Double-Heading Produces Larger Fruit via Inhibiting EjFWLs Expression and Promoting Cell Division at the Early Stage of Loquat Fruit Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
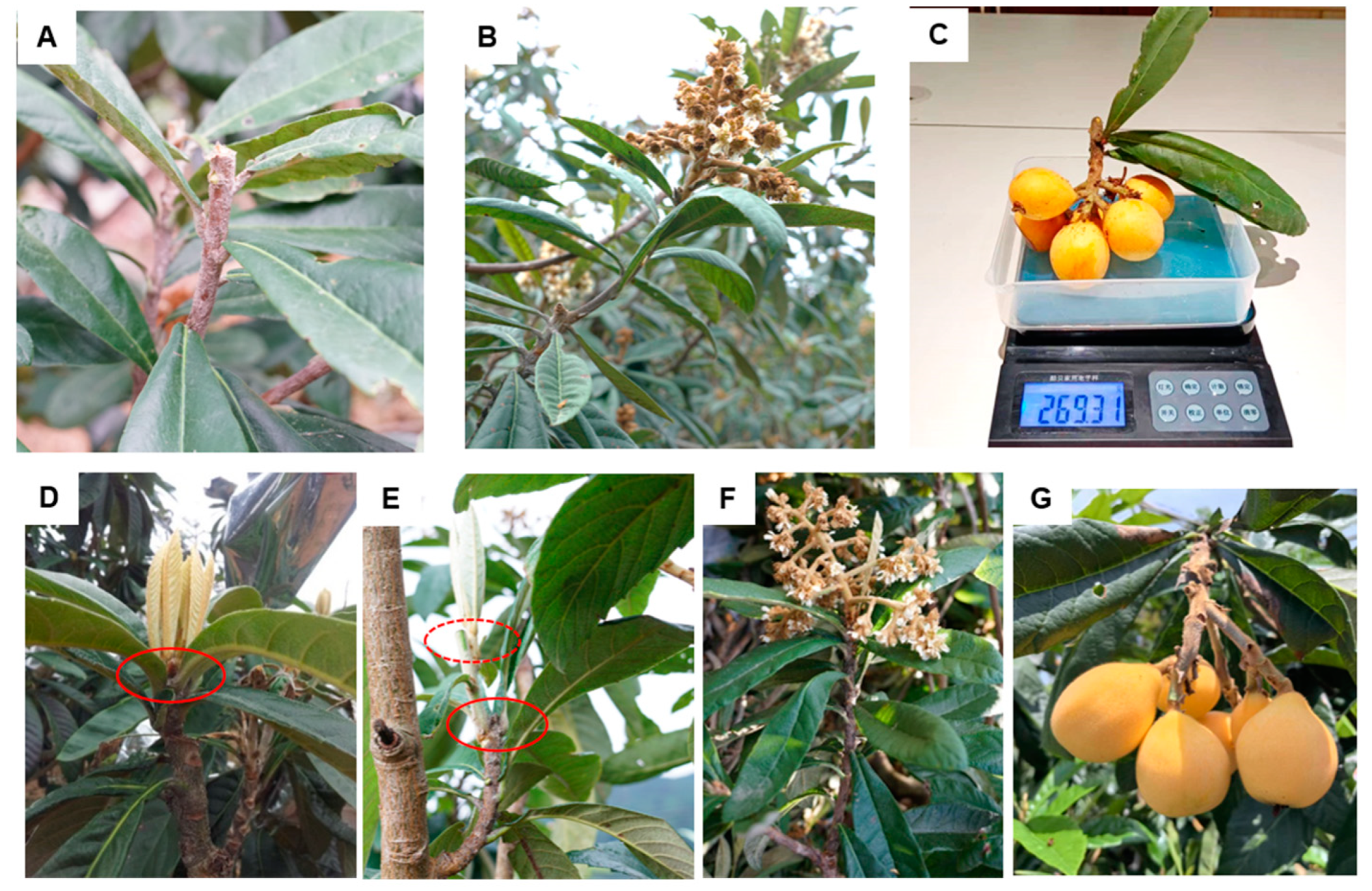
2.2. A Novel Pruning Method for Vigorous Shoot Preparing and Larger Fruit Development
2.3. Tree Vegetative Trait Measurement
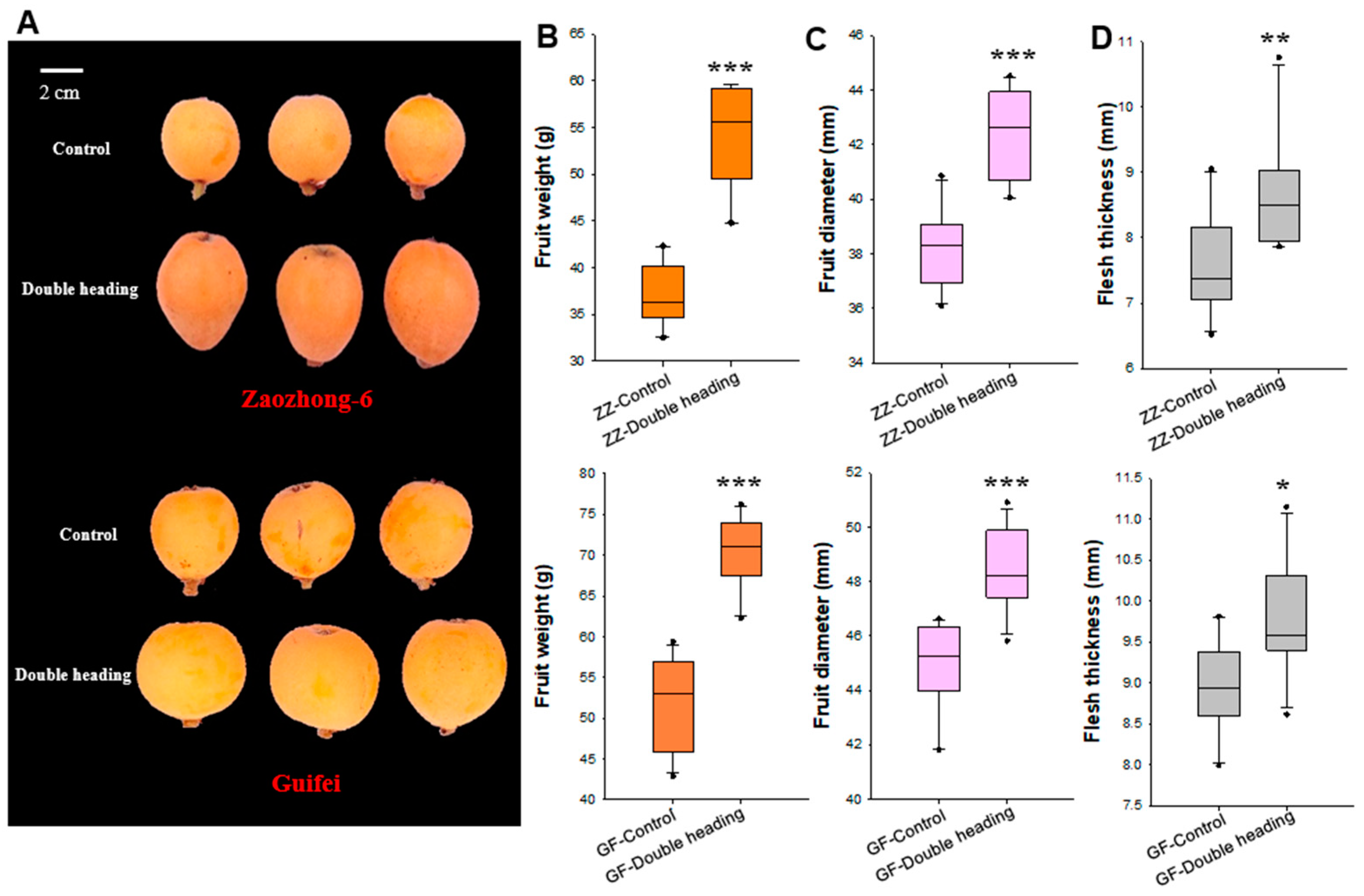
2.4. Fruit Size Measurement
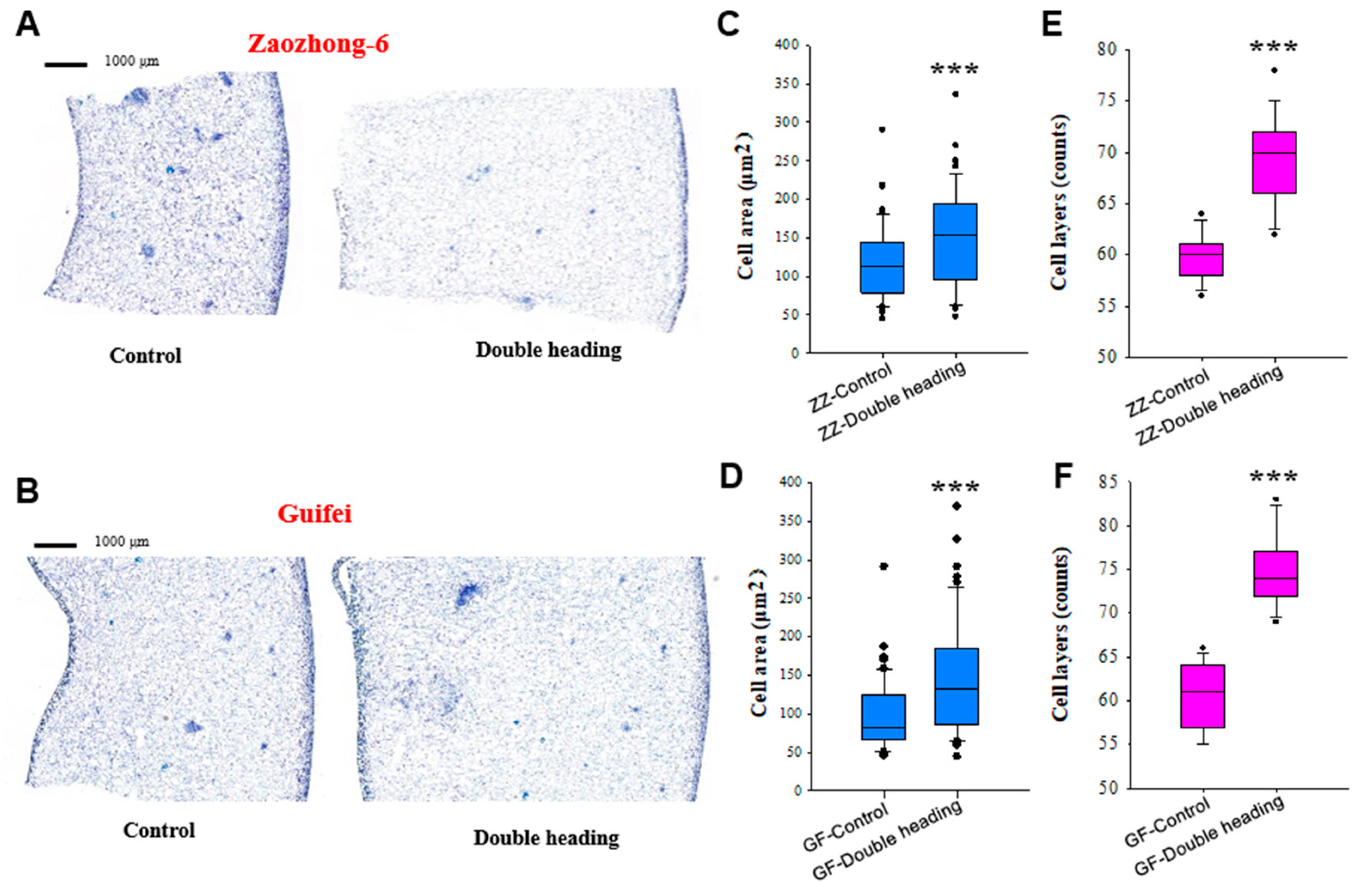
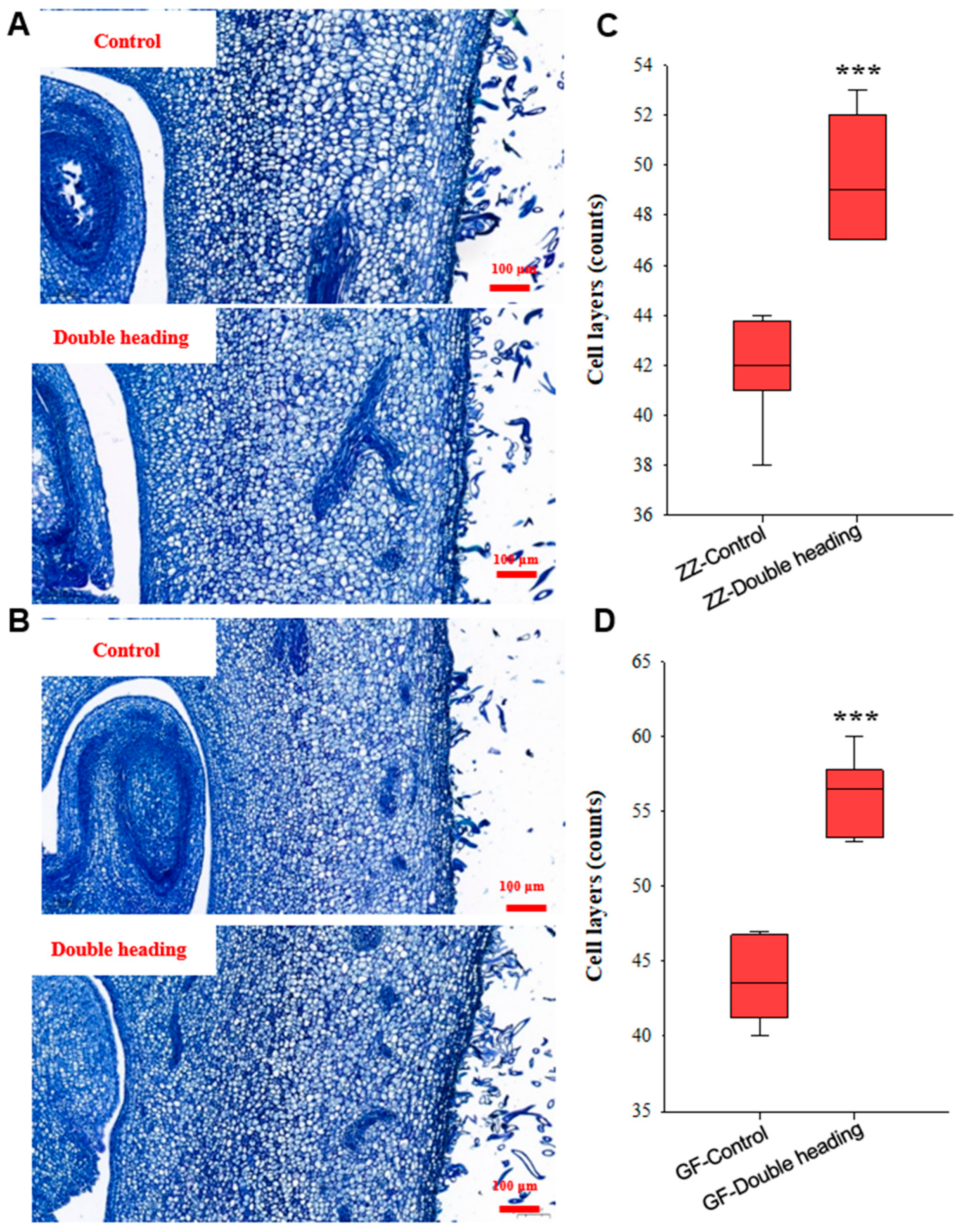
2.5. Section Preparation and Cell Measurement
2.6. Gene Expression Analysis
2.7. Data Analysis
3. Results
3.1. Double-Heading Promotes Shoot Growth
3.2. Double-Heading Enhances Loquat Fruit Enlargement
3.3. Double Back-Cutting Promotes Both Cell Division and Cell Expansion
3.4. Double Back-Cutting Notably Promotes Receptacle Cell Division during Flower Bud Development
3.5. Double Back-Cutting Inhibits EjFWL1/2 Expression during Flower Bud Growth Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, S.; Huang, X.; Cuevas, J.; Janick, J. Loquat: An ancient fruit crop with a promising future. Chron. Hortic. 2007, 47, 12–15. [Google Scholar]
- Zhang, L.; Yu, H.; Lin, S.; Gao, Y. Molecular characterization of FT and FD homologs from Eriobotrya deflexa Nakai forma Koshunensis. Front. Plant Sci. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, Y. Collection of Illustration for Eriobotrya Plants; Science Press: Beijing, China, 2016. [Google Scholar]
