Abstract
Food losses (FL) and waste (FW) occur throughout the food supply chain. These residues are disposed of on landfills producing environmental issues due to pollutants released into the air, water, and soil. Several research efforts have focused on upgrading FL and FW in a portfolio of added-value products and energy vectors. Among the most relevant research advances, biotechnological upgrading of these residues via fermentation has been demonstrated to be a potential valorization alternative. Despite the multiple investigations performed on the conversion of FL and FW, a lack of comprehensive and systematic literature reviews evaluating the potential of fermentative processes to upgrade different food residues has been identified. Therefore, this article reviews the use of FL and FW in fermentative processes considering the composition, operating conditions, platforms, fermentation product application, and restrictions. This review provides the framework of food residue fermentation based on reported applications, experimental, and theoretical data. Moreover, this review provides future research ideas based on the analyzed information. Thus, potential applications and restrictions of the FL and FW used for fermentative processes are highlighted. In the end, food residues fermentation must be considered a mandatory step toward waste minimization, a circular economy, and the development of more sustainable production and consumption patterns.
1. Introduction
Strategies and efforts for promoting sustainable development have been affected by emerging worldwide issues such as COVID-19 and the Russia and Ukraine war [1]. In addition, fuel price fluctuation, food price increases, and high unemployment rates have caused a great impact in different world regions [2]. On the other hand, social inequality and hunger have caused food insecurity and poverty in countries with low economic development (low and lower middle income) [3]. Consequently, the accomplishment of the Sustainable Development Goals (SDGs) proposed by the United Nations (UN) in 2015 has been delayed [4]. Likewise, unsustainable production and consumption patterns have contributed to climate change and biodiversity loss since the current linear economy model releases large amounts of pollutants (e.g., greenhouse gases and untreated industrial effluents) [5]. For this reason, worldwide efforts are guided to developing alternatives for promoting the efficient use of resources [6].
Worldwide estimations indicate that about 30% of food products are considered waste (food residues—FR). In the first links of the Food Value Chains (FVC), about 13.3% of the produced food is lost (i.e., harvest, slaughter, post-harvest, transformation, or manufacturing), and 17% is discarded at the consumer level [7]. FL are included the byproducts generated in food production with little or no added value (e.g., cheese whey, spent brewery yeast, expired juice) [8,9]. According to the Food and Agriculture Organization of the United Nations (FAO), countries with low economic development generate more food losses [9]. The main contributing factors are poor logistics in FVC, and climate change that affects food production. These issues should be addressed to reduce food losses [10]. In addition, the agricultural and agro-industrial sectors are key for the development of countries with low economic growth. Countries with high economic development (upper middle and high income) generate more food waste [9]. The amount and type of food waste generated depends on socio-cultural practices and economic conditions [11]. The current disposal of FR in landfills or incinerators are generating emissions to water, land, and air [12]. Therefore, improving FR management would mitigate these effects and promote the efficient use of resources. However, FR characteristics cannot be generalized to evaluate alternative uses. Food losses (FL) generated in the stages before marketing and consumption present a homogeneous composition since these residues come from a specific value chain. On the other hand, food waste (FW) is a grouping of waste from different products generated in different value chains [13]. The composition of the FW depends on factors such as socio-economic and cultural conditions and the seasonality of food [14].
The FL and FW can be upgraded into added-value products promoting an efficient and sustainable use of resources, bio-economy development, and generating a circular economy within the FVC [15]. In fact, reducing FL and FW is a goal stipulated in the SDG 12 (responsible consumption and production) that also allows linking other aspects such as food security, nutrition, and environmental sustainability “reduce per capita global FLW at the retail and consumer level by half and reduce the loss of food throughout production and the supply chain including post-harvest loss by 2030” [16]. The consequences of meeting this goal would allow the achievement of better food security, a nutrition improvement (SDG 2), and an environmental effects reduction (SDGs 11, 13, 14, and 15). However, the search for new alternatives for the disposal of FL and FW requires the development of other objectives such as the generation of clean and affordable energy (SDG 7), infrastructure, industry and innovation (SDG 9), economic growth and decent employment (SDG 8), reducing inequality (SDG 10) and ending poverty (SDG 1).
FL and FW valorization strategies have been addressed to produce energy vectors such as biomethane, biogas, ethanol, hydrogen, and butanol and products with high added value such as volatile fatty acids, biomaterials, biofertilizers, growth enhancers, organic acids, among others [17]. Other recently investigated FL and FW valorization routes with great potential have been aimed at the production of food grades such as omega-3 oil [18]. Furthermore, expired food has been researched for producing added-value products since the composition after a degradation procedure tends to be constant and high in valuable components [19]. FL and FW are globally and generally made up of carbohydrates (35.5–60%), protein (21.9–30%), oils, fats, and organic acids (3.9–65%) [18,20]. However, the composition of these residues is complex, and varies depending on factors such as the fruit degree maturity, productive process type among others [21]. The carbohydrate and protein fractions can be hydrolyzed to obtain fermentable sugars and oligomers [22]. Fermentable sugars are used in fermentative processes with yeasts, fungi, and bacteria [15]. On the other hand, lipids are a fraction of FL and FW with great potential to be used in fermentative processes using aquatic protists (i.e., single-celled eukaryotes that range from algae to heterotrophic flagellates) [23].
Despite the multiple investigations performed on the conversion of FL and FW, there is a lack of comprehensive and systematic reviews of the literature aimed at evaluating the fermentative processes to obtain different products. This article reviews the use of FL and FW in fermentative processes. The definition and classification of FL and FW based on the links of the FVC are presented. The variability of the chemical characterization of the FL and FW is analyzed from the point of view of chemical composition, and total and volatile solids content. Then, a review of the trends of fermentation products obtained from FL and FW and the main product platforms is presented. Finally, the observed potentials and restrictions, and future directions are elucidated.
2. Food Waste: Definition, Classification Based on Value Chain Link and Chemical Composition
2.1. Definition and Classification
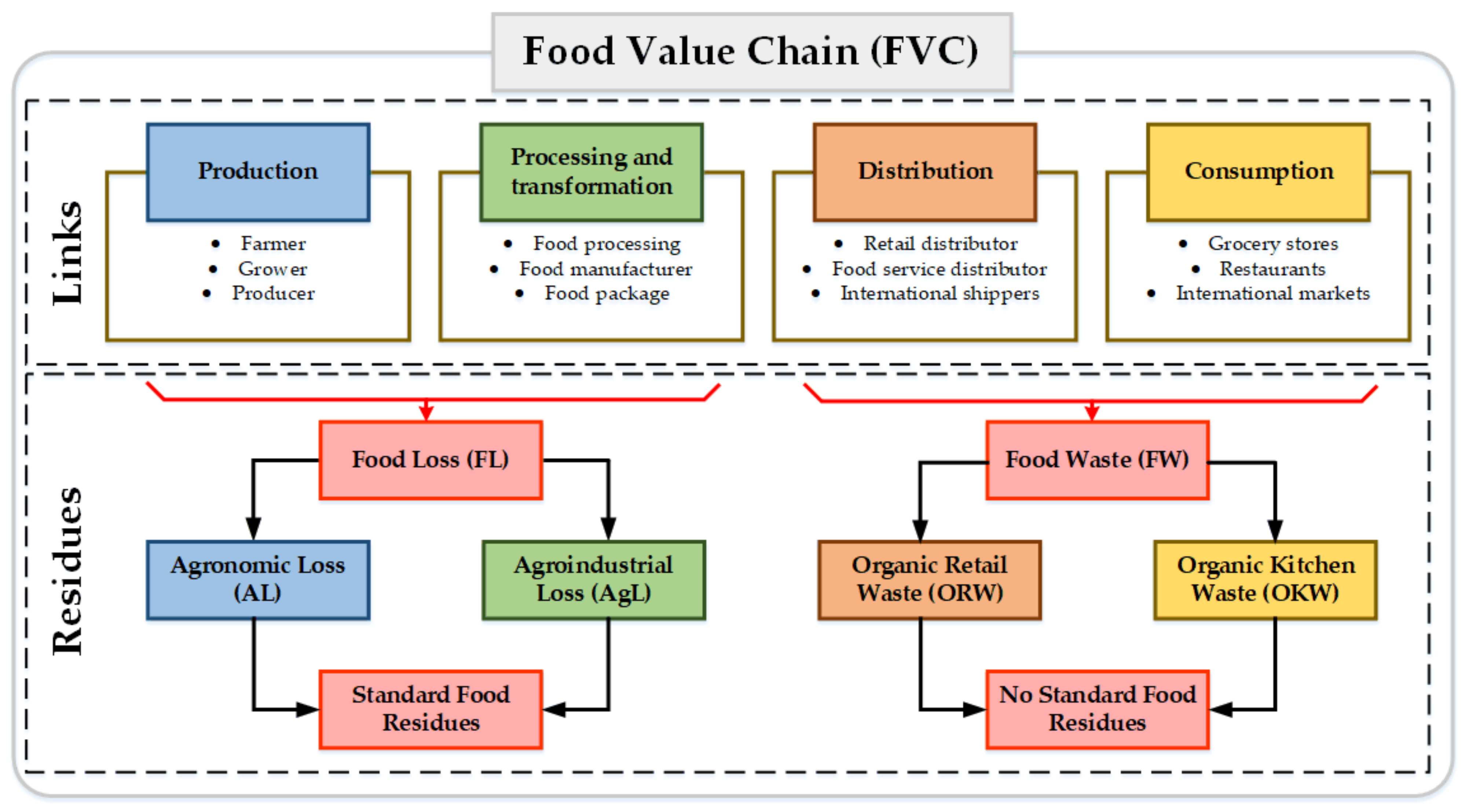
Several definitions and classifications for FW are found in the open literature. However, a clear difference between the residues produced in the agronomic, industrial processing, commercialization, and consumption stages has not been provided [24]. Therefore, an alternative for classifying FW is to consider the FVC links. In general terms, the FVC is composed of four global links: (i) production, (ii) manufacturing and processing, (iii) distribution, and (iv) consumption (see Figure 1).
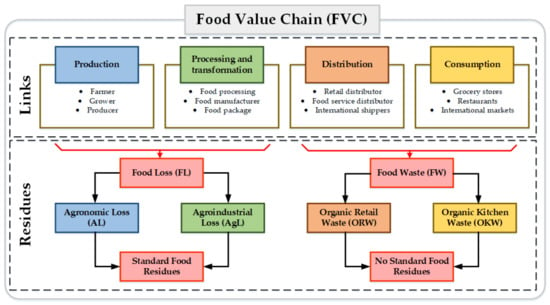
Figure 1.
Classification of Food Loss and Waste based on the value chain.
According to the FAO, “Food Loss (FL) is the decrease in the quantity or quality of food resulting from decisions and actions of food suppliers in the supply chain, excluding retailers, food service providers, and consumers” [9]. All food discarded or incinerated from harvest (slaughter, recovery) to transformation or marketing is considered to be FL. The FL obtained in agricultural and farming production, post-harvest, handling, slaughter, storage, and process distribution and transformation can be grouped as Agronomic Loss (AL). These FLs correspond to the waste generated in a single FVC location. For example, the first three stages of the fruit FVC occur in the same crop location or neighboring areas. In the fruit agricultural production stage, stems, leaves, roots, and fruit are generated with low-quality standards (overripe), flowers, and dung. The FL generated in the processing, packaging, and distribution stages can be grouped as Agro-industrial Loss (AgL). In the case of fruit FVC, these stages generate residues such as peel, seeds and liquid, and solid residues.
On the other hand, “FW is the decrease in the quantity or quality of food resulting from decisions and actions of retailers, food services and consumer” [9]. FW is the food mass decrease within the distribution and consumption links. FW can be classified as organic retail waste (ORW) and organic kitchen food waste (OKFW). This type of waste contains agronomic and agro-industrial products such as vegetables, fruit, farming products, or processed products. The main characteristic of this waste is non-standardized composition. FW includes the following items [7]:
- Food or products that deviate from the end goal: Products with physical and organoleptic properties that do not meet the standard.
- Food or products discarded by retailers or consumers: Expired expiration date.
- Food or food fragments, and edible and inedible products that are discarded in domestic kitchens, food establishments, or markets.
FL and FW can be grouped as food residues (FR). Different authors have supported this classification. For instance, Oliveira et al. [25] reported a systematic review aimed at finding potential alternatives to FL and FW upgrading in the circular bio-economy framework. Moreover, Lucifero [26] analyzed the FL and FW generation issue based on European Union legislation, since European countries have been looking for more environmentally friendly disposal alternatives. Finally, Aschemann-Witzel et al. [27] defined upcycled food based on the FL and FW concepts. Then, the above-mentioned classification has been used in the open literature to refer to the same issues related to FR generation.
An alternative way to classify FR depends on composition variability. Then, standard food residues and non-standard food residues can be identified. Standard food residues do not present changes in composition over time and are generated only in FVC. Agronomic and agro-industrial residues are classified as standard food waste. Non-standard food residues present changes in composition based on the socio-economic and cultural context. Non-standard food residues composition groups the residues generated within the distribution and consumption links. Several reports have supported this classification since a similar classification based on homogeneous and heterogeneous FR has been reported [28].
2.2. Chemical Characterization of Food Waste
FL and FW have a diverse composition, but carbohydrates, proteins, and lipids have been considered to be the most important and representative fractions of these raw materials. Carbohydrates, proteins, and lipids are the source of primary platforms such as sugars, amino acids, fatty acids, and glycerol after hydrolysis. Table 1 and Table 2 present FL and FW chemical characterization.
The composition of both FL and FW depends on the origin source. Table 1 and Table 2 show the composition of different FL and FW. In comparison to the FW, the FL (agronomic and agro-industrial residues) are characterized more completely mainly in terms of fibers, extractives, and ash. Fractions such as fats, pectin and starch are randomly characterized depending on the raw material. However, this leads to incomplete compositions and wrong composition normalizations (wrong fraction concentration calculation). On the other hand, FWs are characterized and studied considering a specific waste generated in a specific sector (e.g., kitchen, dinner shop, or canteen) and in specific locations (e.g., university restaurants, cafeterias in a specific shopping center). representing a limitation for a global valuation of FW. The main reason for this is that composition variability is not considered to propose harvesting routes. The composition of the FL generated in a production chain presents less variability compared to the FW.
Biotechnology conversions include all enzymatic fermentation and saccharification routes [29]. Cellulose, hemicellulose, starch, and pectin fractions are used in FL and FW transformation processes [30]. Thus, the type of products to be obtained considers food, organic molecules, biofuels, and biomaterials [31]. Probiotics and single-cell protein from FL have been extensively studied [32]. Organic molecules such as citric acid, lactic acid, succinic acid, and ascorbic acid have been obtained from FL and FW using cellulose and hemicellulose fractions (fermentable sugars) [29].

Table 1.
Chemical composition of some FW.
Table 1.
Chemical composition of some FW.
| Chemical Composition * | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fraction | Precooked Food Waste [33] | Fruit Peel Waste [34] | Fresh Vegetable Waste [35] | Rice, Vegetables and Meats [36] | Household Food Waste [37] | Restaurant Food Waste [37] | Kitchen Waste [38] | Kitchen Waste [38] | Organic Municipal Solid Waste [39] | Kitchen Garbage [33] | Municipal Solid Waste [40] |
| Moisture ** | 82.8% | N.R. | 89.81% | 81.9% | N.R. | 71.6% | 80.3% | 61.3% | N.R. | 75.2% | 85.7% |
| Total sugar *** | N.R. | N.R. | N.R. | 44.6% | N.R. | N.R. | 59.8% | 85.2% | 35.0% | 37.9% | 46.6% |
| Starch | 41.5% | N.R. | N.R. | 39.0% | 33.1% | 30.7% | N.R. | N.R. | N.R. | 34.8% | 31.2% |
| Cellulose | 2.1% | 21.5% | 26.9% | N.R. | 31.9% | 16.1% | N.R. | N.R. | 28.8% | N.R. | 2.6% |
| Hemicellulose | N.R. | 11.8% | 51.4% | N.R. | 35.0% | 3.1% | N.R. | N.R. | 22.7% | N.R. | N.R. |
| Lignin | N.R. | 8.0% | 21.7% | N.R. | N.R. | N.R. | 0.8% | N.R. | N.R. | N.R. | N.R. |
| Carbohydrates | 56.4% | 58.7% | N.R. | N.R. | N.R. | 50.0% | N.R. | N.R. | N.R. | N.R. | N.R. |
| Fats | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | 15.7% | 7.9% | 9.7% | 13.7% | N.R. |
| Protein | N.R. | N.R. | N.R. | 16.4% | N.R. | N.R. | 21.8% | 5.4% | N.R. | 11.9% | 19.6% |
| Pectin | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | 2.7% | N.R. | N.R. |
| Ash | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | 1.9% | 1.5% | 1.1% | 1.7% | N.R. |
| Total and volatile solids | |||||||||||
| Fraction | Precooked food waste [41] | Fruit peel waste [42] | Fresh vegetable waste [41] | Rice, Vegetables, and meats [43] | Organic fraction of municipal solid waste [44] | Household food waste [45] | Cafeteria food waste [45] | Kitchen waste [45] | Organic municipal solid waste [46] | Kitchen garbage [47] | Municipal solid waste [48] |
| Moisture | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | 76.6% | 90.3% | 81.0% | N.R. | 74.0% |
| Total solid | 30.4% | 9.7% | 12.2% | 53.6% | 24.8% | 50.6% | 51.8% | 52.2% | 51.4% | 52.4% | 54.0% |
| Volatile solid | 69.6% | 90.3% | 87.8% | 46.4% | 75.2% | 49.4% | 48.2% | 47.8% | 48.6% | 47.6% | 46.0% |
| Ratio carbon to nitrogen | |||||||||||
| Fraction | Precooked food waste [41] | Fruit peel waste [49] | Fresh vegetable waste [41] | Rice, Vegetables, and meats [43] | Organic fraction of municipal solid waste [44] | Household food waste [45] | Cafeteria food waste [45] | Kitchen waste [45] | Organic municipal solid waste [46] | Kitchen garbage [47] | Municipal solid waste [48] |
| C/N | 21.69 | 21.10 | 5.65 | 21.7 | 12.6 | 18.00 | 23.00 | 11.00 | 13.20 | 24.50 | 14.80 |
* Chemical composition is reported in dry base; ** Initial moisture of raw material; *** Extractives content in raw material; N.R.: Not reported.

Table 2.
Chemical composition of some FL.
Table 2.
Chemical composition of some FL.
| Chemical Composition (%) * | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fraction | Fermented Cheese Whey [50] | Apple Pomace [51] | Watermelon Rinds [52] | Potato Peels [53] | Coffee Cut-Stem [54] | Oil Palm Rachis [55] | Plantain Peel [56] | Avocado Seed [57] | Tomato Pomace [58] | Carrot Waste [51] | Sugarcane Bagasse [29] | Orange Peel [31] | Wastewater from Cheese Whey [59] | Rice Husk [60] |
| Moisture ** | 6.9% | 4.7% | 10.6% | 65.0% | 4.00% | N.R. | N.R. | 46.10% | 84.7% | 5.5% | N.R. | 77.38% | N.R. | N.R. |
| Total Sugar *** | N.R. | N.R. | N.R. | N.R. | 1.7% | 15.6% | 10.4% | 38.9% | N.R. | N.R. | 8.6% | 21.4% | 25.3% | 8.1% |
| Cellulose | N.R. | 7.3% | 15.5% | 25.8% | 32.4% | 42.0% | 10.5% | N.R. | 27.8% | 27.2% | 31.5% | 19.3% | N.R. | 28.8% |
| Hemicellulose | N.R. | 20.8% | 17.8% | 5.5% | 13.8% | 23.1% | 12.8% | 34.8% | 33.6% | 11.2% | 21.5% | 11.4% | N.R. | 12.3% |
| Lignin | N.R. | 17.4% | 7.8% | 6.6% | 46.6% | 17.3% | 2.6% | 12.0% | 16.2% | 29.2% | 27.2% | 2.0% | N.R. | 24.3% |
| Carbohydrates | 86.2% | 46.9% | 43.4% | 28.8% | N.R. | N.R. | N.R. | N.R. | 13.8% | 29.0% | 8.6% | 21.4% | N.R. | 8.1% |
| Starch | N.R. | N.R. | N.R. | 31.7% | N.R. | N.R. | 45.6% | N.R. | 2.4% | N.R. | N.R. | N.R. | N.R. | N.R. |
| Pectin | N.R. | 7.6% | 15.5% | 1.6% | N.R. | N.R. | N.R. | N.R. | 6.1% | 3.4% | N.R. | 15.0% | N.R. | N.R. |
| Fats | 0.9% | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | 5.7% | N.R. | N.R. | N.R. | 3.7% | 43.4% | N.R. |
| Protein | 12.9% | N.R. | N.R. | N.R. | 4.6% | N.R. | 7.7% | 6.2% | N.R. | N.R. | N.R. | 4.0% | 3.8% | 2.9% |
| Ash | N.R. | N.R. | N.R. | N.R. | 0.9% | 2.0% | 10.4% | 2.5% | N.R. | N.R. | 2.5% | 1.7% | 27.5% | 15.5% |
| Total and volatile solids | ||||||||||||||
| Fraction | Fermented cheese whey [61] | Apple pomace [62] | Watermelon rinds [63] | Potato peels [64] | Coffee cut-stem [65] | Oil palm rachis [66] | Plantain peel [67] | Avocado seed [68] | Tomato pomace [69] | Carrot waste [70] | Sugarcane bagasse [30] | Orange peel [32] | Wastewater from cheese whey [71] | Rice husk [72] |
| Moisture | N.R. | N.R. | 26.9% | N.R. | N.R. | N.R. | N.R. | 13.2% | 83.14% | N.R. | N.R. | 79.8% | N.R. | N.R. |
| Total solid | 67.3% | 17.3% | 55.3% | 9.1% | 50.2% | 45.1% | 11.0 | 50.3% | 15.4% | 9.8% | 56.0% | 51.1% | 50.4% | 52.6% |
| Volatile solid | 32.7% | 82.7% | 44.7% | 90.9% | 49.8% | 54.9% | 89.0 | 49.7% | 84.6% | 90.2% | 44.0% | 48.9% | 49.6% | 47.4% |
| Ratio of carbon and nitrogen | ||||||||||||||
| Fraction | Fermented cheese whey [61] | Apple pomace [73] | Watermelon rinds [74] | Potato peels [75] | Coffee cut-stem [76] | Oil palm rachis [55] | Plantain peel [67] | Avocado seed [77] | Tomato pomace [78] | Carrot waste [79] | Sugarcane bagasse [30] | Orange peel [80] | Wastewater from cheese whey [71] | Rice husk [72] |
| C/N | 8.13 | 61.89 | 42.76 | 10.7 | 48.9 | 62.8 | 39.0 | 57.5 | 11.00 | 27.00 | 33.69 | 33.83 | 5.8 | 38.48 |
* Chemical composition is reported in dry base; ** Initial moisture of raw material; *** Extractives content in raw material; N.R.: No Reported.
