Diversity of Crithmum maritimum L. from Salento Coastal Area: A Suitable Species for Domestication
Abstract
:1. Introduction
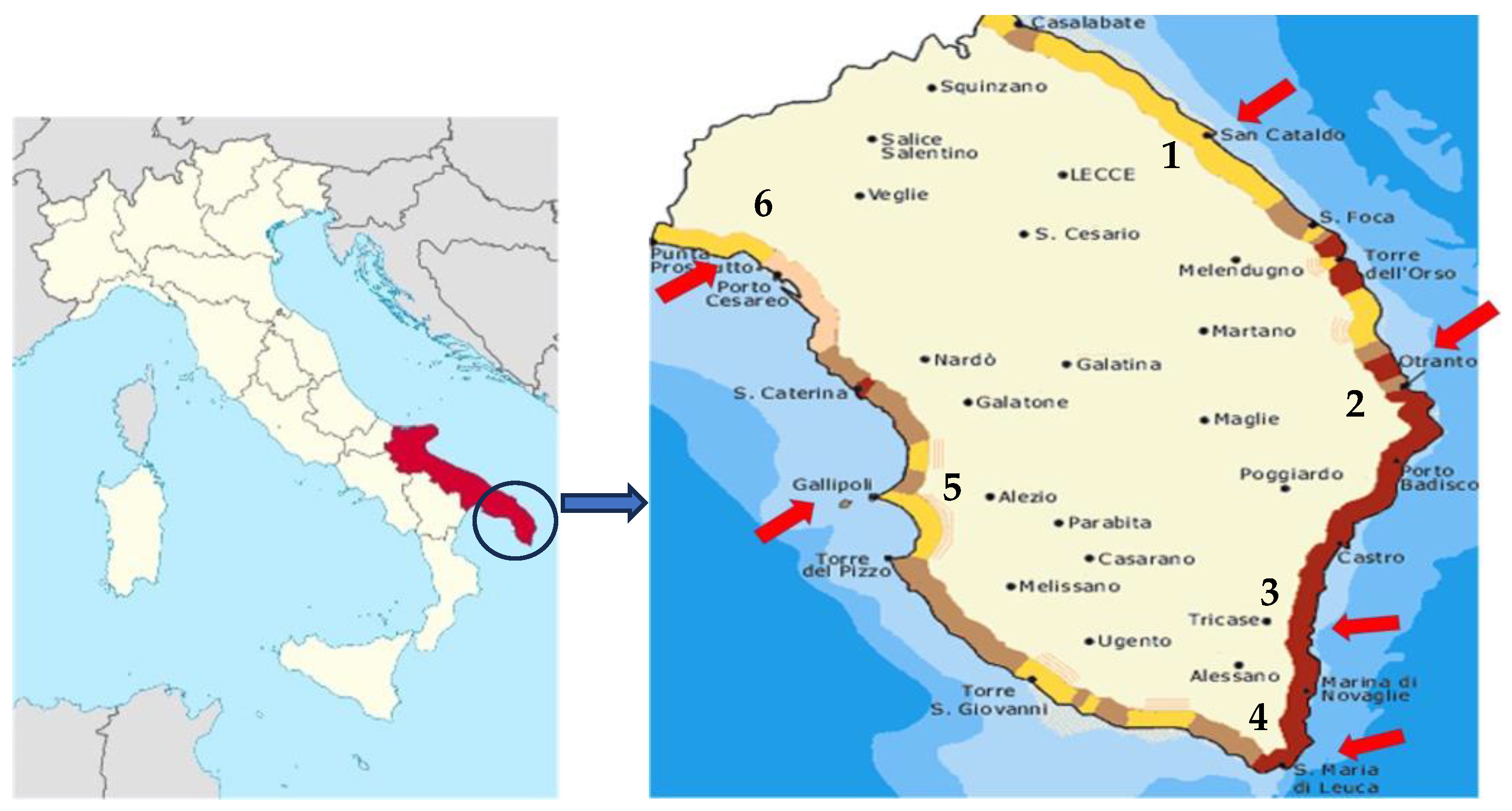
2. Materials and Methods
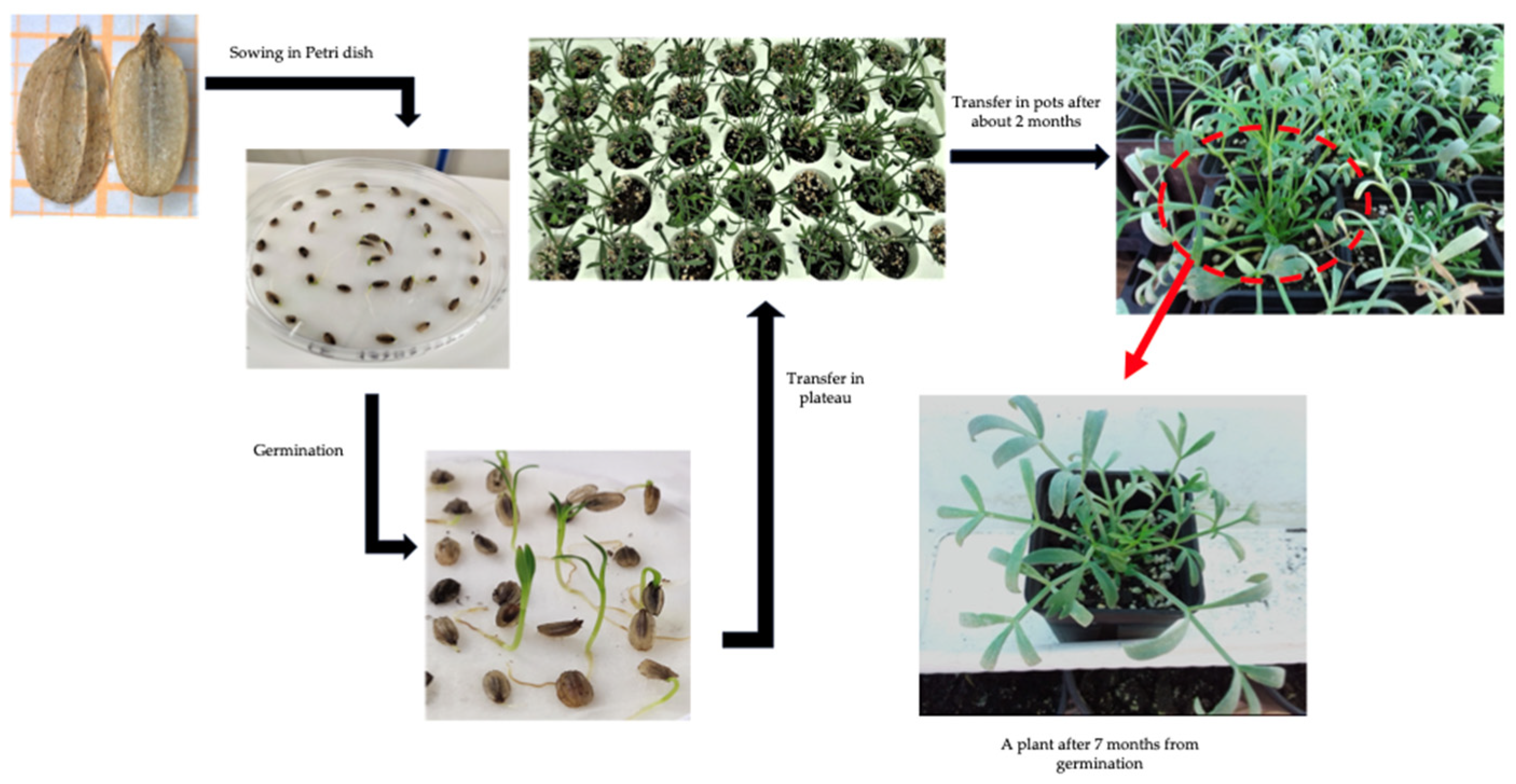
2.1. Plant Material and Experimental Conditions
2.2. Germination Rate
2.3. Analysis of the Volatile Compounds
2.4. Agglomerative Hierarchical Cluster (AHC) Analysis of Volatile Organic Compounds
3. Results
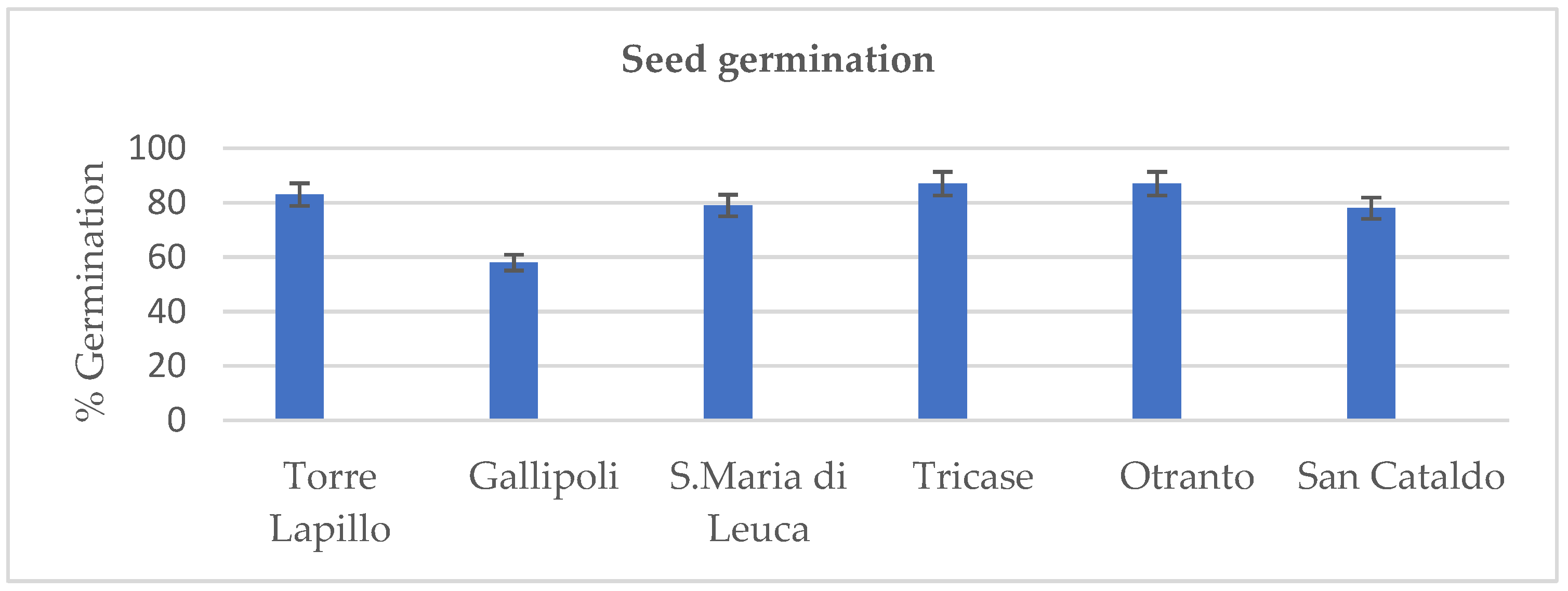
3.1. Seed Germination
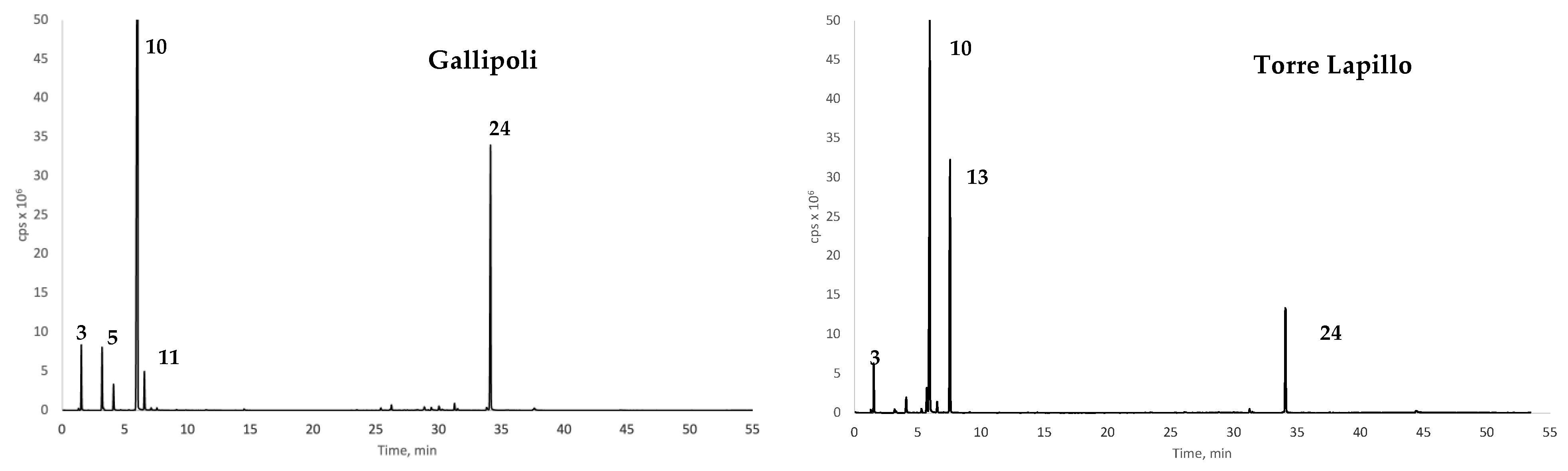
3.2. Volatile Organic Compounds
Agglomerative Hierarchical Cluster Analysis on VOC Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atia, A.; Barhoumi, Z.; Mokded, R.; Abdelly, C.; Smaoui, A. Enviromental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J. Med. Plants Res. 2011, 5, 3564–3571. [Google Scholar]
- Tardío, J.; de Cortes Sánchez-Mata, M.; Morales, R.; Molina, M.; García-Herrera, P.; Morales, P.; Díez-Marqués, C.; Fernández-Ruiz, V.; Cámara, M.; Pardo-de-Santayana, M.; et al. Ethnobotanical and food composition monographs of selected Mediterranean wild edible plants. In Mediterranean Wild Edible Plants; Sánchez-Mata, M., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; pp. 273–470. [Google Scholar] [CrossRef]
