In Vitro Mycotoxin Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Mycotoxin Saccharomyces cerevisiae Adsorption and Desorption Assessments
2.3. Mycotoxin Extraction and Detection
2.4. Saccharomyces cerevisiae Mycotoxin Biotransformation
3. Results and Discussion
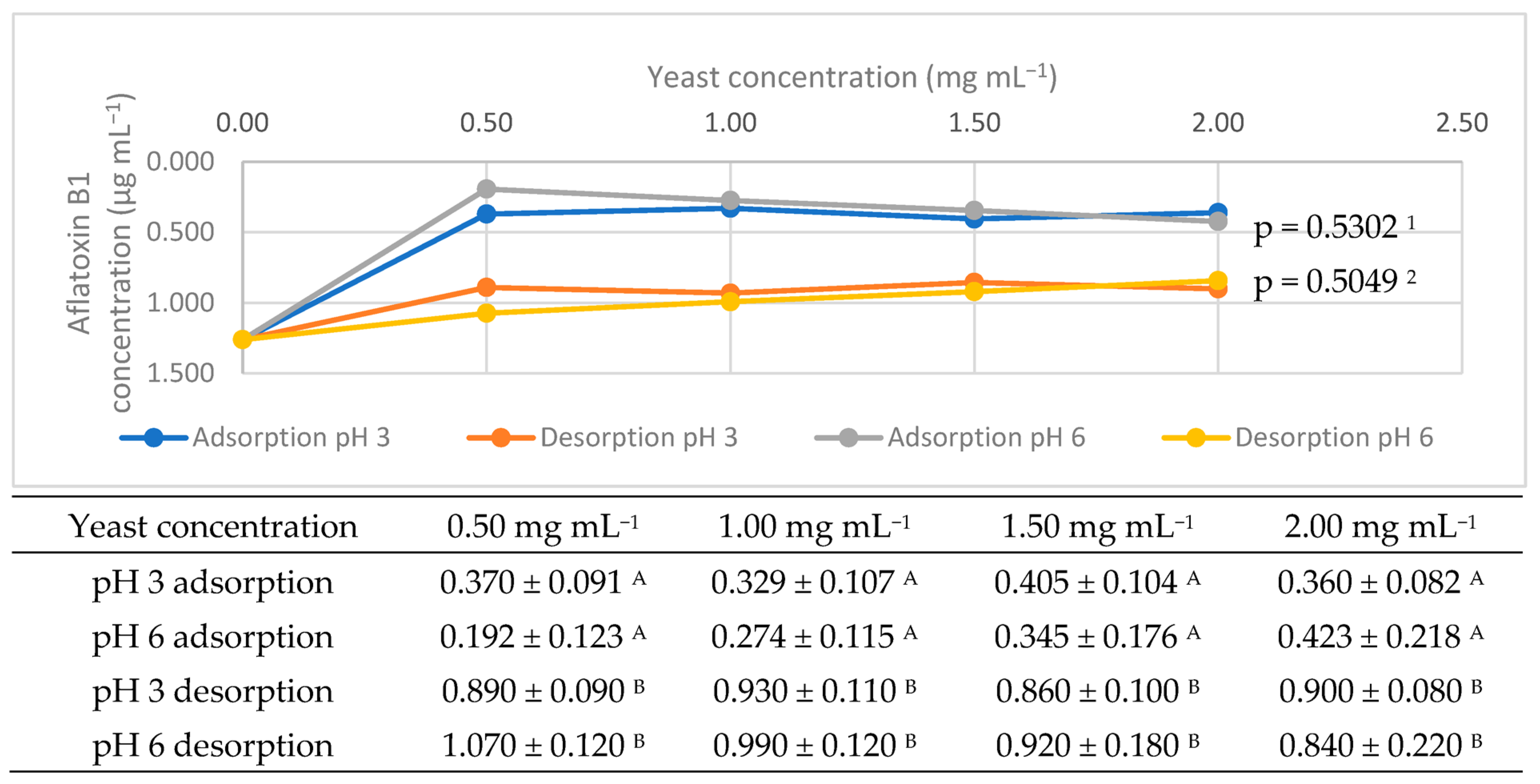
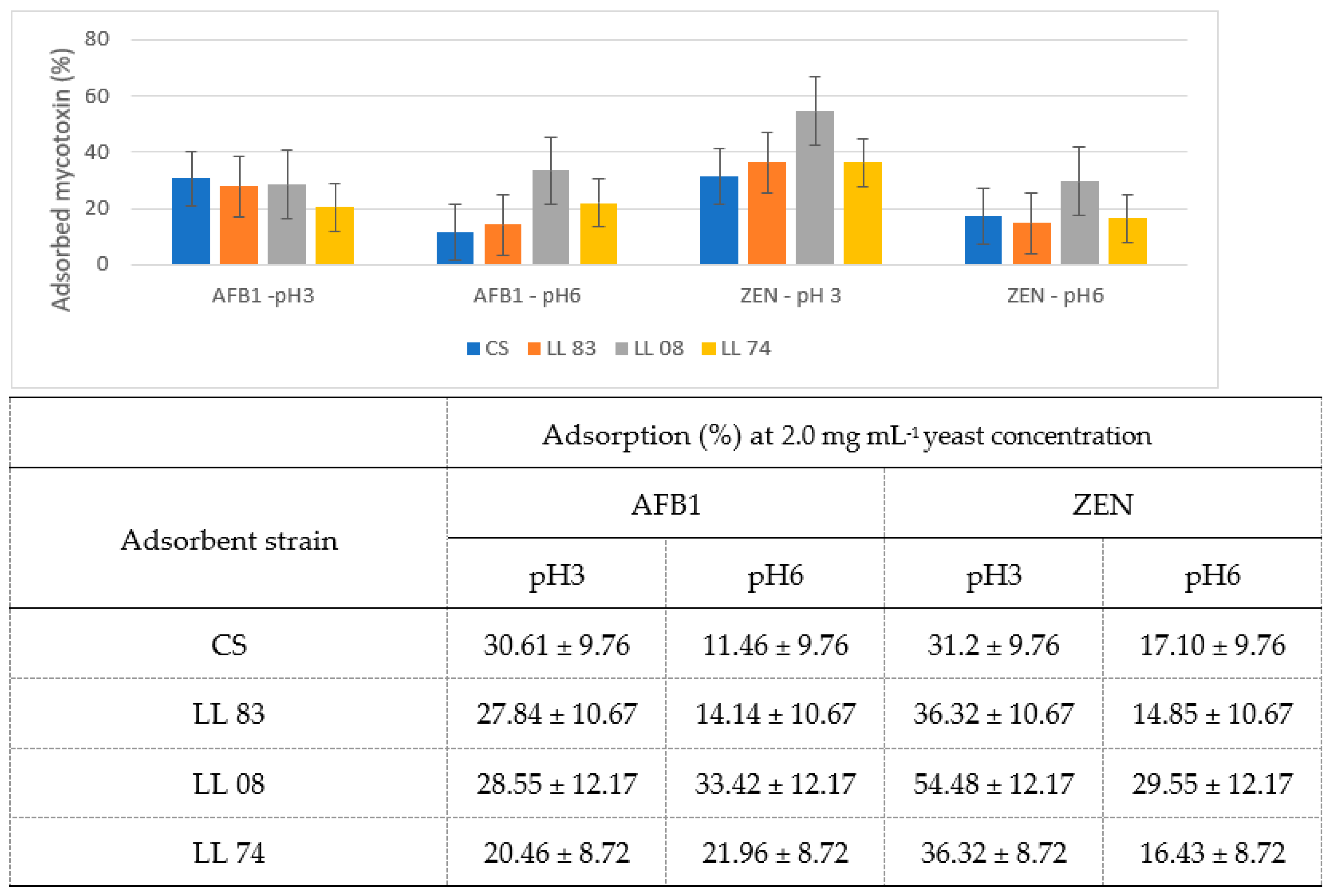
3.1. Adsorptive Potential
3.2. Biotransformation Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peles, F.; Sipos, P.; Kovács, S.; Győri, Z.; Pócsi, I.; Pusztahelyi, T. Biological control and mitigation of aflatoxin contamination in commodities. Toxins 2021, 1, 104. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Meneely, J.P.; Haughey, S.A.; Dean, M.; Wall, P.; Zhang, G.; Baker, B.; Elliott, C.T. Risk assessments for the dietary intake aflatoxins in food: A systematic review (2016–2022). Food Control 2023, 149, 109687. [Google Scholar] [CrossRef]
- Keller, L.A.M.; Aronovich, M.; Keller, K.M.; Castagna, A.A.; Cavaglieri, L.R.; Rosa, C.A.R. Incidence of Mycotoxins (AFB1 and AFM1) in Feeds and Dairy Farms from Rio de Janeiro State, Brazil. Veterinary. Medicine 2016, 1, 29–35. [Google Scholar]
- Kabak, B. Aflatoxins in foodstuffs: Occurrence and risk assessment in Turkey. J. Food Compost. Anal. 2021, 96, 103734. [Google Scholar] [CrossRef]
- Conteçotto, A.C.T.; Pante, G.C.; Castro, J.C.; Souza, A.A.; Lini, R.S.; Romoli, J.C.Z.; Abreu Filho, B.A.; Mikcha, J.M.G.; Mossini, S.A.G.; Machinski Junior, M. Occurrence, exposure evaluation and risk assessment in child population for aflatoxin M1 in dairy products in Brazil. Food Chem. Toxicol. 2021, 148, 111913. [Google Scholar] [CrossRef]
- Pitt, J.I. Toxigenic fungi and mycotoxins. Br. Med. J. 2000, 56, 184–192. [Google Scholar]