- Su, W.; Jing, Y.; Lin, S.; Yue, Z.; Yang, X.; Xu, J.; Wu, J.; Zhang, Z.; Xia, R.; Zhu, J.; et al. Polyploidy underlies co-option and diversification of biosynthetic triterpene pathways in the apple tribe. Proc. Natl. Acad. Sci. USA 2021, 118, e2101767118. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, C.; Chen, K. Nutritional and composition of fruit cultivars: Loquat (Eriobotrya japonica Lindl.). In Nutritional Composition of Fruit Cultivar; Monique, S.J.S., Preedy, V.R., Eds.; Academic Press: Waltham, MA, USA; Elsevier: Amsterdam, The Netherlands, 2016; pp. 371–394. [Google Scholar]
- Su, W.; Shao, Z.; Wang, M.; Gan, X.; Yang, X.; Lin, S. EjBZR1 represses fruit enlargement by binding to the EjCYP90 promoter in loquat. Hortic. Res. 2021, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zhang, H. Fruit Tree Flora of China: Longan and Loquat Section; China Agriculture and Forestry Press: Beijing, China, 1996. [Google Scholar]
- Huang, J.; Xu, X.; Zheng, S. Zaozhong-6, a new loquat variety with extremely early maturity and large fruit size. China Fruits 1993, 4, 4–6. (In Chinese) [Google Scholar]
- Lin, S. Fruit scientific research in new china in the past 70 years: Loquat. J. Fruit Sci. 2019, 36, 1421–1428. (In Chinese) [Google Scholar]
- Yang, M.; Lin, L.; Fan, D.; Song, W.; Peng, Z.; Zhong, S.; Ou, J.; Wang, J.; Liu, Y.; Wei, D.; et al. Progress in cross breeding of loquat in china. Chin. Seed Ind. 2021, 10, 31–35. (In Chinese) [Google Scholar]
- Song, H.; He, X.; Qiao, Y.; Huang, B.; Qiu, X.; Hu, Y.; Xu, S.; Lin, S. Hybridization of ‘Zaozhong No.6’ and big-fruit Spanish loquat cultivars and fruit evalution of the hybrids. J. South. China Agric. Univ. 2015, 36, 65–70. (In Chinese) [Google Scholar]
- Fukuda, S.; Ishimoto, K.; Terakami, S.; Toshiya Yamamoto and Naofumi Hiehata. Genetic mapping of the loquat canker resistance gene Pse-C in loquat (Eriobotrya japonica). Sci. Hortic. 2016, 200, 19–24. [Google Scholar] [CrossRef]