- Renna, M. Reviewing the prospects of sea fennel (Crithmum maritimum L.) as emerging vegetable crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef]
- Böer, B.; Khan, M.A.; Marcum, K.B. World Halophyte Garden: Economic Dividends with Global Significance. In Sabkha Ecosystems. Tasks for Vegetation Science; Khan, M.A., Böer, B., Öztürk, M., Al Abdessalaam, T.Z., Clüsener-Godt, M., Gul, B., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 47, pp. 335–336. [Google Scholar] [CrossRef]
- Kraouia, M.; Nartea, A.; Maoloni, A.; Osimani, A.; Garofalo, C.; Fanesi, B.; Ismaiel, L.; Aquilanti, L.; Pacetti, D. Sea Fennel (Crithmum maritium L.) as an emerging crop for the manufacturing of innovative foods and nutraceuticals. Molecules 2023, 28, 4741. [Google Scholar] [CrossRef]
- Nabel, N.; Boudries, H.; Chougui, N.; Loupassaki, S.; Souagui, S.; Burlo, F.; Hernandez, F.; Carbonell-Barrachina, A.A.; Madani, K.; Larbat, R. Biological activities and secondary compound composition from Crithmum maritimum aerial parts. Int. J. Food Prop. 2017, 20, 1843–1855. [Google Scholar] [CrossRef]
- Sanchez-Faure, A.; Calvo, M.M.; Perez-Jimenez, J.; Martín-Diana, A.B.; Rico, D.; Montero, M.P.; Gomz-Guillen, M.D.C.; Lopez-Caballero, M.E.; Martinez-Alvarez, O. Exploring the potential of common iceplant, seaside arrowgrass and sea fennel as edible halophytic plants. Food Res. Int. 2020, 137, 109613. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Lupidi, G.; Cianfaglione, K.; Dauvergne, X.; Bruno, M.; Benelli, G. Efficacy of sea fennel (Crithmum maritimum L., Apiaceae) essential oils against Culex quinquefasciatus Say and Spodoptera littoralis (Boisd.). Ind. Crops Prod. 2017, 109, 603–610. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Cérantola, S.; Talarmin, H.; Le Meur, C.; Le Floch, G.; Magné, C. New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract. Food Chem. Toxicol. 2010, 48, 553–557. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Fritschi, F.B.; Sengupta, S.; Fichman, Y.; Azad, R.K.; Mittler, R. The impact of water deficit and heat stress combination on the molecular response, physiology, and seed production of soybean. Physiol. Plant. 2021, 172, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible halophytes and halo-tolerant species in Apulia region (Southeastern Italy): Biogeography, traditional food use and potential sustainable crops. Plants 2023, 12, 549. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M. The use of the sea fennel as a new spice-colorant in culinary preparations. Int. J. Gastr. Food Sci. 2013, 1, 111–115. [Google Scholar] [CrossRef]
- Giungato, P.; Renna, M.; Rana, R.; Licen, S.; Barbieri, P. Characterization of dried and freeze-dried sea fennel (Crithmum maritimum L.) samples with headspace gas-chromatography/mass spectrometry and evaluation of an electronic nose discrimination potential. Food. Res. Int. 2019, 115, 65–72. [Google Scholar] [CrossRef]
- Mancianti, F.; Ebani, V.V. Biological activity of essential oils. Molecules 2020, 25, 678. [Google Scholar] [CrossRef] [PubMed]
- De Agostini, A.; Robustelli della Cuna, F.S.; Cortis, P.; Cogoni, A.; Sottani, C.; Soddu, F.; Sanna, C. Volatile organic compounds (VOCs) diversity in the Orchid Himantoglossum robertianum (Loisel.) P. Delfore from Sardinia (Italy). Diversity 2023, 14, 1125. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Migliorini, P.; Gkisakis, V.; Gonzalvez, V.; Raigón, M.D.; Bàrberi, P. Agroecology in Mediterranean Europe: Genesis, State and Perspectives. Sustainability 2018, 10, 2724. [Google Scholar] [CrossRef]
- Zafeiropoulou, V.; Tomou, E.-M.; Douros, A.; Skaitsa, H. The effect of successive harvesting on the volatile constituents of two essential oils of cultivated populations of sea fennel (Crithmum maritimum L.) in Greece. J. Essent. Oil Bear. Plants 2021, 24, 1–11. [Google Scholar] [CrossRef]
