Non-Structural Carbohydrate Content and C:N:P Stoichiometry in Houpoea officinalis Flowers in Response to Development Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Flower Collection and Measurements
2.3. Determination of C, N, and P Concentrations
2.4. Determination of NSC Concentrations
2.5. Statistical Analysis
3. Results
3.1. Variations of Morphological Indicators and Biomass Allocation
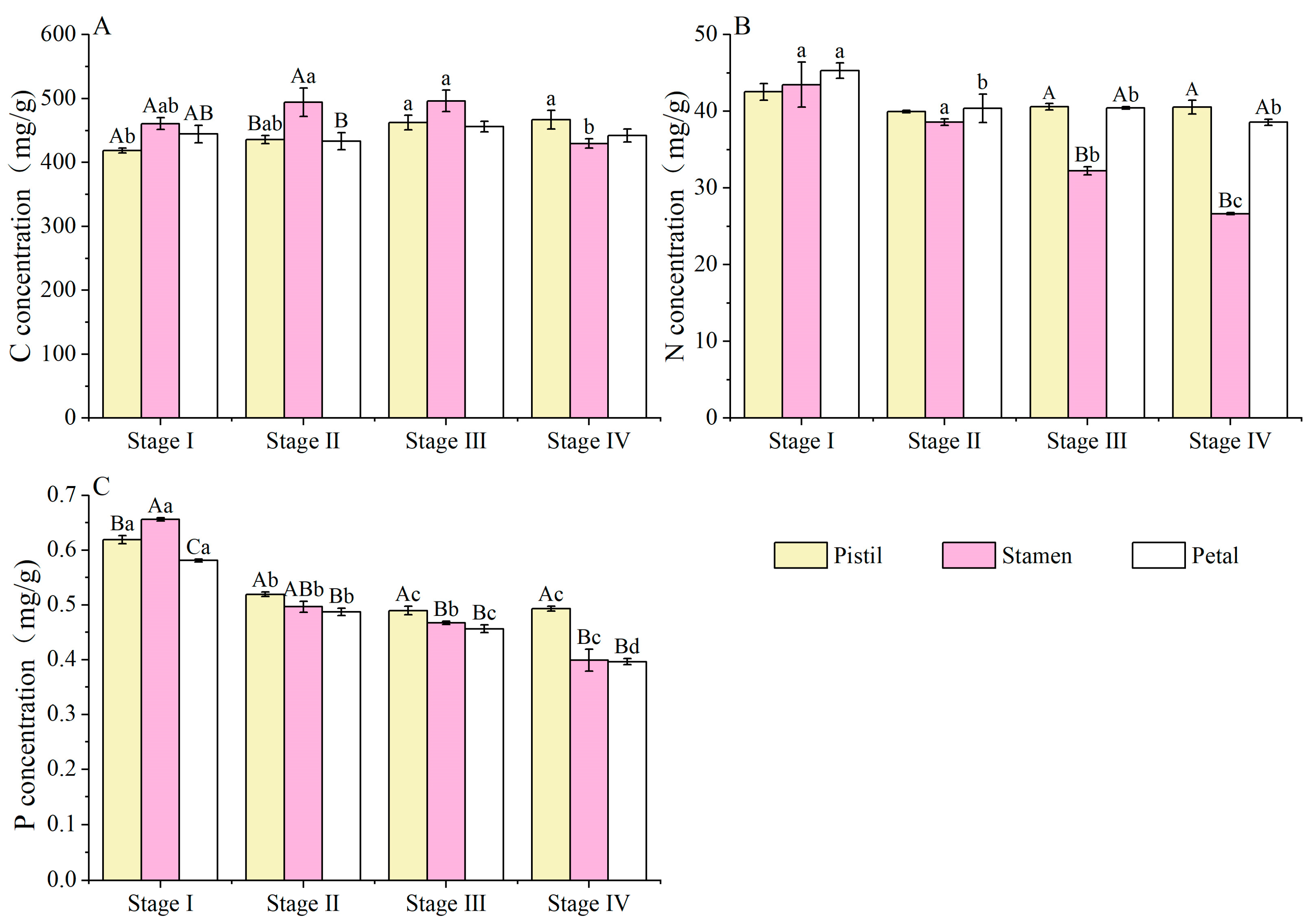
3.2. C, N and P Concentrations
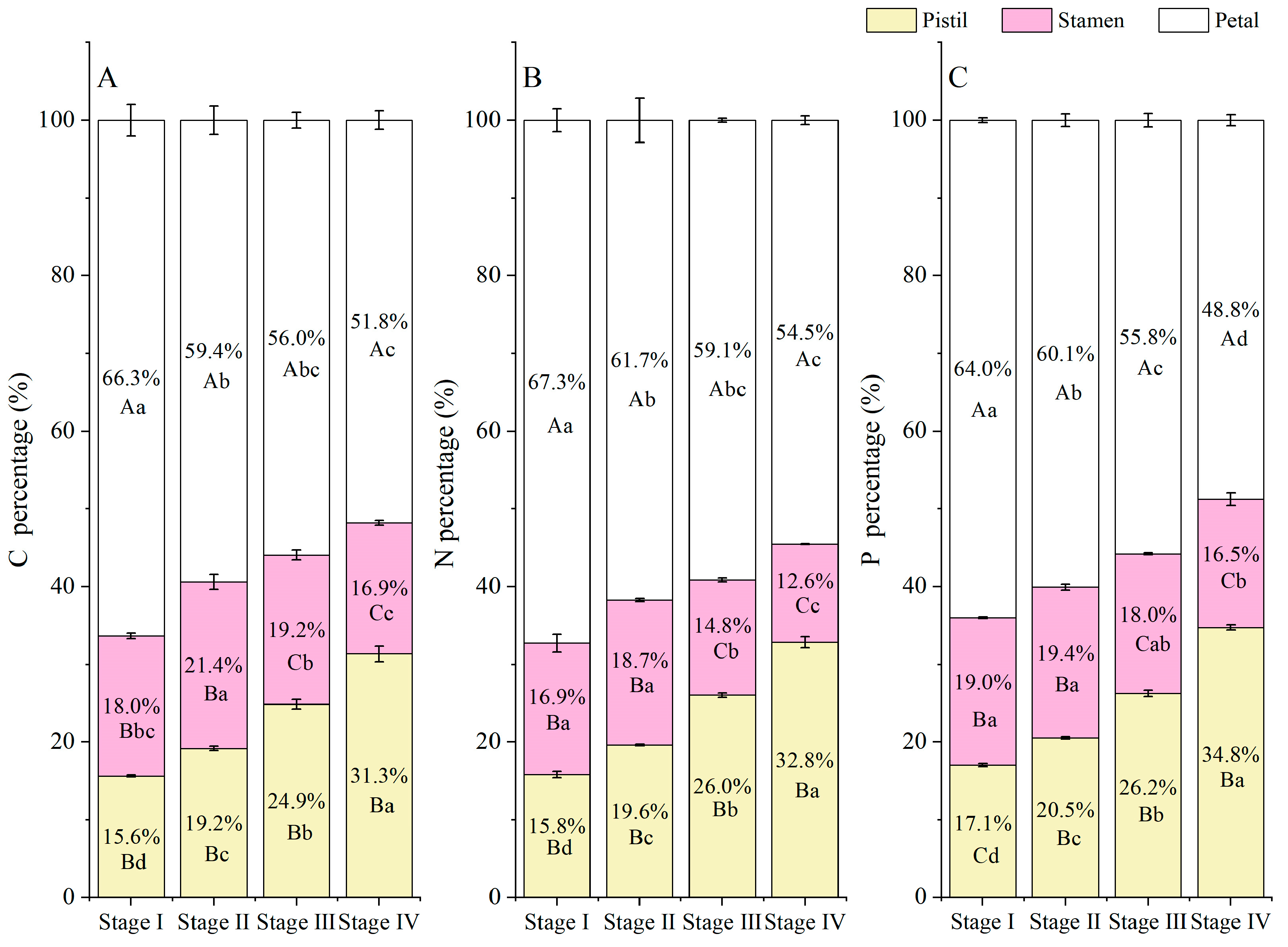
3.3. Element (C, N, and P) Accumulation and Allocation Proportion
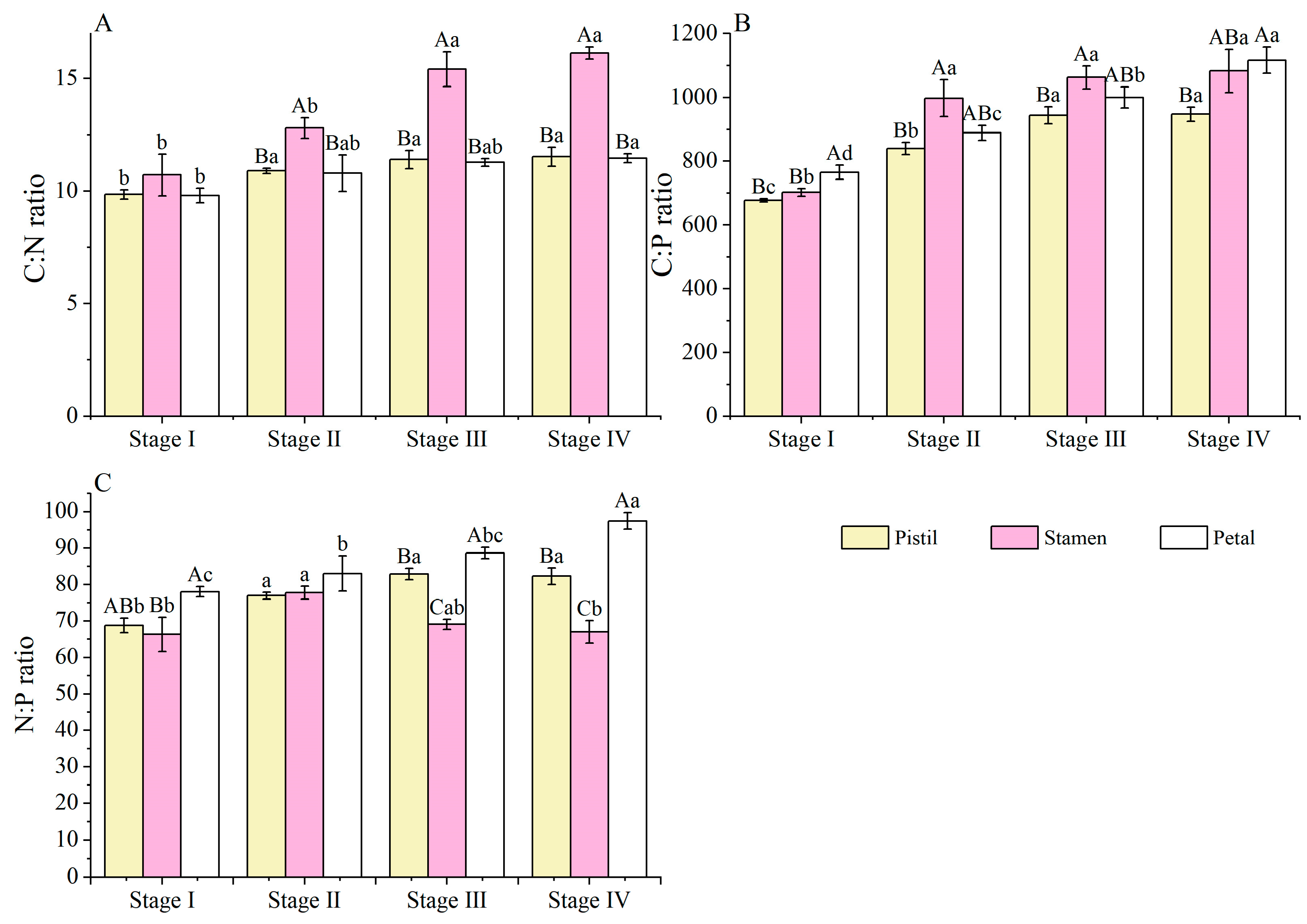
3.4. C, N, and P Stoichiometric Ratio
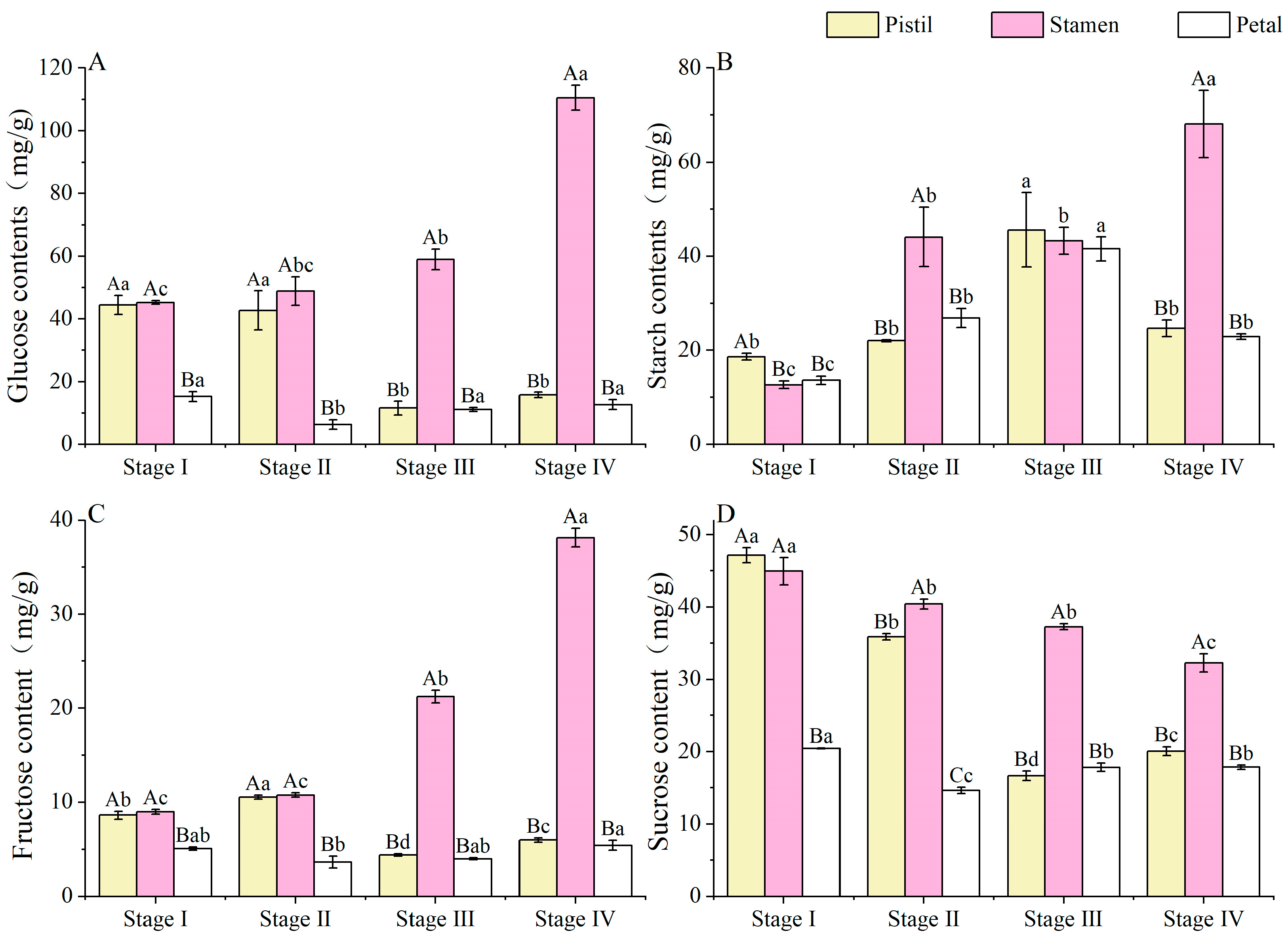
3.5. Non-Structural Sugar (NSCs) Contents
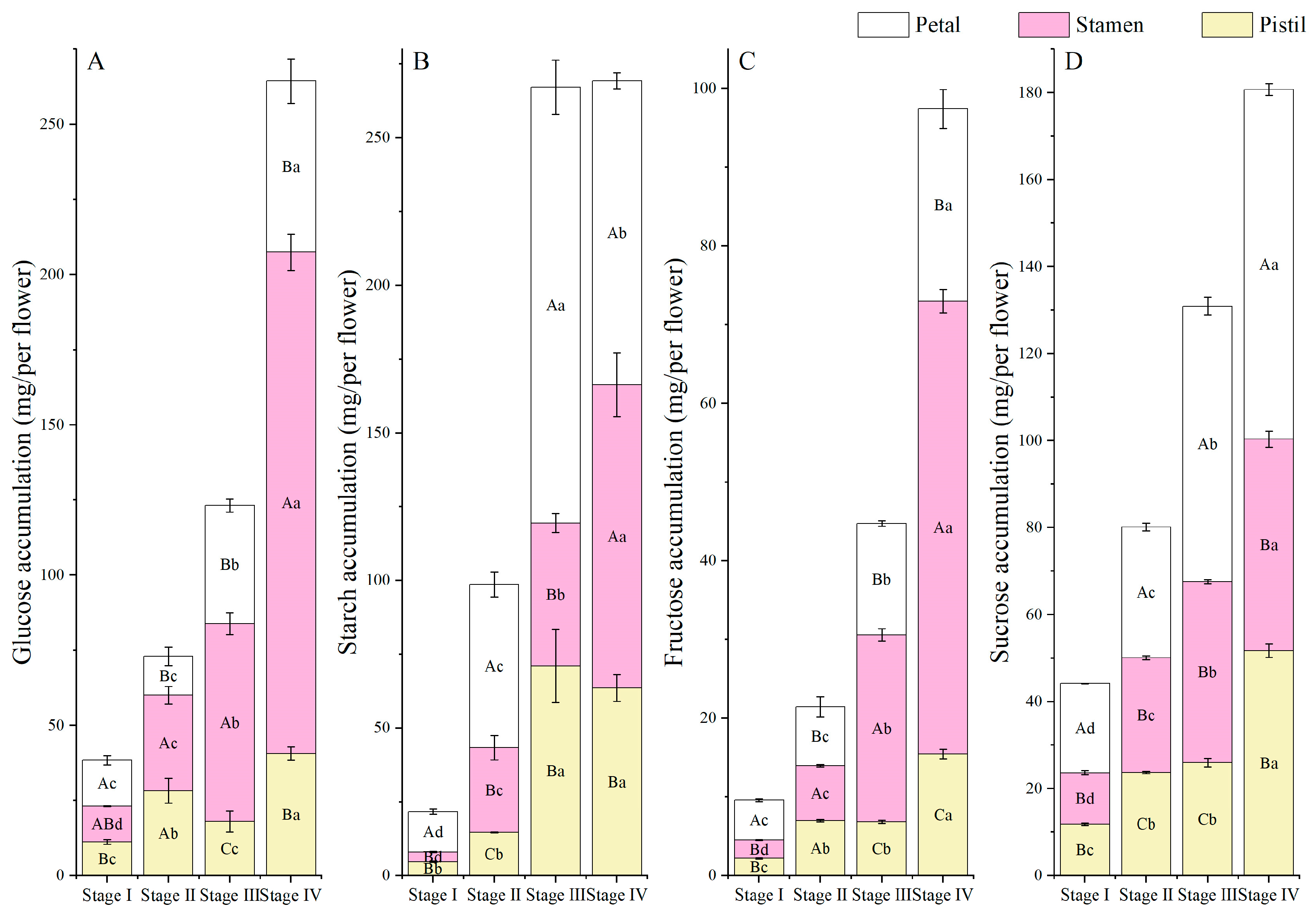
3.6. NSC Accumulation and Allocation Proportion
3.7. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Önder, S.; Tonguç, M.; Erbaş, S.; Önder, D.; Mutlucan, M. Investigation of Phenological, Primary and Secondary Metabolites Changes during Flower Developmental of Rosa Damascena. Plant Physiol. Biochem. 2022, 192, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.R.; Bischoff, M.; Lord, J.M.; Robertson, A.W. Flower Color Influences Insect Visitation in Alpine New Zealand. Ecology 2010, 91, 2638–2649. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, W.G.; Kamdee, C. Flower Opening and Closure: An Update. J. Exp. Bot. 2014, 65, 5749–5757. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yang, Y.; Wang, H.; Qian, W.; Hu, Y.; Gao, S.; Liao, H. The Variations of C/N/P Stoichiometry, Endogenous Hormones, and Non-Structural Carbohydrate Contents in Michelia maudiae ‘Rubicunda’ Flower at Five Development Stages. Horticulturae 2023, 9, 1198. [Google Scholar] [CrossRef]