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health. 2020, 17, 423. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of Oral Exposure to Aflatoxin B1-Induced Renal Dysfunction, Oxidative Stress, and Cell Apoptosis in Mice Kidney by Curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef]
- Forsythe, S.J. Microbiologia da Segurança Alimentar; ArtMed: Porto Alegre, Brazil, 2002; 422p. [Google Scholar]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, ZENralenone. Crit. Rev. Food Sci. Nutr. 2019, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, J.; Chen, Y.; Zhu, J. Zearalenone and Its Masked Forms in Cereals and Cereal-Derived Products: A Review of the Characteristics, Incidence, and Fate in Food Processing. J. Fungi 2022, 8, 976. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Yuan, L.; Li, J. Complicated interactions between bio-adsorbents and mycotoxins during mycotoxin adsorption: Current research and future prospects. Trends Food Sci. Technol. 2020, 96, 127–134. [Google Scholar] [CrossRef]
- Keller, L.A.M.; Abrunhosa, L.; Keller, K.M.; Rosa, C.A.R.; Cavaglieri, L.R.; Venâncio, A. ZENralenone and its derivatives α-ZENralenol and β-ZENralenol decontamination by Saccharomyces cerevisiae strains isolated from bovine forage. Toxins 2015, 7, 3297–3308. [Google Scholar] [CrossRef] [Green Version]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecchini, F.; Morassut, M.; Saiz, J.C.; Moruno, E.G. Anthocyanins enhance yeast’s adsorption of Ochratoxin A during the alcoholic fermentation. Eur. Food Res. Technol. 2019, 245, 309–314. [Google Scholar] [CrossRef]
- Goncalves, B.L.; Rosim, R.E.; de Oliveira, C.A.F.; Corassin, C.H. The in vitro ability of different Saccharomyces cerevisiae-based products to bind aflatoxin B1. Food Control 2015, 47, 298–300. [Google Scholar] [CrossRef]
