Changes in Soil Properties Under the Influence of Microplastics in Plastic and Open Field Production in Three Serbian Valleys
Abstract
:1. Introduction
2. Materials and Methods
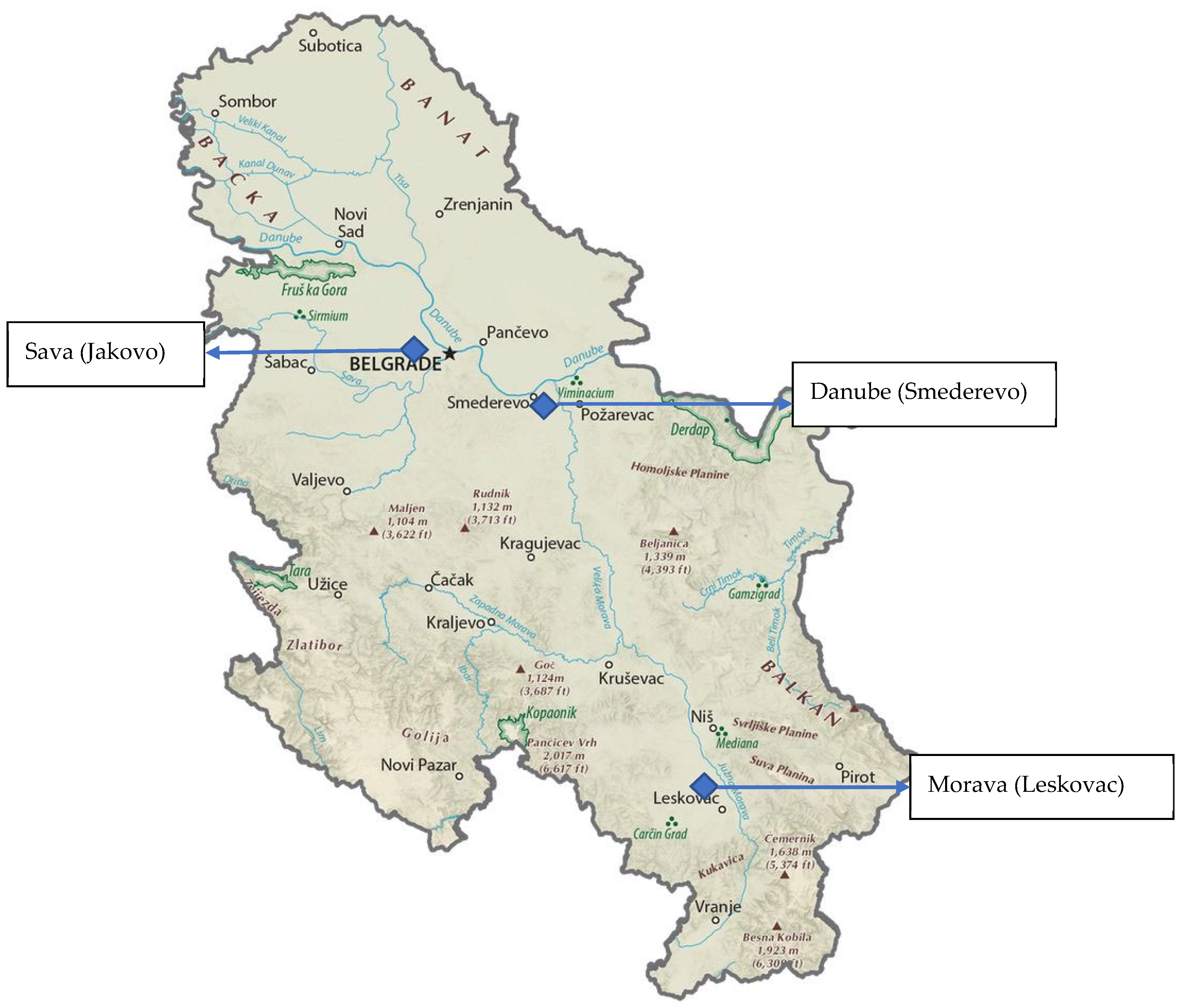
2.1. Site Description and Sampling
2.2. Analytical Methods
2.3. Statistics
3. Results
3.1. Content of Microplastic Particles <5 mm
3.2. Soil Chemical Characteristics
3.3. Content of Microelements
3.4. Soil Physical Properties
3.5. Potentially Mineralizable Carbon Measured via Sequential Soil Microbial Respiration
4. Discussion
4.1. Soil Chemical Characteristics
4.2. Soil Physical Properties
4.3. Soil Organic Carbon
4.4. Soil Labile Organic Carbon
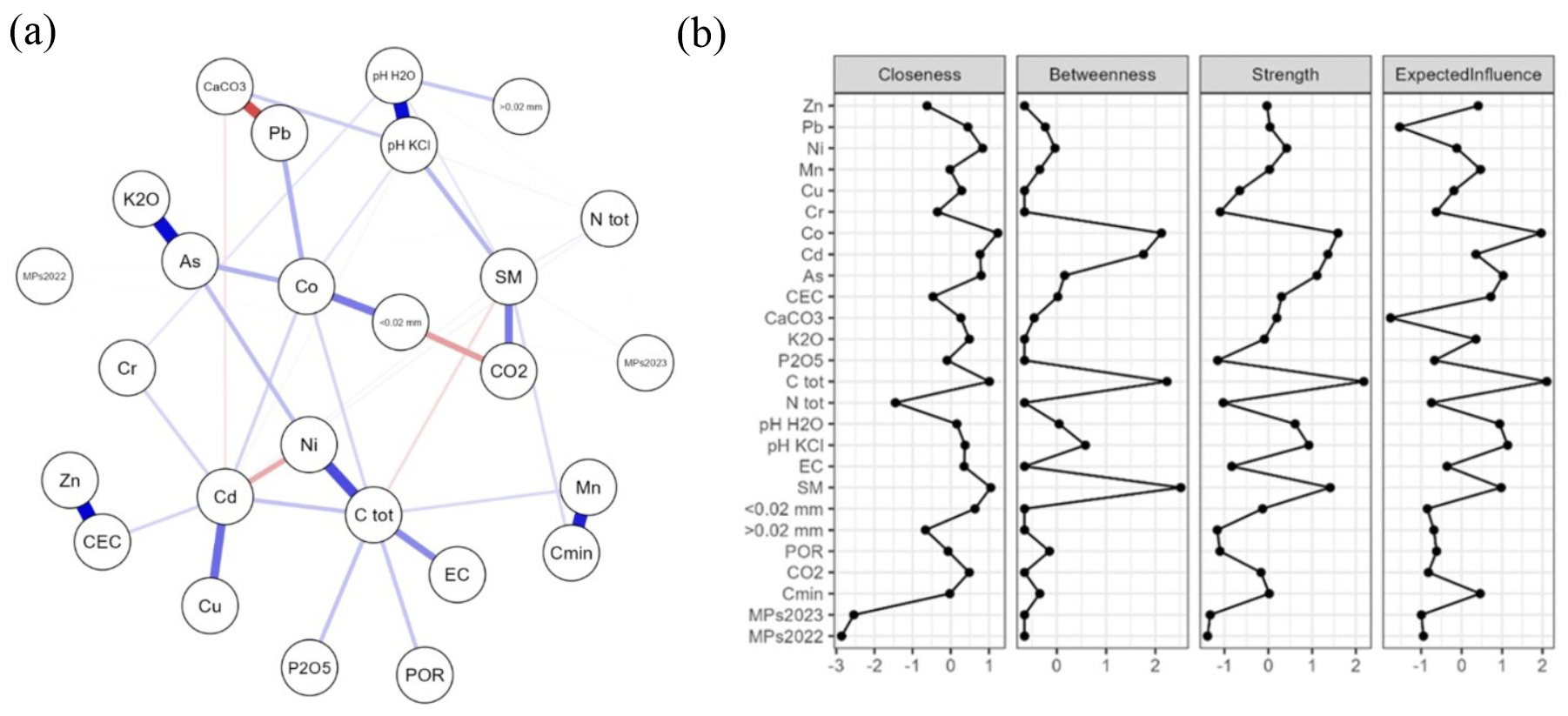
4.5. Network Visualization and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| PMC | Potentially mineralizable carbon |
| NA | Network analysis |
| PTEs | Potentially toxic elements |
References
- Zhang, L.; Wei, H.; Zhang, K.; Li, Z.; Li, F.-M.; Zhang, F. Plastic film mulching increases crop yields and reduces global warming potential under future climate change. Agric. For. Meteorol. 2024, 349, 109963. [Google Scholar] [CrossRef]
- Hofmann, T.; Ghoshal, S.; Tufenkji, N.; Adamowski, J.F.; Bayen, S.; Chen, Q.; Demokritou, P.; Flury, M.; Hüffer, T.; Ivleva, N.P.; et al. Plastics can be used more sustainably in agriculture. Commun. Earth Environ. 2023, 4, 332. [Google Scholar] [CrossRef]
- Salama, K.; Geyer, M. Plastic Mulch Films in Agriculture: Their Use, Environmental Problems, Recycling and Alternatives. Environments 2023, 10, 179. [Google Scholar] [CrossRef]