In recent years, pectin has emerged as a platform product for the synthesis of mucic acid, l-galactonic acid, l-ascorbic acid, 1-4 biotanedium, and ethanol [80]. This product synthesis is carried out from galacturonic acid, the main compound of pectin. Genetically modified micro-organisms have been used to synthesize galacturonic acid and to produce the above-mentioned products. Fibrous and pectic fractions of FL and FW have also been used to synthesize biofuels such as ethanol, butanol, and biogas [57]. Different types of processes, such as saccharification and simultaneous fermentation, have been implemented [81]. Different enzyme concentrations, types of micro-organism, and pretreatment stages (chemical and physical conversions such as steam exploitation, acid hydrolysis, steam distillation) have been applied to produce organic molecules and biofuels [60].
Fermentative processes upgrade hydrolysis products to other added-value platforms using micro-organisms and mild operating conditions (i.e., temperature: 30–50 °C and pressure: atmospheric) [82]. Then, the process energy intensity is low compared to thermochemical and catalytic pathways. Nevertheless, maximum titers and downstream processing (i.e., platform chemical separation from fermentation broth) must be considered before proposing a complete valorization of FL and FW.
3. Trends of Fermentation Products Obtained from Food-Waste Valorization
3.1. Classification Based on Micro-Organism, Mode of Cultivation, Water Activity, Oxygen Requirement, Nutrient Metabolism and Number of Inoculums
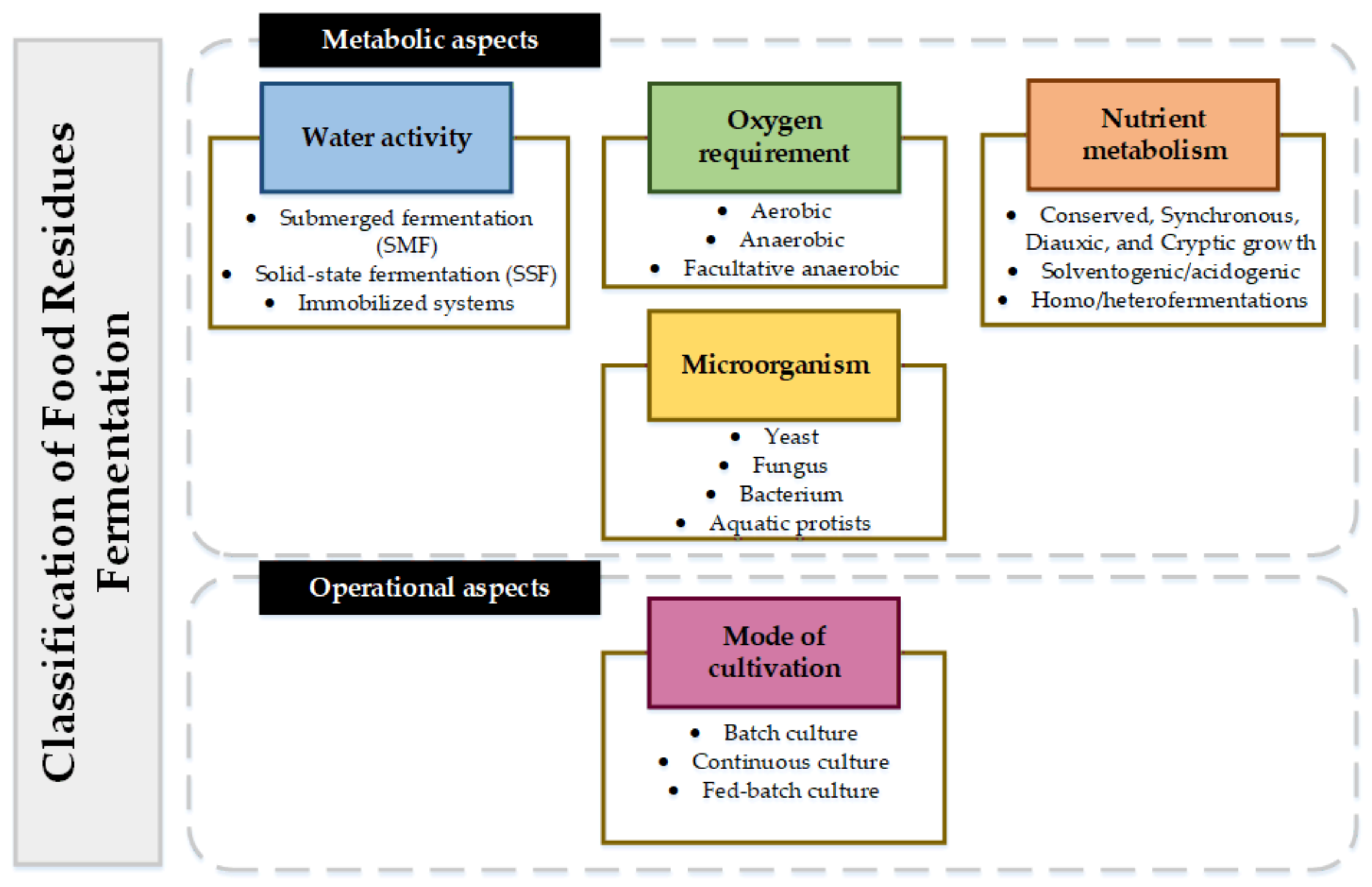
Fermentation is a valorization route with high application, high efficiency, and strong robustness (technological readiness level), and presents a wide range of possible value-added products. Fermentation can be classified considering different metabolic aspects such as water activity, oxygen requirements, and nutrient metabolism. According to technical aspects, fermentations can also be classified considering the reactor type or cultivation mode (i.e., continuously stirred tank reactors, single-stage or batch reactors, electro reactors, among others). Figure 2 presents the classification of fermentative processes:
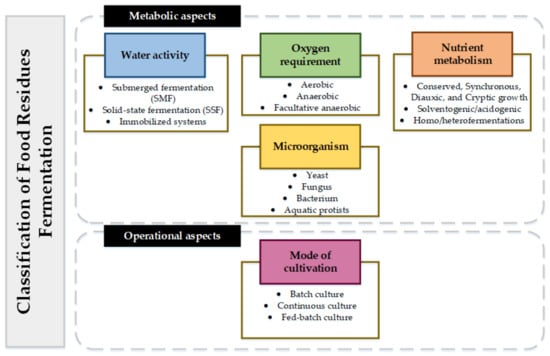
Figure 2.
Classification of FR fermentation.
Micro-organisms are classified as yeasts, fungi, bacteria, and aquatics protists. Aquatics protists are unicellular eukaryotic micro-organisms that can range from algae to heterotrophic flagellates [83]. One of the main characteristics of aquatics protists is the ability to synthesize between 20% w/w and 70% w/w lipids based on [84] cell weight. Depending on culture conditions, some aquatics protists can increase lipid production with high C/N [85] ratios. Among the lipids that protists aquatics can synthesize are saturated and unsaturated fatty acids with chain lengths between 4 and 28 such as omega-3, α-linolenic acid, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), among others [18].
In terms of oxygen requirement, micro-organisms can be classified into aerobic, anaerobic, and facultative anaerobes [86]. Micro-organisms demanding oxygen are aerobic. Anaerobic micro-organisms carry out fermentations without oxygen. Finally, facultative micro-organisms are anaerobic in the cell-growth phase and aerobic in the production phase.
The water activity of fermentation is classified into submerged fermentation (SMF) and solid-state fermentation (SSF). The difference between SMF and SSF is the type of substrate solution used (i.e., solid in SMF, liquid in SSF) [87]. In SSF, the micro-organism growth and the obtained product are carried out in solid particles with little moisture [88]. Some advantages of SSF over SMF are the low operating costs due to less use of water and reagents, fewer separation and purification steps, low energy consumption, and use of non-sterile substrates (non-sterile conditions for the process) [89]. In addition, SSF is carried out with simple technologies [90]. However, SSF has several challenges, such as mass and heat transfer on large scales, poor reproducibility, aeration of the substrate, and poor operational control due to the lack of reaction kinetics [87]. Another disadvantage of SSF is the number of micro-organisms and substrates that can be used in this type of fermentation [91]. The most studied micro-organisms to be used in SSF are fungi and yeasts [91]. This is why SMF is the most sought-after for obtaining commercial products.
Nutrient metabolism (physiological status) of micro-organisms in fermentations has different types of growth: conserved, synchronous, diauxic, or cryptic [92]. On the other hand, fermentations can be classified as solventogenic (i.e., production of solvents) or acidogenic (i.e., production of acids) [93] depending on the product type. When considering the metabolic pathways, homofermentative and heterofermentative processes can be identified. Homofermentative processes make use of metabolism under a substrate to obtain the product. Heterofermentative processes use several metabolic pathways for the synthesis of different substrates or nutrients [94].
Finally, fermentation can be carried out in batch, continuous, or fed-batch mode, depending on the operational aspects [95]. Selecting the type of fermentation and the type of micro-organism is a crucial step for FL and FW valorizing. The type of inoculum (micro-organism), pH, oxidation potential, organic loading rate, nutrients, and substrate type defines process productivity and yield. This section presents a systematic review of the fermentation of different types of FL and FW, considering micro-organisms, operating conditions, water activity, oxygen requirements, and yields to obtain different types of products.
3.2. Pretreatment and Sacarification Stage (Upstream)
Upstream from FL and FW fermentation are the pretreatment and saccharification processes to obtain fermentable sugars. The main objectives of FL and FW pretreatments are (i) to improve mass transfer, (ii) to reduce or to eliminate toxic compounds, (iii) to increase accessibility, and (iv) to increase digestibility [96,97]. The pretreatment selection is based on the FL and FW characteristics and the subsequent processing routes [98]. FL and FW are pretreated with combined physical, thermal and chemical processes [99].
The physical and mechanical pretreatments are grinding, cutting, and blending to increase the surface area [100]. The objective of the thermal pretreatments is to reduce the moisture, help to solubilize sugars, dilate the cell membrane and/or sterilize the biomass [101]. In this pretreatment type, the recalcitrance and chemical composition of biomass are affected. Chemical pretreatments are methods such as hydrolysis with acids (e.g., sulfuric acid, phosphoric acid, hydrochloric acid) or bases (e.g., NaOH, sodium sulfite, hydrogen peroxide). The chemical pretreatment objective is to solubilize the hemicellulose (acid hydrolysis) and the lignin (alkaline pretreatment), protein, and lipids [102]. The operating conditions of the chemical pretreatments of FL and FW must be studied to avoid the formation of inhibitory compounds (i.e., furanic and/or phenolic compounds) [103].
The type of pretreatment that is carried out for FL and FW differs from the fermentation and the chemical composition. Chemical pretreatments are used more in FL than in FW due to the differences in the amount of lignin (see Table 1). Another type of FL pretreatment considers the extraction of bioactive compounds or essential oil from fractions such as lipids, fats, volatile and extractive compounds (see Table 2). The extraction of these compounds for FW has not been widely analyzed. The main reason for this is the limitations of applications for the acquisition of bioactive compounds from FW. The pretreatments for FW are mainly physical and thermal.
Saccharification or enzymatic hydrolysis in FL and FW has been studied using a wide range of enzymes such as α-amylase, cellulases, xylanases, pectinases, proteases, chitinase, lactases, among others [104]. Research on the use of enzyme cocktails in FL and FW focuses on the evaluation of operating conditions (i.e., temperature, pH, agitation, incubation time, enzyme concentration, substrate concentration, among others) to obtain mainly sugars [105]. The enzyme cocktail selection for FL and FW depends on the nature and composition of the residue [106]. For FL, the production of fermentable sugars has been investigated from the most relevant raw material fractions (i.e., cellulose, pectin, and starch) [107]. For example, for agro-industrial residues such as sugarcane bagasse, the enzymatic hydrolysis step is aimed at breaking down the cellulose [108]. For orange peel waste, enzymatic hydrolysis aims to degrade the cellulose and pectin fraction [109]. For residues of amylase origin, the cellulose and starch fractions constitute fermentable sugars sources [110]. On the other hand, the production of fermentable sugars from FW presents a more complex challenge due to its versatility in its chemical composition (see Table 1) [111]. It is necessary to consider different types of enzymes (or enzyme cocktails) to hydrolyze the FW.
The obtained products from FL and FW fermentation can be grouped into energy vectors, biomaterials, organic acids, aromatic compounds, food and feeds, enzymes, and other compounds.
3.3. Products Obtained from Food-Waste Fermentation
3.3.1. Energy Production
The anaerobic digestion of FL and FW is a complex process that encompasses four phases carried out by a bacteria consortium (i.e., hydrolysis, acidogenesis, acetogenesis, and methanogenesis) for biogas production. Biogas is a mixture of methane (CH4), carbon dioxide (CO2) and other gases traces such as nitrogen (N2), oxygen (O2) and hydrogen sulfide (H2S) [112]. The hydrolysis phase is the conversion of complex organic molecules into simple chains (e.g., fatty acids to glycerol and short chains fatty acids, cellulose to cellobiose and glucose). Acidogenesis converts organic molecules into volatile fatty acids (VFA). In the acetogenesis phase, VFAs are converted to acetic acid, H2, and CO2. Finally, in methanogenesis, the generated products in acetogenesis are converted into CH4 and CO2. The variability of the CH4 yields is related to the composition of the FL and FW (i.e., content of carbohydrates, proteins, lipids) and to previous pretreatments stages [113]. In addition, the bacteria consortium in anaerobic digestion requires essential nutrients (i.e., nitrogen, phosphorous, magnesium, sodium, calcium, manganese, and cobalt) that directly affect methane production yields [114]. The yield of methane production by FW digestion is between 0.14 and 0.47 m3 CH4/kg-VS of raw material [114]. Chew et al. [112] analyzed the most influential factors for biogas production considering the characteristics of the substrate and the operating conditions. The challenges presented by anaerobic digestion depending on the substrate (i.e., FL and FW) is the optimal carbon and nitrogen (C/N) ratio to maximize CH4 production [115]. In general, the optimal C/N ratio is between 20 and 30. Lower C/N ratios lead to a higher nitrogen content substrate, which promotes VFA production. However, FW can have a complex C/N ratio from 3 to 55 [116]. Several studies have shown that codigestion of FL and FW improves biogas production by 40–50% [113]. Boua-llagui et al. [117] evaluated the biogas production from FW (fruit and vegetables) and slaughterhouse residues, eliminating between 73 and 89% of the total VS with a biogas yield of 0.3–0.73 m3/kg-VS. Parawira et al. [118] analyzed the production of biogas from potato residues and sugar beet leaves, obtaining a yield of 0.68 m3 CH4/kg-VS. Another limitation and challenge that anaerobic digestion presents is the operating conditions, such as the organic load rate, pH, temperature, moisture content, quality and quantity of the inoculum, agitation, and retention time. All the above influence biogas production [114]. These conditions limit the biodegradability of FL and FW, limiting the accessibility and nutritional balance of anaerobic digestion. Other authors such as Srisowmeya et al. [119], Lytras et al. [120] and Dalke et al. [121] evaluated the main opportunities and limitations of the anaerobic digestion of FL and FW considering aspects of the raw material and operating conditions.
Hydrogen (H2) is a sustainable and renewable fuel from biological, thermochemical, and chemical routes [122]. The biological route is carried out by bacteria-synthesizing enzymes of the hydrogenase and nitrogenase type by photo-fermentation and dark fermentation [123]. H2 production results from the breakdown of carbohydrates by acidogenic and acetogenic metabolic pathways [124]. Photo-fermentation and dark H2 fermentation are sensitive to operating conditions such as pH, temperature, partial product pressure, substrate concentration, and VFA content [125]. Some authors specify that the protein content of the substrate should be increased to improve the production of hydrogen by micro-organisms [126]. In this sense, greater C/N ratio values than 20 present lower H2 production yields [127]. Mohd et al. [128] defined an optimal C/N ratio to be between 20 and 21 according to the systematic review for assessing FL and FW. Some of the limiting factors in FL and FW photo-fermentations and dark fermentations are the pretreatment stages of the substrate and inoculum (i.e., heating between 80 and 140 °C), pH (between 5 and 6 in continuous fermentations and 7 in batch fermentations), and temperature (i.e., thermophilic conditions between 50 and 60 °C) [123]. The inoculum for the production of H2 needs thermal shocks and chemicals [129]. Table 3 shows some yields obtained from the processing of FL and FW to obtain H2.
Biohythane is a mixture of CH4 and H2 in a 1:4 ratio, considered to be a fuel with good caloric efficiency [130]. Biohythane arises as a solution for pure H2 due to storage problems [131]. CH4 makes it possible to reduce the flammability of H2, improving the storage problem. Biohythane production has been studied at laboratory and semi-pilot scale with an anaerobic bacteria consortium [132]. Table 3 presents some biohytane production yields from FL. In the case of FW, biohythane production has not been analyzed.
Ethanol is one of the most researched energy vectors for the use of FL and FW [133,134]. On the other hand, butanol has emerged as a possible substitute for ethanol [135]. Unlike the production of biogas, H2, and biohytane, the production of ethanol and butanol is carried out using fermentable sugars as a substrate. For this reason, the pretreatment and saccharification stage is essential for ethanol and butanol production yields.

Table 3.
Bioenergy products obtained from FL and FW fermentation.
Table 3.
Bioenergy products obtained from FL and FW fermentation.
| Micro-Organism | Water Activity | Oxygen Requirement | Product | Substrate | Operational Conditions | Yields and Productivity | Ref. |
|---|---|---|---|---|---|---|---|
| Candida rugosa | SMF | Anaerobic | Biogas | Food loss: Meat industry waste (pig meat) | Batch reactor volume: 80 mL; Inoculum concentration: 8 gVS/L; Temperature: 37 °C; Stirring rate: 150 rpm; pH: 7.0 | Biogas: 50.60%wt (Biomethane: 707 L/kg-VS) | [136] |
| Pig slurry (Firmicutes and Bacteroidetes) | SMF | Anaerobic | Biogas | Food loss: Tomato pomace (TP) and vegetable sludge (VS) | Anaerobic digester volume: 300 L; Semicontinuous mode; pH: 6.3–7.8; Temperature: 35 °C; Substrate load ratio: 70%:30% (TP and VS: Pig slurry); Stirring rate: 8–10 rpm; Total solids concentration: 6%; Total operating time: 110 days | Biogas: 60%wt | [137] |
| Agro-industrial wastewater treatment sludge | SMF | Anaerobic | Biogas | Food waste: Frozen food factory including fresh vegetable waste (VW) and precooked food waste (FW) | Batch reactor volume: 200 mL; pH: 7.0–8.0 Temperature: 35 °C; HRT: 31 days OLR: 3.5 gCOD/L-d; Sludge concentration: 22.2% wet basis; VW concentration: 32.3% wet basis; FW concentration: 45.5% wet basis | Biogas: 57%wt | [41] |
| Clostridium acetobutylicum | SSF | Anaerobic | Biohydrogen | Food loss: Defatted rice bran | Batch fermentation; Fermentation volume: 200 mL; Temperature: 34 °C pH: 5.5; Inoculum amount: 12.5%(v/v) | Biohydrogen: 10.55%wt (Biohydrogen: 117.24 mL/g sugar consumed) | [138] |
| Wastewater from chemical treatment | SSF | Anaerobic | Hydrogen | Food waste: Canteen-based composite food waste | Acidophilic microenvironment was used; Batch reactor was used; Fermentation volume: 180 mL; Temperature: 29 °C; pH: 6.0; Time: 71 h | Hydrogen: 14.06%wt (Hydrogen: 69.95 mmol) | [122] |
| Buttiauxella sp. 4, Rahnella sp. 10, and Raoultella sp. 47 | SSF | Anaerobic | Hydrogen | Food waste: Vegetable waste | Substrate particle size: 5 mm; Fermentation volume: 50 mL; Inoculum concentration: 10%v/v; Temperature: 28 °C; pH: 6.70; Stirring rate: 120 rpm | Hydrogen: 0.771%wt (Hydrogen: 85.65 mlH2/gVS) | [139] |
| Micro-organisms from wastewater of sweet potato processing factory | SSF | Aerobic | Hydrogen | Food waste: Kitchen waste and white rice | CSTR fermentation; Fermentation volume: 3 L; Temperature: 55 °C; pH: 5.4; Stirring rate: 160 rpm; Fermentation time: 20 days | Hydrogen: 0.255%wt (Hydrogen: 1.27 mmol/gCOD) | [140] |
| Anaerobic digester sludge | SSF | Anaerobic | Hydrogen and methane | Food waste: Potato waste, kitchen garbage, and soybean pulp | CSTR fermentation; Volume for hydrogenesis: 1 L; Volume for methanogenesis: 5 L; Time for hydrogenesis: 2 days; Time for methanogenesis: 10 days; Temperature: 55 °C; pH:5.5; Inoculum load: Added to fill ¼ volume of reactor | VS of Potato waste Hydrogen: 0.765%wt (Hydrogen: 85 mL/gVS) Methane: 22.206%wt (Methane: 338 mL/gVS) VS of Kitchen garbage Hydrogen: 0.594%wt (Hydrogen: 66 mL/gVS) Methane: 23.91%wt (Methane: 364 mL/gVS) VS of Soybean pulp Hydrogen: 0.18%wt (Hydrogen: 20 mL/gVS) Methane: 21.61%wt (Methane: 329 mL/gVS) | [125] |
| Sludge from anaerobic treatment of vinasse | SMF | Anaerobic | Biohythane (hydrogen and methane) | Food loss: Coffee residues | CSTR reactor; Fermentation volume: 4.3 L HRT: 55 days; OLR: 0.19 kg-VS-m3/d Temperature: 55 °C; pH: 5.5–6.0; Stirring rate: 2000 rpm | H2: 34.45%wt (H2: 30–40% in volume) CH4: 44.01%wt (CH4: 70% in volume) | [141] |
| Thermoanaerobacterium for hydrogen stage and Methanosarcina sp. for methane stage | SSF | Anaerobic | Biohythane (hydrogen and methane) | Food loss: Palm oil mill effluent (POME) | Temperature: 55 °C; Hydraulic retention time for hydrogen (HRT): 2 days; Organic loading rate for hydrogen (OLR): 27.5 gCOD/L-d; HRT for methane: 10 days OLR: 5.5 gCOD/L-d; pH: 5.0–6.5; POME is mixed with CH4 at a ratio of 1:1 before feeding the reactor tank | Biohythane: 38.60%wt (Biohythane: 1.93 L/L-d) Composition H2: 11%wt CO2: 37%wt CH4: 52%wt | [142] |
| Saccharomyces cerevisiae | SSF | Anaerobic | Ethanol | Food loss: Pineapple waste | Fermentation volume: 1 L; Substrate concentration: 3 g/L; Glucose content: 4.43%wt; Inoculum concentration: 3 g/L Temperature: 30 °C; pH: 4.0; Incubation period: 3 days | Ethanol: 8.7%wt | [143] |
| Pichia stipitis | SMF | Aerobic | Ethanol | Food loss: Defatted rice bran | Batch fermentation; Fermentation media: 300 mL in 500 mL flask; Stirring rate: 200 rpm; pH: 5.5; Temperature: 30 °C; Culture medium: 5.0 g/L xylose and glucose; Inoculum volume: 8.5 mL; Initial sugars: 33.55 g/L | Ethanol: 42%wt (Ethanol: 0.42 g/g sugar used) | [144] |
| Zymomonas mobilis—ZM4 | SMF | Anaerobic | Ethanol | Food waste from a local supermarket | Fermentation volume: 3 L; Glucose concentration in medium: 200 g/L; Substrate load: 50 kg; Water load: 25 kg Glucoamylase: 20,000 U/g; Temperature: 50 °C; pH: 4.0; Stirring rate: 100 rpm; Time: 6 h | Ethanol: 50%wt (Ethanol: 50 g/g glucose) | [145] |
| Clostridium saccharoperbutylacetonicum | SMF | Anaerobic | Acetone, butanol, ethanol, acetic acid, and butyric acid | Food loss: Rice bran | Batch fermentation; Fermentation volume: 150 mL; Substrate concentration: 10%(w/v); Initial pH: 6.5; Initial temperature: 30 °C; Fermentation time: 120 h; Inoculum concentration: 10%(w/v) | Acetone: 0.23%wt; Butanol: 2.31%wt; Ethanol: 0.21%wt; Acetic acid: 1.24%wt; Butyric acid: 1.90%wt Acetone: 0.23; Butanol: 2.31; Ethanol: 0.21; Acetic acid: 1.24; Butyric acid: 1.90(g/L) | [146] |
| Clostridium kluyver | SMF | Anaerobic | Ethanol, acetate, and butyrate | Food waste: Fruit vegetable waste (FVW) | FVW was smashed and homogenized. Batch fermentation; Fermentation volume: 1 L; Volatile solids concentration: 50 g/L; Inoculum to substrate ratio; Temperature: 35 °C; pH: 6.3 | Ethanol: 1.6%wt Acetate: 10.8%wt Butyrate: 6.6%wt (g/L) Ethanol: 0.8 Acetate: 5.4 Butyrate: 3.3 | [147] |
| Clostridium beijerinckii | SMF | Anaerobic | Acetone, butanol, and ethanol | Food waste: | Fermentation volume: 150 mL; Food-waste medium load: 50 mL; Stock buffer solution: KH2PO4, 0.5 g/L; K2HPO4, 0.5 g/L; and NH4CH3CO2, 2.2 g/L; Temperature: 35 °C pH: 6.0; Incubation period: 72 h | Acetone: 4.0%wt Butanol: 7.7%wt Ethanol: 0.7%wt Acetone: 4.0 g/100 g Butanol: 7.7 g/100 g Ethanol: 0.7 g/100 g | [148] |
| Clostridium sp. strain—BOH3 | SMF | Anaerobic | Acetone, butanol, and hydrogen | Food waste from local food courts in Singapore | Fermentation volume: 160 mL; Substrate concentration: 60 g/L; Temperature: 35 °C pH: 5.0–5.2; Incubation time: 20 h; Stirring rate: 130 rpm | Butanol: 8.00%wt Acetone: 3.00%wt Hydrogen: 0.016%wt (Hydrogen: 0.08 mmol/g) | [149] |
| Clostridium saccharoperbutylacetonicum | IS | Anaerobic | Acetone, butanol, and ethanol | Food waste: Bakery wastes, fruit wastes, and vegetables wastes | Food wastes were homogenized in a blender; Fermentation volume: 245 mL. Immobilized-cell system: Activated carbon; Activated carbon particle size: 3–6 mm; Dilution rate: 0.10 h−1; Temperature: 30 °C; Time: 12 h | Butanol: 10.47%wt (Butanol: 10.47 g/L) ABE: 43%wt | [150] |
SSF: Solid-state fermentation, SMF: Submerged fermentation, IS: Immobilized systems.
