Manure. Sustainability 2022, 14, 6826. [Google Scholar] [CrossRef]
- Tong, S.; Liu, H.; Feng, C.; Chen, N.; Zhao, Y.; Xu, B.; Zhao, J.; Zhu, M. Stimulation Impact of Electric Currents on Heterotrophic Denitrifying Microbial Viability and Denitrification Performance in High Concentration Nitrate-Contaminated Wastewater. J. Environ. Sci. 2019, 77, 363–371. [Google Scholar] [CrossRef]
- Pandit, S.; Savla, N.; Sonawane, J.M.; Sani, A.M.; Gupta, P.K.; Mathuriya, A.S.; Rai, A.K.; Jadhav, D.A.; Jung, S.P.; Prasad, R. Agricultural Waste and Wastewater as Feedstock for Bioelectricity Generation Using Microbial Fuel Cells: Recent Advances. Fermentation 2021, 7, 169. [Google Scholar] [CrossRef]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Startup of Electromethanogenic Microbial Electrolysis Cells with Two Different Biomass Inocula for Biogas Upgrading. ACS Sustain. Chem. Eng. 2017, 5, 8852–8859. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.-M. Overview of electro-fermentation technology for recycling agricultural waste. Interciencia 2021, 46, 91–212. [Google Scholar]
- Kumar, P.; Chandrasekhar, K.; Kumari, A.; Sathiyamoorthi, E.; Kim, B. Electro-Fermentation in Aid of Bioenergy and Biopolymers. Energies 2018, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Mirziev, S.I.; Belostockij, D.E.; Milyukov, V.A. Effect of zeolite application on substrate conversion with high nitrogen content [Effekt vneseniya ceolitov na konversiyu substrata s vysokim soderzhaniem azota]. Bull. Kazan Univ. Technol. 2017, 20, 146–149. [Google Scholar]
- Segi, I. Methods of Soil Microscopy [Metody Pochvennoj Mikroskopii]; Kolos: Port Louis, Mauritius, 1983. [Google Scholar]
- Bahtiyarova, Y.V.; Minnullin, R.R.; Galkin, V.I. Fundamentals of Chemical Experiments and Amusing Experiments in Chemistry: Textbook for Universities and Schools [Osnovy Himicheskogo Eksperimenta i Zanimatel’nye Opyty po Himii: Uchebnoe Posobie dlya vuzov i shkol]; Kazan University Press: Kazan, Russia, 2014. [Google Scholar]
- Kuznecov, A.E.; Gradova, N.B.; Lushnikov, S.V. Applied Ecobiotechnology [Prikladnaya Ekobiotekhnologiya: Uchebnoe Posobie], 2nd ed.; BINOM. Laboratoriya Znaniy: Moscow, Russia, 2012. [Google Scholar]
- Chubur, V.; Danylov, D.; Chernysh, Y.; Plyatsuk, L.; Shtepa, V.; Haneklaus, N.; Roubik, H. Methods for Intensifying Biogas Production from Waste: A Scientometric Review of Cavitation and Electrolysis Treatments. Fermentation 2022, 8, 570. [Google Scholar] [CrossRef]
- Reznikov, K.M.; Kolesnikov, P.D.; Kovalenko, I.V. Biological and pharmacological effects of ionized liquids with different redox potentials [Biologicheskie i farmakologicheskie effekty ionizirovannyh zhidkostej s razlichnym okislitel’no-vosstanovitel’nym potencialom]. Eurasian Union Sci. 2016, 30, 62–68. [Google Scholar]
- Chernysh, Y.Y.; Shtepa, V.N.; Plyatsuk, L.D.; Chubur, V.S.; Danylov, D.V. Anaerobic Digestion Combined with Electrolysis of Poultry Manure and Activated Sludge Inoculum. In Problemele Energeticii Regionale; Institute of Power Engineering: Moldova, Kishinau, 2022; Volume 2, pp. 101–113. [Google Scholar]
- Shtepa, V.N.; Zaec, N.A.; Alekseevskij, D.G. Use of electrolysis processes in reagent-free water treatment: Removal of hydrogen sulfide, organic iron, synthetic surfactants [Ispol’zovanie elektroliznyh processov v bezreagentnoj vodoochistke: Udalenie serovodoroda, organicheskogo zheleza, sinteticheskih poverhnostno-aktivnyh veshchestv]. Energy Autom. 2021, 2, 52–68. [Google Scholar]
- Yi, J.; Dong, B.; Jin, J.; Dai, X. Effect of Increasing Total Solids Contents on Anaerobic Digestion of Food Waste under Mesophilic Conditions: Performance and Microbial Characteristics Analysis. PLoS ONE 2014, 9, e102548. [Google Scholar] [CrossRef] [PubMed]
- Akunna, J.C.; Bizeau, C.; Moletta, R. Nitrate Reduction by Anaerobic Sludge Using Glucose at Various Nitrate Concentrations: Ammonification, Denitrification and Methanogenic Activities. Environ. Technol. 1994, 15, 41–49. [Google Scholar] [CrossRef]
- Denisov, A.A.; Tarasova, I.I.; Pavlinova, I.I. Optimization of activated sludge biocenosis of livestock treatment facilities to reduce the anthropogenic load on aquatic ecosystems [Optimizaciya biocenozov aktivnogo ila ochistnyh sooruzhenij zhivotnovodcheskih kompleksov dlya snizheniya antropogennoj nagruzki na vodnye ekosistemy]. Izv. Samara Sci. Cent. Russ. Acad. Sci. 2011, 5, 162–164. [Google Scholar]