- Bodor, A.; Feigl, G.; Kolossa, B.; Mészáros, E.; Laczi, K.; Kovács, E.; Perei, K.; Rákhely, G. Soils in distress: The impacts and ecological risks of (micro)plastic pollution in the terrestrial environment. Ecotoxicol. Environ. Saf. 2024, 269, 115807. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Hanif, M.N.; Aijaz, N.; Azam, K.; Akhtar, M.; Laftah, W.A.; Babur, M.; Abbood, N.K.; Benitez, I.B. Impact of microplastics on soil (physical and chemical) properties, soil biological properties/soil biota, and response of plants to it: A review. Int. J. Environ. Sci. Technol. 2024, 21, 10277–10318. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Zhang, J.; Wang, G.; Deng, S.; Bao, R.; Zhang, C.; Syed, T.N.; Wang, B.; Zhou, R.; et al. Plastic Pollution in Agriculture as a Threat to Food Security, the Ecosystem, and the Environment: An Overview. Agronomy 2024, 14, 548. [Google Scholar] [CrossRef]
- Maqbool, A.; Soriano, M.-A.; Gómez, J.A. Macro- and micro-plastics change soil physical properties: A systematic review. Environ. Res. Lett. 2023, 18, 123002. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef]
- Rillig, M.C.; Leifheit, E.; Lehmann, J. Microplastic effects on carbon cycling processes in soils. PLoS Biol. 2021, 19, e3001130. [Google Scholar] [CrossRef]
- Sa’adu, I.; Farsang, A. Plastic contamination in agricultural soils: A review. Environ. Sci. Eur. 2023, 35, 13. [Google Scholar] [CrossRef]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Song, W.; Han, F.; Bao, Z.; Chai, Y.; Wang, L.; Huang, C.; Cheng, H.; Chang, L. Mulching practices improve soil moisture and enzyme activity in drylands, increasing potato yield. Agronomy 2024, 14, 1077. [Google Scholar] [CrossRef]
