Effects of High Temperature & Pressure Pretreatment Process on Methane Production from Cyanobacteria
Abstract
1. Introduction
2. Methods and Materials
2.1. Isolation, Identification, Culture Production, Harvesting and Determination of Culture Specific Parameters of Cyanobacteria
2.2. D. tharense Characterization Analyses
2.3. High Temperature-Pressure Pretreatment Process
2.4. High Temperature-Pressure Pretreatment Process Efficiency Analysis
2.5. Biochemical Methane Potential (BMP)
2.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. D. tharense Characterization Results
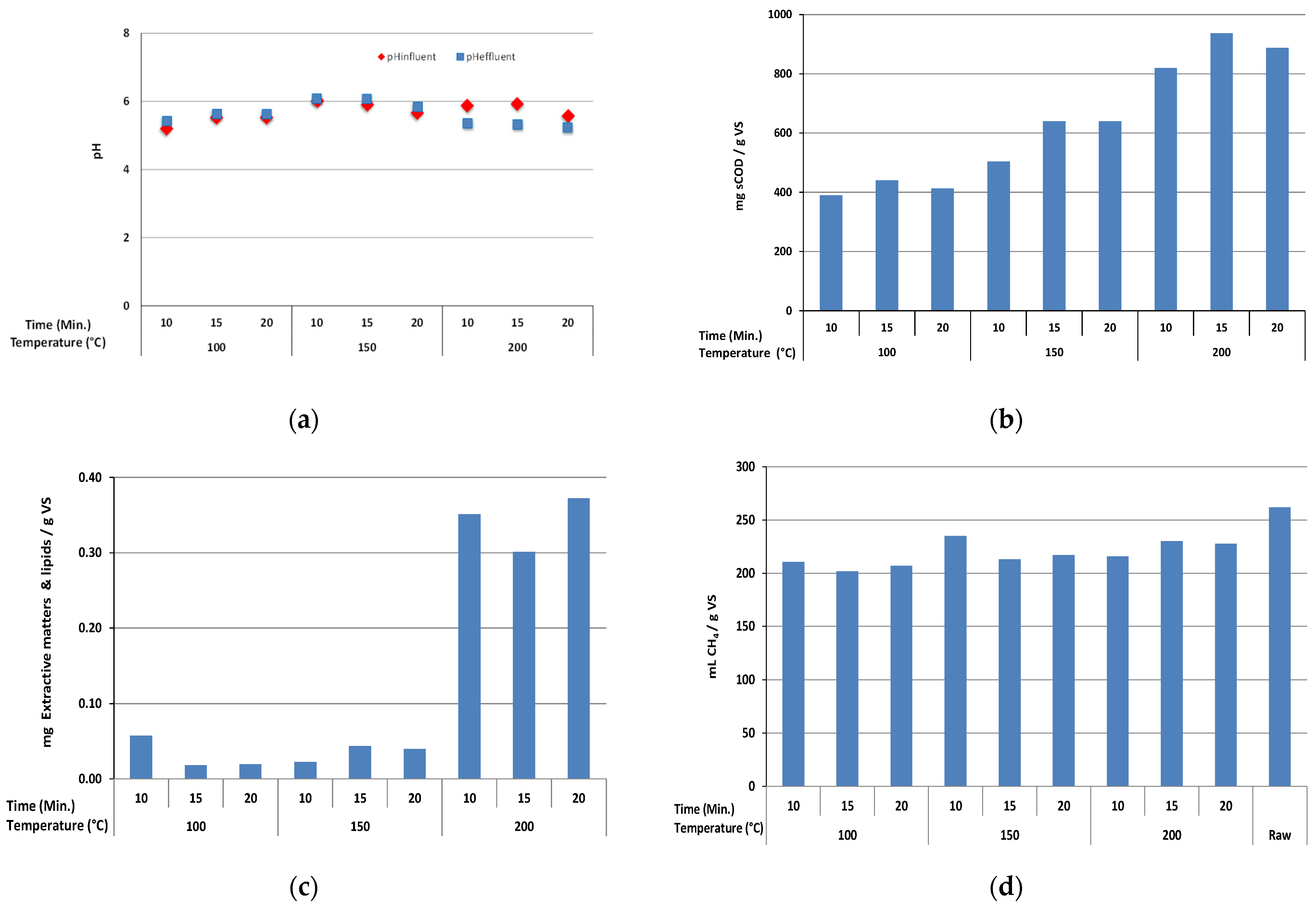
3.2. Effects of HTPP Process on pH, sCOD, Extractables with Lipids and BMP
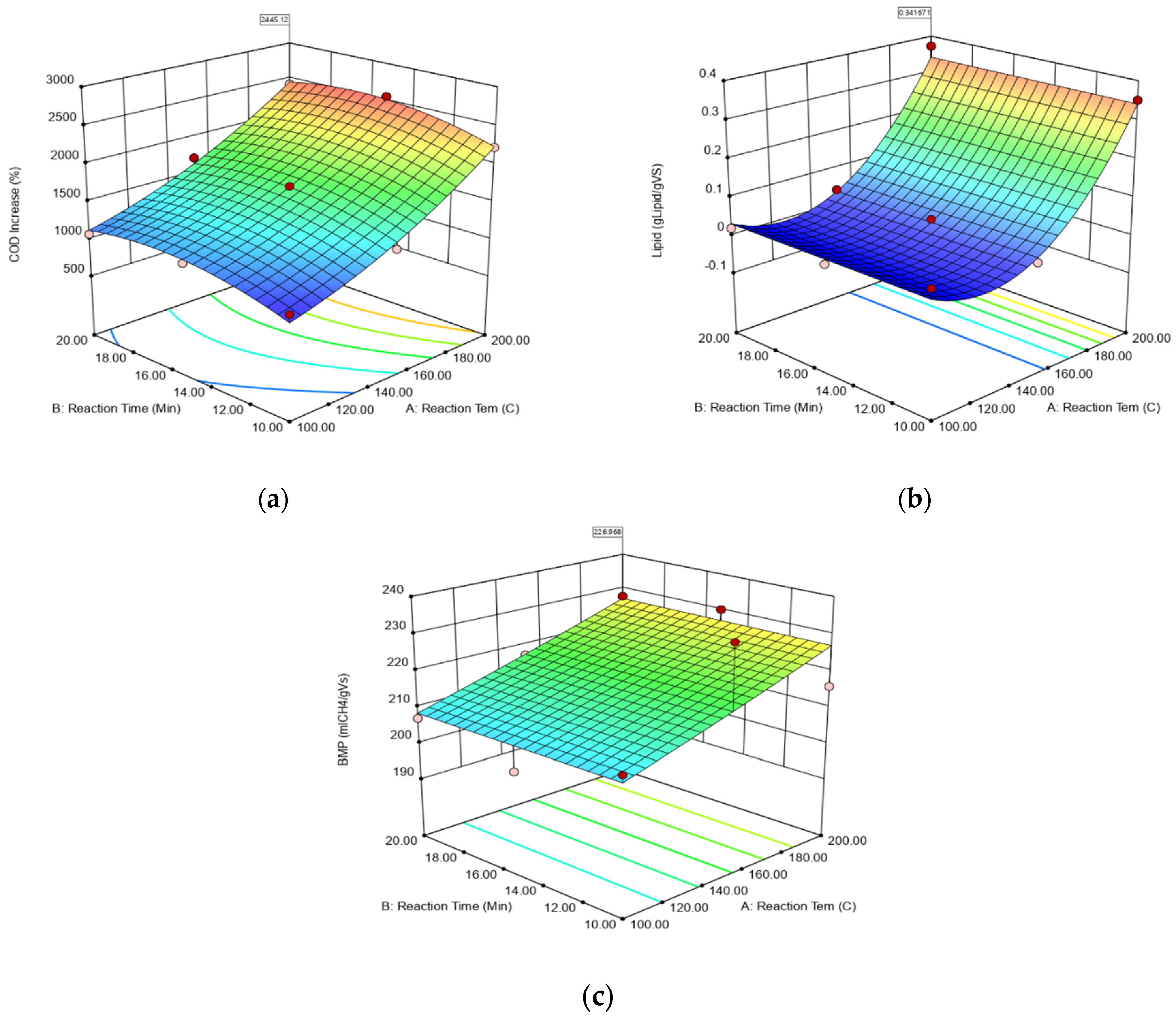
3.3. Modeling of HTPP Process
3.4. Optimization of HTPP Process for Methane Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jankowska, E.; Sahu, A.K.; Oleskowicz-Popiel, P. Biogas from Microalgae: Review on Microalgae’s Cultivation, Harvesting and Pretreatment for Anaerobic Digestion. Renew. Sustain. Energy Rev. 2017, 75, 692–709. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.N.; Banecjee, U.C. Bioactive Compounds from Cyanobacteria and Microalgae: An Overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Ehimen, E.A.; Holm-Nielsen, J.B.; Poulsen, M.; Boelsmand, J.E. Influence of Different Pre-Treatment Routes on the Anaerobic Digestion of a Filamentous Algae. Renew. Energy 2013, 50, 476–480. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic Digestion of Microalgae as a Necessary Step to Make Microalgal Biodiesel Sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as Substrates for Fermentative Biogas Production in a Combined Biorefinery Concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- Keymer, P.; Ruffell, I.; Pratt, S.; Lant, P. High Pressure Thermal Hydrolysis as Pre-Treatment to Increase the Methane Yield during Anaerobic Digestion of Microalgae. Bioresour. Technol. 2013, 131, 128–133. [Google Scholar] [CrossRef]
- Diltz, R.; Pullammanappallil, P. Biofuels from Algae. In Liquid, Gaseous and Solid Biofuels—Conversion Techniques; IntechOpen: London, UK, 2013; p. 348. [Google Scholar]
- Low, E.W.; Chase, H.A. Reducing Production of Excess Biomass during Wastewater Treatment. Water Res. 1999, 33, 1119–1132. [Google Scholar] [CrossRef]