- Fu, X.; Feng, C.; Wang, C.; Yin, X.; Lu, P.; Grierson, D.; Xu, C.; Chen, K. Involvement of multiple phytoene synthase genes in tissue- and cultivar-specific accumulation of carotenoids in loquat. J. Exp. Bot. 2014, 65, 4679–4689. [Google Scholar] [CrossRef]
- Yang, X.; Liu, C.; Lin, S. Genetic relationships in Eriobotrya species as revealed by amplified fragment length polymorphism (AFLP) markers. Sci. Hortic. 2009, 122, 264–268. [Google Scholar] [CrossRef]
- Wen, G.; Dang, J.; Xie, Z.; Wang, J.; Jiang, P.; Guo, Q.; Liang, G. Molecular karyotypes of loquat (Eriobotrya japonica) aneuploids can be detected by using SSR markers combined with quantitative PCR irrespective of heterozygosity. Plant Methods 2020, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K.; Kono, A.; Atsuda, Y.T.; Akoda, K.S. Some factors affecting fruit weight of loquat. Trop. Agric. 1994, 38, 286–292. (In Japanese) [Google Scholar]
- Bu, H.; Yu, W.; Yuan, H.; Yue, P.; Wei, Y.; Wang, A. Endogenous auxin content contributes to larger size of apple fruit. Front. Plant Sci. 2020, 11, 592540. [Google Scholar] [CrossRef] [PubMed]
- Agustí, M.; Gariglio, N.; Juan, M.; Almela, V.; Mesejo, C.; Martínez-Fuentes, A. Effect of branch scoring on fruit development in loquat. J. Hortic. Sci. Biotechnol. 2015, 80, 370–374. [Google Scholar] [CrossRef]
- Reig, C.; Mesejo, C.; Martínez-Fuentes, A.; Iglesias, D.J.; Agustí, M. Fruit load and root development in field-grown loquat trees (Eriobotrya japonica Lindl). J. Plant Growth Regul. 2012, 32, 281–290. [Google Scholar] [CrossRef]
- Agusti, M.; Juan, M.; Almela, V.; Gariglio, N. Loquat fruit size is increased through the thinning effect of Naphthaleneacetic Acid. Plant Growth Regul. 2000, 31, 167–171. [Google Scholar] [CrossRef]