The production of ethanol and butanol has been evaluated by considering different types of micro-organisms. Saccharomyces cerevisiae is the most common yeast used for ethanol production from FL and FW due to high productivity and oxygen requirements (i.e., facultative anaerobic) and yields close to the theoretical value [151]. Zymomonas mobilis is another micro-organism (bacteria type) with great applicability to produce ethanol due to its anaerobic growth capacity, tolerance to inhibition by substrate, and metabolism through Entner (via Doudoroff) which promotes more biomass consumption and an increase in ethanol production using more carbon sources (e.g., glucose and xylose) [152]. Several alternatives have been evaluated for ethanol production from cocultures that promote enzyme production or the ability to use different substrates simultaneously. Ntaikou et al. [153] evaluated the cocultivation with Pichia stipitescepa CECT 1922 and S. Cerevisiae CECT 1339 for ethanol production from potato waste using hexoses and pentoses, obtaining yields of 0.15 L/g of raw material.
3.3.2. Biomaterials
Fermentation processes using FL and FW as raw materials can produce biomaterials such as biopolymers and biosurfactants. These products are biocompatible and biodegradable [154]. Then, several industrial applications related to the cosmetic, pharmaceutical, cleaning, medicine, and food sectors have been identified [155]. Moreover, the environmental impact of producing these biomaterials is low compared to those produced by chemical synthesis. Nevertheless, biopolymer and biosurfactant production has a common issue related to raw materials. Indeed, carbon sources (e.g., sugars and glycerol) have a relatively high cost since energy crops have been used (e.g., corn and wheat) [156]. On the other hand, synthetic materials have a more competitive price in the market. Table 4 presents information related to biomaterial production using different micro-organisms and operating conditions.
Research has aimed at producing biopolymers and biosurfactants when using FL (i.e., second-generation raw materials) as raw material [157]. Upstream processing is required since lignocellulosic materials have high recalcitrance for direct upgrading. Then, pretreatment must be done to reduce crystallinity and to improve enzymes and micro-organism accessibility. Despite the advances in this research field, FL has not been implemented at the commercial level to produce these biomaterials. On the other hand, FW is an interesting raw material from a theoretical point of view due to the high content of carbohydrates and fats. Nevertheless, few research papers have been published supporting or claiming reliable and feasible biomaterial production using FW. Thus, this is a research field highlighted by the authors since new methodologies and strategies for including FW-based processes are required. The main drawbacks of this raw material are related to non-standard composition, titer variability, and possible inhibition. On the other hand, downstream processing also requires a deep analysis due to a comprehensive study on product purification still being lacking.
Biopolymers are naturally produced by Gram-positive and Gram-negative bacteria (e.g., Bacillus sp., Pseudomonas sp., Clostridium sp., Azotobacter sp.) [156]. Polyhydroxyalkanoates (PHAs) are produced by SSF and SMF. Poly 3-hydroxybutyric acid (PHB) is the most common PHA. Several process configurations have been explored to improve PHB production [158]. Indeed, batch, repeated batch, fed-batch, fed-batch with cells recycling, one-stage chemostat, two-stage chemostat, and multi-stage chemostat have been proposed for improving process productivity. Higher yields and productivities can be obtained using continuous processes since operating conditions such as carbon-to-nitrogen molar ratio, temperature, pH, and substrate concentration can be monitored.

Table 4.
Biomaterials obtained from FL and FW fermentation.
Table 4.
Biomaterials obtained from FL and FW fermentation.
| Micro-Organism | Water Activity | Oxygen Requirement | Product | Substrate | Operational Conditions | Yields and Productivity | Ref. |
|---|---|---|---|---|---|---|---|
| Pleurotus sapidus | SSF | Anaerobic | Nanocellulose | Food loss: Sunflower seed hulls | Control medium: 2 g/L malt extract, 0.2 g/L yeast extract, and 1 g/L saccharose. Temperature: 85 °C; Fermentation time: 3 h | Microcrystalline cellulose: 63.4%wt Nanocrystalline cellulose: 66.7%wt | [159] |
| Trichoderma reesei | SSF | Aerobic | Xylose | Food loss: Brewers spent grain | Fermentation: 3 days; pH: 7.00; Temperature: 30 °C; Brewers spent grain: 20 g/L; Stirring rate: 180 rpm; Inoculum concentration: 1 × 106 spores/mL; Sterile solution: 0.8%(w/v) NaCl, 0.05%(w/v) | Xylose: 3.83%wt (Xylose: 38.3 mg/g of Brewers’ spent grain) | [160] |
| Bacillus amyloliquefaciens | SSF | Aerobic | Poly γ-glutamic acid | Food loss: Soybean dregs | Continuous batch fermentation; Fermentation volume: 5 L; Initial moisture of substrate: 70%; Substrate load: 400 g (375.6 g fresh soybean dregs and 24.4 g molasses meal); Inoculum size: 12%; Fermentation temperature: 30 °C Initial pH: 8.0; Fermentation time: 72 h | Poly γ-glutamic acid: 6.58%wt (Poly γ-glutamic acid: 65.79 g/kg) | [161] |
| Activated sludge | SMF | Anaerobic | Polyhydroxyalkanoates (PHA) | Food loss: Fermented cheese whey | Fermentation volume: 500 mL; Inoculum: Cheese whey ratio: 1:5; Inoculum load: 50 g Total Suspended Solids/L; Incubation time: 96 h; Stirring rate: 150 rpm; Fermentation temperature: 37 °C; pH: 5.5 | PHA: 28.2%wt (PHA: 28.2 g/L) | [61] |
| Xanthomonas citri | SSF | Aerobic | Xanthan | Food loss: Potato peel | Substrate load: 50 g; Temperature: 28 °C pH: 7.2; Time: 24 h | Xanthan: 5.80%wt (Xanthan: 2.90 g/50 g peel) | [162] |
| Haloferax mediterranei | SSF | Aerobic | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): (P(3HB-co-3HV)) | Food loss: Cheese whey | Batch fermentation mode; Fermentation volume: 2 L; Temperature: 37 °C; pH: 7.2 Air flow rate: 1 vvm; Dissolved oxygen concentration: 20%; Stirring rate: 200–800 rpm | P(3HB-co-3HV): 9.6%wt (P(3HB-co-3HV): 9.6 g/L) | [163] |
| Recombinant Escherichia coli | SSF | Aerobic | Polyhydroxybutyrate (PHB) | Food loss: Corn steep liquor | Fermentation volume: 50 mL; Inoculum concentration: 0.01 g dry cell/L; Temperature: 37 °C; pH: 7.2; Antifoam concentration: 30µL/L; Incubation period: 48 h | PHB: 6.12%wt (PHB: 6.12 g/L) | [164] |
SSF: Solid-state fermentation, SMF: Submerged fermentation, IS: Immobilized systems.
Moreover, the continuous process contributes to economic feasibility due to high productivity and specific growth rates. Nevertheless, continuous fermentation trends towards microbial contamination [157]. Thus, a careful study of the possible implementation of this operating mode should be done. In addition, techno-economic and environmental analyses of different PHB process configurations need to be analyzed due to the lack of data in the open literature. Indeed, a deep analysis considering kinetic modeling and micro-organism recirculation should be analyzed to discover washout conditions and possible pathways for improving yields (see Table 4). Regarding raw materials used for PHB production, FL has been reported in the literature. For instance, potato processing waste, sugarcane bagasse, waste frying oil, palm oil mill effluent, and olive oil mill waste have been used to produce PHB. Titers vary from 5 g/L to 70 g/L. Finally, FW was also studied at the experimental level using diary waste, but a low yield was reported (i.e., 0.28 g/L) [154].
Xanthan gum (an anionic biopolymer) is another fermentation product. This compound has applications in the food and beverage industries. Xanthan gum concentration after the fermentation process is in the range of 2–4% [165]. In line with PHA and biosurfactant production, Xanthan gum fermentation requires strict conditions, since this process is sensitive to pH changes and inhibitor presence. Then, the same hotspots listed for biopolymers and biosurfactants can be applied here.
3.3.3. Organic Acids
Organic acids such as lactic acid, succinic acid, itaconic acid, acetic acid, and 3-Hydroxypropionic acid (3-HPA) are potential products obtained from FL and FW fermentation [166]. These organic acids have been considered to be building blocks/platforms since further processing can be applied to obtain high-added-value products (e.g., biomaterials). Organic acids constitute a key role in producing and commercializing several final products for food, pharmaceutical, and biomaterials industries. Research and development (R&D) has focused more on upgrading FL than FW since FL has a standard/homogenous composition over time compared to FW. Several research studies have addressed efforts to obtain the above-mentioned organic acids when using FL, such as winery, grape, and corn residues [167]. Nevertheless, FW upgrading via a fermentation process has been studied due to the high content of carbohydrates and lipids in the raw material.
Table 5 presents the yields, micro-organism, and organic acid type derived from FL and FW fermentation.
Lactic acid production has been one of the most studied processes since the demand for this organic acid has increased over the years. Indeed, lactic acid produced by fermentation is preferred over chemically produced lactic acid. This organic acid is the unique monomer used to produce biodegradable polymers (e.g., polylactic acid) [168]. Several wild and engineered strains have upgraded carbon sources such as glucose and glycerol. Solid submerged and solid-state fermentations have been applied at an industrial level as alternatives for this organic acid production.

Table 5.
Organic acids obtained from FL and FW fermentation.
Table 5.
Organic acids obtained from FL and FW fermentation.
| Micro-Organism | Water Activity | Oxygen Requirement | Product | Substrate | Operational Conditions | Yields and Productivity | Ref. |
|---|---|---|---|---|---|---|---|
| Penicillium echinulatum | SSF | Anaerobic | Lactic acid | Food loss: Rice husk | Fermentation volume: 1.0 L; pH: 6.0; Nutrients solution (g/L): proteose peptone, 10.0; yeast extract, 5.0; ammonium citrate, 2.0; and dipotassium phosphate, 2.0.; Temperature: 30 °C | Lactic acid: 12.69%wt (Lactic acid: 12.69 g/L) | [169] |
| Lactobacillus rhamnosus | SSF | Anaerobic | Lactic acid | Food loss: Brewer spent grains | Batch fermentation; Stirring rate: 15 rpm Fermentation volume: 300 mL; Brewer spent grains: 200 mL; Brewer yeast: 50 g/L Inoculum concentration: 5%(v/v); Temperature: 37 °C; pH: 6.2 | Lactic acid: 89%wt | [170] |
| Lactobacillus paracasei | SSF | Anaerobic | Lactic acid | Food loss: Molasses-enriched potato stillage | Batch fermentation (48 h) and Fed-batch fermentation (24 h); 200 mL of stillage + 32 g of molasses; pH: 6.5; Adaption media (g/L): Peptone, 10; meat extract, 10; yeast extract, 5; K2HPO4, 2; C2H9NaO5, 5; C6H17N3O7, 2, and 1 L of distillated water | Lactic acid: 89.0%wt (Lactic acid: 0.89 g/g) | [171] |
| Bacillus coagulans | SSF | Anaerobic | L-lactic acid | Food loss: Defatted rice bran | Batch fermentation; Fermentation volume: 1 L; Substrate load: 20%(w/w); Temperature: 52 °C; Stirring rate: 400 rpm; pH: 6; Inoculum concentration: 6%(v/v); Fermentation time: 25 h | Lactic acid yield: 90%wt Lactic acid productivity: 33.7%wt (Lactic acid productivity: 2.7 g/L-h) | [172] |
| Lacticaseibacillus rhamnosus and Aspergillus niger | SSF and SMF | Aerobic | Lactic acid | Food loss: Dry corn stover | SSF: Fermentation time: 250 mL; Glucoamylase: 8%(w/w); Inoculum: 6%(v/v); Substrate: Chopped to a sized of 3–5 cm; Substrate load: 25 g (dry basis); Temperature: 35 °C; pH: 4.6; Stirring rate: 120 rpm; Time: 96 h SF: Fermented substrate was mixed with glucoamylase: 1850 U/g; pH: 5.3 | Lactic acid: 45.5%wt (Lactic acid: 45.5%w/w) | [173] |
| Yarrowia lipolytica | SSF | Aerobic | Citric acid, mannitol, arabitol, and erythritol | Food loss: Olive-mill wastewater | Fermentation volume: 250 mL; Substrate concentration: 8.98 g/L; Stirring rate: 180 rpm; Peptone: 1.0 g/L; Yeast extract: 1.0 g/L; pH: 5.0–6.0; Temperature: 28 °C; Fermentation time: 191 h | Citric acid: 27.0%wt Mannitol: 15.0%wt Arabitol: 5.0%wt Erythritol: 23.0%wt (g/g of raw material) Citric acid: 0.27 Mannitol: 0.15 Arabitol: 0.05 Erythritol: 0.23 | [174] |
| Aspergillus ornatus and Alternaria alternata | SSF | Aerobic | Citric acid | Food loss: Apple Pomace and peanut shell | Fermentation volume: 500 mL; Substrate load: 25 g; Substrate mixture ratio: 50:50; Moisture content: 50%; Nutritional ingredient: Arginine; Temperature: 30 °C pH: 5.0; Time: 48 h; Stirring rate: 180 rpm | Citric acid: 5.28%wt (Citric acid: 2.64 mg/mL) | [175] |
| Digested sludge inoculum | SSF | Anaerobic | Propionic acid | Food loss: Winery waste | CSTR fermentation; Fermentation volume: 50 L; Substrate: Inoculum ratio: 1:1; Inoculum concentration: 5 gVVS/L; Temperature: 35 °C; pH: 5.5 | Propionic acid: 50.7%wt (Propionic acid: 1.0 gCOD/L) | [176] |
| Digested sludge inoculum | SSF | Anaerobic | Propionic acid | Food loss: Meat and bone meal | Batch fermentation; Fermentation volume: 500 mL; Substrate concentration: 10 g COD/L; Inoculum concentration: 5 gVVS/L; Temperature: 55 °C; pH: 5.5; Fermentation time: 10 day | Propionic acid: 14.3%wt (Propionic acid: 1.43 gCOD/L) | [176] |
| Aspergillus awamori and Aspergillus oryzae. Actinobacillus succinogenes and Escherichia coli | SSF | Anaerobic | Succinic acid | Food loss: Mixed food waste and Bakery waste | Enzymatic hydrolysis: Substrate load: 8.5 g (dry weight); Inoculum concentration: A. awamori, 4.6 × 105 spores/mL; A. oryzae, 6.3 × 105 spores/mL; Temperature: 30 °C; Incubation period: 7 days Bacterial fermentation: Fermentation volume: 2.5 L; Hydrolysate volume: 1.5 L Inoculum size: 10%(v/v); Initial glucose concentration: 58 g/L; Temperature: 37 °C Stirring rate: 150 rpm; Time: 6 h | Succinic acid: 22.4%wt (Succinic acid: 22.4%w/w) | [177] |
| Actinobacillus succinogenes | SSF | Anaerobic | Succinic acid | Food Loss Bread loss | Bread was cut into cube: 1 cm3 Substrate load: 10 g; Inoculum concentration: 2.85 × 107 spores/mL; Temperature: 30 °C pH: 6.6–6.8; CO2 flow rate: 0.5 vvm; Stirring rate: 300 rpm; Time: 3 h | Succinic acid: 55.0%wt (Succinic acid: 0.55 g/g bread) | [178] |
| Rhizopus oryzae | SMF | Aerobic | Fumaric acid | Food loss: Apple pomace | Fermentation volume: 142.1 mL; Substrates concentration: 40 g/L of total solids; pH: 6; Temperature: 30 °C; Stirring rate: 200 rpm; Incubation time: 72 h | Fumaric acid: 3.58%wt (Fumaric acid: 0.35 g/L-h) | [179] |
| Rhizopus oryzae | SSF | Aerobic | Fumaric acid | Food loss: Apple pomace | Substrate load: 250 g; Inoculum concentration: 1 × 107 spores/g dry weight; Temperature: 30 °C; Time: 21 days | Fumaric acid: 5.2%wt | [179] |
| Rhizopus oryzae | SSF | Aerobic | Fumaric acid | Food waste: Obtained from university canteen | Substrate load: 106.36 g/L. Solid-liquid ratio: 1:10; Inoculum concentration: 20%(v/v); Temperature: 30 °C; Stirring speed: 180 rpm. Pressure: 4 MPa. Time: 150 min | Fumaric acid: 23.94%wt | [180] |
| Rhizopus arrhizus | SSF | Anaerobic | Fumaric acid | Food waste: Obtained from the dining hall university | Fermentation volume: 50 mL; Substrate particle size: 2–5 mm; Substrate load: 4.58 g/L; Temperature: 30 °C; Stirring speed: 200 rpm; Time: 168 h. Glucose: 80 g/L; | Fumaric acid: 32.68%wt | [181] |
| Bacillus subtilis | SSF | Anaerobic | Indole-3-acetic acid | Food loss: Cassava fibrous residue | Fermentation volume: 27 mL; Substrate load: 20 g; Moisture: 70% achieved with peptone (N 0.15%); Inoculum concentration: 1 × 106 CFU/mL); Temperature: 30 °C; pH: 7.00; Fermentation time: 10 days | Indole-3-acetic acid: 75.96%wt (Indole-3-acetic acid: 22.8 µg/g) | [182] |
| Providencia sp. | SSF | Anaerobic | Indole-3-acetic acid | Food waste: Obtained from food-waste compost | Fermentation volume: 200 mL; Inoculum concentration: 2% (v/v); NaCl concentration 4% (w/v); Temperature 25 ℃; Initial pH = 5; L-tryptophan concentration 3.0 g/L; Time: 12 h | Indole-3-acetic acid: 96.65%wt | [183] |
| Bacillus pumilus | SMF | Anaerobic | Gallic acid, protocatechuic acid, and p-Coumaric acid | Food loss: Soybean fermented food | Fermentation volume: 90 mL; Temperature: 37 °C; Inoculum concentration: 5%(w/w); Fermentation time: 60 h | Gallic acid: 37.8%wt Protocatechuic acid: 46.3%wt p-Coumaric acid: 1.5 × 10−4%wt (mg/kg) Gallic acid: 1012 Protocatechuic acid: 4.63 p-Coumaric acid: 0.15 | [184] |
| Ganoderma lipsiense | SSF | Anaerobic | Caffeic acid | Food waste: Obtained from university canteen | Fermentation volume: 75 mL; Medium moisture: 51%; mycelium suspension volume: 15 mL; Temperature: 28 °C; Time: 49 days; Substrate load: 25 g | Caffeic acid: 68.92%wt | [185] |
| Pediococcus Pentosaceus | SSF | Anaerobic | Ellagic acid | Food Loss Cloudberry juice | Fermentation volume: 15 L; Inoculum load: 1 × 106 CFU/g; Temperature: 30 °C; Incubation period: 14 days; Stirring rate: 130 rpm | Ellagic acid: 85%wt | [186] |
SSF: Solid-state fermentation, SMF: Submerged fermentation, IS: Immobilized systems.