- Passos, F.; Hernández-Mariné, M.; García, J.; Ferrer, I. Long-Term Anaerobic Digestion of Microalgae Grown in HRAP for Wastewater Treatment. Effect of Microwave Pretreatment. Water Res. 2014, 49, 351–359. [Google Scholar] [CrossRef]
- Lee, J.; Cho, D.H.; Ramanan, R.; Kim, B.H.; Oh, H.M.; Kim, H.S. Microalgae-Associated Bacteria Play a Key Role in the Flocculation of Chlorella Vulgaris. Bioresour. Technol. 2013, 131, 195–201. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Timmers, R.A.; Ballesteros, M.; González-Fernández, C. Enhancing Methane Production of Chlorella vulgaris via Thermochemical Pretreatments. Bioresour. Technol. 2013, 149, 136–141. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Influence of Hydrothermal Pretreatment on Microalgal Biomass Anaerobic Digestion and Bioenergy Production. Water Res. 2015, 68, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Alzate, M.E.; Muñoz, R.; Rogalla, F.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biochemical Methane Potential of Microalgae: Influence of Substrate to Inoculum Ratio, Biomass Concentration and Pretreatment. Bioresour. Technol. 2012, 123, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Kula, T.; Kessler, B.A.; Hong, Y.; Bouwer, E.J.; Betenbaugh, M.J.; Allnutt, F.C.T. Mixed Trophic State Production Process for Microalgal Biomass with High Lipid Content for Generating Biodiesel and Biogas. Bioenergy Res. 2014, 7, 1174–1185. [Google Scholar] [CrossRef]
- Fardinpoor, M.; Perendeci, N.A.; Yılmaz, V.; Taştan, B.E.; Yılmaz, F. Effects of Hydrodynamic Cavitation-Assisted NaOH Pretreatment on Biofuel Production from Cyanobacteria: Promising Approach. Bioenergy Res. 2022, 15, 289–302. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Microalgae Autoflocculation: An Alternative to High-Energy Consuming Harvesting Methods. J. Appl. Phycol. 2013, 25, 991–999. [Google Scholar] [CrossRef]
- Schwede, S.; Kowalczyk, A.; Gerber, M.; Span, R. Anaerobic Co-Digestion of the Marine Microalga Nannochloropsis Salina with Energy Crops. Bioresour. Technol. 2013, 148, 428–435. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Thermal Pretreatment to Improve Methane Production of Scenedesmus Biomass. Biomass Bioenergy 2012, 40, 105–111. [Google Scholar] [CrossRef]