- Liu, Q.; Lv, J.; Ying, Z.; Shi, S.; Zhang, Z. Study on renovation pruning in loquat. J. Zhejiang Agric. Univ. 1994, 20, 33–37. [Google Scholar]
- Lin, S.; Sharpe, R.H.; Janick, J. Loquat: Botany and horticulture. In Horticulture Review; Janick, J., Ed.; Wiley: Hoboken, NJ, USA, 1999. [Google Scholar]
- Frary, A.; Nesbitt, T.C.; Frary, A.; Grandillo, S.; Knaap, E.V.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B.; et al. Fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef]
- Dahan, Y.; Rosenfeld, R.; Zadiranov, V.; Irihimovitch, V. A Proposed conserved role for an avocado fw2.2-like gene as a negative regulator of fruit cell division. Planta 2010, 232, 663–676. [Google Scholar] [CrossRef]
- Li, Z.; He, C. Physalis floridana Cell Number Regulator1 encodes a cell membrane-anchored modulator of cell cycle and negatively controls fruit size. J. Exp. Bot. 2015, 66, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zeng, B.; Luo, S.-P.; Li, X.-G.; Wu, B.; Li, J. Cloning, localization and expression analysis of two Fw2.2-Like genes in small- and large-fruited pear species. J. Integr. Agric. 2016, 15, 282–294. [Google Scholar] [CrossRef]
- Su, W.; Zhu, Y.; Zhang, L.; Yang, X.; Gao, Y.; Lin, S. The cellular physiology of loquat (Eriobotrya japonica Lindl.) fruit with a focus on how cell division and cell expansion processes contribute to pome morphogenesis. Sci. Hortic. 2017, 224, 142–149. [Google Scholar] [CrossRef]
- Su, W.; Zhang, L.; Jiang, Y.; Huang, T.; Chen, X.; Liu, Y.; Wu, J.; Yang, X.; Lin, S. EjFWLs are repressors of cell division during early fruit morphogenesis of loquat. Sci. Hortic. 2021, 287, 110261. [Google Scholar] [CrossRef]