Fermentation yields range between 0.45 g of lactic acid/g of glucose to 0.97 g of lactic acid/g of glucose in batch, fed-batch, and continuous mode [187]. Currently, lactic acid is produced from corn at the industrial level. Raw materials (i.e., carbon and nitrogen sources) account for 35% of the total operating cost of the process [166]. Then, alternative raw materials have been proposed as a strategy to decrease operational expenditure. Despite the recent advances related to lactic acid production using FW, the most suitable raw materials for processing are FL due to the standard composition and management. Then, agricultural and agro-industrial residues are attractive for implementation.
3-HPA is a structural isomer of lactic acid. Nevertheless, large-scale production using fermentative pathways has not been implemented commercially since hotspots related to titer, yield, and productivity must be overcome. Research efforts have been made to demonstrate the feasible production of 3-HPA when using different carbon sources, such as C6 sugars and glycerol [188]. Fermentation for producing 3-HPA involves using recombinant strains such as E. coli and K. pneumonia due to the need to improve hotspots related to metabolism [189]. This organic acid can be upgraded to acrylic acid, which has several applications in health care products, adhesives, and metal lubricants. In addition, 3-HPA is used to produce superabsorbent polymers [166]. The study of 3-HPA production still requires more effort to ensure a cheap raw material. FW has been proposed as an ideal substrate since these materials are rich in carbohydrates and fatty acids. However, few literature reports have addressed 3-HPA production using this raw material.
Finally, other organic acids such as citric acid, succinic acid, malic acid, and acetic acid also can be produced via FL or FW fermentation. These processes have been widely reported in the open literature. Nevertheless, chemical routes have been implemented instead of fermentative processes since high conversions are obtained when using synthetic chemical raw materials.
3.3.4. Aromatic Compounds
Aromatic compound production is carried out using three routes: (i) chemical synthesis; (ii) direct extraction from a natural matrix; and (iii) direct extraction from biotechnological processes (e.g., microbial cultures, use of enzymes and microbial cell cultures) [190]. The direct extraction of a natural matrix and the biotechnological route have gained great importance in recent years [191]. The main reason for this is that the aromatic compounds obtained by these routes can be labeled as “natural” and friendly to the environment [192].
The microbial culture route for aromatic compound production is carried out by bioconversion/biotransformation and fermentation [193]. In biotransformation, the micro-organism converts a precursor into an interest product through one or more reactions [194]. The transformation is performed by an enzyme complex that catalyzes the biotransformation reaction. Sale et al. [193] evaluated the ferulic acid biotransformation to vanillin from stereoselective or regioselective changes. This type of biotechnological route starts from the modification in the genomes of micro-organisms [195]. An example of this is the genetic modification of Escherichia coli with the gene-encoding lipoxygenase (obtained from Pleurotus sapidus) to convert valencene into the grapefruit flavor nootkatone [196]. The FRs analyzed under this transformation route are the AL (FL). However, biotransformation processes have only been analyzed using precursors, and not biomass directly [195].
Table 6 presents different studies aimed at the production of aromatic compounds from FL:

Table 6.
Aromatic compounds obtained from FL and FW fermentation.
In the fermentation routes to produce aromatic compounds, a complete metabolic pathway is involved [193]. The carbohydrate catabolism is carried out to produce primary metabolites and aromatic compounds [205]. One of the main limitations of aromatic compound production by fermentation is the need to support the initial growth of the micro-organism with glucose [206]. These limitations have been overcome with the implementation of SSF and mixing different FL. Aggelopoulos et al. [90] evaluated the production of volatile aroma compounds from AL mixed with Kluyveromyces marxiaunus and S. cerevisiae.
3.3.5. Foods and Feeds
Fermentative processes are applied for the production of foods (e.g., beverages), food additives (e.g., sweeteners), and feeds (e.g., protein and fibers). Fermentative processes play a key role in the food industry since microbial fermentation can help improve organoleptic properties by improving flavor and aroma. Cocoa bean fermentation is one of the most important stages for producing chocolate [207]. Indeed, cocoa bean fermentation contributes to the development of the chocolate flavor since a series of micro-organisms (e.g., bacteria and yeast) produce a wide range of organic compounds with special characteristics. Then, fermentation can be considered to be a fundamental activity in the food and feed industrial sector. Certainly, food fermentation is considered to be a food-processing technology due to process complexity and understanding [208].
Similar applications can be found in the open literature to produce yogurt, beer, cider, and wine, among others. On the other hand, carbon source (e.g., sugar) fermentation when using different micro-organisms can lead to the production of food additives such as sweeteners (e.g., all sugar alcohols), pigments, antibiotics, and amino acids [209]. Food additives can be produced using FL and FW as raw materials since both feedstocks have a high fermentable substrates content. Regarding FL, cane molasses has been used to produce isomaltulose using engineered Yarrowia lipolytica strain. A maximum titer and yield of 161.2 g/L and 0.96 g/g were obtained in fed-batch mode after an operation time of 3.5 days [210]. Another example is related to the sugar alcohol production (i.e., mannitol, xylitol, sorbitol, and erythritol). These compounds are produced using yeasts and bacteria. Sugar alcohol production has been researched widely when using different raw materials [211]. Nevertheless, most cases related to sweetener production are associated with FL since few research reports have studied FW as a potential raw material. This fact can be attributed to the raw material origin, as human products must guarantee security. Indeed, consumers probably would not consume sweeteners if they knew the raw materials used in the production process (e.g., organic kitchen food waste). Finally, feed products such as soluble dietary fiber can be produced via food-waste fermentation using Aspergillus oryzae [212]. These kinds of products are produced to improve the nutritional properties of feed.
Table 7 presents a list of substrates and micro-organisms used for food and feed production through fermentation.

Table 7.
Foods and feeds obtained from FL and FW fermentation.
3.3.6. Enzymes
Enzymes are essential when considering biomass processing, since these proteins break down structural polymer linkages to produce oligomers (e.g., disaccharides) and monomers (e.g., monomeric sugars). Indeed, enzymes have been the basis for proposing biomass upgrading to classical products such as ethanol and lactic acid [219]. Commercial enzymes constitute a cocktail/mixture of different proteins with specific functions. Commercial cellulases (e.g., Celluclast 1.5 L or CellicCTec2) are a mixture of endo-beta-1,4-glucanase (1,4-beta-D-glucan-glucanohydrolaza, E.C.3.2.1.4), exo-beta-1,4-glucanase (1,4-beta-D-glucan cellobiohydrolase, E.C.3.2.1.91) and beta-1,4-glucosidase (cellobiohydrolase, beta-D-glucoside glucohydrolase, E.C.3.2.1.21). This cocktail degrades cellulose to produce glucose for further fermentation. Nevertheless, few reports have focused on the improvement of the enzyme production process due to already existing and standard procedures implemented at the industrial level. In addition, few reports have focused on reviewing substrates used to produce enzymes. Therefore, this section aims to describe the enzyme production process, substrates, fermentation mode based on water activity, hotspots, and potential improvements based on the literature.
Enzymes are produced using fungus as a micro-organism. Aspergillus niger and Trichoderma ressei are the most used fungus to produce hydrolases (e.g., aminopeptidase, arabinofuranosidase, catalase, cellulase, glucanase, lactase, pectinase, and xylanase) [220]. Another fungus has been used to produce a wide variety of hydrolases (see Table 8). Hydrolases are the most common enzymes applied in biomass upgrading processes. The operating conditions of the fermentation process should be monitored to control pH and temperature, since proteins tend to denature. Enzyme production processes involve nine stages: (i) raw materials conditioning; (ii) sterilization; (iii) inoculum addition; (iv) fermentation; (v) flocculation; (vi) filtration or centrifugation; (vii) ultrafiltration; (viii) stabilization; and (ix) bacterial filtration [221]. Regardless of the fermentation type (i.e., SSF or SF), the above-mentioned steps could be considered to be similar stages. SSF has been the most applied process for enzyme production (see Table 8). Process optimization for the increase of productivities and yields has been addressed to improve the capability of fungus to degrade substrates based on mutagenesis and screening, the addition of stronger promoters, and multicopy strains [221]. Regarding downstream processing, membrane chromatography has been assessed as an alternative for fast enzyme purification. Nevertheless, this process has not been implemented at a commercial level.
FL and FW can be used as substrates in the enzyme production process (see Table 8). Indeed, Steudler et al. [222] reported the potential implementation of agricultural and agro-industrial residues as a substrate for enzyme production. High enzyme production yields were acquired when using wheat bran and straw. However, other raw materials have proved to be excellent substrates (e.g., orange peel waste) [221]. Therefore, related research aiming to find cheap and efficient raw materials for enzyme production is needed since the costs can account for more than 15% of the total operating costs of a bioprocess (e.g., cellulosic ethanol and lactic acid) [223]. Moreover, research and development must be addressed to obtain higher enzyme amounts and different activities. Indeed, DuPont published the patent US 8765.442 B2 describing a process for the production of an enzyme product with a plurality of enzyme activities obtained by fermentation of an Aspergillus sp. strain [224]. The patent describes the use of wheat brand (FL) to obtain an enzyme product comprising endo-polygalacturonase activity, exo-polygalacturonase activity, pectinesterase activity, pectin lyase activity, cellulase activity, xylanase activity, and arabinanase activity.

Table 8.
Enzymes obtained from FL and FW fermentation.
Table 8.
Enzymes obtained from FL and FW fermentation.
| Micro-Organism | Water Activity | Oxygen Requirement | Product | Substrate | Operational Conditions | Yields and Productivity | Ref. |
|---|---|---|---|---|---|---|---|
| Penicillium simplicissimum, | SSF | Aerobic | Lipase | Food loss: Castor bean waste; Jatropha curcas seed cake; Sugarcane bagasse, sunflower seed, and olive oil | Substrate particle size: 0.42–1.18 mm; Substrate load: 20 g; Temperature: 30 °C; Relative moisture: 95%; Fermentation time: 96 h | Lipase: 35.5%wt (Lipase: 155 U/g) | [225] |
| Lysinibacillus sp. | SSF | Aerobic | Laccase | Food waste: Whear bran | Fermentation volume: 250 mL; Inoculum volume: 0.5 mL; Temperature: 37 °C; Fermentation time: 72 h: Stirring rate: 160 rpm | Laccase: 18.9%wt | [226] |
| Bacillus halodurans | SSF | Aerobic | Fibrinolytic enzyme | Food waste: Banana peel, black gram husk, cow dung, paddy straw, rice bran, and wheat bran | Fermentation volume: 100 mL; Moisture: 80%; Temperature: 50 °C; pH: 8.32; Substrate load: 5.0 g; Inoculum concentration: 2–12% | Fibrinolytic enzyme: 13.7%wt (Fibrinolytic enzyme: 6851 U/g) | [227] |
| Aspergillus brasiliensis | SMF | Aerobic | Pectin lyase | Food waste: Corn steep liquor and orange peel | Agro-industrial medium: 160 g/L orange peel, 150 g/L corn steep liquor, and 300 g/L; Temperature: 30 °C; Initial pH: 5.5; Fermentation time: 100 h; Stirring rate: 180 rpm | Pectin lyase: 15.3%wt (Pectin lyase: 46 U/mL) | [228] |
| Fusarium oxysporum | SSF | Anaerobic | Protease | Food loss: Agro-industrial waste rice bran | Fermentation volume: 250 mL; Initial moisture content: 50%(w/w); Substrate load: 10 g; Temperature: 35 °C; pH: 7.0; Incubation period: 7 days; Stirring rate: 2000 rpm | Protease: 32.7%wt (Protease: 70.5 U/g) | [229] |
| Aspergillus niger | SSF | Anaerobic | Polygalactouronase | Food loss: Wheat bran, Coffee pulp | Wheat bran load: 1.5 g; Coffee pulp load: 23.1 g; Glucose concentration: 20 g/L; Distilled water volume: 70 mL; Temperature: 30 °C; pH: 4.5; Inoculum: 2 × 106 spores/mL; Time: 72 h; Stirring rate: 300 rpm | Polygalactouronase: 14.6%wt (Polygalactouronase: 515 U/L) | [230] |
| Bacillus subtilis | SSF | Aerobic | α-amylase | Food loss: Banana peel | Fermentation volume: 100 mL; Temperature: 35 °C; pH: 7.0; Incubation time: 24 h; Substrate moisture: 80%; | α-amylase: 6.38%wt (α-amylase: 7.26 U/mL/min) | [231] |
| Streptomyces sp. | SSF | Anaerobic | Cellulase | Food loss: Fruit waste | Substrate load: 10 g; Temperature: 40 °C pH: 5.0; Incubation period: 4 days | Cellulase: 13.6%wt (Cellulase: 20 U/mL/min) | [232] |
| Aspergillus awamori | SSF | Aerobic | Cellulase, xylanase, exo-polygalacturonase, and endo-polygalacturonase | Food loss: Grape pomace | Fermentation volume: 100 mL; Substrate load: 10 g; Medium moisture: 60% Inoculum concentration: 5 × 105 spores/g Temperature: 30 °C Incubation period: 25 h | Cellulase: 10%wt; Xylanase: 40%wt; Exo-polygalacturonase: 25%wt; Endo-polygalacturonase: 0.03%wt (IU/g dry substrate) Cellulase: 10; Xylanase: 40; Exo-polygalacturonase: 25; Endo-polygalacturonase: 0.03 | [233] |
| Penicillium viridicatum | SSF | Anaerobic | Polygalacturonase | Food loss: Wheat bran | Fermentation volume: 250 mL; Substrate load: 5 g; Inoculum load: 1 × 107 spores/g dry substrate; Temperature: 55 °C; Time: 14 days | Polygalacturonase: 30%wt (Polygalacturonase: 30 U/g) | [234] |
| Penicillium viridicatum | SSF | Anaerobic | Pectin lyase | Food loss: Orange bagasse | Fermentation volume: 250 mL; Substrate load: 5 g; Temperature: 50 °C; Time: 14 days | Pectin lyase: 40.0%wt (Pectin lyase: 2000 U/g) | [234] |
| Aspergillus oryzae | SSF | Aerobic | Pectinase | Food loss: Citrus pulp and sugarcane bagasse | Packed-bed bioreactor; Substrate load: 15 kg; Citrus pulp, 51.6%wt; sugarcane bagasse, 48.4%wt; Inoculum concentration: 4 × 107 spores/g dry substrate; Temperature: 30 °C; Aeration rate: 100 mL/min; Incubation time: 48 h | Pectinase: 2.46%wt (Pectinase: 37 U/g) | [235] |
| Penicillium chrysogenum and Trichhoderma viride | SSF | Anaerobic | Tannin acyl hydrolase or Tannase | Food waste: Grape peel | Fermentation volume: 250 mL; pH: 5.5; Temperature: 30 °C; Substrate load: 5 g; Incubation period: 96 h | Tannase: 0.96%wt (Tannase: 84 U/g/min) | [236] |
SSF: Solid-state fermentation, SMF: Submerged fermentation, IS: Immobilized systems.
Despite efforts to improve process yields, techno-economic assessment of enzyme production processes analyzing the influence of the composition, and downstream process configuration, has not been reported widely in the open literature. Then, an uncertainty related to real enzyme production costs based on different raw materials will continue. This research field can be explored with the aid of simulation tools.
3.3.7. Other Organic Compounds
Table 9 shows other organic compounds (e.g., volatile compounds, AGV, vitamins, antibiotics, and herbicide) obtained through FL and FW fermentation processes. The production of volatile compounds (i.e., esters, alcohols, carbonyl compounds, and organic acids) has been investigated from FL (AL and AgL) by mixing substrates to enhance carbohydrate content [90].
VFA (i.e., acetic, propionic, butyric, valeric, and hexanoic acids) are important intermediates during the acidogenesis stage in anaerobic digestion [237]. VFA are considered to be a platform product for the recent biosurfactants, bioflocculants and biopolymers [238]. VFA production can be influenced by operating conditions such as substrate concentration (specifically the C/N ratio), solid retention times, temperature, and pH, mainly. Unlike the anaerobic digestion directed to the production of biogas, the production of VFA is carried out in the first 36 h in batch processes [239]. In relation to the temperature, VFA production is favored between 25 and 55 °C. For FW, higher VFA production yields have been reported under acidic conditions (e.g., 5.25 to 11) [240]. In fact, higher VFA production yields have been reported when the pH is controlled at 6 during the entire digestion time.
In SSF, a wide range of products that can be obtained from substrates such as FL have been studied. Among these products, vitamin B complex (biological precursor/provitam of vitamin D2), pigments, and flavor synthesis has been considered [241]. Antibiotics production using SSF with FL has been analyzed from micro-organisms such as Streptomyces marinensi and S. fradiae. However, the main constraints for antibiotics production by SSF are the high production costs and low production yields. Pesticides have been products that have been studied from the FL fermentation. The most analyzed substrates are mainly grouped in AL and AgL such as potato peel and coffee peel, to be used for pest control in banana, sugar cane, coffee, and soybean crops. Some of the analyzed micro-organisms are Beauveria bassiana and Colleto-trichum truncatum.
Another type of process that has been investigated regarding FL fermentation has been the obtaining of antioxidants, antipigmentants and phenols. Razak et al. [242] evaluated the fermentation of rice bran using A. oryzae to obtain extracts with antioxidant and antiaging capacity. Liu et al. [243] analyzed the particle size and concentration of rice bran from Rhizopus oryzae for the production of biomass, protein, and phenolic compounds.

Table 9.
Other organic compounds obtained from FL and FW fermentation.
Table 9.
Other organic compounds obtained from FL and FW fermentation.
| Micro-Organism | Water Activity | Oxygen Requirement | Product | Substrate | Operational Conditions | Yields and Productivity | Ref. |
|---|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae | SSF | Anaerobic | Esters, alcohols, carbonyl compounds, and organic acids. | Food loss: Orange pulp, molasses, brewer spent grains | Orange pulp volume: 100 mL; Molasses volume: 50 mL; Brewer spent grains: 70 g pH: 5.5; Temperature: 30 °C; Pressure: 1.5 Atm | Ethanol: 56.8%wt; 2,6-dimethyl-2- heptanol: 1.36%wt; 1-octanol: 0.10%wt; Phenylethyl alcohol: 3.1%wt; 3-heptanone: 0.19%wt; 2-heptanone: 0.021%wt; 2-methyl-4-heptanone: 0.0926%wt; 5-nonanone: 0.0052%wt; Cyclohexane- 1,1,3,5- tetramethyl: 0.0031%wt (mg/kg) Ethanol: 135.6; 2,6-dimethyl-2- heptanol: 35.8; 1-octanol: 10.2 Phenylethyl alcohol: 31.2; 3-heptanone: 19.0 2-heptanone: 20.6; 2-methyl-4-heptanone: 926.8; 5-nonanone: 51.8 Cyclohexane- 1,1,3,5- tetramethyl: 31.0 | [90] |
| Kluyveromyces marxianus | SSF | Anaerobic | Esters, alcohols, and carbonyl compounds. | Food loss: Orange pulp, molasses, potato pulp, whey, and brewer’s spent grains | Orange pulp volume: 100 mL; Molasses volume: 10 mL; Potato pulp volume: 10 mL; Whey volume: 30 mL; Brewer spent grains: 80 g; Distillate water: 50 mL pH: 7; Temperature: 30 °C; Pressure: 1.5 Atm | Methyl palmitate: 85.7%wt (Methyl palmitate: 85.7 g/100 g fat) Methyl oleate: 3.6%wt (Methyl oleate: 3.6 g/100 g fat) Methyl linoleate: 9.0%wt (Methyl linoleate: 9.0 g/100 g fat) Ethyl acetate: 0.0012%wt (Ethyl acetate: 12.4 mg/kg) | [90] |
| Yarrowia lipolytica | SMF | Aerobic | Isomaltulose and lipids | Food loss: Sugarcane molasses | Fed-batch fermentation; Fermentation volume: 6.0 L; Inoculum concentration: 5.0%(v/v); Fermentation time: 80 h; Aeration time: 50 L/min; Stirring rate: 300 rpm; Temperature: 30 °C; pH: 6.0; Pretreated sugarcane molasses: 350 g/L Corn steep liquor: 1.0 g/L | Isomaltulose: 96.0%wt (Isomaltulose: 96%w/w) Lipids: 20.9%wt (Lipids: 12.2 g/L) | [210] |
| Thermomyces Lanuginosus | SSF | Aerobic | Volatile acids | Food waste: Hull-less pumpkin, flax, and hemp oil cakes | Batch fermentation; Fermentation volume in glass jars: 670 mL; Substrate load: 50 g; Substrate moisture: 60%; Temperature: 45 °C; Fermentation time: 9 days; No agitation required.; Inoculum load: 6 mm diameter suspended in 10 mL of water | Hull-less pumpkin oil cake Volatile acids: 58.9%wt (Volatile acids: 22 U/mL) Flax oil cake Volatile acids: 22.8%wt (Volatile acids: 17 U/mL) Hemp pumpkin Volatile acids: 9.38%wt (Volatile acids: 7 U/mL) | [244] |
| Cryptococcus albidus sp. Aerius | SMF | Anaerobic | Ergosterol (Biological precursor/provitam of vitamin D2) | Food waste: Dairy waste(whey) | Fermentation volume: 2500 L; Fermentation time: 96 h; Temperature: 25 °C | Ergosterol: 50%wt | [245] |
| Amycolatopsis Mediterranean | SSF | Aerobic | Rifamycin (Antiobiotics) | Food loos: Coconut oil cake, groundnut oil cake, ground nut shell and rice husk | Initial moisture content: 60%; Inoculum concentration: 8%(w/w); Substrate particle size: 1.4–1.6 mm; Initial pH: 8.0 Temperature: 32 °C; Incubation period: 10 days | Rifamycin: 0.146%wt (Rifamycin: 1.46 mg/g dry substrate) | [246] |
| Streptomyces fradiae | SSF | Aerobic | Neomycin (Antiobiotics) | Food loss: Apple pomace, cotton seed meal, soybean powder and wheat bran | Fermentation volume: 250 mL; Substrate load: 10 g; Substrate particle size: 1.2 mm; Initial moisture: 70%; Temperature: 30 °C pH: 8.0; Time: 5 days | Neomycin: 2.77%wt (Neomycin: 27,658 µg/g substrate) | [247] |
| Sporidiobolus salmonicolor | SSF | Anaerobic | Astaxanthin (pigment) | Food waste: Wheat waste | Fermentation volume: 250 mL; Substrate load: 100 g; Fermentation temperature: 23 °C; Moisture content: 90%; pH: 7.0 | Astaxanthin: 0.0061%wt (Astaxanthin: 60.54 µg astaxanthin/g wheat wastes) | [248] |
| Phoma sp. | SSF | Aerobic | Bioherbicide | Food loss: Soybean bran, bagasse, and corn steep liquor | Fermentation volume: 500 mL; Substrate load: 10 g; Moisture content: 70%wt.; Soybean bran load: 30%wt.; Corn steep liquor: 20%wt.; Temperature: 28 °C; Time: 5 days | The produced bioherbicide contributed to plants height up to 11.46 cm and root length of 10.94 cm | [249] |
| Bacillus subtilis | SSF | Anaerobic | Biosurfactant | Food loss: Orange peels extract, potato peels extract, banana peels extract, and bagasse extract | Nutritive medium (g/L): NO3NH4, 1.0; KH2PO4, 6.0; MgSO4, 0.1; Fermentation temperature: 30 °C; pH: 7.0; Time: 24 h; Stirring rate: 12,000 rpm | Orange peel extract: 8.9%wt Potato peel extract: 22.0%wt Banana peel extract: 49.0%wt Bagasse extract: 12.7%wt (g/L) Orange peel extract: 0.089; Potato peel extract: 0.022; Banana peel extract: 0.049; Bagasse extract: 0.127 | [250] |
SSF: Solid-state fermentation, SMF: Submerged fermentation, IS: Immobilized systems.