- Golub, N.; Kozlovets, O.; Voiyevoda, D. Technology of Anaerobic-Aerobic Purification of Wastewater from Nitrogen Compounds after Obtaining Biogas. East.-Eur. J. Enterp. Technol. 2016, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Dubovik, O.S.; Markevich, R.M. Improvement of biotechnologies of nitrogen and phosphorus removal from municipal wastewater [Sovershenstvovanie biotekhnologij udaleniya azota i fosfora iz gorodskih stochnyh vod]. Tr. BGTU. Seriya 2 Him. Tekhnologii Biotekhnologiya Geoekologiya 2016, 4, 232–238. [Google Scholar]
- Tartakovsky, B.; Mehta, P.; Bourque, J.-S.; Guiot, S.R. Electrolysis-Enhanced Anaerobic Digestion of Wastewater. Bioresour. Technol. 2011, 102, 5685–5691. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Gu, J.; Zhao, Q.; Liu, Y. COD Capture: A Feasible Option towards Energy Self-Sufficient Domestic Wastewater Treatment. Sci. Rep. 2016, 6, 25054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Liu, W.; Shi, Y.; Wang, B.; Huang, C.; Liu, C.; Wang, A. Microbial Electrolysis Enhanced Bioconversion of Waste Sludge Lysate for Hydrogen Production Compared with Anaerobic Digestion. Sci. Total Environ. 2021, 767, 144344. [Google Scholar] [CrossRef]
- Bo, T.; Zhu, X.; Zhang, L.; Tao, Y.; He, X.; Li, D.; Yan, Z. A New Upgraded Biogas Production Process: Coupling Microbial Electrolysis Cell and Anaerobic Digestion in Single-Chamber, Barrel-Shape Stainless Steel Reactor. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef]
- Timmers, P.H.A.; Welte, C.U.; Koehorst, J.J.; Plugge, C.M.; Jetten, M.S.M.; Stams, A.J.M. Reverse Methanogenesis and Respiration in Methanotrophic Archaea. Archaea 2017, 2017, 1654237. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhao, J.; Chen, L.; Liu, Y.; Zuo, J. Methanogenic Community Structure in Simultaneous Methanogenesis and Denitrification Granular Sludge. Front. Environ. Sci. Eng. 2018, 12, 10. [Google Scholar] [CrossRef]
- Xu, J.; Bu, F.; Zhu, W.; Luo, G.; Xie, L. Microbial Consortiums of Hydrogenotrophic Methanogenic Mixed Cultures in Lab-Scale Ex-Situ Biogas Upgrading Systems under Different Conditions of Temperature, PH and CO. Microorganisms 2020, 8, 772. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.A.; Bryant, M.P.; Wolin, M.J. Characteristics of S organism isolated from Methanobacillus omelianskii. J. Bacteriol. 1972, 109, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Appels, L.; Lauwers, J.; Degrève, J.; Helsen, L.; Lievens, B.; Willems, K.; van Impe, J.; Dewil, R. Anaerobic Digestion in Global Bio-Energy Production: Potential and Research Challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Hofmann, T. Recirculating aquaculture system-based salmon farming. Field Actions Sci. Rep. 2019, 20, 85–87. [Google Scholar]
- Preena, P.G.; Kumar, V.J.R.; Singh, I.S.B. Nitrification and denitrification in recirculating aquaculture systems: The processes and players. Rev. Aquac. 2021, 13, 2053–2075. [Google Scholar] [CrossRef]
- Yogev, U.; Barnes, A.; Gross, A. Nutrients and Energy Balance Analysis for a Conceptual Model of a Three Loops off Grid, Aquaponics. Water 2016, 8, 589. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shtepa, V.; Balintova, M.; Shykunets, A.; Chernysh, Y.; Chubur, V.; Plyatsuk, L.; Junakova, N. Intensification of Waste Valorization Techniques for Biogas Production on the Example of Clarias gariepinus Droppings. Fermentation 2023, 9, 225. https://doi.org/10.3390/fermentation9030225
Shtepa V, Balintova M, Shykunets A, Chernysh Y, Chubur V, Plyatsuk L, Junakova N. Intensification of Waste Valorization Techniques for Biogas Production on the Example of Clarias gariepinus Droppings. Fermentation. 2023; 9(3):225. https://doi.org/10.3390/fermentation9030225
Chicago/Turabian StyleShtepa, Vladimir, Magdalena Balintova, Aliaksei Shykunets, Yelizaveta Chernysh, Viktoriia Chubur, Leonid Plyatsuk, and Natalia Junakova. 2023. "Intensification of Waste Valorization Techniques for Biogas Production on the Example of Clarias gariepinus Droppings" Fermentation 9, no. 3: 225. https://doi.org/10.3390/fermentation9030225