- Nascimento, J.P.B.; Vieira, D.C.M.; Meiado, M.V. Ex situ conservation of Brazilian Cacti. Gaia Sci. 2015, 9, 111–116. [Google Scholar]
- Negro, C.; Dimita, R.; Min Allah, S.; Miceli, A.; Luvisi, A.; Blando, F.; De Bellis, L.; Accogli, R. Phytochemicals and Volatiles in Developing Pelargonium ‘Endsleigh’ Flowers. Horticulturae 2021, 7, 419. [Google Scholar] [CrossRef]
- Dimita, R.; Min Allah, S.; Luvisi, A.; Greco, D.; De Bellis, L.; Accogli, R.; Mininni, C.; Negro, C. Volatile compounds and total phenolic content of Perilla frutescens at microgreens and mature stages. Horticulturae 2022, 8, 71. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Li, Z.G.; Tian, W.L.; Fang, X.M.; Su, S.K.; Peng, W.J. Differential Volatile Organic Compounds in Royal Jelly Associated with Different Nectar Plants. J. Integr. Agric. 2016, 15, 1157–1165. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Magné, C. Effect of salinity and chemical factors on seed germination in the halophyte Crithmum maritimum L. Plant Soil 2008, 313, 83–87. [Google Scholar] [CrossRef]
- Nimac, A.; Lazarević, B.; Petek, M.; Vidak, M.; Šatović, Z.; Carović-Stanko, K. Effects of Salinity and Seed Priming on Germination of Sea Fennel (Crithmum maritimum L.). Agric. Conspec. Sci. 2018, 38, 181–185. Available online: https://hrcak.srce.hr/203017 (accessed on 24 November 2023).