- Wei, S.; Liu, H.; Li, J.; Ren, T.; Xie, J. Metabolite Variations of Sugars, Organic Acids, Fatty Acids and Amino Acids in Flower Buds of Zingiber Mioga Roscoe at Different Developmental Stages. J. Food Compos. Anal. 2023, 116, 105050. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Önder, D.; Erbaş, S.; Mutlucan, M. Flower Color and Carbohydrate Metabolism Changes during the Floral Development of Rosa Damascena. S. Afr. J. Bot. 2023, 156, 234–243. [Google Scholar] [CrossRef]
- Ågren, G.I. Stoichiometry and Nutrition of Plant Growth in Natural Communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Li, L.; Sheen, J. Dynamic and Diverse Sugar Signaling. Curr. Opin. Plant Biol. 2016, 33, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Y.; Xu, G. How does nitrogen shape plant architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef]
- Prathap, V.; Kumar, A.; Maheshwari, C.; Tyagi, A. Phosphorus homeostasis: Acquisition, sensing, and long-distance signaling in plants. Mol. Biol. Rep. 2022, 49, 8071–8086. [Google Scholar] [CrossRef]
- Poorter, H.; Remkes, C.; Lambers, H. Carbon and Nitrogen Economy of 24 Wild Species Differing in Relative Growth Rate. Plant Physiol. 1990, 94, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yu, G.; Wang, Q.; Wang, R.; Zhang, J.; Liu, C.; He, N. Conservative Allocation Strategy of Multiple Nutrients among Major Plant Organs: From Species to Community. J. Ecol. 2020, 108, 267–278. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Slik, J.W.F.; Cao, K. Leaf Element Concentrations of Terrestrial Plants across China Are Influenced by Taxonomy and the Environment. Glob. Ecol. Biogeogr. 2012, 21, 809–818. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global Patterns of Plant Leaf N and P in Relation to Temperature and Latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P Stoichiometry of Organisms and Ecosystems in a Changing World: A Review and Perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, M. Linkages of C: N: P Stoichiometry between Soil and Leaf and Their Response to Climatic Factors along Altitudinal Gradients. J. Soils Sediments 2019, 19, 1820–1829. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Qian, W.-Z.; Yi, L.; Ye, Y.-L.; Gu, T.; Gao, S.; Cao, G.-X. Nutrient Composition and Antioxidant Activity of Cercis Chinensis Flower in Response to Different Development Stages. Horticulturae 2023, 9, 961. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the Roles of Nonstructural Carbohydrates in Forest Trees—From What We can Measure to What We Want to Know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Song, X.; Peng, C.; Zhou, G.; Gu, H.; Li, Q.; Zhang, C. Dynamic Allocation and Transfer of Non-Structural Carbohydrates, a Possible Mechanism for the Explosive Growth of Moso Bamboo (Phyllostachys heterocycla). Sci. Rep. 2016, 6, 25908. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Ann. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Wang, Y.; Funnell, K.A.; Eason, J.R.; Morgan, E.R.; Woolley, D.J. Osmotic Regulation via Carbohydrate Metabolism Drives Petal Expansion and Floret Opening in Gentian ‘Showtime Spotlight. ’ Sci. Hortic. 2016, 211, 19–25. [Google Scholar] [CrossRef]
- Cho, L.-H.; Pasriga, R.; Yoon, J.; Jeon, J.-S.; An, G. Roles of Sugars in Controlling Flowering Time. J. Plant Biol. 2018, 61, 121–130. [Google Scholar] [CrossRef]
- Yamada, K.; Norikoshi, R.; Suzuki, K.; Imanishi, H.; Ichimura, K. Determination of Subcellular Concentrations of Soluble Carbohydrates in Rose Petals during Opening by Nonaqueous Fractionation Method Combined with Infiltration–Centrifugation Method. Planta 2009, 230, 1115–1127. [Google Scholar] [CrossRef]