- Cho, S.; Park, S.; Seon, J.; Yu, J.; Lee, T. Evaluation of Thermal, Ultrasonic and Alkali Pretreatments on Mixed-Microalgal Biomass to Enhance Anaerobic Methane Production. Bioresour. Technol. 2013, 143, 330–336. [Google Scholar] [CrossRef]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of Microalgae to Improve Biogas Production: A Review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef]
- Bermúdez Menéndez, J.M.; Arenillas, A.; Menéndez Díaz, J.Á.; Boffa, L.; Mantegna, S.; Binello, A.; Cravotto, G. Optimization of Microalgae Oil Extraction under Ultrasound and Microwave Irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1779–1784. [Google Scholar] [CrossRef]
- Ertit Taştan, B.; Duygu, E.; Dönmez, G. Boron Bioremoval by a Newly Isolated Chlorella sp. and Its Stimulation by Growth Stimulators. Water Res. 2012, 46, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Perendeci, N.A.; Yılmaz, V.; Ertit Taştan, B.; Gökgöl, S.; Fardinpoor, M.; Namlı, A.; Steyer, J.P. Correlations between Biochemical Composition and Biogas Production during Anaerobic Digestion of Microalgae and Cyanobacteria Isolated from Different Sources of Turkey. Bioresour. Technol. 2019, 281, 209–216. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Loewus, F.A. Improvement in Anthrone Method for Determination of Carbohydrates Errors in Volumetric Analysis Arising from Adsorption. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Waterborg, J.H.; Matthews, H.R. The Lowry Method for Protein Quantitation. Protein Protoc. Handb. 1984, 1, 7–9. [Google Scholar]
- Bridoux, G.; Dhulster, P.; Manem, J. Analyse des Graisses Dans les Stations d’épuration. Tech. Sci. Méthodes Génie Urbain Génie Rural 1994, 5, 257–262. [Google Scholar]
- Lesteur, M.; Bellon-Maurel, V.; Gonzalez, C.; Latrille, E.; Roger, J.M.; Junqua, G.; Steyer, J.P. Alternative Methods for Determining Anaerobic Biodegradability: A Review. Process Biochem. 2010, 45, 431–440. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Theisen, H.; Vigil, S. Integrated Solid Waste Management. Engineering Principles and Management Issues; McGraw-Hill: New York, NY, USA, 1993; ISBN 0070632375. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Mottet, A.; Steyer, J.P.; Déléris, S.; Vedrenne, F.; Chauzy, J.; Carrère, H. Kinetics of Thermophilic Batch Anaerobic Digestion of Thermal Hydrolysed Waste Activated Sludge. Biochem. Eng. J. 2009, 46, 169–175. [Google Scholar] [CrossRef]
- Perendeci, N.A.; Gökgöl, S.; Orhon, D. Impact of Alkaline H2O2 Pretreatment on Methane Generation Potential of Greenhouse Crop Waste under Anaerobic Conditions. Molecules 2018, 23, 1794. [Google Scholar] [CrossRef]