- Bischoff, A.; Müller-Schärer, H. Testing population differentiation in plant species- how important are environmental maternal effects. Oikos 2010, 3, 445–454. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Mattana, E.; Acosta, A.T.R.; Bacchetta, G. Seed germination responses to varying environmental conditions and provenances in Crucianella maritima L., a threatened costal species. Comp. Rend. Biol. 2012, 335, 26–31. [Google Scholar] [CrossRef]
- Marchioni-Ortu, A.; Bocchieri, E. A study of the germination responses of a Sardinian population of sea fennel (Crithmum maritimum). Can. J. Bot. 1984, 62, 1832–1835. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangi, M.M.; Fan, Y. Volatile terpenoids; multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–810. [Google Scholar] [CrossRef]
- Jallali, I.; Megdiche, W.; Oueslati, S.; Smaoui, A.; Abdelly, C.; Ksouri, R. Changes in phenolic composition and antioxidant activities of the edible halophyte Crithmum maritimum L. with physiological stage and extraction method. Acta Physiol. Plant. 2012, 34, 1451–1459. [Google Scholar] [CrossRef]
- Atia, A.; Debez, A.; Barhoumi, Z.; Abdell, C. Localization and composition of seed oils of Crithmum maritimum L. (Apiaceae) Afr. J. Biotechnol. 2010, 9, 6482–6485. [Google Scholar]
- Burczyk, J.; Wierzchowska-Renke, K. Geographic and Environmental Influences on the Variation of Essential Oil and Coumarins in Crithmum maritimum L. J. Herbs Spices Med. Plants 2002, 4, 305–311. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J.D. Antioxidant and antimicrobial activity of Foeniculum vulagare and Crithmum maritimum essential oils. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef]
- Senatore, F.; Napolitano, F.; Ozcan, M. Composition and antibacterial activity of the essential oil from Crithmum maritimum L. (Apiaceae) growing wild in Turkey. Flavour Fragr. J. 2000, 15, 186–189. [Google Scholar] [CrossRef]
- Politeo, O.; Popovic, M.; Bratincevic, M.V.; Kovacevic, K.; Urlic, B.; Mekinic, I.G. Chemical profiling of Sea Fennel (Crithmum maritimum L., Apiaceae) essential oils and their isolation residual waste-water. Plants 2023, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Pateira, L.; Nogueira, T.; Antunes, A.; Venâncio, F.; Tavares, R.; Capelo, J. Two chemotypes of Crithmum maritimum L. from Portugal. Flavour Fragr. J. 1999, 14, 333–343. [Google Scholar] [CrossRef]
- Chizzola, R. Essential oil composition of wild growing Apiaceae from Europe and the Mediterranean. Nat. Prod. Commun. 2010, 5, 1477–1492. [Google Scholar] [CrossRef] [PubMed]
- Mundina, M.; Vila, R.; Tomi, F.; Tomàs, X.; Ciccío, J.F.; Adzet, T.; Casanova, J.; Canigueral, S. Composition and chemical polymorphism of the essential oils from Piper lanceaefolium. Biochem. Syst. Ecol. 2001, 29, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, B.; Maxia, A.; Piras, A.; Porcedda, S.; Tuveri, E.; Goncalvess, M.J.; Cavaleiros, C.; Salgueiros, L. Isolation of Crithmum maritimum L. volatile oil by supercritical carbon dioxide extraction and biological assays. Nat. Prod. Res. 2007, 21, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Polatoglu, K.; Karakoc, O.C.; Yucel Yucel, Y.; Gucel, S.; Demirci, B.; Baser, K.H.C.; Demirci, F. Insecticidal activity of edible Crithmum maritimum L. essential oil against Coleopteran and Lepidopteran insects. Ind. Crops Prod. 2016, 89, 383–389. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, E.; Buzon-Duran, L.; Andres-Juan, C.; Lorenzo-Vidal, B.; Martin-Gil, J.; Martin-Ramos, P. Physicochemical characterization of Crithmum maritimum L. and Daucus carota subsp. gummifer (Syme) Hook.fil. and their antimicrobial activity against apple tree and grapevine phytopathogens. Agronomy 2021, 11, 886. [Google Scholar] [CrossRef]
- Pasias, I.N.; Ntakoulas, D.D.; Raptopoulou, K.; Gardeli, C.; Proestos, C. Chemical composition of essential oils of aromatic and medicinal herbs cultivated in Greece-Benefits and Drawbacks. Foods 2021, 10, 2354. [Google Scholar] [CrossRef]
- Senatore, F.; De Feo, V. Essential oil of a possible new chemotype of Crithmum maritimum L. growing in Campania (Southern Italy). Flavour Fragr. J. 1994, 9, 305–307. [Google Scholar] [CrossRef]
- Jallali, I.; Hannachi, H.; Zaouali, Y.; Smaoui, A.; Abdelly, C.; Ksouri, R. Crithmum maritimum L. volatile compound’s diversity through Tunisian populations: Use of a plant organ-based statistical approach for chemotype identification. Chem Biodivers. 2023, 20, e202300827. [Google Scholar] [CrossRef]
- Murata, K.M.; Iida, D.; Ueno, Y.; Samukawa, K.; Ishizaka, Y.; Kotake, T.; Matsuda, H. Novel polyacetylene derivatives and their inhibitory activities on acetylcholinesterase obtained from Panax ginseng roots. Nat. Med. 2017, 71, 114–122. [Google Scholar] [CrossRef]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C(17)-polyacetyenes on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/limonene (accessed on 24 November 2023).