Biosurfactant demand is increasing over time. Indeed, the global market for surfactants was valued at USD 43.6 billion in 2017 and is predicted to reach USD 66.4 billion by 2025 (i.e., the compound annual growth rate of 5.4% (2018–2025)) [251]. Synthetic surfactants are bulk chemicals with market prices of about 2 USD/kg, whereas biosurfactant costs were estimated between 5 and 20 USD/kg [252,253]. Therefore, 90% of the total costs are assigned to purification costs (80%) and substrate costs (10%) [156]. Potential uses for biosurfactants are numerous and include applications in the food (shelf life), cosmetic (care products), pharmaceutical (anti-adhesives), and agricultural (biocontrol) sectors [252,253]. Amphipathic biosurfactant molecules consist of a hydrophilic and hydrophobic moiety(s). Biosurfactants can be classified as glycolipids (e.g., rhamnolipids) and lipopeptides (e.g., surfactin). Pseudomonas aeruginosa and Bacillus subtilis produce glycolipids and lipopeptides, respectively [156]. Henket et al. [254] reported the maximum theoretical di-rhamnolipid production using different carbon sources based on ATP balancing. Indeed, maximum yields of 0.52 g/g, 0.59 g/g, 0.64 g/g, and 1.26 g/g can be obtained when using glucose, glycerol, soybean oil, and stearic acid as carbon sources. Although the techno-economic and environmental assessment reported in the open literature related to biosurfactant production is wide, several issues need to be studied to increase the economic feasibility of the process by reducing production costs. For instance, Sarubbo et al. [156] highlighted the following critical issues: (i) biosurfactant production under non-sterile conditions; (ii) upstream processing stages decrease; (iii) mechanical design of bioreactors for improving mass and energy transfer phenomena; (iv) new alternatives for product purification; (v) life-cycle assessment involving raw materials sources (i.e., cradle-to-gate approach); and (vi) sustainability assessment based on technical, economic, environmental, and social indicators.
3.4. Main Platform Products Generated in Fermentation of Food Residues
Platform products are those molecules with a potential to be used for the production of a wide range of added-value products. These products can be obtained using both FL and FW via fermentation, enzymatic hydrolysis, catalytic upgrading, and thermochemical processing [255]. Sugars (e.g., glucose), organic acids (e.g., lactic acid, succinic acid), alcohols (glycerol), and furans (e.g., furfural and 5-hydroxymethylfurfural) have been considered to be the most important platform chemicals produced using FL and FW. Several authors have researched yields, operating conditions, and techno-economic prefeasibility assessment of platform chemical production, and further upgrading using FL and FW as raw materials [256,257]. On the other hand, different reviews have been addressed to analyze and to describe the most important platform chemicals without considering production pathways [255,258]. Therefore, this section aims to give an overview on platform molecule production via fermentation.
Sugars platforms are upgraded via fermentation to other chemical platforms such as lactic acid, succinic acid, citric acid, and volatile fatty acids (VFA). Other studies have reported high titers and yields of lactic acid, succinic acid, and citric acid fermentations. For instance, Peinemann et al. [259] reported a titer of 74 g/L with a yield of 0.57 g of lactic/g of glucose using Streptococcus sp. in a continuous mode. These fermentation products have been further upgraded to polyhydroxyalkanoates (PHAs) such as polylactic acid (PLA) and polyhydroxybutirate (PHB) (See Table 5). These PHAs can be obtained after catalytic and fermentative processes, respectively. Indeed, PHB production using VFA derived from waste frying oil anaerobic fermentation was studied by Vu et al. [260].
A glycerol platform can be fermented to malic acid, itaconic acid, 1,3 propanediol, and oxalic acid using yeast (e.g., Candida sp. and Yarrowia sp.) and fungus (e.g., Aspergillus sp.) [261]. These fermentation processes can potentially upgrade the crude glycerol from biodiesel production and increase process sustainability. High titers, yields, and productivities can be obtained using wild strains. For instance, Juy et al. [262] reported a fermentation productivity of 0.19 g/L/h with a yield of 0.44 g itaconic acid/g of glycerol after six days. Nevertheless, low growing rates and sensitive operating conditions have been reported. Therefore, recombinant micro-organisms such as Escherichia coli, Saccharomyces cerevisiae, Corynebacterium glutamicum, and Yarrowia lipolytica can be used to overcome these drawbacks [263]. Engineering micro-organisms has led to the acquisition of higher yields than those acquired when using wild or native strains. For instance, Harder et al. [264] reported a titer of 32 g/L of itaconic acid with a yield of 0.68 mol itaconic acid/mol of glucose after five days.
Amino acid and fatty acid platforms are fermented to obtain VFA. The fermentation process is carried out under anaerobic conditions. VFA are considered platform products, since the chemical industry has used carboxylic acids as precursors to different products such as esters, ketones, alkanes, alcohols, aldehydes, biofuels, and PHAs (see Table 5) [265]. Parameters such as FL and FW composition, fermentation temperature, and oxygen concentration are key factors for obtaining high titers and yields. For instance, an increase in temperature from 35 °C to 55 °C reduce the VFA concentration from 17 g/L to 11 g/L [266].
Platform molecule production from FL and FW via fermentation opens the possibilities of upgrading these residues into a portfolio of high-added-value products, since different processes such as polymerization, catalytic upgrading, and fermentation can be combined in the same facility. This conceptual statement has been studied and supported by several authors, since different experimental and simulation procedures have been reported in the open literature [267]. Table 10 presents platform molecules derived from FW and the possible upgrading to high-added-value products.

Table 10.
Platforms and products from FL and FW fermentation.
4. Systematic Analysis of the Reviewed Information
The use of FL and FW promotes sustainable agro-industrial development and the transition to a circular economy model. These residues could contribute significantly to non-renewable original raw material demand for energy vectors and value-added products. Chemical composition is one of the key factors that defines the transformation route and the type of product to be obtained, as can be observed from Table 1 and Table 2. FL generation in the first links of the FVC presents a standard chemical composition, unlike FW. FW is residue that evolved different products from the basic family basket being governed by socio-economic conditions and cultures (i.e., context). This residue diversification that groups FW causes great variability in chemical composition by increasing complexity in fermentation processes analysis. On the other hand, Teigiserova et al. [271] established that FL is generated in a specific link of the FVC (i.e., the residues are the same despite the seasonality of the crops or livestock) where the chemical composition is not affected to a great extent. For this reason, FLs have been studied and evaluated more in fermentative processes than FW. In addition, FWs generally have higher types of matter fractions (e.g., starch, pectin, fat, among others) than FLs. For example, FLs such as leaves and stems generated in the producer link do not present significant starch and pectin content.
Energy production from FL and FW by fermentative processes has two trends in the use of raw material (see Table 3). The first trend starts from the direct use of waste from a micro-organism consortium. The second trend is the use of fermentable sugars obtained upstream (i.e., pretreatment and saccharification). Zou et al. [260] reviewed the critical points and evaluated the future aspects for the valorization and upgrading of FL and FW to multiple bio-energies based on the second trends. The production of energy vectors by direct FL and FW fermentation aims to obtain biogas and hydrogen. In this type of fermentation, the origin of the raw material is not one of the limiting factors in the process, i.e., FL and FW have been evaluated without restrictions due to the variability in chemical composition. This is because the micro-organism consortium synthesizes the necessary enzymes to carry out the hydrolysis step in situ for the fermentation process. Nevertheless, it is necessary to address more studies to analyze the metabolic pathway of the consortium to define which are the bottlenecks of micro-organisms on the chemical composition of FL and FW. Zhu et al. [268] analyzed the effective micro-organism consortium to produce energy vectors using sewage sludge applied on anaerobic fermentation employing biomass as direct raw material for the substrate. For fermentations that start from the use of fermentable sugars, the type of energy vectors studied are ethanol, butanol, and hydrogen. In fermentations to produce hydrogen from fermentable sugars, the analyzed micro-organisms are specific (i.e., they are not a consortium). Thus, for this type of fermentation, FL is analyzed more. However, a great deal of research has been done addressing the production of fermentable sugars.
Biomaterial production has been evaluated to a greater extent from FL, as presented in Table 4. The production of bioplastics is one of the most analyzed fermentative routes for the valorization of residues (AL and AgL). The current trend for bioplastics production from FL is studied by Chong et al. [269]. Other trends in FL fermentation are the production of nanocellulose and xanthan. FW presents less tendency for biomaterial production by fermentative routes. Some of the factors that limit the use of FW is the variability of foods that comprise it. Due to the heterogeneity of the FW, inhibitors can be generated in the fermentative pathways that affect the metabolism of micro-organisms. This trend was observed in the production of aromatic compounds, enzymes, and other organic compounds such as antibiotics, pigments and bioherbicides (see Table 7 and Table 8). The valorization of FL for the synthesis of this type of compound has been evaluated considering the mixture of agricultural and agro-industrial residues (AL and AgL). In the fermentative processes to obtain enzymes, the most evaluated residues are those obtained in the food agro-industry.
As shown in Table 5, in the fermentative processes for organic acid production, FL and FW have been analyzed equally. However, in more specific fermentations such as fumaric acid fermentation, indole-3-acetic acid, gallic acid, and ellagic acid, this trend is not true. Micro-organisms involved for these compound syntheses are generally isolated or genetically modified, for which the restriction in the use of substrates and the inhibitors presence constitute a crucial factor. Deckers et al. [272] published and overview of genetically modified micro-organisms for the high value-added production using fermentative process.
5. Restrictions of Food-Waste Fermentation Processes
FL and FW impact not only generates problems in terms of inadequate management of residues resulting in environmental issues, but also in terms of the misuse of resources such as land, water, and energy, among others. Problems related to the variability of the composition and the structural complexity of the FWs are some of the main challenges. Thus, criteria such as FW nature, due to population-diversified eating habits, the collecting moment, and the place of generation are some of the most relevant restrictions for the FW valorization routes during the search process.
In addition, logistical problems attributed to collection and transport issues constitute a limitation to put into practice the valorization of FW. The main reason is that FW is found mixed with other municipal solid waste and non-biogenic waste. In countries of low and medium economic development, the disposition and management of the FW are not adequate, and it is disorganized. Therefore, FL and FW valorization depends on the availability of value chains, the correctly organized collection of FWs and classification strategies. In this sense, government entities must generate strategies and campaigns to organize food security. In the case of FL, industries and the actors of the other CV links must devise collection and transport protocols since the production of FL is segregated in most cases. Then, food processing side-streams are a suitable raw material for decentralized upgrading or in situ valorization in food-processing facilities.
One of the alternatives for FL and FW recovery is to implement routes that are viable on a small and medium scale, from which vulnerable communities could benefit. Structured recovery method strategies must be implemented with FL and FW. One of the future research studies should focus on optimizing and establishing large- or medium-scale production processes that are cheaper and more efficient. The main restriction for FW fermentation processes is presented in Table 11.

Table 11.
Restriction for the FL and FW use in fermentation processes.
6. Potential of Food-Waste Fermentation Processes
FL and FW are alternative raw materials to be used in fermentation processes, since several building blocks (e.g., sugars, fatty acids, proteins, etc.) and secondary products (e.g., colorants, biomaterials) can be obtained. Thus, FR are a potential source of a great variety of commodities, fine chemicals, and specialty chemicals, decreasing the environmental impact caused by the production and use of synthetic molecules. Strategies to ensure the possible use of FL and FW in different industrial sectors must be addressed to replace oil-based products. Indeed, FR bioconversion contributes to overcoming issues such as (i) energy transition and energy security, (ii) food security, (ii) circular economy implementation, (iii) the establishment of sustainable consumption and production patterns, (iv) non-renewable resource dependency, and (v) sustainability of existing processes.
Energy transition and security can be improved in developed and developing countries using FL and FW as raw materials in thermochemical or fermentative processes. Biotechnological upgrading of FL and FW contributes to reducing the consumption of oil-based fuels in the transport and industrial sectors, since high volumes of biofuels can be produced. High titers, yields, and productivities are possible when using FL and FW as substrates since a high amount of carbohydrates are present. On the other hand, biogas production is projected as an integral process for FL, and FW upgrading due to the low raw material conditioning required. The bioconversion of FR provides energy security due to the diversification of the energy matrix through the production of heat and power from energy vectors. FW has the potential to provide high amounts of energy due to the large amounts produced by dairy. Nevertheless, more efforts are needed to implement FL and FW processing facilities.
The food security of a region/country can be improved via FL and FW valorization since identifying most contributing factors related to residue generation can be used as a starting point for minimizing food value chain inefficiencies. In addition, food security is proven since decreasing FL and FW through bioconversion processes led to a reduction in the overall residue generation per capita. Thus, less food is lost at the end of production chains. Linked to food security, FL and FW upgrading to obtain alternative products allows the implementation of a circular economy model in the FVC, since an efficient use of the residues is proposed.
Consumers are aware of the environmental impact of the current linear economy scheme. The bio-based products market has increased in the past few years. This fact encourages the research and development of FL and FW as raw materials to be upgraded through fermentation processes due to the possibility of producing similar molecules to those derived from synthetic routes. Thus, these residues can potentially promote the research and implementation of alternative raw materials in industrial processes. In this way, FL and FW can be highlighted as potential sources of added-value products since availability, logistics, and technological maturity are present today. On the other hand, using these renewable resources can potentially decrease the extraction, use, and upgrading of non-renewable resources, improving the environmental impact of different productive chains. Finally, FL and FW upgrading through the fermentative process contributes to the enhancement of the sustainability of existing processes, since residues are used to produce new products that can be commercialized. This behavior allows for the improvement of economic feasibility, since more revenues can be perceived. In this way, the integral use of all FR fractions can promote the creation of new green industries with optimal processing conditions.
The techno-economic analysis of fermentative routes for RF recovery is one of the main types of research that must be addressed to guarantee economic viability. Reducing operating and capital costs, increasing production yields, improving the separation and purification of metabolites, and making full use of RF are some of the strategies that make fermentation routes economically viable [273].
Regarding the potential of FL and FW as raw materials for different bioprocesses, anaerobic digestion has been considered to be an efficient solution for treating and upgrading FW, since conditioning and pretreatment are not mandatory. The anaerobic digestion process has the advantages of a low generation of greenhouse gas emissions (GHG), a high potential for producing heat and power in cogeneration units, and a high potential for obtaining biofertilizers or organic growth promoters. The anaerobic digestion process can be seen as a fundamental base for FW upgrading, since a consortium of micro-organisms allows for the production of several added-value products and platform molecules. Indeed, anaerobic digestion is considered to be a robust process because the raw material chemical composition is not necessarily constant over time. For this reason, several authors have identified this process as a key step for upgrading a mixture of residues. Products such as methane, hydrogen, and volatile fatty acids have been identified as potential building blocks for further upgrading into biomaterials, food, feed, and energy.
FL upgrading has been addressed for specific products since fermentation processes have evolved from different perspectives. For instance, engineered micro-organisms have been developed as an alternative for increasing titers, yields, and productivity. Moreover, fermentation process configuration and technological development have been analyzed as potential alternatives for improving efficiencies and reducing energy consumption. Therefore, the production of biosurfactants, biopolymers, agrochemicals, and food additives tends to be more feasible at an industrial scale, and FL is a promising feedstock to be upgraded via biotechnological processes, since these raw materials can reduce current operating costs. Furthermore, the integration of FL can produce ideal substrates for further processing. The bioconversion of mixed FL for producing single-cell protein, enzymes, amino feeds, volatile compounds, and feed additives has not been exploited. The bioconversion of mixed FL substrates from micro-organisms such as yeasts, fungi, bacteria, and algae presents several advantages, such as: (i) the possibility of generating complete substrates without the need to add nutrients to the medium; (ii) the capacity of small-scale agro-industries to exploit their waste in situ; (iii) the improvement of the nutritional value of livestock feed. Then, the above-mentioned applications are potential pathways for improving FL implementation in existing production processes. One of the potentials of FL and FW is addressed in obtaining food oils such as omega-3, since this kind of compound can increase the economic feasibility of fermentative processes. This research can prompt the study of new micro-organisms (e.g., aquatic protists), since high yields and titers can be obtained at small and medium scales.
Non-conventional products such as pigments, herbicides, biopolymers, food additives, and pharmaceutical products can be increased by implementing FL and FW as raw materials. Furthermore, fermentative processes of several substrates can boost the use of micro-organisms in all industries, contributing to industry decarbonization goals.
7. Conclusions and Future Directions
FL and FW valorization is one of the most promising alternatives to promote SDG accomplishment. FL and FW upgrading processes promote the efficient use of resources, mitigating the environmental impact of current disposal methods (i.e., incineration and landfill). Fermentation can be profiled as an alternative with a high potential to valorize FR. However, fermentation process applications for upgrading FL and FW are limited to the composition of these residues and process specificity. However, not all fermentation processes can be applied to the FR (i.e., FL) generated in the first links of the value chain due to the current technological context. However, anaerobic digestion is a promising route for implementing these links. In addition, energy could be provided in non-interconnected areas with a service failure. On the other hand, the processes to obtain organic acids must be applied considering factors such as FR flow, the supply chain, and logistics. For the processes aimed at producing aromatic compounds, enzymes, antibiotics, pigments, and bioherbicides, the analysis and evaluation should maximize yields and productivity and facilitate the use of more complex FR such as FW.
Author Contributions
Conceptualization, M.O.-S., P.-J.I.-G., A.F.A.-R. and C.A.C.A.; formal analysis, C.A.C.A.; investigation, M.O.-S., P.-J.I.-G. and A.F.A.-R.; writing—original draft preparation, M.O.-S. and P.-J.I.-G.; writing—review and editing, M.O.-S., P.-J.I.-G., A.F.A.-R. and C.A.C.A.; supervision, C.A.C.A.; funding acquisition, C.A.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This paper is the result of the research work developed through the project: PROGRAMA DE INVESTIGACIÓN RECONSTRUCCIÓN DEL TEJIDO SOCIAL EN ZONAS DE POS-CONFLICTO EN COLOMBIA SIGP Coded: 57579 within the research project “COMPETENCIAS EMPRESARIALES Y DE INNOVACIÓN PARA EL DESARROLLO ECONÓMICO Y LA INCLUSIÓN PRODUCTIVA DE LAS REGIONES AFECTADAS POR EL CONFLICTO COLOMBIANO” SIGP Coded: 58907. Funded within the framework of Colombia Científica, Contract No. FP44842-213-2018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the result.
References
- Allam, Z.; Bibri, S.E.; Sharpe, S.A. The Rising Impacts of the COVID-19 Pandemic and the Russia–Ukraine War: Energy Transition, Climate Justice, Global Inequality, and Supply Chain Disruption. Resources 2022, 11, 99. [Google Scholar] [CrossRef]
- FAO. Food Security and Nutrition in the World the State of Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO: Rome, Italy, 2022. [CrossRef]
- Pollard, C.M.; Booth, S. Food Insecurity and Hunger in Rich Countries—It Is Time for Action against Inequality. Int. J. Environ. Res. Public Health 2019, 16, 1804. [Google Scholar] [CrossRef] [PubMed]
- The Sustainable Development Goals Report. Available online: https://unstats.un.org/sdgs (accessed on 2 July 2021).
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Sustainable Energy Transition for Renewable and Low Carbon Grid Electricity Generation and Supply. Front. Energy Res. 2022, 9, 1032. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Martín-García, B.; Verardo, V.; Romano, R.; Sacchi, R.; Masi, P. New Biotechnological Production of EPA by Pythium Irregulare Using Alternative Sustainable Media Obtained from Food Industry By-Products and Waste. Sustainability 2023, 15, 1147. [Google Scholar] [CrossRef]
- FAO. Food Loss and Waste and the Right to Adequate Food: Making the Connection; FAO: Rome, Italy, 2018; p. 48, Licence: CC BY-NC-SA 3.0 IGO.
- Calicioglu, O.; Flammini, A.; Bracco, S.; Bellù, L.; Sims, R. The Future Challenges of Food and Agriculture: An Integrated Analysis of Trends and Solutions. Sustainability 2019, 11, 222. [Google Scholar] [CrossRef]
- Hermanussen, H.; Loy, J.-P.; Egamberdiev, B. Determinants of Food Waste from Household Food Consumption: A Case Study from Field Survey in Germany. Environ. Res. Public Health 2022, 19, 14253. [Google Scholar] [CrossRef]
- Trabold, T.A.; Nair, V. Conventional Food Waste Management Methods. In Sustainable Food Waste-to-Energy Systems; Academic Press: New York, NY, USA, 2018. [Google Scholar]
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding Food Loss and Waste-Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef]
- Nguyen, K.L.P.; Chuang, Y.H.; Chen, H.W.; Chang, C.C. Impacts of Socioeconomic Changes on Municipal Solid Waste Characteristics in Taiwan. Resour. Conserv. Recycl. 2020, 161, 104931. [Google Scholar] [CrossRef]
- Sarker, A.; Ghosh, M.K.; Islam, T.; Bilal, M.; Nandi, R.; Raihan, M.L.; Hossain, M.N.; Rana, J.; Barman, S.K.; Kim, J.-E. Sustainable Food Waste Recycling for the Circular Economy in Developing Countries, with Special Reference to Bangladesh. Sustainability 2022, 14, 12035. [Google Scholar] [CrossRef]
- FAO. Food Loss and Food Waste. Available online: http://www.fao.org/food-loss-and-food-waste/flw-data (accessed on 2 March 2021).