- Yang, S.J.; Lee, B.T.; Kim, S.O.; Park, S. Review of microplastics in soils: State-of-the-art occurrence, transport, and investigation methods. J. Soils Sediments 2024, 24, 779–792. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, T.; Huang, F.; Zeng, Y.; Cui, Y.; Chen, J.; White, J.C.; Fang, L. Micro/nanoplastics pollution poses a potential threat to soil health. Glob. Chang. Biol. 2024, 30, e17470. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-H.; Chang, S.; Hong, S.H.; Shim, W.J. Microplastics as a vector of hydrophobic contaminants: Importance of hydrophobic additives. Integr. Environ. Assess. Manag. 2017, 13, 494–499. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Kim, S.; Sarkar, B.; Oleszczuk, P.; Sang, M.K.; Haque, M.N.; Ok, Y.S. Effects of microplastics on the terrestrial environment: A critical review. Environ. Res. 2022, 209, 112734. [Google Scholar] [CrossRef]
- Mikavica, I.; Ranđelović, D.; Ilić, M.; Obradović, M.; Stojanović, J.; Mutic, J. Distribution of microplastics in (sub)urban soils of Serbia and Cd, As, and Pb uptake by Capsella bursa-pastoris (L.) Medik. Chemosphere 2024, 363, 142891. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ingraffia, R.; de Souza Machado, A.A. Microplastic Incorporation into Soil in Agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef]
- Tiwari, E.; Sistla, S. Agricultural plastic pollution reduces soil function even under best management practices. PNAS Nexus 2024, 3, 433. [Google Scholar] [CrossRef]
- Grujíc, T.; Saljnikov, E.; Mutavdžíc, D.; Jovkovíc, M.; Stefanovíc, S.; Miladinovíc, V.; Krnjajíc, S.; Belanovíc Simíc, S.; Marjanovíc, Ž. Impact of Microplastics on Forest Soil Properties in Pollution Hotspots in Alluvial Plains of Large Rivers (Morava, Sava, and Danube) of Serbia. Forests 2025, 16, 363. [Google Scholar] [CrossRef]
- SRPS ISO 10694:2005; Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). Institute for Standardisation of Republic of Serbia: Belgrade, Serbia, 2005.