- Mursiti, S.; Lestari, N.; Wahyu Ningsih, T. The activity of D-limonene from sweet orange peel (Citrus sinensis L.) extract as a natural insecticide controller of bedbugs (Cimex cimicidae). Orient. J. Chem. 2019, 35, 1420–1425. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/gamma-Terpinene (accessed on 24 November 2023).
- Weedmaps. Available online: https://weedmaps.com/learn/the-plant/sabinene (accessed on 23 November 2023).
- Koeduka, T.; Watanabe, B.; Shirahama, K.; Nakayasu, M.; Suzuki, S.; Furuta, T.; Suzuki, H.; Matsui, K.; Kosaka, T.; Ozaki, S. Biosynthesis of dillapiole/apiole in dill (Anethum graveolens): Characterization of regioselective phenylpropene O-methyltransferase. Plant J. 2023, 113, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G. Cytochrome P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999, 29, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Belzile, A.S.; Majerus, S.L.; Podeszfinski, C.; Guiller, G.; Durst, T.; Arnason, J.T. Dillapiol derivatives as synergists: Structure-activity relationship analysis. Pest. Biochem. Physiol. 2000, 66, 33–40. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Yoshinari, T.; Shams-Ghahfarokhi, M.; Rezaee, M.B.; Nagasawa, H.; Sakuda, S. Dillapiol and Apiol as specific inhibitors of the biosynthesis of aflatoxin G1 in Aspergillus parasiticus. Biosci. Biotechnol. Biochem. 2007, 71, 2329–2332. [Google Scholar] [CrossRef]
- Wu, K.H.; Lee, W.J.; Cheng, T.C.; Chang, H.W.; Chen, L.C.; Chen, C.C.; Lien, H.M.; Lin, T.N.; Ho, Y.S. Study of the antitumor mechanisms of apiole derivatives (AP-02) from Petroselinum crispum through induction of G0/G1 phase cell cycle arrest human COLO 205 cancer cells. BMC Complem. Altern. Med. 2019, 19, 188. [Google Scholar] [CrossRef]
- FooDB. Available online: foodb.ca/compounds/FDB001465 (accessed on 24 November 2023).