- Norikoshi, R.; Imanishi, H.; Ichimura, K. Changes in Cell Number, Osmotic Potential and Concentrations of Carbohydrates and Inorganic Ions in Tweedia caerulea during Flower Opening. J. Jpn. Soc. Hortic. Sci. 2013, 82, 51–56. [Google Scholar] [CrossRef]
- Lebon, G.; Wojnarowiez, G.; Holzapfel, B.; Fontaine, F.; Vaillant-Gaveau, N.; Clement, C. Sugars and Flowering in the Grapevine (Vitis vinifera L.). J. Exp. Bot. 2008, 59, 2565–2578. [Google Scholar] [CrossRef]
- Boldingh, H.L.; Alcaraz, M.L.; Thorp, T.G.; Minchin, P.E.H.; Gould, N.; Hormaza, J.I. Carbohydrate and Boron Content of Styles of ‘Hass’ Avocado (Persea americana Mill.) Flowers at Anthesis can Affect Final Fruit Set. Sci. Hortic. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Luo, H.; Wu, H.; Yu, X.; Zhang, X.; Lu, Y.; Fan, J.; Tang, L.; Wang, Z. A Review of the Phytochemistry and Pharmacological Activities of Magnoliae officinalis Cortex. J. Ethnopharmacol. 2019, 236, 412–442. [Google Scholar] [CrossRef]
- Poivre, M.; Duez, P. Biological Activity and Toxicity of the Chinese Herb Magnolia officinalis Rehder & E. Wilson (Houpo) and Its Constituents. J. Zhejiang Univ. Sci. B 2017, 18, 194–214. [Google Scholar] [CrossRef]
- Niu, L.; Hou, Y.; Jiang, M.; Bai, G. The Rich Pharmacological Activities of Magnolia officinalis and Secondary Effects Based on Significant Intestinal Contributions. J. Ethnopharmacol. 2021, 281, 114524. [Google Scholar] [CrossRef]
- Verlinden, S. Changes in Mineral Nutrient Concentrations in Petunia Corollas during Development and Senescence. HortScience 2003, 38, 71–74. [Google Scholar] [CrossRef]