- Oliveira, A.A.; Keller, K.M.; Deveza, M.V.; Keller, L.A.M.; Dias, E.O.; Martini-Santos, B.J.; Rosa, C.A.R. Effect of three different anti-mycotoxin additives on broiler chickens exposed to aflatoxin B1. Arch. Med. Vet. 2015, 47, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Ochratoxin A removal by yeasts after exposure to simulated human gastrointestinal conditions. J. Food Sci. 2016, 81, 2756–2760. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, J.G.; Liu, B.; Wang, Z.L.; Yuan, Y.H.; Yue, T.L. Effect of yeast cell morphology, cell wall physical structure and chemical composition on patulin adsorption. PLoS ONE 2015, 10, e0136045. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Liu, X.J.; Liu, Y.; Han, Y.Q.; Li, J. Exogenous calcium ions enhance patulin adsorption capability of Saccharomyces cerevisiae. J. Food Prot. 2019, 82, 1390–1397. [Google Scholar] [CrossRef]
- Pfliegler, W.P.; Pusztahelyi, T.; Pócsi, I. Mycotoxins-prevention and decontamination by yeasts. J. Basic Microbiol. 2015, 55, 805–818. [Google Scholar] [CrossRef]
- Silva, F.C.; Chalfoun, S.M.; Batista, L.R.; Santos, C.; Lima, N. Taxonomia polifásica para identificação de Aspergillus seção flavi: Uma revisão. Rev. Eletrônica Sala Aula Foco 2015, 1, 18–40. [Google Scholar] [CrossRef]
- Bovo, F.; Corassin, C.H.; Rosim, R.E.; Oliveira, C.A.F. Efficiency of Lactic Acid Bacteria Strains for Decontamination of Aflatoxin M1 in Phosphate Buffer Saline Solution and in Skimmed Milk. Food Bioprocess Technol. 2013, 6, 2230–2234. [Google Scholar] [CrossRef]