- Isah, S.; Ozbay, G. Valorization of Food Loss and Wastes: Feedstocks for Biofuels and Valuable Chemicals. Front. Sustain. Food Syst. 2020, 4, 82. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Oliviero, M.; Sacchi, R.; Masi, P. Sustainable Production of Food Grade Omega-3 Oil Using Aquatic Protists: Reliability and Future Horizons. New Biotechnol. 2021, 62, 32–39. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Kachrimanidou, V. Advances in Food and by-Products Processing towards a Sustainable Bioeconomy. Foods 2019, 8, 425. [Google Scholar] [CrossRef]
- Pour, F.H.; Makkawi, Y.T. A Review of Post-Consumption Food Waste Management and Its Potentials for Biofuel Production. Energy Rep. 2021, 7, 7759–7784. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Feng, K.; Liu, J. Oriented Fermentation of Food Waste towards High-Value Products: A Review. Energies 2020, 13, 5638. [Google Scholar] [CrossRef]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and by-Products through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef]
- De Oliveira, M.M.; Lago, A.; Dal’ Magro, G.P. Food Loss and Waste in the Context of the Circular Economy: A Systematic Review. J. Clean Prod. 2021, 294, 126284. [Google Scholar] [CrossRef]
- Lucifero, N. Food Loss and Waste in the EU Law between Sustainability of Well-Being and the Implications on Food System and on Environment. Agric. Agric. Sci. Procedia 2016, 8, 282–289. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Asioli, D.; Banovic, M.; Perito, M.A.; Peschel, A.O.; Stancu, V. Defining Upcycled Food: The Dual Role of Upcycling in Reducing Food Loss and Waste. Trends Food Sci. Technol. 2023, 132, 132–137. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Guillemin, G.J.; Gupta, V.K.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Bioethanol Production from Food Wastes Rich in Carbohydrates. Curr. Opin. Food Sci. 2022, 43, 71–81. [Google Scholar] [CrossRef]
- Haghdan, S.; Renneckar, S.; Smith, G.D. Sources of Lignin. Lignin Polym. Compos. 2016, 10, 1–11. [Google Scholar]
- Vats, N.; Khan, A.A.; Ahmad, K. Observation of Biogas Production by Sugarcane Bagasse and Food Waste in Different Composition Combinations. Energy 2019, 185, 1100–1105. [Google Scholar] [CrossRef]
- Mariana, O.S.; Camilo, S.T.J.; Ariel, C.A.C. A Comprehensive Approach for Biorefineries Design Based on Experimental Data, Conceptual and Optimization Methodologies: The Orange Peel Waste Case. Bioresour. Technol. 2021, 325, 124682. [Google Scholar] [CrossRef]
- Martín, M.A.; Siles, J.A.; Chica, A.F.; Martín, A. Biomethanization of Orange Peel Waste. Bioresour. Technol. 2010, 101, 8993–8999. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, H.; Xu, W.; Gong, L.; Zhang, W.; Zou, D. Ethanol Production from Kitchen Garbage Using Response Surface Methodology. Biochem. Eng. J. 2008, 39, 604–610. [Google Scholar] [CrossRef]
- Edwiges, T.; Frare, L.; Mayer, B.; Lins, L.; Mi Triolo, J.; Flotats, X.; de Mendonça Costa, M.S.S. Influence of Chemical Composition on Biochemical Methane Potential of Fruit and Vegetable Waste. Waste Manag. 2018, 71, 618–625. [Google Scholar] [CrossRef]
- Rodríguez-Valderrama, S.; Escamilla-Alvarado, C.; Rivas-García, P.; Magnin, J.P.; Alcalá-Rodríguez, M.; García-Reyes, R.B. Biorefinery Concept Comprising Acid Hydrolysis, Dark Fermentation, and Anaerobic Digestion for Co-Processing of Fruit and Vegetable Wastes and Corn Stover. Environ. Sci. Pollut. Res. 2020, 27, 28585–28596. [Google Scholar] [CrossRef]
- Zhang, L.; Jahng, D. Long-Term Anaerobic Digestion of Food Waste Stabilized by Trace Elements. Waste Manag. 2012, 32, 1509–1515. [Google Scholar] [CrossRef]
- Dhiman, S.; Mukherjee, G. Present Scenario and Future Scope of Food Waste to Biofuel Production. J. Food Process Eng. 2021, 44, e13594. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Koike, Y.; Liu, K.; An, M.Z.; Morimura, S.; Wu, X.L.; Kida, K. Ethanol Production from Kitchen Waste Using the Flocculating Yeast Saccharomyces Cerevisiae Strain KF-7. Biomass Bioenergy 2008, 32, 1037–1045. [Google Scholar] [CrossRef]
- Lissens, G.; Klinke, H.; Verstraete, W.; Ahring, B.; Thomsen, A.B. Wet Oxidation Treatment of Organic Household Waste Enriched with Wheat Straw for Simultaneous Saccharification and Fermentation into Ethanol. Environ. Technol. 2004, 25, 647–655. [Google Scholar] [CrossRef]
- Aǧdaǧ, O.N.; Sponza, D.T. Effect of Alkalinity on the Performance of a Simulated Landfill Bioreactor Digesting Organic Solid Wastes. Chemosphere 2005, 59, 871–879. [Google Scholar] [CrossRef]
- Carucci, G.; Carrasco, F.; Trifoni, K.; Majone, M.; Beccari, M. Anaerobic Digestion of Food Industry Wastes: Effect of Codigestion on Methane Yield. J. Environ. Eng. 2005, 131, 1037–1045. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ahmad, D.; Soning, C. Bio-Hydrogen Generation from Mixed Fruit Peel Waste Using Anaerobic Contact Filter. Int. J. Hydrog. Energy 2007, 32, 4754–4760. [Google Scholar] [CrossRef]
- Abudi, Z.N.; Hu, Z.; Sun, N.; Xiao, B.; Rajaa, N.; Liu, C.; Guo, D. Batch Anaerobic Co-Digestion of OFMSW (Organic Fraction of Municipal Solid Waste), TWAS (Thickened Waste Activated Sludge) and RS (Rice Straw): Influence of TWAS and RS Pretreatment and Mixing Ratio. Energy 2016, 107, 131–140. [Google Scholar] [CrossRef]
- Browne, J.D.; Allen, E.; Murphy, J.D. Assessing the Variability in Biomethane Production from the Organic Fraction of Municipal Solid Waste in Batch and Continuous Operation. Appl. Energy 2014, 128, 307–314. [Google Scholar] [CrossRef]
- Xiguang Chen, R.T.; Romano, R.Z. Anaerobic Digestion of Food Wastes for Biogas Production. Int. J. Agric. Biol. Eng. 2010, 3, 61–72. [Google Scholar]
- Zhang, L.; Lee, Y.W.; Jahng, D. Anaerobic Co-Digestion of Food Waste and Piggery Wastewater: Focusing on the Role of Trace Elements. Bioresour. Technol. 2011, 102, 5048–5059. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Tan, T. Batch and Semi-Continuous Anaerobic Digestion of Food Waste in a Dual Solid-Liquid System. Bioresour. Technol. 2013, 145, 10–16. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of Food Waste as Feedstock for Anaerobic Digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The Anaerobic Co-Digestion of Food Waste and Cattle Manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Karam, M.C.; Hosri, C.; Hussain, R.; Barbar, R.; Gaiani, C.; Scher, J. Effect of Whey Powder Rehydration and Dry-Denaturation State on Acid Milk Gels Characteristics. J. Food Process Preserv. 2017, 41, e13200. [Google Scholar] [CrossRef]
- Nawirska, A.; Kwaśniewska, M. Dietary Fibre Fractions from Fruit and Vegetable Processing Waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.A.; Ahmed, A.R. Utilization of Watermelon Rinds and Sharlyn Melon Peels as a Natural Source of Dietary Fiber and Antioxidants in Cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Adhikari, H.; Rai, S.K. Production of Alkaline Protease by a Thermophilic Bacillus Subtilis under Solid-State Fermentation (SSF) Condition Using Imperata Cylindrica Grass and Potato Peel as Low-Cost Medium: Characterization and Application of Enzyme in Detergent Formulation. Biochem. Eng. J. 2008, 39, 353–361. [Google Scholar] [CrossRef]
- Triana, C.F.; Quintero, J.A.; Agudelo, R.A.; Cardona, C.A.; Higuita, J.C. Analysis of Coffee Cut-Stems (CCS) as Raw Material for Fuel Ethanol Production. Energy 2011, 36, 4182–4190. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C. Oil Palm Rachis Gasification for Synthesis Gas Production Oil Palm Rachis Gasification for Synthesis Gas Production; Universidad Nacional de Colombia: Manizales, Colombia, 2017. [Google Scholar]
- Alonso-Gómez, L.A.; Solarte-Toro, J.C.; Bello-Pérez, L.A.; Cardona-Alzate, C.A. Performance Evaluation and Economic Analysis of the Bioethanol and Flour Production Using Rejected Unripe Plantain Fruits (Musa paradisiaca L.) as Raw Material. Food Bioprod. Process. 2020, 121, 29–42. [Google Scholar] [CrossRef]
- García-Vargas, M.C.; Contreras, M.D.M.; Castro, E. Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts. Appl. Sci. 2020, 10, 8195. [Google Scholar] [CrossRef]
- Del Valle, M.; Cámara, M.; Torija, M.E. Chemical Characterization of Tomato Pomace. J. Sci. Food Agric. 2006, 86, 1232–1236. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Verardo, V.; Martín-García, B.; di Pierro, P.; Sorrentino, A.; Baselice, M.; Oliviero, M.; Sacchi, R.; Masi, P. Formulation of New Media from Dairy and Brewery Wastes for a Sustainable Production of Dha-Rich Oil by Aurantiochytrium Mangrovei. Mar. Drugs 2022, 20, 39. [Google Scholar] [CrossRef]
- Raj, T.; Kapoor, M.; Gaur, R.; Christopher, J.; Lamba, B.; Tuli, D.K.; Kumar, R. Physical and Chemical Characterization of Various Indian Agriculture Residues for Biofuels Production. Energy Fuels 2015, 29, 3111–3118. [Google Scholar] [CrossRef]
- Colombo, B.; Sciarria, T.P.; Reis, M.; Scaglia, B.; Adani, F. Polyhydroxyalkanoates (PHAs) Production from Fermented Cheese Whey by Using a Mixed Microbial Culture. Bioresour. Technol. 2016, 218, 692–699. [Google Scholar] [CrossRef]
- JEWELL, W.J.; CUMMINGS, R.J. Apple Pomace Energy and Solids Recovery. J. Food Sci. 1984, 49, 407–410. [Google Scholar] [CrossRef]
- Ofomatah, A.; Obasi, E. Biogas optimization potentials of cow dung, pig dung and poultry droppings with sugar cane bagasse and water melon peel. FUW Trends Sci. Technol. J. 2017, 2, 267–270. [Google Scholar]
- Mahmood, A.U.; Greenman, J.; Scragg, A.H. Orange and Potato Peel Extracts: Analysis and Use as Bacillus Substrates for the Production of Extracellular Enzymes in Continuous Culture. Enzyme Microb. Technol. 1998, 22, 130–137. [Google Scholar] [CrossRef]
- Neves, L.; Oliveira, R.; Alves, M.M. Anaerobic Co-Digestion of Coffee Waste and Sewage Sludge. Waste Manag. 2006, 26, 176–181. [Google Scholar] [CrossRef]
- Aubrey, M.L.L.; Chin, C.F.S.; Seelan, J.S.S.; Chye, F.Y.; Lee, H.H.; Rakib, M.R.M. Conversion of Oil Palm By-Products into Value-Added Products through Oyster Mushroom (Pleurotus ostreatus) Cultivation. Horticulturae 2022, 8, 1040. [Google Scholar] [CrossRef]
- Bardiya, N.; Somayaji, D.; Khanna, S. Biomethanation of Banana Peel and Pineapple Waste. Bioresour. Technol. 1996, 58, 73–76. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C. Sustainability Assessment of Different Biorefinery Schemes to Enhance the Development of Post-Conflict Areas in the Colombian Context: The Montes de Maria Case; Universidad Nacional de Colombia: Manizales, Colombia, 2022. [Google Scholar]
- Deressa, L.; Libsu, S.; Chavan, R.B.; Manaye, D.; Dabassa, A. Production of Biogas from Fruit and Vegetable Wastes Mixed with Different Wastes. Environ. Ecol. Res. 2015, 3, 65–71. [Google Scholar] [CrossRef]
- Verrier, D.; Roy, F.; Albagnac, G. Two-Phase Methanization of Solid Vegetable Wastes. Biol. Wastes 1987, 22, 163–177. [Google Scholar] [CrossRef]
- Carlini, M.; Castellucci, S.; Moneti, M. Biogas Production from Poultry Manure and Cheese Whey Wastewater under Mesophilic Conditions in Batch Reactor. Energy Procedia 2015, 82, 811–818. [Google Scholar] [CrossRef]
- Jabeen, M.; Zeshan; Yousaf, S.; Haider, M.R.; Malik, R.N. High-Solids Anaerobic Co-Digestion of Food Waste and Rice Husk at Different Organic Loading Rates. Int. Biodeterior. Biodegrad. 2015, 102, 149–153. [Google Scholar] [CrossRef]
- Kennedy, M.; List, D.; Lu, Y.; Foo, L.Y.; Newman, R.H.; Sims, I.M.; Bain, P.J.S.; Hamilton, B.; Fenton, G. Apple Pomace and Products Derived from Apple Pomace: Uses, Composition and Analysis. In Analysis of Plant Waste Materials. Modern Methods of Plant Analysis; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Hassan, D.U.; Abdulsalam, S. Assessement of Bio-Fertilizer Quality of Anaerobic Digestion of Watermelon Peels and Cow Dung. Chem. Biomol. Eng. 2017, 2, 135–141. [Google Scholar]
- Liang, S.; McDonald, A.G. Anaerobic Digestion of pre-Fermented Potato Peel Wastes for Methane Production. Waste Manag. 2015, 46, 197–200. [Google Scholar] [CrossRef]
- Colantoni, A.; Paris, E.; Bianchini, L.; Ferri, S.; Marcantonio, V.; Carnevale, M.; Palma, A.; Civitarese, V.; Gallucci, F. Spent Coffee Ground Characterization, Pelletization Test and Emissions Assessment in the Combustion Process. Sci. Rep. 2021, 11, 5119. [Google Scholar] [CrossRef]
- Sánchez, F.; Araus, K.; Domínguez, M.P.; Miguel, G.S. Thermochemical Transformation of Residual Avocado Seeds: Torrefaction and Carbonization. Waste Biomass Valorization 2016, 8, 2495–2510. [Google Scholar] [CrossRef]
- Roussis, I.; Kakabouki, I.; Folina, A.; Konstantas, A.; Travlos, I.; Bilalis, D. Effects of Tomato Pomace Composts on Yield and Quality of Processing Tomato (Lycopersicon esculentum Mill.). Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2019, 76, 250–257. [Google Scholar] [CrossRef]
- Kaur, P.; Ghoshal, G.; Jain, A. Bio-Utilization of Fruits and Vegetables Waste to Produce β-Carotene in Solid-State Fermentation: Characterization and Antioxidant Activity. Process Biochem. 2019, 76, 155–164. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit Peel Waste: Characterization and Its Potential Uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Rashama, C.; Ijoma, G.N.; Matambo, T.S. Appraising Different Models for Predicting Biomethane Potential: The Case of Avocado Oil Processing by-Products. J. Mater. Cycles Waste Manag. 2021, 23, 409–415. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Kuo, C.-H.; Sun, P.-P.; Dong, C.-D. Agro-Industrial Food Waste as a Low-Cost Substrate for Sustainable Production of Industrial Enzymes: A Critical Review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- Caron, D.A.; Worden, A.Z.; Countway, P.D.; Demir, E.; Heidelberg, K.B. Protists Are Microbes Too: A Perspective. ISME J. 2009, 3, 4–12. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty Acid Biosynthesis in Microorganisms Being Used for Single Cell Oil Production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part I: Biochemistry of Single Cell Oil Production. Eur. J. Lipid Sci. Technol. 2011, 113, 807–815. [Google Scholar] [CrossRef]
- Minh, P.N.N.; Trung Le, T.; van Camp, J.; Raes, K. Valorization of Waste and by-Products from the Agrofood Industry Using Fermentation Processes and Enzyme Treatments. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Doriya, K.; Jose, N.; Gowda, M.; Kumar, D.S. Solid-State Fermentation vs Submerged Fermentation for the Production of L-Asparaginase. Adv. Food Nutr. Res. 2016, 78, 115–135. [Google Scholar]
- Sharma, R.; Oberoi, H.S.; Dhillon, G.S. Fruit and Vegetable Processing Waste: Renewable Feed Stocks for Enzyme Production. In Agro-Industrial Wastes as Feedstock for Enzyme Production: Apply and Exploit the Emerging and Valuable Use Options of Waste Biomass; Academic Press: New York, NY, USA, 2016. [Google Scholar]
- Troiano, D.; Orsat, V.; Dumont, M.J. Solid-State Co-Culture Fermentation of Simulated Food Waste with Filamentous Fungi for Production of Bio-Pigments. Appl. Microbiol. Biotechnol. 2022, 106, 4029–4039. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid State Fermentation of Food Waste Mixtures for Single Cell Protein, Aroma Volatiles and Fat Production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef]
- Yazid, N.A.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef]
- Chandrasekaran, M. Valorization of Food Processing by-Products; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Pavlostathis, S.G. Kinetics and Modeling of Anaerobic Treatment and Biotransformation Processes. In Comprehensive Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 6. [Google Scholar]
- Laëtitia, G.; Pascal, D.; Yann, D. The Citrate Metabolism in Homo- and Heterofermentative LAB: A Selective Means of Becoming Dominant over Other Microorganisms in Complex Ecosystems. Food Nutr. Sci. 2014, 05, 953–969. [Google Scholar] [CrossRef]
- Rajpurohit, H.; Eiteman, M.A. Nutrient-Limited Operational Strategies for the Microbial Production of Biochemicals. Microorganisms 2022, 10, 2226. [Google Scholar] [CrossRef] [PubMed]
- Dharma Patria, R.; Rehman, S.; Vuppaladadiyam, A.K.; Wang, H.; Lin, C.S.K.; Antunes, E.; Leu, S.Y. Bioconversion of Food and Lignocellulosic Wastes Employing Sugar Platform: A Review of Enzymatic Hydrolysis and Kinetics. Bioresour. Technol. 2022, 352, 127083. [Google Scholar] [CrossRef] [PubMed]
- Gallego-García, M.; Moreno, A.D.; Manzanares, P.; Negro, M.J.; Duque, A. Recent Advances on Physical Technologies for the Pretreatment of Food Waste and Lignocellulosic Residues. Bioresour. Technol. 2023, 369, 128397. [Google Scholar] [CrossRef] [PubMed]
- Parthiba Karthikeyan, O.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.C.; Carrere, H. Pretreatment of Food Waste for Methane and Hydrogen Recovery: A Review. Bioresour. Technol. 2018, 249, 1025–1039. [Google Scholar] [CrossRef]
- Fisgativa, M.; Saoudi, M.; Tremier, A. Impact of an aerobic pre-treatment on the anaerobic biodegradability of food waste. In Proceedings of the 6th International Conference on Engineering for Waste and Biomass Valorisation, Albi, Frace, 23 May 2016. [Google Scholar]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of Feedstock Pretreatment Strategies for Improved Anaerobic Digestion: From Lab-Scale Research to Full-Scale Application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Balasubramanian, R.; Wong, J.W.C. Pretreatment of Organic Solid Substrates for Bioenergy and Biofuel Recovery. In Current Developments in Biotechnology and Bioengineering: Solid Waste Management; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Alino, J.H.L.; Bastos, J.A.; Remor, P.V.; Frare, L.M.; Orssatto, F.; Damaceno, F.M.; Edwiges, T. Alkaline Pretreatment and Pre-Hydrolysis Using Acidic Biowastes to Increase Methane Production from Sugarcane Bagasse. Methane 2022, 1, 189–200. [Google Scholar] [CrossRef]
- Gundupalli, M.P.; Bhattacharyya, D. Recovery of Reducing Sugar from Food Waste: Optimization of Pretreatment Parameters Using Response Surface Methodology. In Biofuels and Bioenergy (BICE2016) International Conference, Bhopal, India, 23–25 February 2016; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Torres-León, C.; Chávez-González, M.L.; Hernández-Almanza, A.; Martínez-Medina, G.A.; Ramírez-Guzmán, N.; Londoño-Hernández, L.; Aguilar, C.N. Recent Advances on the Microbiological and Enzymatic Processing for Conversion of Food Wastes to Valuable Bioproducts. Curr. Opin. Food Sci. 2020, 38, 40–45. [Google Scholar] [CrossRef]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Górnaś, P.; Seglina, D. Non-Waste Technology through the Enzymatic Hydrolysis of Agro-Industrial by-Products. Trends Food Sci. Technol. 2018, 77, 64–76. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Sustainable Bioconversion of Food Waste into High-Value Products by Immobilized Enzymes to Meet Bio-Economy Challenges and Opportunities—A Review. Food Res. Int. 2019, 123, 226–240. [Google Scholar] [CrossRef]
- Mazlan, N.A.; Samad, K.A.; Wan Yussof, H.; Saufi, S.M.; Jahim, J. Xylooligosaccharides from Potential Agricultural Waste: Characterization and Screening on the Enzymatic Hydrolysis Factors. Ind. Crops Prod. 2018, 129, 575–584. [Google Scholar] [CrossRef]