- Tanksley, S.D. The genetic, developmental and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004, 16, S181–S189. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yin, D.; Chen, C.; Yu, D.; Han, W. Optimal design of plant canopy based on light interception: A case study with loquat. Front. Plant Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Agustí, M.; Gariglio, N.; Castillo, A.; Juan, M.; Almela, V. Improvement of loquat fruit quality. In Proceedings of the First International Symposium on Loquat, Valencia, Spain, 11–13 April 2002; Badenes, M.L., Llácer, G., Eds.; CIHEAM: Zaragoza, Spain, 2003; pp. 81–85. [Google Scholar]
- Ding, C.; Zhang, H. The effect of plant hormones on fruit development of loquat. Acta Hortic. Sin. 1988, 15, 148–154. (In Chinese) [Google Scholar]
- Hussain, T.; Irfan, A.; Ijaz, A.; Mehwish, L.; Tahir, A.M.; Adeel, A.; Ghazal, R.; Sana, A.; Waqas, N.; Aysha, M.; et al. Naveed Muhammad Saqib and Ali Imran. Evaluation of fruit bunch bagging techniques for improvement of loquat fruit quality. J. Appl. Res. Plant Sci. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Myers, S.C.; Ferree, D.C. Influence of time of summer pruning and limb orientation on yield, fruit size, and quality of vigorous ‘Delicious’ apple tress. J. Am. Soc. Hortic. Sci. 1983, 108, 630–633. [Google Scholar] [CrossRef]
- Al-Saif, A.M.; Abdel-Aziz, H.F.; Khalifa, S.M.; Elnaggar, I.A.; Abd El-wahed, A.E.; Farouk, M.H.; Hamdy, A.E. Pruning boosts growth, yield, and fruit quality of old Valencia orange trees: A field study. Agriculture 2023, 13, 1720. [Google Scholar] [CrossRef]