- Wang, T.-W.; Tan, J.; Li, L.-Y.; Yang, Y.; Zhang, X.-M.; Wang, J.-R. Combined Analysis of Inorganic Elements and Flavonoid Metabolites Reveals the Relationship between Flower Quality and Maturity of Sophora japonica L. Front. Plant Sci. 2023, 14, 1255637. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Ren, H.; Wang, M.; Qian, W.; Hu, Y.; Yang, Y.; Yu, T.; Zhao, K.; Gao, S. Changes in Growth Parameters, C:N:P Stoichiometry and Non-Structural Carbohydrate Contents of Zanthoxylum armatum Seedling in Response to Five Soil Types. Horticulturae 2024, 10, 261. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal Component Analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Ho, T.-T.; Kwon, A.-R.; Yoon, Y.-J.; Paek, K.-Y.; Park, S.-Y. Endoreduplication Level Affects Flower Size and Development by Increasing Cell Size in Phalaenopsis and Doritaenopsis. Acta Physiol. Plant. 2016, 38, 190. [Google Scholar] [CrossRef]
- Steinacher, G.; Wagner, J. Flower Longevity and Duration of Pistil Receptivity in High Mountain Plants. Flora 2010, 205, 376–387. [Google Scholar] [CrossRef]
- Deng, Y.; Jia, X.; Liang, L.; Gu, C.; Sun, X. Morphological Anatomy, Sporogenesis and Gametogenesis in Flowering Process of Jasmine (Jasminum sambac Aiton). Sci. Hortic. 2016, 198, 257–266. [Google Scholar] [CrossRef]
- Mittal, I.; Jhanji, S.; Dhatt, K.K. Efficacy of Sodium Nitroprusside, a Nitric Oxide Donor, on Vase Life and Postharvest Attributes of Gladiolus Spikes. Acta Physiol. Plant. 2021, 43, 108. [Google Scholar] [CrossRef]
- Jhanji, S.; Kaur, G.; Kaur, R.; Dhatt, U.K. Physiological and Biochemical Changes during Flower Development and Senescence in Chrysanthemum and Gladiolus. Acta Physiol. Plant. 2023, 45, 14. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; van Meeteren, U. Flower Opening and Closure: A Review. J. Exp. Bot. 2003, 54, 1801–1812. [Google Scholar] [CrossRef]
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and Determinants of Carbon Content in Plants: A Global Synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef]