- Balaguer Moya, E.; Cunha, M.L.S.; Prado, C.A.; Turella, S.; da Silva, S.S.; Abou-Hachem, M.; Dragone, G.; dos Santos, J.C.; Mussatto, S.I. Evaluation of Enzymatic Hydrolysis of Sugarcane Bagasse Using Combination of Enzymes or Co-Substrate to Boost Lytic Polysaccharide Monooxygenases Action. Catalysts 2022, 12, 1158. [Google Scholar] [CrossRef]
- Talebnia, F.; Pourbafrani, M.; Lundin, M.; Taherzadeh, M.J. Optimization Study of Citrus Wastes Saccharification by Dilute-Acid Hydrolysis. Bioresources 2008, 3, 108–122. [Google Scholar]
- Souto, L.R.F.; Caliari, M.; Soares Júnior, M.S.; Fiorda, F.A.; Garcia, M.C. Utilization of Residue from Cassava Starch Processing for Production of Fermentable Sugar by Enzymatic Hydrolysis. Food Sci. Technol. 2017, 37, 19–24. [Google Scholar] [CrossRef]
- Hafid, H.S.; Rahman, N.A.; Md Shah, U.K.; Baharudin, A.S. Enhanced Fermentable Sugar Production from Kitchen Waste Using Various Pretreatments. J. Environ. Manag. 2015, 156, 290–298. [Google Scholar] [CrossRef]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.V.N.; Anjum, H.; Chang, C.K.; Show, P.L. Effects of Anaerobic Digestion of Food Waste on Biogas Production and Environmental Impacts: A Review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food Waste-to-Energy Conversion Technologies: Current Status and Future Directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef]
- Hunter, S.M.; Blanco, E.; Borrion, A. Expanding the Anaerobic Digestion Map: A Review of Intermediates in the Digestion of Food Waste. Sci. Total Environ. 2021, 767, 144265. [Google Scholar] [CrossRef]
- Salangsang, M.C.D.; Sekine, M.; Akizuki, S.; Sakai, H.D.; Kurosawa, N.; Toda, T. Effect of Carbon to Nitrogen Ratio of Food Waste and Short Resting Period on Microbial Accumulation during Anaerobic Digestion. Biomass Bioenergy 2022, 162, 106481. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic Digestion of Food Waste—Challenges and Opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Bouallagui, H.; Rachdi, B.; Gannoun, H.; Hamdi, M. Mesophilic and Thermophilic Anaerobic Co-Digestion of Abattoir Wastewater and Fruit and Vegetable Waste in Anaerobic Sequencing Batch Reactors. Biodegradation 2009, 20, 401–409. [Google Scholar] [CrossRef]
- Parawira, W.; Murto, M.; Zvauya, R.; Mattiasson, B. Anaerobic Batch Digestion of Solid Potato Waste Alone and in Combination with Sugar Beet Leaves. Renew. Energy 2004, 29, 1811–1823. [Google Scholar] [CrossRef]
- Srisowmeya, G.; Chakravarthy, M.; Nandhini Devi, G. Critical Considerations in Two-Stage Anaerobic Digestion of Food Waste—A Review. Renew. Sustain. Energy Rev. 2019, 119, 109587. [Google Scholar] [CrossRef]
- Lytras, G.; Lytras, C.; Mathioudakis, D.; Papadopoulou, K.; Lyberatos, G. Food Waste Valorization Based on Anaerobic Digestion. Waste Biomass Valorization 2021, 12, 1677–1697. [Google Scholar] [CrossRef]
- Dalke, R.; Demro, D.; Khalid, Y.; Wu, H.; Urgun-Demirtas, M. Current Status of Anaerobic Digestion of Food Waste in the United States. Renew. Sustain. Energy Rev. 2021, 151, 111554. [Google Scholar] [CrossRef]
- Reddy, M.V.; Chandrasekhar, K.; Mohan, S.V. Influence of Carbohydrates and Proteins Concentration on Fermentative Hydrogen Production Using Canteen Based Waste under Acidophilic Microenvironment. J. Biotechnol. 2011, 155, 387–395. [Google Scholar] [CrossRef]
- Kothari, R.; Singh, D.P.; Tyagi, V.V.; Tyagi, S.K. Fermentative Hydrogen Production—An Alternative Clean Energy Source. Renew. Sustain. Energy Rev. 2012, 16, 2337–2346. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Ban, Q.; He, J.; Jha, A.K. Metabolic Pathways of Hydrogen Production in Fermentative Acidogenic Microflora. J. Microbiol. Biotechnol. 2012, 22, 668–673. [Google Scholar] [CrossRef]
- Chu, C.F.; Xu, K.Q.; Li, Y.Y.; Inamori, Y. Hydrogen and Methane Potential Based on the Nature of Food Waste Materials in a Two-Stage Thermophilic Fermentation Process. Int. J. Hydrog. Energy 2012, 37, 10611–10618. [Google Scholar] [CrossRef]
- Mohan, S.V.; Mohanakrishna, G.; Goud, R.K.; Sarma, P.N. Acidogenic Fermentation of Vegetable Based Market Waste to Harness Biohydrogen with Simultaneous Stabilization. Bioresour. Technol. 2009, 100, 3061–3068. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, D.Y. Fermentative Hydrogen Production from Tofu-Processing Waste and Anaerobic Digester Sludge Using Microbial Consortium. Proc. Bioresour. Technol. 2010, 101, S48–S52. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Mumtaz, T.; Hassan, M.A.; Abd Rahman, N. Food Waste and Food Processing Waste for Biohydrogen Production: A Review. J. Environ. Manag. 2013, 130, 375–385. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Dhar, B.R.; Nakhla, G. Single and Combined Effect of Various Pretreatment Methods for Biohydrogen Production from Food Waste. Int. J. Hydrog. Energy 2011, 36, 11379–11387. [Google Scholar] [CrossRef]
- Rena; Zacharia, K.M.B.; Yadav, S.; Machhirake, N.P.; Kim, S.H.; Lee, B.D.; Jeong, H.; Singh, L.; Kumar, S.; Kumar, R. Bio-Hydrogen and Bio-Methane Potential Analysis for Production of Bio-Hythane Using Various Agricultural Residues. Bioresour. Technol. 2020, 309, 123297. [Google Scholar] [CrossRef]
- Meena, R.A.A.; Rajesh Banu, J.; Yukesh Kannah, R.; Yogalakshmi, K.N.; Kumar, G. Biohythane Production from Food Processing Wastes—Challenges and Perspectives. Bioresour. Technol. 2020, 298, 122449. [Google Scholar] [CrossRef]
- Shanmugam, S.; Mathimani, T.; Rene, E.R.; Edwin Geo, V.; Arun, A.; Brindhadevi, K.; Pugazhendhi, A. Biohythane Production from Organic Waste: Recent Advancements, Technical Bottlenecks and Prospects. Int. J. Hydrog. Energy 2021, 46, 11201–11216. [Google Scholar] [CrossRef]
- Anwar Saeed, M.; Ma, H.; Yue, S.; Wang, Q.; Tu, M. Concise Review on Ethanol Production from Food Waste: Development and Sustainability. Environ. Sci. Pollut. Res. 2018, 25, 28851–28863. [Google Scholar] [CrossRef]
- Bibra, M.; Samanta, D.; Sharma, N.K.; Singh, G.; Johnson, G.R.; Sani, R.K. Food Waste to Bioethanol: Opportunities and Challenges. Fermentation 2022, 9, 8. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wu, C.; Zhu, W.; Wang, Q. A Review on Characteristics of Food Waste and Their Use in Butanol Production. Rev. Environ. Health 2019, 34, 447–457. [Google Scholar] [CrossRef]
- Cavaleiro, A.J.; Ferreira, T.; Pereira, F.; Tommaso, G.; Alves, M.M. Biochemical Methane Potential of Raw and pre-Treated Meat-Processing Wastes. Bioresour. Technol. 2012, 129, 519–525. [Google Scholar] [CrossRef]
- Ros, M.; de Souza Oliveira Filho, J.; Perez Murcia, M.D.; Bustamante, M.A.; Moral, R.; Coll, M.D.; Lopez Santisima-Trinidad, A.B.; Pascual, J.A. Mesophilic Anaerobic Digestion of Pig Slurry and Fruit and Vegetable Waste: Dissection of the Microbial Community Structure. J. Clean. Prod. 2017, 156, 757–765. [Google Scholar] [CrossRef]
- Azman, N.F.; Abdeshahian, P.; Kadier, A.; Nasser Al-Shorgani, N.K.; Salih, N.K.M.; Lananan, I.; Hamid, A.A.; Kalil, M.S. Biohydrogen Production from De-Oiled Rice Bran as Sustainable Feedstock in Fermentative Process. Int. J. Hydrog. Energy 2016, 41, 145–156. [Google Scholar] [CrossRef]
- Marone, A.; Massini, G.; Patriarca, C.; Signorini, A.; Varrone, C.; Izzo, G. Hydrogen Production from Vegetable Waste by Bioaugmentation of Indigenous Fermentative Communities. Int. J. Hydrog. Energy 2012, 37, 5612–5622. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, S.L.; Chen, I.C.; Cheng, S.S. Starch Hydrolysis Characteristics of Hydrogen Producing Sludge in Thermophilic Hydrogen Fermentor Fed with Kitchen Waste. Int. J. Hydrog. Energy 2009, 34, 7435–7440. [Google Scholar] [CrossRef]
- Pinto, M.P.M.; Mudhoo, A.; de Alencar Neves, T.; Berni, M.D.; Forster-Carneiro, T. Co–Digestion of Coffee Residues and Sugarcane Vinasse for Biohythane Generation. J. Environ. Chem. Eng. 2018, 6, 146–155. [Google Scholar] [CrossRef]
- Seengenyoung, J.; Mamimin, C.; Prasertsan, P.; O-Thong, S. Pilot-Scale of Biohythane Production from Palm Oil Mill Effluent by Two-Stage Thermophilic Anaerobic Fermentation. Int. J. Hydrog. Energy 2019, 44, 3347–3355. [Google Scholar] [CrossRef]
- Hossain, A.B.M.S.; Fazliny, A.R. Creation of Alternative Energy by Bio-Ethanol Production from Pineapple Waste and the Usage of Its Properties for Engine. Afr. J. Microbiol. Res. 2010, 4, 813–819. [Google Scholar]
- Chandel, A.K.; Narasu, M.L.; Rudravaram, R.; Pogaku, R.; Rao, L.V. Bioconversion of De-Oiled Rice Bran (DORB) Hemicellulosic Hydrolysate into Ethanol by Pichia Stipitis NCM3499 under Optimized Conditions. Int. J. Food Eng. 2009, 5, 1. [Google Scholar] [CrossRef]
- Ma, K.; Ruan, Z.; Shui, Z.; Wang, Y.; Hu, G.; He, M. Open Fermentative Production of Fuel Ethanol from Food Waste by an Acid-Tolerant Mutant Strain of Zymomonas Mobilis. Bioresour. Technol. 2016, 203, 295–302. [Google Scholar] [CrossRef]
- Al-Shorgani, N.K.N.; Kalil, M.S.; Yusoff, W.M.W. Biobutanol Production from Rice Bran and De-Oiled Rice Bran by Clostridium Saccharoperbutylacetonicum N1-4. Bioprocess Biosyst. Eng. 2012, 35, 817–826. [Google Scholar] [CrossRef]
- Yu, J.; Huang, Z.; Wu, P.; Zhao, M.; Miao, H.; Liu, C.; Ruan, W. Performance and Microbial Characterization of Two-Stage Caproate Fermentation from Fruit and Vegetable Waste via Anaerobic Microbial Consortia. Bioresour. Technol. 2019, 284, 398–405. [Google Scholar] [CrossRef]
- Poe, N.E.; Yu, D.; Jin, Q.; Ponder, M.A.; Stewart, A.C.; Ogejo, J.A.; Wang, H.; Huang, H. Compositional Variability of Food Wastes and Its Effects on Acetone-Butanol-Ethanol Fermentation. Waste Manag. 2020, 107, 150–158. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Su, G.; He, J. Enhanced Direct Fermentation from Food Waste to Butanol and Hydrogen by an Amylolytic Clostridium. Renew. Energy 2020, 153, 522–529. [Google Scholar] [CrossRef]
- Jin, Q.; An, Z.; Damle, A.; Poe, N.; Wu, J.; Wang, H.; Wang, Z.; Huang, H. High Acetone-Butanol-Ethanol Production from Food Waste by Recombinant Clostridium Saccharoperbutylacetonicum in Batch and Continuous Immobilized-Cell Fermentation. ACS Sustain. Chem. Eng. 2020, 8, 9822–9832. [Google Scholar] [CrossRef]
- Magyar, M.; da Costa Sousa, L.; Jayanthi, S.; Balan, V. Pie Waste—A Component of Food Waste and a Renewable Substrate for Producing Ethanol. Waste Manag. 2017, 62, 247–254. [Google Scholar] [CrossRef]
- He, M.X.; Wu, B.; Qin, H.; Ruan, Z.Y.; Tan, F.R.; Wang, J.L.; Shui, Z.X.; Dai, L.C.; Zhu, Q.L.; Pan, K.; et al. Zymomonas Mobilis: A Novel Platform for Future Biorefineries. Biotechnol. Biofuels 2014, 7, 101. [Google Scholar] [CrossRef]
- Ntaikou, I.; Menis, N.; Alexandropoulou, M.; Antonopoulou, G.; Lyberatos, G. Valorization of Kitchen Biowaste for Ethanol Production via Simultaneous Saccharification and Fermentation Using Co-Cultures of the Yeasts Saccharomyces Cerevisiae and Pichia Stipitis. Bioresour. Technol. 2018, 263, 75–83. [Google Scholar] [CrossRef]
- Ranganathan, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Utilization of Food Waste Streams for the Production of Biopolymers. Heliyon 2020, 6, e04891. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.S.; Wong, J.W.C.; Bui, X.T. A Critical Review on Various Feedstocks as Sustainable Substrates for Biosurfactants Production: A Way towards Cleaner Production. Microb. Cell Fact. 2021, 20, 120. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; da Silva, M.G.C.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, Properties, Applications, Trends, and General Perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Chakrapani, G.; Zare, M.; Ramakrishna, S. Biomaterials from the Value-Added Food Wastes. Bioresour. Technol. Rep. 2022, 19, 101181. [Google Scholar] [CrossRef]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Postemsky, P.D.; Bidegain, M.A.; Lluberas, G.; Lopretti, M.I.; Bonifacino, S.; Inés Landache, M.; Zygadlo, J.A.; Fernández-Lahore, M.; Omarini, A.B. Biorefining via Solid-State Fermentation of Rice and Sunflower by-Products Employing Novel Monosporic Strains from Pleurotus Sapidus. Bioresour. Technol. 2019, 289, 121692. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.; Silvério, S.C.; Rodrigues, L.R. One-Step Process for Producing Prebiotic Arabino-Xylooligosaccharides from Brewer’s Spent Grain Employing Trichoderma Species. Food Chem. 2019, 270, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Tang, B.; Wang, Q.; Xu, Z.; Sun, L.; Ma, J.; Li, S.; Xu, H.; Lei, P. The Bio-Processing of Soybean Dregs by Solid State Fermentation Using a Poly Γ-Glutamic Acid Producing Strain and Its Effect as Feed Additive. Bioresour. Technol. 2019, 291, 121841. [Google Scholar] [CrossRef] [PubMed]
- Vidhyalakshmi, R.; Vallinachiyar, C.; Radhika, R. Production of Xanthan from Agro-Industrial Waste. J. Adv. Sci. Res. 2012, 3, 56–59. [Google Scholar]
- Pais, J.; Serafim, L.S.; Freitas, F.; Reis, M.A.M. Conversion of Cheese Whey into Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) by Haloferax Mediterranei. New Biotechnol. 2016, 33, 224–230. [Google Scholar] [CrossRef]
- Nikel, P.I.; Pettinari, M.J.; Méndez, B.S.; Galvagno, M.A. Statistical Optimization of a Culture Medium for Biomass and Poly(3-Hydroxybutyrate) Production by a Recombinant Escherichia Coli Strain Using Agroindustrial Byproducts. Int. Microbiol. 2005, 8, 243–250. [Google Scholar]
- Drioli, E.; Giorno, L. Membrane Contactors and Integrated Membrane Operations. In Comprehensive Membrane Science and Engineering; Academic Press: New York, NY, USA, 2010; Volume 4. [Google Scholar]
- Merrylin, J.; Kannah, R.Y.; Banu, J.R.; Yeom, I.T. Production of Organic Acids and Enzymes/Biocatalysts from Food Waste. In Food Waste to Valuable Resources: Applications and Management; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Wang, S.; Xu, C.; Song, L.; Zhang, J. Anaerobic Digestion of Food Waste and Its Microbial Consortia: A Historical Review and Future Perspectives. Int. J. Environ. Res. Public Health 2022, 19, 9519. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent Trends in Lactic Acid Biotechnology: A Brief Review on Production to Purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Montipó, S.; Ballesteros, I.; Fontana, R.C.; Liu, S.; Ballesteros, M.; Martins, A.F.; Camassola, M. Bioprocessing of Rice Husk into Monosaccharides and the Fermentative Production of Bioethanol and Lactate. Cellulose 2019, 26, 7309–7322. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljević, M.; Kocić-Tanackov, S.; Marković, R.; Djukić-Vuković, A.; Mojović, L. Use of Spent Brewer’s Yeast in L-(+) Lactic Acid Fermentation. J. Inst. Brew. 2019, 125, 357–363. [Google Scholar] [CrossRef]
- Mladenović, D.; Pejin, J.; Kocić-Tanackov, S.; Djukić-Vuković, A.; Mojović, L. Enhanced Lactic Acid Production by Adaptive Evolution of Lactobacillus Paracasei on Agro-Industrial Substrate. Appl. Biochem. Biotechnol. 2019, 187, 753–769. [Google Scholar] [CrossRef]
- Alexandri, M.; Neu, A.K.; Schneider, R.; López-Gómez, J.P.; Venus, J. Evaluation of Various Bacillus Coagulans Isolates for the Production of High Purity L-Lactic Acid Using Defatted Rice Bran Hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 1321–1329. [Google Scholar] [CrossRef]
- Wang, X.Q.; Wang, Q.H.; Ma, H.Z.; Yin, W. Lactic Acid Fermentation of Food Waste Using Integrated Glucoamylase Production. J. Chem. Technol. Biotechnol. 2009, 84, 139–143. [Google Scholar] [CrossRef]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of Added-Value Chemical Compounds through Bioconversions of Olive-Mill Wastewaters Blended with Crude Glycerol by a Yarrowia Lipolytica Strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef]
- Ali, S.R.; Anwar, Z.; Irshad, M.; Mukhtar, S.; Warraich, N.T. Bio-Synthesis of Citric Acid from Single and Co-Culture-Based Fermentation Technology Using Agro-Wastes. J. Radiat. Res. Appl. Sci. 2016, 9, 57–62. [Google Scholar] [CrossRef]
- Esteban-Gutiérrez, M.; Garcia-Aguirre, J.; Irizar, I.; Aymerich, E. From Sewage Sludge and Agri-Food Waste to VFA: Individual Acid Production Potential and up-Scaling. Waste Manag. 2018, 77, 203–212. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Qi, Q.; Gao, C.; Lin, C.S.K. Mixed Food Waste as Renewable Feedstock in Succinic Acid Fermentation. Appl. Biochem. Biotechnol. 2014, 174, 1822–1833. [Google Scholar] [CrossRef]
- Leung, C.C.J.; Cheung, A.S.Y.; Zhang, A.Y.Z.; Lam, K.F.; Lin, C.S.K. Utilisation of Waste Bread for Fermentative Succinic Acid Production. Biochem. Eng. J. 2012, 65, 10–15. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. A Fermentative Approach towards Optimizing Directed Biosynthesis of Fumaric Acid by Rhizopus Oryzae 1526 Utilizing Apple Industry Waste Biomass. Fungal. Biol. 2015, 119, 1279–1290. [Google Scholar] [CrossRef]
- Fan, T.; Liu, X.; Zhao, R.; Zhang, Y.; Liu, H.; Wang, Z.; Wang, F.; Nie, K.; Deng, L. Hydrolysis of Food Waste by Hot Water Extraction and Subsequent Rhizopus Fermentation to Fumaric Acid. J. Environ. Manag. 2020, 270, 110954. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, J.; Wang, M.; Wang, W.; Deng, L.; Nie, K.; Yue, X.; Wang, F.; Tan, T. Food Waste Fermentation to Fumaric Acid by Rhizopus Arrhizus RH7-13. Appl. Biochem. Biotechnol. 2016, 180, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.R.; Ray, R.C. Optimization of Cultural Conditions and Their Statistical Interpretation for Production of Indole-3-Acetic Acid by Bacillus Subtilis CM5 Using Cassava Fibrous Residue. J. Sci. Ind. Res. 2008, 97, 622–628. [Google Scholar]
- Fan, Y.; Yu, K.; Zheng, H.; Chen, Y.; Zhao, R.; Li, Y.; Zheng, Z. A High-Yielding Strain of Indole-3-Acetic Acid Isolated from Food Waste Compost: Metabolic Pathways, Optimization of Fermentation Conditions, and Application. Environ. Technol. 2022, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Hong, S.Y.; Math, R.K.; Lee, J.H.; Kambiranda, D.M.; Kim, J.M.; Islam, S.M.A.; Yun, M.G.; Cho, J.J.; Lim, W.J.; et al. Biotransformation of Phenolics (Isoflavones, Flavanols and Phenolic Acids) during the Fermentation of Cheonggukjang by Bacillus Pumilus HY1. Food Chem. 2009, 114, 413–419. [Google Scholar] [CrossRef]
- Costa, T.M.; Kaufmann, V.; Paganelli, C.J.; Siebert, D.A.; Micke, G.A.; Alberton, M.D.; Tavares, L.B.B.; de Oliveira, D. Kinetic Identification of Phenolic Compounds and Potential Production of Caffeic Acid by Ganoderma Lipsiense in Solid-State Fermentation. Bioprocess Biosyst. Eng. 2019, 42, 1325–1332. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Juvonen, R.; Kössö, T.; Truchado, P.; Westerlund-Wikström, B.; Leppänen, T.; Moilanen, E.; Oksman-Caldentey, K.M. Fermentation and Dry Fractionation Increase Bioactivity of Cloudberry (Rubus chamaemorus). Food Chem. 2016, 197, 950–958. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.-M.; Lebaka, V.R.; Wee, Y.-J. Lactic Acid for Green Chemical Industry: Recent Advances in and Future Prospects for Production Technology, Recovery, and Applications. Fermentation 2022, 8, 609. [Google Scholar] [CrossRef]
- Matsakas, L.; Hrůzová, K.; Rova, U.; Christakopoulos, P. Biological Production of 3-Hydroxypropionic Acid: An Update on the Current Status. Fermentation 2018, 4, 13. [Google Scholar] [CrossRef]
- Jers, C.; Kalantari, A.; Garg, A.; Mijakovic, I. Production of 3-Hydroxypropanoic Acid from Glycerol by Metabolically Engineered Bacteria. Front. Bioeng. Biotechnol. 2019, 7, 124. [Google Scholar] [CrossRef]
- Longo, M.A.; Sanromán, M.A. Production of Food Aroma Compounds: Microbial and Enzymatic Methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar] [CrossRef]