- SRPS ISO 13878:2005; Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (Elemental Analysis). Institute for Standardization: Belgrade, Serbia, 2005.
- SRPS ISO 10390:2022; Soil, Treated Biowaste and Sludge—Determination of pH. SRPS, Institute for Standardisation of Republic of Serbia: Belgrade, Serbia, 2022.
- Burt, R. Kellogg Soil Survey Laboratory Methods Manual; United States Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2014. [Google Scholar]
- SRPS ISO 10693:2005; Soil Quality—Determination of Carbonate Content, Volumetric Method. SRPS, Institute for Standardisation of Republic of Serbia: Belgrade, Serbia, 2005.
- Riehm, H. The ammonium lactate acetic acid method for the determination of easily soluble phosphoric acid in carbonate-containing soil. Agrochimica 1958, 3, 49–65. [Google Scholar]
- Dane, J.H.; Topp, G.C. Methods of Soil Analysis: Part 4 Physical Methods; SSSA Book Series No. 5; Soil Science Society of America: Madison, WI, USA, 2002; p. 1692. [Google Scholar]
- ISO-11272:1993; Soil Quality—Determination of Dry Bulk Density. International Organization for Standardization: Geneva, Switzerland, 1993.
- Folegatti, M.V.; Sano, E.E.; Nascimento, P.C. Sampling equipment for soil bulk density determination tested in a Kandiudalfic Eutrudox and a Typic Hapludox. Soil. Sci. Soc. Am. J. 2001, 65, 1723–1728. [Google Scholar]
- Bošnjak, Ð. Methods of Research and Determination of Physical Properties of Soil; Yugoslav Society for Soil Studies, Soil Physics Commission: Novi Sad, Serbia, 1997; pp. 17–20, 59–60. [Google Scholar]
- Horwath, W.R.; Paul, E.A. Microbiological and Biochemical Properties. In Methods of Soil Analysis; Part II; SSSA; Soil Science Society of America: Madison, WI, USA, 1996; Chapter 38; pp. 836–859. [Google Scholar]
- Mrvić, V.V.; Saljnikov, E.; Sikirić, B.; Jaramaz, D. Concentration, Background Values and Limits of Potential Toxic Elements in Soils of Central Serbia. In Advances in Understanding Soil Degradation; Saljnikov, E., Mueller, L., Lavrishchev, A., Eulenstein, F., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Iqbal, B.; Zhao, T.; Yin, W.; Zhao, X.; Xie, Q.; Khan, K.Y.; Du, D. Impacts of soil microplastics on crops: A review. Appl. Soil Ecol. 2023, 181, 104680. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic shape, polymer type, and concentration affect soil properties and plant biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef]
- Indurthi, S.; Sarma, I.; Vara Vinod, D. Horticultural Innovations Elevating Crop Yields and Agricultural Sustainability for a Flourishing Future. Plant Cell Biotech. Mol. Biol. 2024, 25, 22–44. [Google Scholar] [CrossRef]