- Patel, A.; Gami, B.; Patel, P.; Patel, B. Microalgae: Antiquity to Era of Integrated Technology. Renew. Sustain. Energy Rev. 2017, 71, 535–547. [Google Scholar] [CrossRef]
- Assunção, M.F.G.; Amaral, R.; Martins, C.B.; Ferreira, J.D.; Ressurreição, S.; Santos, S.D.; Varejão, J.M.T.B.; Santos, L.M.A. Screening Microalgae as Potential Sources of Antioxidants. J. Appl. Phycol. 2017, 29, 865–877. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Giménez, J.B.; Bouzas, A.; Carrere, H.; Steyer, J.P.; Ferrer, J.; Seco, A. Assessment of Cross-Flow Filtration as Microalgae Harvesting Technique Prior to Anaerobic Digestion: Evaluation of Biomass Integrity and Energy Demand. Bioresour. Technol. 2018, 269, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Al hattab, M.; Ghaly, A. Microalgae Oil Extraction Pre-Treatment Methods: Critical Review and Comparative Analysis Fundamentals of Renewable Energy and Applications. J. Fundam. Renew. Energy Appl. 2015, 5, 1–26. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. Chlorella vulgaris vs Cyanobacterial Biomasses: Comparison in Terms of Biomass Productivity and Biogas Yield. Energy Convers. Manag. 2015, 92, 137–142. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Magdalena, J.A.; Muylaert, K.; Gonzalez-Fernandez, C. High methane yields in anaerobic digestion of the cyanobacterium Pseudanabaena sp. Algal Res. 2019, 44, 101689. [Google Scholar] [CrossRef]
- Choudhary, P.; Assemany, P.P.; Naaz, F.; Bhattacharya, A.; De Siqueira Castro, J.; do Couto Couto, E.D.A.; Calijuri, M.L.; Pant, K.K.; Malik, A. A review of biochemical and thermochemical energy; onversion routes of wastewater grown algal biomass. Sci. Total Environ. 2020, 726, 137961. [Google Scholar] [CrossRef]


| Independent Variables | The Coded Levels | ||
|---|---|---|---|
| Low Level (−1) | Middle Level (0) | High Level (+1) | |
| Reaction temperature (°C) | 100 | 150 | 200 |
| Reaction time (min) | 10 | 15 | 20 |
| Parameters | D. tharanse |
|---|---|
| Dry weight, X (g L−1) | 3.61 ± 0.26 |
| Chlorophyll a, Chl a (µg mL−1) | 0.73 ± 0.24 |
| Specific growth rate (µ (day−1) | 0.36 ± 0.007 |
| Maximum productivity, Pmax (g L−1.day−1) | 0.35 ± 0.027 |
| CO2 fixation rate, FCO2 (g day−1) | 0.06 ± 0.005 |
| Parameter | D. tharense |
|---|---|