- Matias, P.; Barrote, I.; Azinheira, G.; Continella, A.; Duarte, A. Citrus pruning in the Mediterranean climate: A review. Plants 2023, 12, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Pramanick, K.K.; Kashyap, P.; Kishore, D.K.; Shajtma, Y.P. Effect of summer pruning and CPPU on yield and quality of kiwi fruit (Actinidia deliciosa). J. Environ. Biol. 2015, 36, 351–356. [Google Scholar] [PubMed]
- Gomasta, J.; Sarker, B.C.; Haque, M.A.; Anwari, A.; Mondal, S.; Uddin, M.S. Pruning techniques affect flowering, fruiting, yield and fruit biochemical traits in guava under transitory sub-tropical conditions. Heliyon 2024, 10, e30064. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Malladi, A. Higher growth of the apple (Malus × domestica Borkh.) fruit cortex is supported by resource intensive metabolism during early development. BMC Plant Biol. 2020, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, X.; Jiang, J.; Huang, C.; Xu, J.; Zhang, Z.; Liu, Y. Correlationship between bearing base shoot character with form quality of fruit of zaozhong6 loquat. Fujian J. Agric. Sci. 2002, 17, 33–37. (In Chinese) [Google Scholar]
- Cuevas, J.; Salvador-Sola, F.J.; Gavilán, J.; Lorente, N.; Hueso, J.J.; González-Padierna, C.M. Loquat fruit sink strength and growth pattern. Sci. Hortic. 2003, 98, 131–137. [Google Scholar] [CrossRef]
- Wang, L.; Shi, L.; Wang, H.; Huang, X. Size of early and late blossoming loquat fruits and their growth and development temperature. Guizhou Agric. Sci. 2009, 37, 150–151. (In Chinese) [Google Scholar]
- Chen, Q.; Jing, D.; Wang, S.; Xu, F.; Bao, C.; Luo, M.; Guo, Q. The putative role of the NAC transcription factor EjNACL47 in cell enlargement of loquat (Eriobotrya japonica Lindl.). Horticulturae 2021, 7, 323. [Google Scholar] [CrossRef]