- Pereyra, C.M.; Gil, S.; Cristofolini, A.; Bonci, M.; Makita, M.; Monge, M.P.; Montenegro, M.A.; Cavaglieri, L.R. The production of yeast cell wall using an agroindustrial waste influences the wall thickness and is implicated on the aflatoxin B1 adsorption process. Food Res. Int. 2018, 111, 306–313. [Google Scholar] [CrossRef]
- Aazami, M.H.; Nasri, M.H.F.; Mojtahedi, M.; Mohammadi, S.R. In vitro aflatoxin B1 binding by the cell wall and (1→3)-β-d-glucan of baker’s yeast. J. Food Prot. 2018, 81, 670–676. [Google Scholar] [CrossRef]
- Hamza, Z.; El-Hashash, M.; Aly, S.; Hathout, A.; Soto, E.; Sabry, B.; Ostroff, G. Preparation and characterization of yeast cell wall beta-glucan encapsulated humic acid nanoparticles as an enhanced aflatoxin B1 binder. Carbohydr. Polym. 2019, 203, 185–192. [Google Scholar] [CrossRef]
- Prapapanpong, J.; Udomkusonsri, P.; Mahavorasirikul, W.; Choochuay, S.; Tansakul, S. In vitro studies on gastrointestinal monogastric and avian models to evaluate the binding efficacy of mycotoxin adsorbents by liquid chromatography-tandem mass spectrometry. J. Adv. Vet. Anim. Res. 2019, 6, 125–132. [Google Scholar] [CrossRef]
- Martínez, J.; Hernández-Rodríguez, M.; Méndez-Albores, A.; Téllez-Isaías, G.; Jiménez, E.M.; Nicolás-Vázquez, M.I.; Ruvalcaba, R.M. Computational Studies of Aflatoxin B1 (AFB1): A Review. Toxins 2023, 15, 153. [Google Scholar] [CrossRef]
- NCBI. National Center for Biotechnology Information. PubChem Compound Summary for CID 15558498, Aflatoxin M1. 2022a. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aflatoxin-M1 (accessed on 24 March 2023).
- NCBI. National Center for Biotechnology Information. PubChem Compound Summary for CID 5281576, ZENralenone. 2022b. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/ZENralenone (accessed on 24 March 2023).