- Gong, Z.; Sheng, M.; Zheng, X.; Zhang, Y.; Wang, L. Ecological Stoichiometry of C, N, P and Si of Karst Masson Pine Forests: Insights for the Forest Management in Southern China. Sci. Total Environ. 2024, 912, 169490. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S. N:P Ratios in Terrestrial Plants: Variation and Functional Significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Kim, N.; Han, W.; Guo, Y.; Han, T.; Du, E.; Fang, J. Effects of Nitrogen and Phosphorus Supply on Growth Rate, Leaf Stoichiometry, and Nutrient Resorption of Arabidopsis thaliana. Plant Soil 2015, 388, 147–155. [Google Scholar] [CrossRef]
- Guilpart, N.; Metay, A.; Gary, C. Grapevine Bud Fertility and Number of Berries per Bunch Are Determined by Water and Nitrogen Stress around Flowering in the Previous Year. Eur. J. Agron. 2014, 54, 9–20. [Google Scholar] [CrossRef]
- Lau, T.-C.; Stephenson, A.G. Effects of Soil Phosphorus on Pollen Production, Pollen Size, Pollen Phosphorus Content, and the Ability to Sire Seeds in Cucurbita pepo (Cucurbitaceae). Sex. Plant Reprod. 1994, 7, 215–220. [Google Scholar] [CrossRef]
- Atasay, A.; Akgül, H.; Uçgun, K.; Şan, B. Nitrogen Fertilization Affected the Pollen Production and Quality in Apple Cultivars “Jerseymac” and “Golden Delicious”. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2013, 63, 460–465. [Google Scholar] [CrossRef]
- Ye, T.; Li, Y.; Zhang, J.; Hou, W.; Zhou, W.; Lu, J.; Xing, Y.; Li, X. Nitrogen, Phosphorus, and Potassium Fertilization Affects the Flowering Time of Rice (Oryza sativa L.). Glob. Ecol. Conserv. 2019, 20, e00753. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E.; Casal, S. Phytochemical Characterization of Borago officinalis L. and Centaurea cyanus L. during Flower Development. Food Res. Int. 2019, 123, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, S.; Tafazoli, E.; Dokhani, S.; Rahemi, M.; Emam, Y. Changes in Carbohydrate Contents in Shoot Tips, Leaves and Roots of Strawberry (Fragaria×ananassa Duch.) during Flower-Bud Differentiation. Sci. Hortic. 2007, 113, 255–260. [Google Scholar] [CrossRef]
- Hedhly, A.; Vogler, H.; Schmid, M.W.; Pazmino, D.; Gagliardini, V.; Santelia, D.; Grossniklaus, U. Starch Turnover and Metabolism during Flower and Early Embryo Development. Plant Physiol. 2016, 172, 2388–2402. [Google Scholar] [CrossRef]
- Sood, S.; Vyas, D.; Nagar, P.K. Physiological and Biochemical Studies during Flower Development in Two Rose Species. Sci. Hortic. 2006, 108, 390–396. [Google Scholar] [CrossRef]
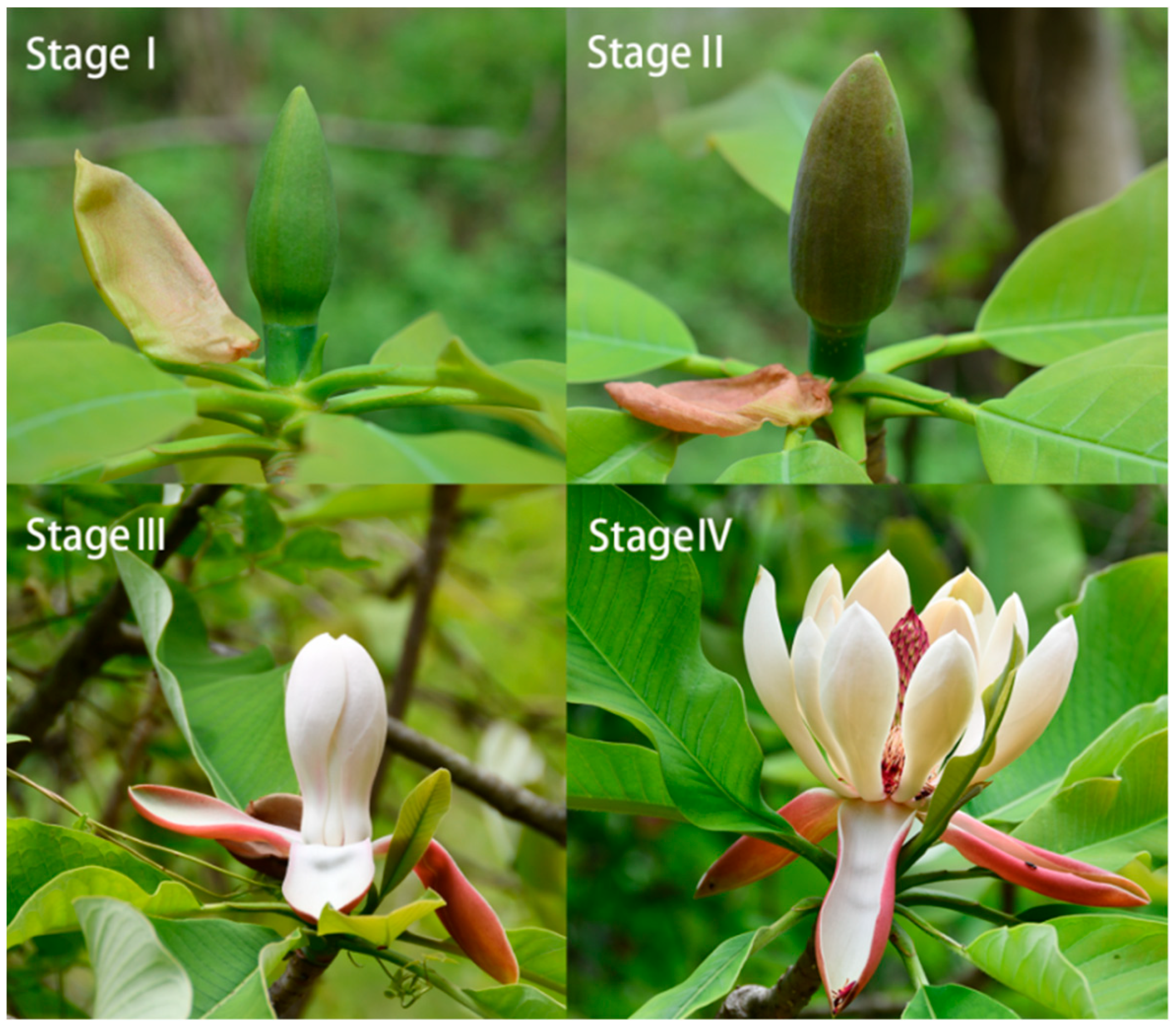



| Stage | Pistil | Stamen | Petal | |||
|---|---|---|---|---|---|---|
| Length (cm) | Width (cm) | Length (cm) | Width (cm) | Length (cm) | Width (cm) | |
| I | 5.00 ± 0.15 b | 1.34 ± 0.08 a | 1.88 ± 0.04 c | 0.22 ± 0.01 c | 6.28 ± 0.14 d | 3.13 ± 0.13 c |
| II | 5.51 ± 0.16 b | 1.96 ± 0.06 a | 2.17 ± 0.06 b | 0.26 ± 0.02 bc | 7.40 ± 0.07 c | 3.49 ± 0.12 c |
| III | 7.08 ± 0.21 a | 2.09 ± 0.05 a | 2.74 ± 0.10 a | 0.35 ± 0.01 a | 8.70 ± 0.15 b | 4.80 ± 0.09 b |
| IV | 7.31 ± 0.24 a | 2.15 ± 0.07 a | 2.56 ± 0.12 a | 0.30 ± 0.03 ab | 11.3 ± 0.32 a | 5.62 ± 0.14 a |
| Stage | Pistil | Stamen | Petal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fresh Weight/g | Dry Weight/g | RWC (%) | Fresh Weight/g | Dry Weight/g | RWC (%) | Fresh Weight/g | Dry Weight/g | RWC (%) | |
| I | 1.90 ± 0.09 c | 0.25 ± 0.01 c | 86.8 a | 1.74 ± 0.15 c | 0.26 ± 0.02 d | 84.8 a | 7.35 ± 0.50 d | 1.01 ± 0.08 d | 86.3 a |
| II | 4.15 ± 0.58 c | 0.65 ± 0.08 c | 84.1 b | 3.60 ± 0.51 b | 0.65 ± 0.09 c | 81.9 b | 16.3 ± 1.39 c | 2.07 ± 0.26 c | 87.4 a |
