Exploring the Production of Secondary Metabolites from a Halophyte Tetragonia tetragonoides through Callus Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Combinations of Plant Growth Regulators Used to Induce Callus from NZS Leaves
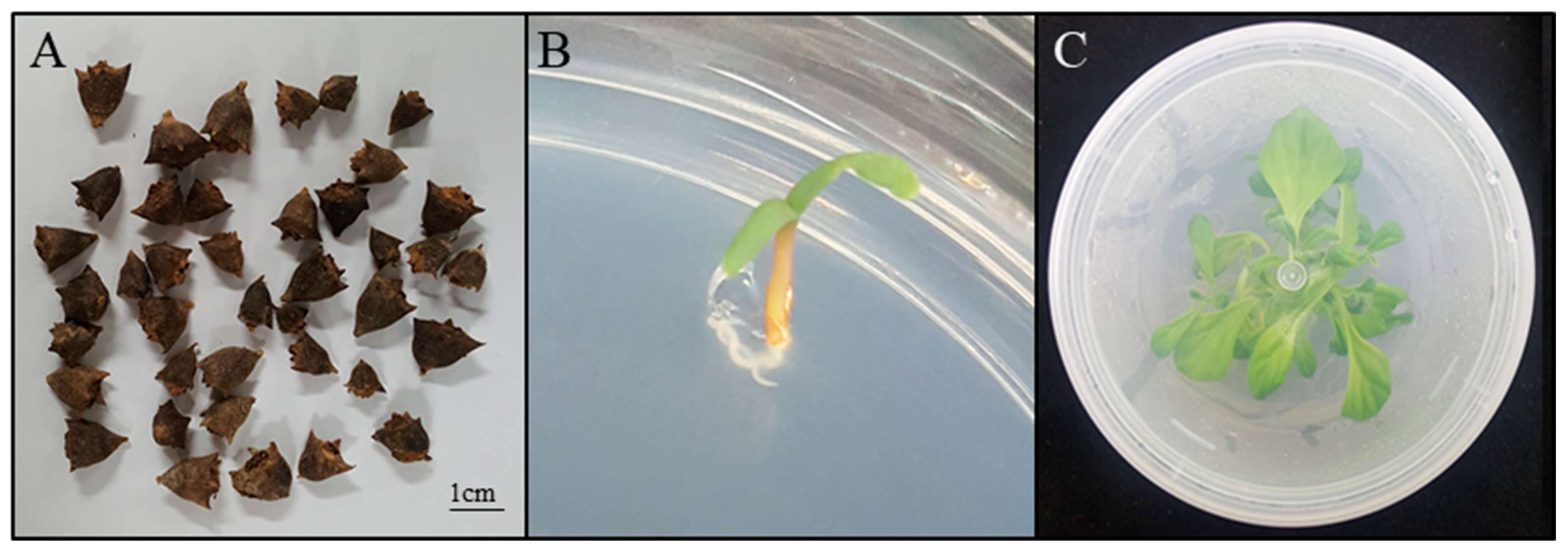
2.2. Explant Material and In Vitro Culture Conditions
2.3. Measuring Total Phenol Content (TPC) and Total Flavonoid Content (TFC) of Selected Callus
2.4. Analysis of Gene Expression Involved in the Biosynthesis of Flavonoids
2.5. Phytochemical Analysis of Selected Callus via GC-MS
2.6. Statistical Analysis
3. Results
3.1. Effect of PGR on Callus Induction
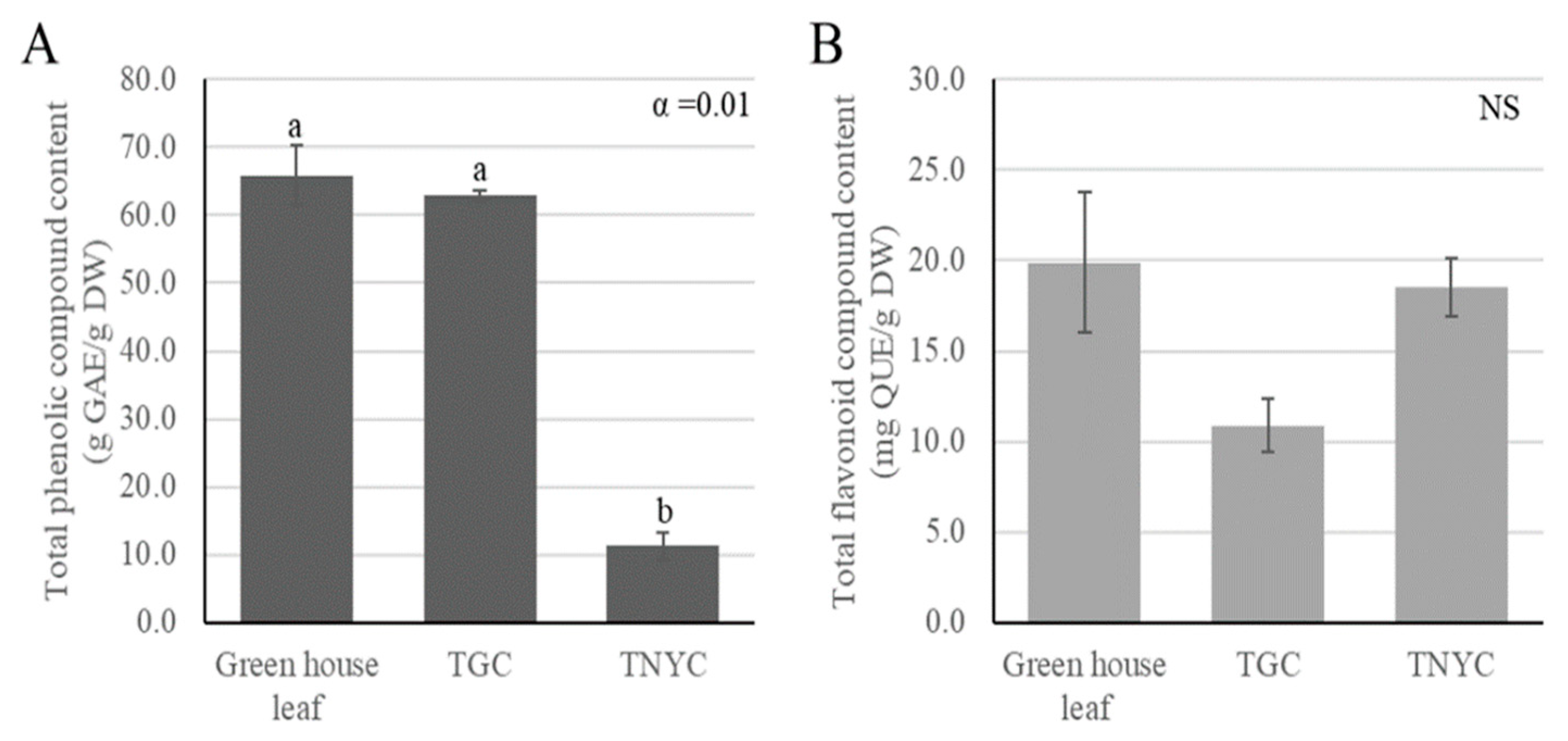
3.2. Total Phenolic and Total Flavonoid Concentration of Selected Callus
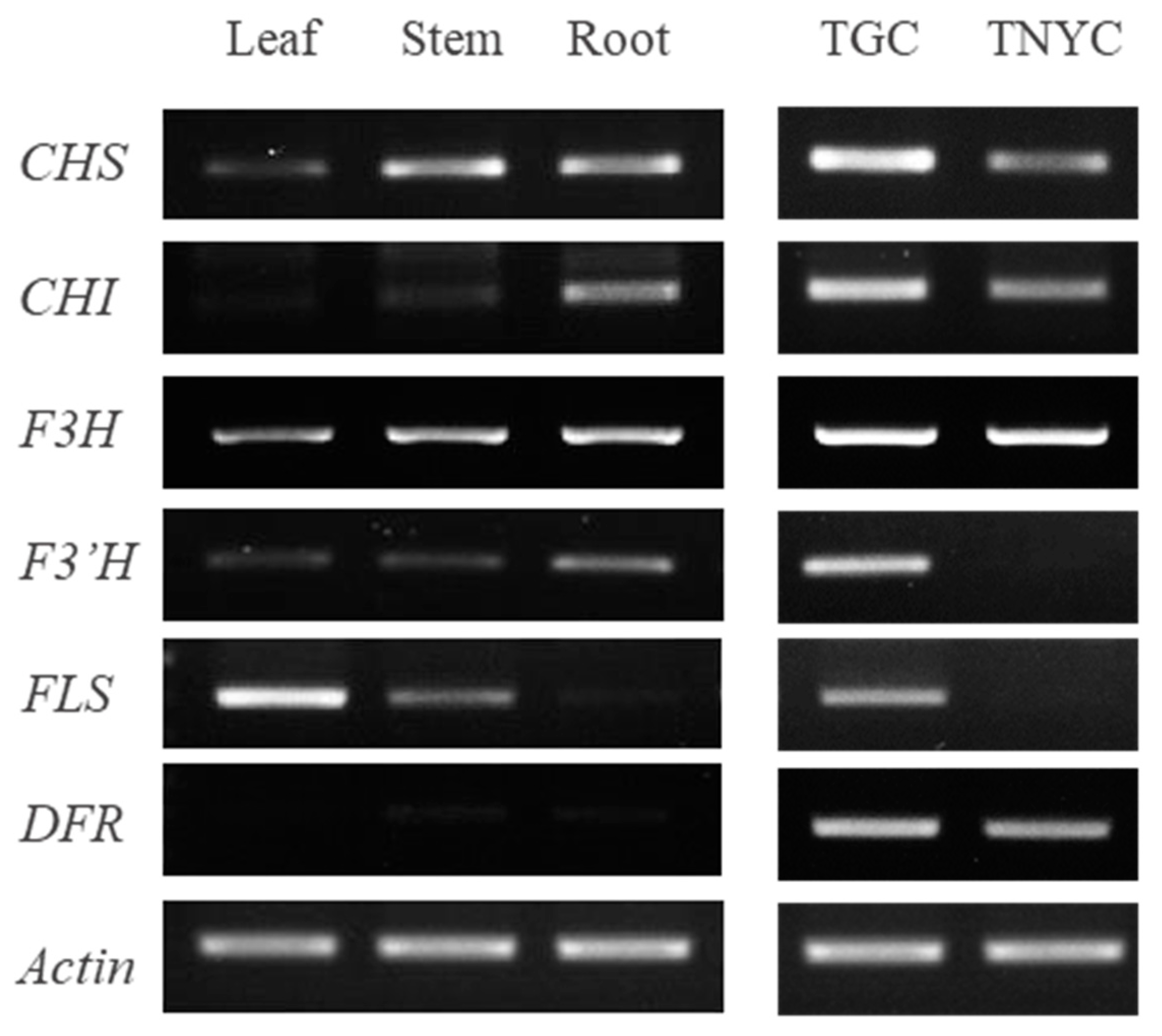
3.3. Comparison of Gene Expressions Related to Flavonoid Biosynthesis
3.4. Analysis of the Phytochemicals of TGC and TNYC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karakas, F.P.; Turker, A.U. Improvement of shoot proliferation and comparison of secondary metabolites in shoot and callus cultures of Phlomis armeniaca by LC-ESI-MS/MS analysis. In Vitro Cell. Dev. Biol.-Plant. 2016, 52, 608–618. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defense mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Plant secondary metabolism: Diversity, function and its evolution. Nat. Prod. Commun. 2008, 3, 1934578X0800300801. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Balandrin, M.F.; Klocke, J.A.; Wurtele, E.S.; Bollinger, W.H. Natural plant chemicals: Sources of industrial and medicinal materials. Science 1985, 228, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental stress and secondary metabolites in plants: An overview. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 153–167. [Google Scholar]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Steward, F.C.; Mapes, M.O.; Mears, K. Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cells. Am. J. Bot. 1958, 45, 705–708. [Google Scholar] [CrossRef]
- Plasson, C.; Michel, R.; Lienard, D.; Saint-Jore-Dupas, C.; Sourrouille, C.; March, G.G.D.; Gomord, V. Production of recombinant proteins in suspension–cultured plant cells. In Recombinant Proteins from Plants: Methods and Protocols; Humana: New York, NY, USA, 2009; pp. 145–161. [Google Scholar]