- Bouaicha, O.; Mimmo, T.; Tiziani, R.; Praeg, N.; Polidori, C.; Lucini, L.; Borruso, L. Microplastics make their way into the soil and rhizosphere: A review of the ecological consequences. Rhizosphere 2022, 22, 100542. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef]
- Yadav, S.; Gupta, E.; Patel, A.; Srivastava, S.; Mishra, V.K.; Singh, P.S.; Barik, S.K. Unravelling the emerging threats of microplastics to agroecosystems. Rev. Environ. Sci. Bio. Technol. 2022, 21, 771–798. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, S.; Zhang, C.; Zhou, Y.; Qin, W. Soil microplastic characteristics and the effects on soil properties and biota: A systematic review and meta-analysis. Environ. Pollut. 2022, 313, 120183. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskiy, G.V.; Kust, G.S.; Sanaev, V.G. Soils in Biosphere and Life of Human; Monograph; Publishing house of the Moscow State Forest University: Moscow, Russia, 2012; p. 584. (In Russian) [Google Scholar]
- Karbozova-Saljnikov, E.; Funakawa, S.H.; Akhmetov, K.; Kosaki, T. Soil organic matter status of Chernozem soil in North Kazakhstan: Effects of summer fallow. Soil Biol. Biochem. 2004, 36, 1373–1381. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Rao, C.S.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil organic carbon dynamics: Impact of land use changes and management practices: A review. Adv. Agron. 2019, 156, 1–107. [Google Scholar]
- Saljnikov, E.; Lavrishchev, A.; Römbke, J.; Rinklebe, J.; Scherber, C.; Wilke, B.-M.; Tóth, T.; Blum, W.E.H.; Behrendt, U.; Eulenstein, F.; et al. Understanding and Monitoring Chemical and Biological Soil Degradation. In Advances in Understanding Soil Degradation; Saljnikov, E., Mueller, L., Lavrishchev, A., Eulenstein, F., Eds.; Innovations in Landscape Research; Springer: Cham, Switzerland, 2021; pp. 75–124. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Q.; Wang, T.; Qiu, G.; Kang, Z.; Zeng, Y.; Yang, X.; Song, N.; Han, X.; Yu, H. Responses of Bacterial Communities to Microplastics: At the Soil Aggregate Level. SSRN Electron. J. 2023. [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef]
- Ren, X.; Tang, J.; Liu, X.; Liu, Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 2020, 256, 113347. [Google Scholar] [CrossRef]
- Dong, H.; Liu, T.; Han, Z.; Sun, Q.; Li, R. Determining time limits of continuous film mulching and examining residual effects on cotton yield and soil properties. J. Environ. Biol. 2015, 36, 677. [Google Scholar]