- Cong, B.; Liu, J.; Tanksley, S.D. Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proc. Natl. Acad. Sci. USA 2002, 99, 13606–13611. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Tian, J.; Li, J.; Wen, Y. Genome-wide identification and expression analysis of the Fw2.2-Like gene family in pear. Horticulturae 2023, 9, 1–16. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.; Deng, C.; Wei, W.; Chen, X.; Lin, H.; Chen, Y.; Xu, Q.; Tong, Z.; Zheng, S.; Jiang, J. Double-Heading Produces Larger Fruit via Inhibiting EjFWLs Expression and Promoting Cell Division at the Early Stage of Loquat Fruit Development. Horticulturae 2024, 10, 793. https://doi.org/10.3390/horticulturae10080793
Su W, Deng C, Wei W, Chen X, Lin H, Chen Y, Xu Q, Tong Z, Zheng S, Jiang J. Double-Heading Produces Larger Fruit via Inhibiting EjFWLs Expression and Promoting Cell Division at the Early Stage of Loquat Fruit Development. Horticulturae. 2024; 10(8):793. https://doi.org/10.3390/horticulturae10080793
Chicago/Turabian StyleSu, Wenbing, Chaojun Deng, Weilin Wei, Xiuping Chen, Han Lin, Yongping Chen, Qizhi Xu, Zhihong Tong, Shaoquan Zheng, and Jimou Jiang. 2024. "Double-Heading Produces Larger Fruit via Inhibiting EjFWLs Expression and Promoting Cell Division at the Early Stage of Loquat Fruit Development" Horticulturae 10, no. 8: 793. https://doi.org/10.3390/horticulturae10080793
APA StyleSu, W., Deng, C., Wei, W., Chen, X., Lin, H., Chen, Y., Xu, Q., Tong, Z., Zheng, S., & Jiang, J. (2024). Double-Heading Produces Larger Fruit via Inhibiting EjFWLs Expression and Promoting Cell Division at the Early Stage of Loquat Fruit Development. Horticulturae, 10(8), 793. https://doi.org/10.3390/horticulturae10080793