- Lee, K.Y.; Shin, J.Y.; Ahn, M.S.; Kim, S.J.; An, H.R.; Kim, Y.J.; Kwon, O.H.; Lee, S.Y. Callus Derived from Petals of the Rosa hybrida Breeding Line 15R-12-2 as New Material Useful for Fragrance Production. Plants 2023, 12, 2986. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme Microb. Technol. 1995, 17, 674–684. [Google Scholar] [CrossRef]
- Anjum, S.; Abbasi, B.H.; Hano, C. Trends in accumulation of pharmacologically important antioxidant-secondary metabolites in callus cultures of Linum usitatissimum L. Plant Cell Tissue Organ Cult. 2017, 129, 73–87. [Google Scholar] [CrossRef]
- Adil, M.; Ren, X.; Kang, D.I.; Thi, L.T.; Jeong, B.R. Effect of explant type and plant growth regulators on callus induction, growth and secondary metabolites production in Cnidium officinale Makino. Mol. Biol. Rep. 2018, 45, 1919–1927. [Google Scholar] [CrossRef]
- Coskun, Y.; Duran, R.E.; Kilic, S. Striking effects of melatonin on secondary metabolites produced by callus culture of rosemary (Rosmarinus officinalis L.). Plant Cell Tissue Organ Cult. 2019, 138, 89–95. [Google Scholar] [CrossRef]
- Wilson, C.; Lesch, S.M.; Grieve, C.M. Growth stage modulates salinity tolerance of New Zealand Spinach (Tetragonia tetragonioides, Pall) and Red Orach (Atriplex hortensis L.). Ann. Bot. 2000, 85, 501–509. [Google Scholar] [CrossRef]
- Kato, M.; Takeda, T.; Ogihara, Y.; Shimu, M.; Nomura, T.; Tomita, Y. Studies on the structure of polysaccharide from Tetragonia tetragonioides. Chem. Pharm. Bull. 1985, 33, 3675–3680. [Google Scholar] [CrossRef]
- Choi, H.J.; Kang, J.S.; Choi, Y.W.; Jeong, Y.K.; Joo, W.H. Inhibitory activity on the diabetes related enzymes of Tetragonia tetragonioides. KSBB J. 2008, 23, 419–424. [Google Scholar]
- Lee, M.A.; Choi, H.J.; Kang, J.S.; Choi, Y.W.; Joo, W.H. Antioxidant activities of the solvent extracts from Tetragonia tetragonioides. J. Life Sci. 2008, 18, 220–227. [Google Scholar] [CrossRef]
- Choi, H.J.; Yee, S.T.; Kwon, G.S.; Joo, W.H. Anti-inflammatory and anti-tumor effects of Tetragonia tetragonoides extracts. Microbiol. Biotechnol. Lett. 2015, 43, 391–395. [Google Scholar] [CrossRef]
- Choi, H.J.; Yee, S.T.; Joo, W.H. Antidiabetic activity of polysaccharide extract from Tetragonia tetragonoides in streptozotocin-induced diabetic mice. J. Life Sci. 2017, 27, 579–583. [Google Scholar] [CrossRef]
- Kim, D.S.; Ko, B.S.; Ryuk, J.A.; Park, S. Tetragonia tetragonioides protected against memory dysfunction by elevating hippocampal amyloid-β deposition through potentiating insulin signaling and altering gut microbiome composition. Int. J. Mol. Sci. 2020, 21, 2900. [Google Scholar] [CrossRef]