| pH | 7.4 |
| Total solid, TS (g kg−1) | 97.4 ± 6.56 |
| Volatile solid, VS (g kg−1) | 91 ± 4.28 |
| Sugar (mg gVS−1) | 1099.8 ± 28.93 |
| Extractive matter and lipids, (%) | 1.2 ± 0.30 |
| Total Kjeldahl nitrogen, TKN (mg gVS−1) | 709.8 ± 28.98 |
| Total protein (mg L−1) | 28.1 ± 1.27 |
| Soluble protein (mg L−1) | 4.4 ± 0.40 |
| Total organic carbon, TOC (%) | 48.9 |
| Total chemical oxygen demand, tCOD (mg L−1) | 355 ± 53.03 |
| Soluble chemical oxygen demand, sCOD (mg L−1) | 106 ± 5.66 |
| Total phosphorus, TP (mg L−1) | 5.1 ± 0.02 |
| Elemental Analysis (%) | |
| C | 50.17 |
| H | 7.07 |
| N | 5.87 |
| S | 0.48 |
| C/N ratio | 8.55 |
| Calorific value—Dulong Equation (Kcal kg−1) | 5220 |
| Species | BMP (mLCH4 gVS−1) | References |
|---|---|---|
| Desertifilum tharense | 261.8 | This study |
| Phormidium animale | 293 | [23] |
| Spirulina maxima | 630–740 | [4] |
| Synechocystis sp. | 220 | [38] |
| Spirulina platensis | 470–690 | [4] |
| Aphanizomenon ovalisporum | 223 | [38] |
| Anabaena planctonica | 187 | [38] |
| sCOD Model COD = −833.19227–5.18428 × Reaction temperature + 236.16508 × Reaction time + 0.061396 × Reaction temperature2 − 7.15996 × Reaction time2 | |||||
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
| Model | 2.807 × 106 | 4 | 7.017 × 105 | 88.13 | 0.0004 |
| A-Reaction temperature | 2.627 × 106 | 1 | 2.627 × 106 | 29.95 | <0.0001 |
| B-Reaction time | 68,476.96 | 1 | 68,476.9 | 8.6 | 0.0427 |
| A2 | 47,118.78 | 1 | 47,118.7 | 5.92 | 0.0718 |
| B2 | 64,081.35 | 1 | 64,081.3 | 8.05 | 0.0470 |
| Residual | 31,851.14 | 4 | 7962.78 | ||
| Cor total | 2.839 × 106 | 8 | |||
| Standard deviation | 89.3 | R2 | 0.9888 | ||
| Mean | 1685.06 | Adj-R2 | 0.9776 | ||
| Coefficient of variation (%) | 5.30 | Pred-R2 | 0.9432 | ||
| Precision | 1.612 × 105 | Adeq Precision | 24.169 | ||
| Extractive Matter and Lipids Model Extractive matter and lipids = +0.93333–0.015070 × Reaction temperature + 6.05600 × 10−5 × Reaction time2 | |||||
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
| Model | 0.19 | 2 | 0.095 | 145.88 | <0.0001 |
| A-Reaction temperature | 0.14 | 1 | 0.14 | 221.28 | <0.0001 |
| A2 | 0.046 | 1 | 0.046 | 70.48 | 0.0002 |
| Residual | 3.930 × 10−3 | 6 | 6.505 × 10−4 | ||
| Cor total | 0.19 | 8 | |||
| Standard deviation | 0.026 | R2 | 0.9798 | ||
| Mean | 0.14 | Adj-R2 | 0.9731 | ||