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Ramos, A.J. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.Y.; Kim, J.; Cheong, D.H.; Hong, H.; Jeong, J.Y.; Kim, B.G. An In Vitro Study on the Efficacy of Mycotoxin Sequestering Agents for Aflatoxin B1, Deoxynivalenol, and Zearalenone. Animals 2022, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, A.; Apajalahti, J.; Kettunen, H.; Ojanperä, S.; Bell, A.N.W.; Keegan, J.D.; Moran, C.A. Efficient Aflatoxin B1 Sequestration by Yeast Cell Wall Extract and Hydrated Sodium Calcium Aluminosilicate Evaluated Using a Multimodal In-Vitro and Ex Vivo Methodology. Toxins 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ogunade, I.M.; Kim, D.H.; Li, X.; Pech-Cervantes, A.A.; Arriola, K.G.; Oliveira, A.S.; Driver, J.P.; Ferraretto, L.F.; Staples, C.R.; et al. Effect of adding clay with or without a Saccharomyces cerevisiae fermentation product on the health and performance of lactating dairy cows challenged with dietary aflatoxin B1. J. Dairy Sci. 2018, 101, 3008–3020. [Google Scholar] [CrossRef] [PubMed]
- Maki, C.R.; Thomas, A.D.; Elmore, S.E.; Romoser, A.A.; Harvey, R.B.; Ramirez, H.A.; Phillips, T.D. Effects of calcium montmorillonite clay and aflatoxin exposure on dry matter intake, milk production, and milk composition. J. Dairy Sci. 2016, 99, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.O.; Rodrigues, R.O.; Ledoux, D.R.; Rottinghaus, G.E.; Borutova, R.; Averkieva, O.; Mcfadden, T.B. Feed additives containing sequestrant clay minerals and inactivated yeast reduce aflatoxin excretion in milk of dairy cows. J. Dairy Sci. 2019, 102, 6614–6623. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.L.; Wang, Y.M.; Zhou, H.L.; Liu, J.X. Effects of dietary adsorbent on milk aflatoxin M1 content and the health of lactating dairy cows exposed to long-term aflatoxin B1 challenge. J. Dairy Sci. 2018, 101, 8944–8953. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Su, R.; Yin, R.; Lai, D.; Wang, M.; Liu, Y.; Zhou, L. Detoxification of Mycotoxins through Biotransformation. Toxins 2020, 12, 121. [Google Scholar] [CrossRef] [Green Version]
- Barrientos-Velázquez, A.L.; Arteaga, S.; Dixon, J.B.; Deng, Y. The effects of pH, pepsin, exchange cation, and vitamins on aflatoxin adsorption on smectite in simulated gastric fluids. Appl. Clay Sci. 2016, 120, 17–23. [Google Scholar] [CrossRef]
- Elliot, C.T.; Connolly, L.; Kolawole, O. Potential adverse effects on animal health and performance caused by the addition of mineral adsorbents to feeds to reduce mycotoxin exposure. Mycotoxin Res. 2019, 36, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Miao, Y.; Sun, Z.; Zheng, S. Simultaneous adsorption of aflatoxin B1 and zearalenone by mono- and di-alkyl cationic surfactants modified montmorillonites. J. Colloid Interface Sci. 2018, 511, 67–76. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y. Review on microbial degradation of ZENralenone and aflatoxins. Grain Oil Sci. Technol. 2020, 3, 117–125. [Google Scholar] [CrossRef]
- Fruhauf, S.; Novak, B.; Nagl, V.; Hackl, M.; Hartinger, D.; Rainer, V.; Labudová, S.; Adam, G.; Aleschko, M.; Moll, W.D.; et al. Biotransformation of the Mycotoxin ZENralenone to Its Metabolites Hydrolyzed ZENralenone (HZEN) and Decarboxylated Hydrolyzed ZENralenone (DHZEN) Diminishes Its Estrogenicity In Vitro and In Vivo. Toxins 2019, 11, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moebus, V.F.; Pinto, L.d.A.; Köptcke, F.B.N.; Keller, K.M.; Aronovich, M.; Keller, L.A.M. In Vitro Mycotoxin Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage. Fermentation 2023, 9, 585. https://doi.org/10.3390/fermentation9070585
Moebus VF, Pinto LdA, Köptcke FBN, Keller KM, Aronovich M, Keller LAM. In Vitro Mycotoxin Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage. Fermentation. 2023; 9(7):585. https://doi.org/10.3390/fermentation9070585
Chicago/Turabian StyleMoebus, Victor Farias, Leonardo de Assunção Pinto, Felipe Braz Nielsen Köptcke, Kelly Moura Keller, Marcos Aronovich, and Luiz Antonio Moura Keller. 2023. "In Vitro Mycotoxin Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage" Fermentation 9, no. 7: 585. https://doi.org/10.3390/fermentation9070585
APA StyleMoebus, V. F., Pinto, L. d. A., Köptcke, F. B. N., Keller, K. M., Aronovich, M., & Keller, L. A. M. (2023). In Vitro Mycotoxin Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage. Fermentation, 9(7), 585. https://doi.org/10.3390/fermentation9070585