- Sunagawa, H.; Agarie, S.; Umemoto, M.; Makishi, Y.; Nose, A. Effect of urea-type cytokinins on the adventitious shoots plant, Mesembryanthemum crystallinum. Plant Prod. Sci. 2007, 10, 47–56. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.H.; Yuk, H.J.; Lee, G.J.; Kim, D.S. Tetragonia tetragonoides (Pall.) Kuntze (New Zealand Spinach) prevents obesity and hyperuricemia in high-fat diet-induced obese mice. Nutrients 2018, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Centeno, M.L.; Rodríguez, A.; Feito, I.; Fernández, B. Relationship between endogenous auxin and cytokinin levels and morphogenic responses in Actinidia deliciosa tissue cultures. Plant Cell Rep. 1996, 16, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Gueye, B.; Morcillo, F.; Collin, M.; Gargani, D.; Overvoorde, P.; Aberlenc-Bertossi, F.; Tranbarger, T.; Sane, D.; Tregear, J.W.; Borgel, A.; et al. Acquisition of callogenic capacity in date palm leaf tissues in response to 2,4-D treatment. Plant Cell Tissue Organ Cult. 2009, 99, 35–45. [Google Scholar] [CrossRef]
- Berejsza-Wysecki, W.; Hrazdin, G. Establishment of callus and cell suspension cultures of raspberry (Rubus idaeus cv. Royalty). Plant Cell Tissue Organ Cult. 1994, 37, 213–216. [Google Scholar] [CrossRef]
- Gaj, M.D. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul. 2004, 43, 27–47. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008; 501p. [Google Scholar]
- Rad, M.M.; Abdossi, V.; Moradi, P.; Rakhshandehroo, F.; Mehrafarin, A. Phytochemical changes of Digitalis purpurea L. in response to polyamines and methyl jasmonate application in callus culture. J. Plant Biochem. Biotechnol. 2022, 31, 310–319. [Google Scholar] [CrossRef]
- Linden, L.; Riikonen, A. Effects of 6-benzylaminopurine, thidiazuron and type of explant on in vitro shoot development of Acer platanoides L. Propag. Ornam. Plants 2006, 6, 201–204. [Google Scholar]
- Jones, M.P.; Yi, Z.; Murch, S.J.; Saxena, P.K. Thidiazuron-induced regeneration of Echinacea purpurea L.: Micropropagation in solid and liquid culture systems. Plant Cell Rep. 2007, 26, 13–19. [Google Scholar] [CrossRef]
- Wang, J.; Bao, M.Z. Plant regeneration of pansy (Viola wittrockiana) ‘Caidie’ via petiole-derived callus. Sci. Hortic. 2007, 111, 266–270. [Google Scholar] [CrossRef]