| III | 6.75 ± 0.87 b | 1.55 ± 0.19 b | 76.9 d | 4.15 ± 0.06 b | 1.12 ± 0.02 b | 73.0 d | 19.7 ± 1.20 b | 3.55 ± 0.21 b | 82.0 c |
| IV | 13.3 ± 1.31 a | 2.58 ± 0.27 a | 80.6 c | 6.52 ±0.64 a | 1.51 ± 0.15 a | 76.9 c | 28.1 ± 0.84 a | 4.50 ± 0.18 a | 84.0 b |
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| Glucose concentration | 0.34909 | 0.3236 | −0.15173 |
| Starch concentration | 0.36917 | −0.10128 | −0.13488 |
| Fructose concentration | 0.40093 | 0.19085 | −0.17745 |
| Sucrose concentration | 0.08265 | 0.4945 | 0.21313 |
| C concentration | 0.11207 | −0.10532 | 0.87582 |
| N concentration | −0.43173 | 0.01485 | 0.08933 |
| P concentration | −0.27832 | 0.42472 | 0.18284 |
| C:N ratio | 0.43122 | −0.01701 | 0.206 |
| C:P ratio | 0.30272 | −0.39901 | 0.15451 |
| N:P ratio | −0.14236 | −0.50285 | −0.08417 |
| Percentage of variance (%) | 44.4% | 35.9% | 13.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Qiu, Y.; Cheng, Y.; Yu, T.; Zhu, M.; Qian, W.; Gao, S.; Zhuang, G. Non-Structural Carbohydrate Content and C:N:P Stoichiometry in Houpoea officinalis Flowers in Response to Development Stages. Horticulturae 2024, 10, 784. https://doi.org/10.3390/horticulturae10080784
Yang Y, Qiu Y, Cheng Y, Yu T, Zhu M, Qian W, Gao S, Zhuang G. Non-Structural Carbohydrate Content and C:N:P Stoichiometry in Houpoea officinalis Flowers in Response to Development Stages. Horticulturae. 2024; 10(8):784. https://doi.org/10.3390/horticulturae10080784
Chicago/Turabian StyleYang, Yao, Yuxian Qiu, Yu Cheng, Ting Yu, Maoyuan Zhu, Wenzhang Qian, Shun Gao, and Guoqing Zhuang. 2024. "Non-Structural Carbohydrate Content and C:N:P Stoichiometry in Houpoea officinalis Flowers in Response to Development Stages" Horticulturae 10, no. 8: 784. https://doi.org/10.3390/horticulturae10080784