| Coefficient of variation (%) | 18.71 | Pred-R2 | 0.9547 | ||
| Precision | 8.782 × 10−3 | Adeq Precision | 21.037 | ||
| BMP Model 1/BMP = +5.19480 × 10−3–3.91018 × 10−6 × Reaction temperature | |||||
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
| Model | 2.293 × 10−7 | 1 | 2.293 × 10−7 | 7.56 | 0.0285 |
| A-Reaction temperature | 2.293 × 10−7 | 1 | 2.293 × 10−7 | 7.56 | 0.0285 |
| Residual | 2.123 × 10−7 | 7 | 32,033 × 10−8 | ||
| Cor total | 4.416 × 10−7 | 8 | |||
| Standard deviation | 1.741 × 10−4 | R2 | 0.5193 | ||
| Mean | 4.608 × 10−3 | Adj-R2 | 0.4506 | ||
| Coefficient of variation (%) | 3.78 | Pred-R2 | 0.2714 | ||
| Precision | 3.218 × 10−3 | Adeq Precision | 4.763 | ||
| Maximum Methane Production at Minimum Cost | |||
|---|---|---|---|
| Parameter | Optimization Conditions | Model Prediction Results | Validation Experiment Results |
| Desirability | 0.834 | ||
| Reaction temperature (°C) | Range | 105.7 | |
| Reaction time (min) | Min (+++++) | 10.7 | |
| BMP (mLCH4 gVS−1) | Max (+++++) | 209.5 | 205.1 |
| Maximum Methane Production | |||
| Parameter | Optimization Conditions | Model Prediction Results | Validation Experiment Results |
| Desirability | 0.92 | ||
| Reaction temperature (°C) | Range | 200 | |
| Reaction time (min) | Range | 20 | |
| BMP (mLCH4 gVS−1) | Max (+++++) | 227.1 | 211.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahan, M.; Fardinpoor, M.; Yılmaz, V.; Yılmaz, F.; Perendeci, N.A. Effects of High Temperature & Pressure Pretreatment Process on Methane Production from Cyanobacteria. Fermentation 2023, 9, 240. https://doi.org/10.3390/fermentation9030240
Şahan M, Fardinpoor M, Yılmaz V, Yılmaz F, Perendeci NA. Effects of High Temperature & Pressure Pretreatment Process on Methane Production from Cyanobacteria. Fermentation. 2023; 9(3):240. https://doi.org/10.3390/fermentation9030240
Chicago/Turabian StyleŞahan, Murat, Mona Fardinpoor, Vedat Yılmaz, Fatih Yılmaz, and N. Altınay Perendeci. 2023. "Effects of High Temperature & Pressure Pretreatment Process on Methane Production from Cyanobacteria" Fermentation 9, no. 3: 240. https://doi.org/10.3390/fermentation9030240
APA StyleŞahan, M., Fardinpoor, M., Yılmaz, V., Yılmaz, F., & Perendeci, N. A. (2023). Effects of High Temperature & Pressure Pretreatment Process on Methane Production from Cyanobacteria. Fermentation, 9(3), 240. https://doi.org/10.3390/fermentation9030240