- Suttle, J.C. Involvement of ethylene in the action of the cotton defoliant thidiazuron. Plant Physiol. 1985, 78, 272–276. [Google Scholar] [CrossRef]
- Pourebad, N.; Motafakkerazad, R.; Kosari-Nasab, M.; Farsad Akhtar, N.; Movafeghi, A. The influence of TDZ concentrations on in vitro growth and production of secondary metabolites by the shoot and callus culture of Lallemantia iberica. Plant Cell Tissue Organ Cult. 2015, 122, 331–339. [Google Scholar] [CrossRef]
- Singh, C.K.; Raj, S.R.; Jaiswal, P.S.; Patil, V.R.; Punwar, B.S.; Chavda, J.C.; Subhash, N. Effect of plant growth regulators on in vitro plant regeneration of sandalwood (Santalum album L.) via organogenesis. Agrofor. Syst. 2016, 90, 281–288. [Google Scholar] [CrossRef]
- Srivastava, S.; Krishna, R.; Sinha, R.P.; Singh, M. TDZ-induced plant regeneration in Brassica oleracea L. var. botrytis: Effect of antioxidative enzyme activity and genetic stability in regenerated plantlets. In Vitro Cell. Dev. Biol.-Plant. 2017, 53, 598–605. [Google Scholar]
- Mok, M.C.; Martin, R.C.; Mok, D.W.S. Cytokinins: Biosynthesis metabolism and perception. In Vitro Cell. Dev. Biol.-Plant. 2000, 36, 102–107. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Coenen, C.; Lomax, T.L. Auxin—Cytokinin interactions in higher plants: Old problems and new tools. Trends Plant Sci. 1997, 2, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Mol, J.; Grotewold, E.; Koes, R. How genes paint flowers and seeds. Trends Plant Sci. 1998, 3, 212–217. [Google Scholar] [CrossRef]
- Deng, J.; Chen, S.; Yin, X.; Wang, K.; Liu, Y.; Li, S.; Yang, P. Systematic qualitative and quantitative assessment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars. Food Chem. 2013, 139, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comtem, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Phatak, S.V.; Heble, M.R. Organogenesis and terpenoid synthesis in Mentha arvensis. Fitoterapia 2002, 73, 32–39. [Google Scholar] [CrossRef]
- Wahyuni, D.K.; Huda, A.; Faizah, S.; Purnobasuki, H.; Wardojo, B.P.E. Effects of light, sucrose concentration and repetitive subculture on callus growth and medically important production in Justicia gendarussa Burm. f. Plant Biotechnol. Rep. 2020, 27, e00473. [Google Scholar]
- Richard, C.; Lescot, M.; Inzé, D.; De Veylder, L. Effect of auxin, cytokinin, and sucrose on cell cycle gene expression in Arabidopsis thaliana cell suspension cultures. Plant Cell Tissue Organ Cult. 2002, 69, 167–176. [Google Scholar] [CrossRef]