- Boccia, F.; Covino, D.; Sarnacchiaro, P. Genetically Modified Food versus Knowledge and Fear: A Noumenic Approach for Consumer Behaviour. Food Res. Int. 2018, 111, 682–688. [Google Scholar] [CrossRef]
- De Felipe, L.O.; de Oliveira, A.M.; Bicas, J.L. Bioaromas—Perspectives for Sustainable Development. Trends Food Sci. Technol. 2017, 62, 141–153. [Google Scholar] [CrossRef]
- Sales, A.; Paulino, B.N.; Pastore, G.M.; Bicas, J.L. Biogeneration of Aroma Compounds. Curr. Opin. Food Sci. 2018, 19, 77–84. [Google Scholar] [CrossRef]
- Ben Akacha, N.; Gargouri, M. Microbial and Enzymatic Technologies Used for the Production of Natural Aroma Compounds: Synthesis, Recovery Modeling, and Bioprocesses. Food Bioprod. Process. 2015, 94, 675–706. [Google Scholar] [CrossRef]
- Carroll, A.L.; Desai, S.H.; Atsumi, S. Microbial Production of Scent and Flavor Compounds. Curr. Opin. Biotechnol. 2016, 37, 8–15. [Google Scholar] [CrossRef]
- Zelena, K.; Krings, U.; Berger, R.G. Functional Expression of a Valencene Dioxygenase from Pleurotus Sapidus in E. Coli. Bioresour. Technol. 2012, 108, 231–239. [Google Scholar] [CrossRef]
- Christen, P.; Bramorski, A.; Revah, S.; Soccol, C.R. Characterization of Volatile Compounds Produced by Rhizopus Strains Grown on Agro-Industrial Solid Wastes. Bioresour. Technol. 2000, 71, 211–215. [Google Scholar] [CrossRef]
- Rossi, S.C.; Vandenberghe, L.P.S.; Pereira, B.M.P.; Gago, F.D.; Rizzolo, J.A.; Pandey, A.; Soccol, C.R.; Medeiros, A.B.P. Improving Fruity Aroma Production by Fungi in SSF Using Citric Pulp. Food Res. Int. 2009, 42, 484–486. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, P.; Sun, Z.; Bai, Y.; Wang, J.; Guo, X. Production of Vanillin from Waste Residue of Rice Bran Oil by Aspergillus Niger and Pycnoporus Cinnabarinus. Bioresour. Technol. 2007, 98, 1115–1119. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-Phenylethanol (Rose Aroma) Production Potential of an Isolated Pichia Kudriavzevii through Solid-State Fermentation. Process. Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Christen, P.; Meza, J.C.; Revah, S. Fruity Aroma Production in Solid State Fermentation by Ceratocystis Fimbriata: Influence of the Substrate Type and the Presence of Precursors. Mycol. Res. 1997, 101, 911–919. [Google Scholar] [CrossRef]
- Medeiros, A.B.P.; Pandey, A.; Vandenberghe, L.P.S.; Pastore, G.M.; Soccol, C.R. Production and Recovery of Aroma Compounds Produced by Solid-State Fermentation Using Different Adsorbents. Food Technol. Biotechnol. 2006, 44, 47–51. [Google Scholar]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast Flavour Production by Solid State Fermentation of Orange Peel Waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; Mahmoud, M.G.; Asker, M.M.S.; Lotfy, S.N. Characterization and Evaluation of Coconut Aroma Produced by Trichoderma Viride EMCC-107 in Solid State Fermentation on Sugarcane Bagasse. Electron. J. Biotechnol. 2015, 18, 5–9. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef]
- Soares, M.; Christen, P.; Pandey, A.; Soccol, C.R. Fruity Flavour Production by Ceratocystis Fimbriata Grown on Coffee Husk in Solid-State Fermentation. Process. Biochem. 2000, 35, 857–861. [Google Scholar] [CrossRef]
- John, W.A.; Böttcher, N.L.; Behrends, B.; Corno, M.; D’souza, R.N.; Kuhnert, N.; Ullrich, M.S. Experimentally Modelling Cocoa Bean Fermentation Reveals Key Factors and Their Influences. Food Chem. 2020, 302, 125335. [Google Scholar] [CrossRef]
- Lavefve, L.; Marasini, D.; Carbonero, F. Microbial Ecology of Fermented Vegetables and Non-Alcoholic Drinks and Current Knowledge on Their Impact on Human Health. Adv. Food Nutr. Res. 2019, 87, 147–185. [Google Scholar] [CrossRef]
- Glibowski, P.; Skrzypczak, K. Microbial Production of Food Ingredients and Additives. Sciencedirect 2017, 5. [Google Scholar]
- Wang, Z.P.; Wang, Q.Q.; Liu, S.; Liu, X.F.; Yu, X.J.; Jiang, Y.L. Efficient Conversion of Cane Molasses Towards High-Purity Isomaltulose and Cellular Lipid Using an Engineered Yarrowia Lipolytica Strain in Fed-Batch Fermentation. Molecules 2019, 24, 1228. [Google Scholar] [CrossRef]
- Park, Y.C.; Oh, E.J.; Jo, J.H.; Jin, Y.S.; Seo, J.H. Recent Advances in Biological Production of Sugar Alcohols. Curr. Opin. Biotechnol. 2016, 37, 105–113. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Villela-Castrejón, J.; Perez-Carrillo, E.; Gómez-Sánchez, C.E.; Gutiérrez-Uribe, J.A. Effects of Solid-State Fungi Fermentation on Phenolic Content, Antioxidant Properties and Fiber Composition of Lime Cooked Maize by-Product (Nejayote). J. Cereal. Sci. 2019, 90, 102837. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Teixeira, J.A.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Aguilar, C.N. Fructo-Oligosaccharides (FOS) Production by Fungal Submerged Culture Using Aguamiel as a Low-Cost by-Product. LWT 2019, 102, 75–79. [Google Scholar] [CrossRef]
- Ng, C.W.C.; Ismail, A.F.; Makhtar, M.M.Z.; Jamaluddin, M.N.F.; Tajarudin, H.A. Conversion of Food Waste via Two-Stage Fermentation to Controllable Chicken Feed Nutrients by Local Isolated Microorganism. Int. J. Recycl. Org. Waste Agric. 2020, 9, 33–47. [Google Scholar] [CrossRef]
- Tropea, A.; Ferracane, A.; Albergamo, A.; Potortì, A.G.; Turco, V.L.; di Bella, G. Single Cell Protein Production through Multi Food-Waste Substrate Fermentation. Fermentation 2022, 8, 91. [Google Scholar] [CrossRef]
- Kosakai, T.; Kato, H.; Sho, C.; Kawano, K.; Iwai, K.I.; Takase, Y.; Ogawa, K.; Nishiyama, K.; Yamasaki, M. Dietary Fermented Products Using Koji Mold and Sweet Potato-Shochu Distillery by-Product Promotes Hepatic and Serum Cholesterol Levels and Modulates Gut Microbiota in Mice Fed a High-Cholesterol Diet. PeerJ 2019, 7, e7671. [Google Scholar] [CrossRef]
- Wang, S.K.; Wang, X.; Tian, Y.T.; Cui, Y.H. Nutrient Recovery from Tofu Whey Wastewater for the Economical Production of Docosahexaenoic Acid by Schizochytrium Sp. S31. Sci. Total Environ. 2020, 710, 136448. [Google Scholar] [CrossRef]
- Watanabe, K.; Arafiles, K.H.V.; Higashi, R.; Okamura, Y.; Tajima, T.; Matsumura, Y.; Nakashimada, Y.; Matsuyama, K.; Aki, T. Isolation of High Carotenoid-Producing Aurantiochytrium Sp. Mutants and Improvement of Astaxanthin Productivity Using Metabolic Information. J. Oleo Sci. 2018, 67, 571–578. [Google Scholar] [CrossRef]
- Van Beilen, J.B.; Li, Z. Enzyme Technology: An Overview. Curr. Opin. Biotechnol. 2002, 13, 338–344. [Google Scholar] [CrossRef]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A Comprehensive Insight into Fungal Enzymes: Structure, Classification, and Their Role in Mankind’s Challenges. J. Fungi 2022, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Arnau, J.; Yaver, D.; Hjort, C.M. Strategies and Challenges for the Development of Industrial Enzymes Using Fungal Cell Factories. In Grand Challenges in Biology and Biotechnology; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Steudler, S.; Werner, A.; Walther, T. It Is the Mix That Matters: Substrate-Specific Enzyme Production from Filamentous Fungi and Bacteria Through Solid-State Fermentation. Adv. Biochem. Eng./Biotechnol. 2019, 169, 51–81. [Google Scholar] [CrossRef] [PubMed]
- Solarte-Toro, J.C.; Romero-García, J.M.; Susmozas, A.; Ruiz, E.; Castro, E.; Cardona-Alzate, C.A. Techno-Economic Feasibility of Bioethanol Production via Biorefinery of Olive Tree Prunings (OTP): Optimization of the Pretreatment Stage. Holzforschung 2019, 73, 3–13. [Google Scholar] [CrossRef]
- Grønfeldt, M.Z. Process for Production of an Enzyme Product. 13/140279. 2009. [Google Scholar]
- Godoy, M.G.; Gutarra, M.L.E.; Castro, A.M.; MacHado, O.L.T.; Freire, D.M.G. Adding Value to a Toxic Residue from the Biodiesel Industry: Production of Two Distinct Pool of Lipases from Penicillium Simplicissimum in Castor Bean Waste. J. Ind. Microbiol. Biotechnol. 2011, 38, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, V.; Khan, M.; Balda, S.; Gupta, N.; Capalash, N.; Sharma, P. Flavonoid-Rich Agro-Industrial Residues for Enhanced Bacterial Laccase Production by Submerged and Solid-State Fermentation. 3 Biotech. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Vijayaraghavan, P.; Vincent, S.G.P.; Arasu, M.V.; Al-Dhabi, N.A. Bioconversion of Agro-Industrial Wastes for the Production of Fibrinolytic Enzyme from Bacillus Halodurans IND18: Purification and Biochemical Characterization. Electron. J. Biotechnol. 2016, 20, 1–8. [Google Scholar] [CrossRef]
- Pili, J.; Danielli, A.; Nyari, N.L.D.; Zeni, J.; Cansian, R.L.; Backes, G.T.; Valduga, E. Biotechnological Potential of Agro-Industrial Waste in the Synthesis of Pectin Lyase from Aspergillus Brasiliensis. Food Sci. Technol. Int. 2017, 24, 97–109. [Google Scholar] [CrossRef]
- Ali, S.S.; Vidhale, N.N. Protease Production by Fusarium Oxysporum in SolidState Fermentation Using Rice Bran. Am. J. Microbiol. Res. 2013, 3, 45–47. [Google Scholar]
- Maldonado, M.C.; Strasser De Saad, A.M. Production of Pectinesterase and Polygalacturonase by Aspergillus Niger in Submerged and Solid State Systems. J. Ind. Microbiol. Biotechnol. 1998, 20, 34–38. [Google Scholar] [CrossRef]
- Unakal, C.; Kallur, R.I.; Kaliwal, B.B. Production of $alpha$-Amylase Using Banana Waste by Bacillus Subtilis under Solid State Fermentation Production of α-Amylase Using Banana Waste by Bacillus Subtilis under Solid State Fermentation. Eur. J. Exp. Biol. 2012, 2, 1044–1052. [Google Scholar]
- Rathnan, R.K.; Ambili, M. Cellulase Enzyme Production by Streptomyces Sp Using Fruit Waste as Substrate. Aust. J. Basic Appl. Sci. 2011, 5, 1114–1118. [Google Scholar]
- Botella, C.; de Ory, I.; Webb, C.; Cantero, D.; Blandino, A. Hydrolytic Enzyme Production by Aspergillus Awamori on Grape Pomace. Biochem. Eng. J. 2005, 26, 100–106. [Google Scholar] [CrossRef]
- Silva, D.; da Silva Martins, E.; da Silva, R.; Gomes, E. Pectinase Production by Penicillium Viridicatum Rfc3 by Solid State Fermentation Using Agricultural Wastes and Agro-Industrial by-Products. Braz. J. Microbiol. 2002, 33, 318–324. [Google Scholar] [CrossRef]
- Biz, A.; Finkler, A.T.J.; Pitol, L.O.; Medina, B.S.; Krieger, N.; Mitchell, D.A. Production of Pectinases by Solid-State Fermentation of a Mixture of Citrus Waste and Sugarcane Bagasse in a Pilot-Scale Packed-Bed Bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
- Paranthaman, R.; Vidyalakshmi, R. Effects of Fungal Co-Culture for the Biosynthesis of Tannase and Gallic Acid from Grape Wastes under Solid State Fermentation. Glob. J. Biotechnol. 2009, 4, 29–36. [Google Scholar]
- Nagarajan, S.; Jones, R.J.; Oram, L.; Massanet-Nicolau, J.; Guwy, A. Intensification of Acidogenic Fermentation for the Production of Biohydrogen and Volatile Fatty Acids—A Perspective. Fermentation 2022, 8, 325. [Google Scholar] [CrossRef]
- Chalima, A.; Oliver, L.; de Castro, L.F.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of Volatile Fatty Acids from Microalgae for the Production of High Added Value Compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef]
- Vázquez-Fernández, A.; Suárez-Ojeda, M.E.; Carrera, J. Review about Bioproduction of Volatile Fatty Acids from Wastes and Wastewaters: Influence of Operating Conditions and Organic Composition of the Substrate. J. Environ. Chem. Eng. 2022, 10, 107917. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile Fatty Acids Production from Food Waste: Effects of PH, Temperature, and Organic Loading Rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.Á. Application of Solid-State Fermentation to Food Industry—A Review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Abd Rashid, N.Y.; Jamaluddin, A.; Sharifudin, S.A.; Abd Kahar, A.; Long, K. Cosmeceutical Potentials and Bioactive Compounds of Rice Bran Fermented with Single and Mix Culture of Aspergillus Oryzae and Rhizopus Oryzae. J. Saudi Soc. Agric. Sci. 2017, 16, 127–134. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; An, X.; Qi, J. Study on the Enhancement of Antioxidant Properties of Rice Bran Using Mixed-Bacteria Solid-State Fermentation. Fermentation 2022, 8, 212. [Google Scholar] [CrossRef]
- Tišma, M.; Tadić, T.; Budžaki, S.; Ostojčić, M.; Šalić, A.; Zelić, B.; Tran, N.N.; Ngothai, Y.; Hessel, V. Lipase Production by Solid-State Cultivation of Thermomyces Lanuginosus on by-Products from Cold-Pressing Oil Production. Processes 2019, 7, 465. [Google Scholar] [CrossRef]
- Németh, Á.; Kaleta, Z. Complex Utilization of Dairy Waste (Whey) in Biorefinery. WSEAS Trans. Environ. Dev. 2015, 11, 80–88. [Google Scholar]
- Vastrad, B.M.; Neelagund, S.E. Optimization of Process Parameters for Rifamycin B Production Under Solid State Fermentation from Amycolatopsis Mediterranean Mtcc 14. Int. J. Curr. Pharm Res. 2012, 4, 101–108. [Google Scholar]
- Vastrad, B.M.; Neelagund, S.E. Optimization and Production of Neomycin from Different Agro Industrial Wastes in Solid State Fermentation. Int. J. Pharm. Sci. Drug Res. 2011, 3, 104–111. [Google Scholar]
- Dursun, D.; Dalgiç, A.C. Optimization of Astaxanthin Pigment Bioprocessing by Four Different Yeast Species Using Wheat Wastes. Biocatal. Agric. Biotechnol. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Klaic, R.; Sallet, D.; Foletto, E.L.; Jacques, R.J.S.; Guedes, J.V.C.; Kuhn, R.C.; Mazutti, M.A. Optimization of Solid-State Fermentation for Bioherbicide Production by Phoma sp. Braz. J. Chem. Eng. 2017, 34, 377–384. [Google Scholar] [CrossRef]
- Rane, A.N.; Baikar, V.V.; Ravi Kumar, D.V.; Deopurkar, R.L. Agro-Industrial Wastes for Production of Biosurfactant by Bacillus Subtilis ANR 88 and Its Application in Synthesis of Silver and Gold Nanoparticles. Front. Microbiol. 2017, 8, 492. [Google Scholar] [CrossRef]
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; de Jesús Cázares-Marinero, J. Biosurfactants: The Green Generation of Speciality Chemicals and Potential Production Using Solid-State Fermentation (SSF) Technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef]
- Lang, S.; Wullbrandt, D. Rhamnose Lipids—Biosynthesis, Microbial Production and Application Potential. Appl. Microbiol. Biotechnol. 1999, 51, 22–32. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Henkel, M.; Müller, M.M.; Kügler, J.H.; Lovaglio, R.B.; Contiero, J.; Syldatk, C.; Hausmann, R. Rhamnolipids as Biosurfactants from Renewable Resources: Concepts for next-Generation Rhamnolipid Production. Process Biochem. 2012, 47, 1207–1219. [Google Scholar] [CrossRef]
- Lee, J.; Chen, W.-H.; Park, Y.-K. Recent Achievements in Platform Chemical Production from Food Waste. Bioresour. Technol. 2022, 366, 128204. [Google Scholar] [CrossRef]
- Trivedi, J.; Bhonsle, A.K.; Atray, N. Processing food waste for the production of platform chemicals. In Refining Biomass Residues for Sustainable Energy and Bioproducts: Technology, Advances, Life Cycle Assessment, and Economics; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Kover, A.; Kraljić, D.; Marinaro, R.; Rene, E.R. Processes for the Valorization of Food and Agricultural Wastes to Value-Added Products: Recent Practices and Perspectives. Syst. Microbiol. Biomanufacturing 2022, 2, 50–66. [Google Scholar] [CrossRef]
- Peinemann, J.C.; Demichelis, F.; Fiore, S.; Pleissner, D. Techno-Economic Assessment of Non-Sterile Batch and Continuous Production of Lactic Acid from Food Waste. Bioresour. Technol. 2019, 289, 121631. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.; Teleky, B.-E.; Szabo, K.; Martău, A.-G.; Ştefănescu, B.E.; Dulf, F.-V.; Vodnar, D.-C. Waste Cooking Oil and Crude Glycerol as Efficient Renewable Biomass for the Production of Platform Organic Chemicals through Oleophilic Yeast Strain of Yarrowia Lipolytica. Environ. Technol. Innov. 2022, 28, 102943. [Google Scholar] [CrossRef]
- Juy, M.I.; Orejas, J.A.; Lucca, M.E. Study of Itaconic Acid Production by Aspergillus Terrus MJL05 Strain with Different Variable. Rev. Colom. Biotechnol. 2010, 12, 187–193. [Google Scholar]
- Saha, B.C. Emerging Biotechnologies for Production of Itaconic Acid and Its Applications as a Platform Chemical. J. Ind. Microbiol. Biotechnol. 2017, 44, 303–315. [Google Scholar] [CrossRef]
- Harder, B.J.; Bettenbrock, K.; Klamt, S. Model-Based Metabolic Engineering Enables High Yield Itaconic Acid Production by Escherichia Coli. Metab. Eng. 2016, 38, 29–37. [Google Scholar] [CrossRef]
- Kim, N.J.; Lim, S.J.; Chang, H.N. Volatile Fatty Acid Platform: Concept and Application. In Emerging Areas in Bioengineering; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Vu, D.H.; Mahboubi, A.; Rood, A.; Heinmaa, I.; Taherzadeh, M.J.; Akesson, D. Thorough Investigation of the Effects of Cultivation Factors on Polyhydroalkanoates (PHAs) Production by Cupriavidus necator from Food Waste-Derived Volatile Fatty Acids. Fermentation 2022, 8, 605. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current Perspectives on Acidogenic Fermentation to Produce Volatile Fatty Acids from Waste. Rev. Environ. Sci. Biotechnol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food Waste Biorefinery: Pathway towards Circular Bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, B.; Jayamuthunagai, J.; Sreejith, R.; Iyyappan, J.; Praveenkumar, R. Techno Economic Analysis of Malic Acid Production Using Crude Glycerol Derived from Waste Cooking Oil. Bioresour. Technol. 2022, 351, 126956. [Google Scholar] [CrossRef]
- Rajendran, N.; Han, J. Integrated Polylactic Acid and Biodiesel Production from Food Waste: Process Synthesis and Economics. Bioresour. Technol. 2022, 343, 126119. [Google Scholar] [CrossRef]
- Rajendran, N.; Han, J. Techno-Economic Analysis and Life Cycle Assessment of Poly (Butylene Succinate) Production Using Food Waste. Waste Manag. 2023, 156, 168–176. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Hamelin, L.; Thomsen, M. Review of High-Value Food Waste and Food Residues Biorefineries with Focus on Unavoidable Wastes from Processing. Resour. Conserv. Recycl. 2019, 149, 413–426. [Google Scholar] [CrossRef]
- Zou, L.; Wan, Y.; Zhang, S.; Luo, J.; Li, Y.Y.; Liu, J. Valorization of Food Waste to Multiple Bio-Energies Based on Enzymatic Pretreatment: A Critical Review and Blueprint for the Future. J. Clean. Prod. 2020, 277, 124091. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, Y.; Zhen, G.; Lu, X.; Xu, S.; Zhang, J.; Gu, L.; Wen, H.; Liu, H.; Zhang, X.; et al. Effective Multipurpose Sewage Sludge and Food Waste Reduction Strategies: A Focus on Recent Advances and Future Perspectives. Chemosphere 2023, 311, 136670. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Khoo, K.S.; Yew, G.Y.; Leong, W.H.; Lim, J.W.; Lam, M.K.; Ho, Y.C.; Ng, H.S.; Munawaroh, H.S.H.; Show, P.L. Advances in Production of Bioplastics by Microalgae Using Food Waste Hydrolysate and Wastewater: A Review. Bioresour. Technol. 2021, 342, 125947. [Google Scholar] [CrossRef]
- Deckers, M.; Deforce, D.; Fraiture, M.A.; Roosens, N.H.C. Genetically Modified Micro-Organisms for Industrial Food Enzyme Production: An Overview. Foods 2020, 9, 326. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Sacchi, R.; Masi, P. Techno-Economic Assessment of DHA-Rich Aurantiochytrium Sp. Production Using Food Industry by-Products and Waste Streams as Alternative Growth Media. Bioresour. Technol. Rep. 2022, 18, 100997. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).