- Chu, J.; Zhou, J.; Wang, Y.; Jones, D.L.; Ge, J.; Yang, Y.; Zeng, Z. Field application of biodegradable microplastics has no significant effect on plant and soil health in the short term. Environ. Pollut. 2023, 316, 120556. [Google Scholar] [CrossRef]
- Kim, S.W.; Jeong, S.-W.; An, Y.-J. Microplastics disrupt accurate soil organic carbon measurement based on chemical oxidation method. Chemosphere 2021, 276, 130178. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic disguising as soil carbon storage. Environ. Sci. Tech. 2018, 52, 6079–6080. [Google Scholar] [CrossRef] [PubMed]
- Čakmak, D.; Perović, V.; Kresović, M.; Pavlović, D.; Pavlović, M.; Mitrović, M.; Pavlović, P. Sources and a Health Risk Assessment of Potentially Toxic Elements in Dust at Children’s Playgrounds with Artificial Surfaces: A Case Study in Belgrade. Arch. Environ. Contam. Toxicol. 2020, 78, 190–205. [Google Scholar] [CrossRef]
- Li, F.; Yang, X.; Zhang, Z.; Jiang, Y.; Gong, Y. Behaviour, ecological impacts of microplastics and cadmium on soil systems: A systematic review. Environ. Techol. Innov. 2024, 35, 103637. [Google Scholar] [CrossRef]
- Klöckner, P.; Reemtsma, T.; Wagner, S. The diverse metal composition of plastic items and its implications. Sci. Total Environ. 2021, 764, 142870. [Google Scholar] [CrossRef]
- Ingraffia, R.; Amato, G.; Iovino, M.; Rillig, M.C.; Giambalvo, D.; Frenda, A.S. Polyester microplastic fibers in soil increase nitrogen loss via leaching and decrease plant biomass production and N uptake. Environ. Res. Lett. 2022, 17, 054012. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, W.; Duan, C.; Zhu, X.; Wu, H.; Zhang, X. Fang Microplastics pollution from different plastic mulching years accentuate soil microbial nutrient limitations. Gondwana Res. 2022, 108, 91–101. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Gordillo, H.; Waldmann, W.; Rillig, M.C. Photodegradation modifies microplastic effects on soil properties and plant performance. J. Appl. Ecol. 2023, 61, 13–24. [Google Scholar] [CrossRef]
- Parvez, M.; Ullah, H.; Faruk, O.; Simon, E.; Czédli, E. Role of Microplastics in Global Warming and Climate Change: A Review. Water Air Soil Pollut. 2024, 235, 201. [Google Scholar] [CrossRef]
- Pérez-Reverón, R.; Álvarez-Méndez, S.J.; Kropp, R.M.; Perdomo-González, A.; Hernández-Borges, J.; Díaz-Peña, F.J. Microplastics in agricultural systems:analytical methodologies and effects on soil quality and crop yield. Agriculture 2022, 12, 1162. [Google Scholar] [CrossRef]
- Rillig, M.C.; Kim, S.W.; Zhu, Y.G. The soil plastisphere. Nat. Rev. Microbiol. 2024, 22, 64–74. [Google Scholar] [CrossRef] [PubMed]


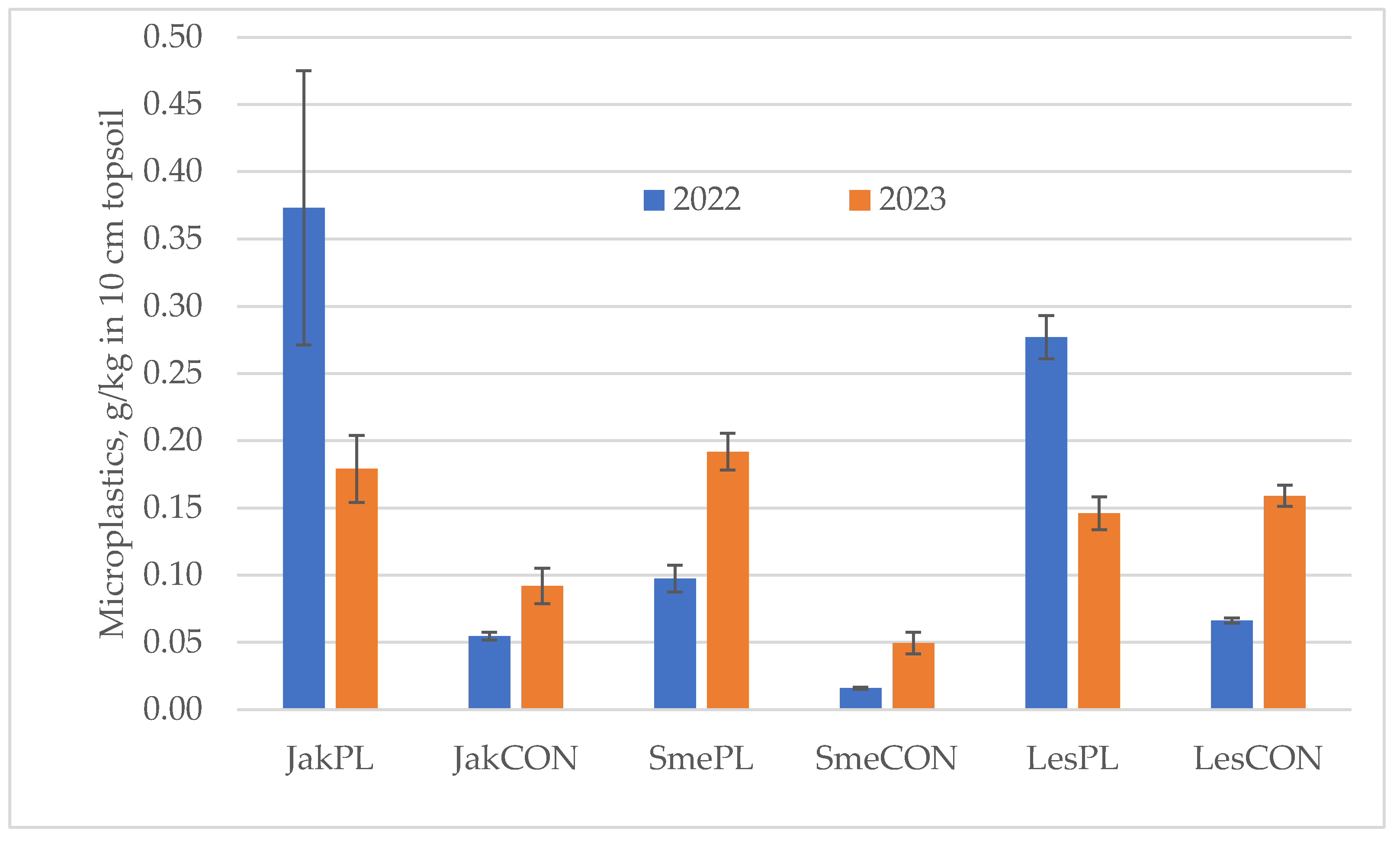