- Garg, S.K.; Jain, A. Fermentative production of 2,3-butanediol: A review. Bioresour. Technol. 1995, 51, 103–109. [Google Scholar] [CrossRef]
- Celińska, E.; Grajek, W. Biotechnological production of 2,3-butanediol—Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Syu, M.J. Biological production of 2,3-butanediol. Appl. Microbiol. Biotechnol. 2001, 55, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Ma, Y.; Shi, Y.; Ma, H.L. 2,3-Butanediol induces brown blotch resistance in creeping bentgrass by strengthening cell wall structure and promoting lignin synthesis of precursor phenolic acid. Acta Physiol. Plant. 2023, 45, 40. [Google Scholar] [CrossRef]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize (Zea mays L.). Physiol. Plant. 2021, 172, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Carlezon, W.A., Jr.; Pliakas, A.M.; Parow, A.M.; Detke, M.J.; Cohen, B.M.; Renshaw, P.F. Antidepressant-like effects of cytidine in the forced swim test in rats. Biol. Psychiatry 2002, 51, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Knapp, S.; Wurtman, R.J. Enhancement of free fatty acid incorporation into phospholipids by choline plus cytidine. Brain Res. 1999, 822, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Hauger, R.L.; Koob, G.F. Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacology 1992, 109, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Radkowski, M.; Zdrodowska, B.; Gomółka-Pawlicka, M. Effect of succinic acid on elimination of Salmonella in chicken meat. J. Food Prot. 2018, 81, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Nissen, M.D.; Lau, E.T.; Cabot, P.J.; Steadman, K.J. Baltic amber teething necklaces: Could succinic acid leaching from beads provide anti-inflammatory effects? BMC Complement. Altern. Med. 2019, 19, 162. [Google Scholar] [CrossRef]
- Harber, K.J.; de Goede, K.E.; Verberk, S.G.; Meinster, E.; de Vries, H.E.; van Weeghel, M.; de Winther, M.P.; Van den Bossche, J. Succinate is an inflammation-induced immunoregulatory metabolite in macrophages. Metabolites 2020, 10, 372. [Google Scholar] [CrossRef]
- Global Succinic Acid Market Analysis & Trends [2013–2017]—Industry Forecast to 2025: $1.76 Billion Growth Opportunities/Investment Opportunities—Research and Markets. Available online: https://ceo.ca/@newswire/global-succinic-acid-market-analysis-trends-2013-2017 (accessed on 6 March 2017).