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a key floral and foliar volatile involved in multiple interactions between plants and other organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M.; Caretto, S.; Mita, G.; Serio, F. Sea fennel (Crithmum maritimum L.): From underutilized crop to new dried product for food use. Gen. Res. Crop Evol. 2017, 64, 205–216. [Google Scholar] [CrossRef]


| No. | Compound Name | Peak Area (%) | RIa | RIb | |||||
|---|---|---|---|---|---|---|---|---|---|
| San Cataldo | Otranto | Tricase | S. Maria di Leuca | Gallipoli | Torre Lapillo | ||||
| 1 | Unknown | 0.6 | 0.3 | 960 | - | ||||
| 2 | Δ3-Carene | 0.4 | 2.6 | 1.5 | 978 | 1005 | |||
| 3 | Cyclofenchene | 1.2 | 3.5 | 4.1 | 981 | 946 | |||
| 4 | Sabinene | 0.2 | 18.0 | 985 | 978 | ||||
| 5 | β-Phellandrene | 3.9 | 0.4 | 1001 | 1004 | ||||
| 6 | β-Pinene | 0.1 | 1005 | 990 | |||||
| 7 | β-Myrcene | 0.7 | 1.2 | 1.8 | 1.9 | 1.7 | 1.7 | 1013 | 992 |
| 8 | α-Terpinene | 0.4 | 1021 | 1014 | |||||
| 9 | Cymene | 2.9 | 1024 | 1020 | |||||
| 10 | D-Limonene | 37.8 | 76.3 | 92.0 | 64.7 | 66.4 | 49.3 | 1028 | 1031 |
| 11 | (c/t) β-Ocimene | 5.0 | 5.6 | 2.4 | 3.1 | 1.0 | 1032 | 1032 | |
| 12 | (c/t) β-Ocimene | 0.3 | 0.3 | 0.2 | 2.4 | 1034 | 1032 | ||
| 13 | γ-Terpinene | 13.3 | 0.8 | 27.9 | 1062 | 1064 | |||
| 14 | Allocimene | 1.2 | 1122 | 1131 | |||||
| 15 | α-Terpineol | 0.3 | 0.1 | 1182 | 1190 | ||||
| 16 | α-Copaene | 0.3 | 1376 | 1376 | |||||
| 17 | β-Caryophyllene | 0.3 | 1418 | 1417 | |||||
| 18 | α-Bergamotene | 0.2 | 1.2 | 0.4 | 1427 | 1438 | |||
| 19 | α-Cedrene | 0.1 | 1433 | 1446 | |||||
| 20 | β-Bisabolene | 0.1 | 1498 | 1505 | |||||
| 21 | β-Sesquiphellandrene | 0.1 | 0.3 | 1522 | 1524 | ||||
| 22 | G ermacrene B | 0.5 | 0.1 | 0.5 | 0.4 | 1556 | 1558 | ||
| 23 | (Dill)apiol | 11.9 | 0.9 | 1678 | 1681 | ||||
| 24 | (Dill)apiol | 22.5 | 0.3 | 5.0 | 20.2 | 11.6 | 1681 | 1681 | |
| 25 | Panaxynone | 4.2 | 2022 | 2018 | |||||
| Total compounds identified % | 97.2 | 87.3 | 100 | 96,8 | 99,3 | 100 | |||
| Monoterpene hydrocarbons | 57.5 | 85.8 | 99.3 | 90.6 | 77.9 | 88 | |||
| Oxygenated monoterpenes | 0.3 | 0.1 | |||||||
| Sesquiterpenes hydrocarbons | 0.8 | 0.3 | 0.6 | 1.2 | 1.2 | 0.4 | |||
| Phenylpropanoids | 34.4 | 1.2 | 5.0 | 20.2 | 11.6 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accogli, R.; Nutricati, E.; De Bellis, L.; Renna, M.; Luvisi, A.; Negro, C. Diversity of Crithmum maritimum L. from Salento Coastal Area: A Suitable Species for Domestication. Horticulturae 2024, 10, 81. https://doi.org/10.3390/horticulturae10010081
Accogli R, Nutricati E, De Bellis L, Renna M, Luvisi A, Negro C. Diversity of Crithmum maritimum L. from Salento Coastal Area: A Suitable Species for Domestication. Horticulturae. 2024; 10(1):81. https://doi.org/10.3390/horticulturae10010081
Chicago/Turabian StyleAccogli, Rita, Eliana Nutricati, Luigi De Bellis, Massimiliano Renna, Andrea Luvisi, and Carmine Negro. 2024. "Diversity of Crithmum maritimum L. from Salento Coastal Area: A Suitable Species for Domestication" Horticulturae 10, no. 1: 81. https://doi.org/10.3390/horticulturae10010081
APA StyleAccogli, R., Nutricati, E., De Bellis, L., Renna, M., Luvisi, A., & Negro, C. (2024). Diversity of Crithmum maritimum L. from Salento Coastal Area: A Suitable Species for Domestication. Horticulturae, 10(1), 81. https://doi.org/10.3390/horticulturae10010081