| Plant Growth Regulators (mg·L−1) | Callus Induction (%) Z | Fresh Weight (g) Z | |||
|---|---|---|---|---|---|
| Zeatin | IAA | NAA | 2,4-D | ||
| 0.5 | 0.5 | 0.0 ± 0.0 b | 0.046 ± 0.002 ij | ||
| 0.5 | 1.0 | 0.0 ± 0.0 b | 0.064 ± 0.002 g–j | ||
| 0.5 | 1.5 | 0.0 ± 0.0 b | 0.065 ± 0.004 g–j | ||
| 0.5 | 2.0 | 60.0 ± 0.0 ab | 0.041 ± 0.003 ij | ||
| 1.0 | 0.5 | 75.0 ± 2.5 ab | 0.053 ± 0.001 h–j | ||
| 1.0 | 1.0 | 0.0 ± 0.0 b | 0.054 ± 0.003 h–j | ||
| 1.0 | 1.5 | 0.0 ± 0.0 b | 0.058 ± 0.001 h–j | ||
| 1.0 | 2.0 | 0.0 ± 0.0 b | 0.069 ± 0.005 g–j | ||
| 1.5 | 0.5 | 65.0 ± 2.5 ab | 0.045 ± 0.004 ij | ||
| 1.5 | 1.0 | 25.0 ± 2.5 b | 0.055 ± 0.004 h–j | ||
| 1.5 | 1.5 | 25.0 ± 2.5 b | 0.058 ± 0.005 h–j | ||
| 1.5 | 2.0 | 0.0 ± 0.0 b | 0.055 ± 0.003 h–j | ||
| 2.0 | 0.5 | 0.0 ± 0.0 b | 0.044 ± 0.001 ij | ||
| 2.0 | 1.0 | 0.0 ± 0.0 b | 0.037 ± 0.000 j | ||
| 2.0 | 1.5 | 0.0 ± 0.0 b | 0.039 ± 0.001 ij | ||
| 2.0 | 2.0 | 0.0 ± 0.0 b | 0.055 ± 0.004 h–j | ||
| 0.5 | 0.5 | 75.0 ± 7.5 ab | 0.070 ± 0.004 g–j | ||
| 0.5 | 1.0 | 80.0 ± 10.0 ab | 0.117 ± 0.010 c–j | ||
| 0.5 | 1.5 | 95.0 ± 2.5 ab | 0.081 ± 0.006 e–j | ||
| 0.5 | 2.0 | 90.0 ± 0.0 ab | 0.207 ± 0.034 a–c | ||
| 1.0 | 0.5 | 65.0 ± 2.5 ab | 0.095 ± 0.013 d–j | ||
| 1.0 | 1.0 | 70.0 ± 5.0 ab | 0.057 ± 0.003 h–j | ||
| 1.0 | 1.5 | 100.0 ± 0.0 ab | 0.081 ± 0.006 e–j | ||
| 1.0 | 2.0 | 90.0 ± 0.0 ab | 0.075 ± 0.004 f–j | ||
| 1.5 | 0.5 | 65.0 ± 7.5 ab | 0.090 ± 0.005 d–j | ||
| 1.5 | 1.0 | 65.0 ± 7.5 ab | 0.124 ± 0.007 c–j | ||
| 1.5 | 1.5 | 70.0 ± 15.0 ab | 0.093 ± 0.011 d–j | ||
| 1.5 | 2.0 | 45.0 ± 17.5 ab | 0.035 ± 0.003 j | ||
| 2.0 | 0.5 | 45.0 ± 17.5 ab | 0.087 ± 0.010 d–j | ||
| 2.0 | 1.0 | 70.0 ± 15.0 ab | 0.076 ± 0.001 f–j | ||
| 2.0 | 1.5 | 65.0 ± 12.5 ab | 0.057 ± 0.001 h–j | ||
| 2.0 | 2.0 | 95.0 ± 2.5 ab | 0.148 ± 0.007 a–g | ||
| 0.5 | 0.5 | 60.0 ± 20.0 ab | 0.176 ± 0.014 a–e | ||
| 0.5 | 1.0 | 100.0 ± 0.0 ab | 0.081 ± 0.004 e–j | ||
| 0.5 | 1.5 | 75.0 ± 2.5 ab | 0.088 ± 0.009 d–j | ||
| 0.5 | 2.0 | 85.0 ± 2.5 ab | 0.044 ± 0.002 ij | ||
| 1.0 | 0.5 | 65.0 ± 17.5 ab | 0.172 ± 0.002 a–f | ||
| 1.0 | 1.0 | 100.0 ± 0.0 a | 0.180 ± 0.008 a–d | ||
| 1.0 | 1.5 | 100.0 ± 0.0 a | 0.234 ± 0.009 a | ||
| 1.0 | 2.0 | 100.0 ± 0.0 a | 0.133 ± 0.010 c–j | ||
| 1.5 | 0.5 | 100.0 ± 0.0 a | 0.228 ± 0.027 ab | ||
| 1.5 | 1.0 | 100.0 ± 0.0 a | 0.139 ± 0.009 c–j | ||
| 1.5 | 1.5 | 100.0 ± 0.0 a | 0.076 ± 0.001 f–j | ||
| 1.5 | 2.0 | 100.0 ± 0.0 a | 0.060 ± 0.004 h–j | ||
| 2.0 | 0.5 | 100.0 ± 0.0 a | 0.161 ± 0.013 a–g | ||
| 2.0 | 1.0 | 100.0 ± 0.0 a | 0.112 ± 0.002 d–j | ||
| 2.0 | 1.5 | 100.0 ± 0.0 a | 0.237 ± 0.004 a | ||
| 2.0 | 2.0 | 100.0 ± 0.0 a | 0.072 ± 0.002 g–j | ||
| Plant Growth Regulators (mg·L−1) | Callus Induction (%) Z | Fresh Weight (g) Z | ||
|---|---|---|---|---|
| 6-BA | NAA | 2,4-D | ||
| 0.0 | 0.0 | 0.0 ± 0.0 d | 0.014 ± 0.001 d | |
| 0.0 | 0.5 | 0.0 ± 0.0 d | 0.016 ± 0.001 d | |
| 0.0 | 1.0 | 0.0 ± 0.0 d | 0.017 ± 0.001 d | |
| 0.0 | 1.5 | 0.0 ± 0.0 d | 0.013 ± 0.001 d | |
| 1.0 | 0.0 | 40.0 ± 2.9 bc | 0.215 ± 0.005 d | |
| 1.0 | 0.5 | 16.7 ± 1.7 b–d | 0.026 ± 0.003 d | |
| 1.0 | 1.0 | 0.0 ± 0.0 b–d | 0.013 ± 0.001 d | |
| 1.0 | 1.5 | 0.0 ± 0.0 cd | 0.015 ± 0.001 d | |
| 1.5 | 0.0 | 26.7 ± 1.7 b–d | 0.211 ± 0.004 d | |
| 1.5 | 0.5 | 10.0 ± 2.9 d | 0.023 ± 0.002 d | |
| 1.5 | 1.0 | 10.0 ± 5.0 b–d | 0.026 ± 0.003 d | |
| 1.5 | 1.5 | 26.7 ± 7.3 cd | 0.035 ± 0.001 d | |
| 2.0 | 0.0 | 16.7 ± 3.3 b–d | 0.139 ± 0.001 d | |
| 2.0 | 0.5 | 3.3 ± 1.7 d | 0.019 ± 0.001 d | |
| 2.0 | 1.0 | 3.3 ± 1.7 b–d | 0.020 ± 0.004 d | |
| 2.0 | 1.5 | 46.7 ± 13.0 b | 0.047 ± 0.007 d | |
| 0.0 | 0.0 | 0.0 ± 0.0 d | 0.013 ± 0.001 d | |
| 0.0 | 0.5 | 86.7 ± 3.3 b | 0.060 ± 0.003 cd | |
| 0.0 | 1.0 | 100.0 ± 0.0 b–d | 0.026 ± 0.015 d | |
| 0.0 | 1.5 | 100.0 ± 0.0 b–d | 0.021 ± 0.004 d | |
| 1.0 | 0.0 | 46.7 ± 3.3 a | 0.124 ± 0.005 bc | |
| 1.0 | 0.5 | 100.0 ± 0.0 a | 0.221 ± 0.001 b | |
| 1.0 | 1.0 | 100.0 ± 0.0 a | 0.211 ± 0.003 b | |
| 1.0 | 1.5 | 100.0 ± 0.0 a | 0.207 ± 0.006 b | |
| 1.5 | 0.0 | 33.3 ± 4.4 a | 0.220 ± 0.002 b | |
| 1.5 | 0.5 | 100.0 ± 0.0 a | 0.344 ± 0.011 a | |
| 1.5 | 1.0 | 100.0 ± 0.0 a | 0.337 ± 0.003 a | |
| 1.5 | 1.5 | 100.0 ± 0.0 a | 0.392 ± 0.034 a | |
| 2.0 | 0.0 | 20.0 ± 7.6 a | 0.210 ± 0.004 b | |
| 2.0 | 0.5 | 100.0 ± 0.0 a | 0.416 ± 0.019 a | |
| 2.0 | 1.0 | 100.0 ± 0.0 a | 0.380 ± 0.027 a | |
| 2.0 | 1.5 | 100.0 ± 0.0 a | 0.375 ± 0.021 a | |
| Peak No. | R.T (min) z | Identified Metabolites | Area (%) y |
|---|---|---|---|
| 1 | 7.5825 | 2,3-Butanediol | 68.55 |
| 2 | 9.3225 | Ethanol | 5.544 |
| 3 | 9.3779 | Cyclopentasiloxane, decamethyl- | - |
| 4 | 11.8558 | Dimethylamine | 5.647 |
| 5 | 17.3404 | Cytidine | 20.258 |
| 6 | 23.0586 | 5,6,8,9-TETRAMETHOXY-2-METHYLPEPERO (3,4,5-JK)-9,10-DIHYDROPHENANTHRACENE | - |
| 7 | 23.3906 | Silane, 1,4-phenylenebis[trimethyl- | - |
| 8 | 23.6858 | Silicone grease, Siliconfett | - |
| 9 | 23.7903 | Silicone grease, Siliconfett | - |
| Peak No. | R.T (min) z | Identified Metabolites | Area (%) y |
|---|---|---|---|
| 1 | 4.5327 | Butanedioic acid | 23.053 |
| 2 | 4.7971 | Ethanol | 1.855 |
| 3 | 5.4305 | Ethanol | - |
| 4 | 6.156 | Ethyl 4-(N-(2-nitro)benzylidene)amino-benzoate | 3.528 |
| 5 | 6.2052 | Cyclotetrasiloxane, octamethyl- | 3.519 |
| 6 | 7.2135 | 5,6,8,9-TETRAMETHOXY-2-METHYLPEPERO (3,4,5-JK)-9,10-DIHYDROPHENANTHRACENE | 1.605 |
| 7 | 7.4165 | Propanoic acid, 3-(acetylthio)-2-methyl- | 3.473 |
| 8 | 7.6316 | Thioacetonitrile | 4.078 |
| 9 | 8.7384 | N-(Methoxycarbonylmethyl)-N-ethylnitrosamine | 4.666 |
| 10 | 8.9659 | 1-PROPANOL-O-D | 2.314 |
| 11 | 9.2549 | 6-Aza-5,7,12,14-tetrathiapentacene | - |
| 12 | 9.7714 | Cyclopentasiloxane, decamethyl- | 1.243 |
| 13 | 10.374 | Ammonia | 1.726 |
| 14 | 10.4785 | 6-Aza-5,7,12,14-tetrathiapentacene | 1.243 |
| 15 | 10.706 | (S)-N-(Ethoxycarbonyl)valine | - |
| 16 | 11.4869 | 2,4,5-Trioxoimidazolidine | 1.588 |
| 17 | 11.5668 | 2,3,4-Tridesoxy-5,6:8,9-di-O-isopropyliden-3-methyl-4-nitro-.beta.-D-manno-4-nonulo-4,7-furanosononitrile | 5.062 |
| 18 | 11.7635 | 1,3,5-Triazine, hexahydro-1,3,5-trimethyl- | 2.855 |
| 19 | 12.0402 | 1H-Imidazole-4-carboxamide, 5-amino- | - |
| 20 | 12.7104 | Ethanamine, N-ethyl-N-nitroso- | 8.237 |
| 21 | 12.858 | L-Alanine, N-glycyl- | 2.35 |
| 22 | 13.2515 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | - |
| 23 | 14.1554 | 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | 2.104 |
| 24 | 15.0654 | 1-(2-Adamantylidene)semicarbazide | - |
| 25 | 15.9877 | 2-Methoxy-4-vinylphenol | 1.529 |
| 26 | 16.7193 | 3-Piperidinol | 14.137 |
| 27 | 16.7501 | 3-Piperidinol | - |
| 28 | 17.3588 | 1,3-Propanediol, 2-ethyl-2-(hydroxymethyl)- | 6.378 |
| 29 | 17.697 | 1-Propanol, 3-amino- | - |
| 30 | 19.6031 | E-1-phenylbutetene | - |
| 31 | 19.7383 | E-1-phenylbutetene | - |
| 32 | 19.7875 | 3,27-Dioxa-2,28-disilanonacosane, 2,2,4,28,28-pentamethyl- | 0.854 |
| 33 | 19.9597 | Phenol, 2,2′-[(1-methyl-1,2-ethanediyl)bis(nitrilomethylidyne)]bis- | 0.854 |
| 34 | 21.1095 | Urea | - |
| 35 | 21.4784 | 4-HYDROXY-3-NITROCOUMARIN | 0.938 |
| 36 | 21.6628 | 4-HYDROXY-3-NITROCOUMARIN | - |
| 37 | 21.8166 | Phenol, 2,2′-[(1-methyl-1,2-ethanediyl)bis(nitrilomethylidyne)]bis- | - |
| 38 | 22.911 | 5,6,8,9-TETRAMETHOXY-2-METHYLPEPERO(3,4,5-JK)-9,10-DIHYDROPHENANTHRACENE | - |
| 39 | 23.1324 | 5,6,8,9-TETRAMETHOXY-2-METHYLPEPERO(3,4,5-JK)-9,10-DIHYDROPHENANTHRACENE | - |
| 40 | 23.2246 | 5,6,8,9-TETRAMETHOXY-2-METHYLPEPERO(3,4,5-JK)-9,10-DIHYDROPHENANTHRACENE | - |
| 41 | 23.4275 | 5,6,8,9-TETRAMETHOXY-2-METHYLPEPERO(3,4,5-JK)-9,10-DIHYDROPHENANTHRACENE | - |
| 42 | 23.5751 | 1,1,1,3,5,5,5-Heptamethyltrisiloxane | - |
| 43 | 23.6058 | 1,3-Bis(trimethylsilyl)benzene | 0.854 |
| 44 | 23.7657 | Silicone grease, Siliconfett | 0.854 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.Y.; Nam, D.-H.; Jeon, Y.; Park, S.U.; Cho, J.; Gulandaz, M.A.; Chung, S.-O.; Lee, G.-J. Exploring the Production of Secondary Metabolites from a Halophyte Tetragonia tetragonoides through Callus Culture. Horticulturae 2024, 10, 244. https://doi.org/10.3390/horticulturae10030244
Lee KY, Nam D-H, Jeon Y, Park SU, Cho J, Gulandaz MA, Chung S-O, Lee G-J. Exploring the Production of Secondary Metabolites from a Halophyte Tetragonia tetragonoides through Callus Culture. Horticulturae. 2024; 10(3):244. https://doi.org/10.3390/horticulturae10030244
Chicago/Turabian StyleLee, Ka Youn, Do-Hyeon Nam, Yongsam Jeon, Sang Un Park, Jongki Cho, Md Ashrafuzzaman Gulandaz, Sun-Ok Chung, and Geung-Joo Lee. 2024. "Exploring the Production of Secondary Metabolites from a Halophyte Tetragonia tetragonoides through Callus Culture" Horticulturae 10, no. 3: 244. https://doi.org/10.3390/horticulturae10030244
APA StyleLee, K. Y., Nam, D. -H., Jeon, Y., Park, S. U., Cho, J., Gulandaz, M. A., Chung, S. -O., & Lee, G. -J. (2024). Exploring the Production of Secondary Metabolites from a Halophyte Tetragonia tetragonoides through Callus Culture. Horticulturae, 10(3), 244. https://doi.org/10.3390/horticulturae10030244