| Pairs | Paired Differences | t | df | Sig. (2-Tailed) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Std. Dev. | Std. Error Mean | 95% Confidence Interval of the Difference | |||||
| Lower | Upper | |||||||
| 2022 | ||||||||
| JakPL–JakCON | 0.21856 | 0.21410 | 0.01470 | 0.07712 | 0.35999 | 3.043 | 8 | 0.006 |
| SmePL–SmeCON | 0.08156 | 0.03503 | 0.01168 | 0.05463 | 0.10848 | 6.984 | 8 | 0.000 |
| LesPL–LesCON | 0.21078 | 0.04437 | 0.01479 | 0.17667 | 0.24488 | 14.25 | 8 | 0.000 |
| 2023 | ||||||||
| JakPL–JakCON | 0.08700 | 0.04590 | 0.01874 | 0.03884 | 0.13516 | 4.643 | 5 | 0.006 |
| SmePL–SmeCON | 0.14250 | 0.02469 | 0.01008 | 0.11659 | 0.16841 | 14.14 | 5 | 0.000 |
| LesPL–LesCON | 0.20700 | 0.03324 | 0.01357 | 0.17212 | 0.24188 | 15.25 | 5 | 0.000 |
| Site | EC | pH | N tot. | C tot. | P2O5 | K2O | CaCO3 | CEC | |
|---|---|---|---|---|---|---|---|---|---|
| µS/cm | KCl | H2O | % | mg/100 g | % | cmol/kg | |||
| 0–15 cm | |||||||||
| JakPL | 509b | 8.43a | 8.79a | 0.196a | 2.66a | 138a | 24.3a | 5.33a | 19.3a |
| JakCON | 295a | 7.68a | 8.22a | 0.364b | 4.05b | 255b | 177b | 4.44a | 29.5b |
| SmePL | 1091d | 7.45a | 7.76a | 0.368b | 3.18b | 177a | 15.1a | 0.44b | 29.3b |
| SmeCON | 775c | 6.88ba | 7.24a | 0.670c | 6.82c | 290b | 279c | 0.44b | 39.8b |
| LesPL | 547b | 7.20a | 7.48a | 0.131a | 1.07d | 94.5a | 47.5a | n.d. | 10.0c |
| LesCON | 343a | 7.71a | 8.04a | 0.250a | 2.32a | 271b | 102d | 0.89b | 17.8a |
| LSD | ** | * | * | ** | ** | * | * | ||
| SE | 56.92 | 0.116 | 0.121 | 0.037 | 0.376 | 15.246 | 17.859 | 0.432 | 2.036 |
| SD | 278.83 | 0.566 | 0.594 | 0.179 | 1.842 | 74.69 | 87.493 | 2.117 | 9.974 |
| Site | As | Cd | Co | Cr | Cu | Mn | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | |||||||||
| 0–15 cm | |||||||||
| JakPL | 8.12a | 0.40a | 11.7a | 48.4a | 20.4a | 531a | 69.7a | 13.9a | 73.0a |
| JakCON | 8.20a | 0.45a | 12.0a | 50.7a | 27.6a | 551a | 70.3a | 16.1a | 115b |
| SmePL | 14.3b | 0.82b | 16.5b | 80.6b | 28.3a | 580a | 111b | 71.0b | 149b |
| SmeCON | 14.8b | 0.94b | 17.0b | 80.5b | 49.0b | 607a | 112b | 86.6b | 257c |
| LesPL | 6.02c | 0.65c | 11.9a | 29.8c | 21.6a | 468a | 22.4c | 67.5b | 117b |
| LesCON | 5.69c | 0.64c | 11.2a | 28.2c | 25.4a | 457a | 22.7c | 59.3b | 135b |
| LSD | ** | *** | * | ** | ** | ** | *** | *** | |
| SE | 0.773 | 0.04 | 0.511 | 4.453 | 2.002 | 12.123 | 7.618 | 5.799 | 11.95 |
| SD | 3.789 | 0.196 | 2.505 | 21.814 | 9.81 | 59.39 | 37.323 | 28.41 | 58.541 |
| Site | Bulk Density | Specific Mass | Porosity | Particle Size Distribution, % | |||||
|---|---|---|---|---|---|---|---|---|---|
| >0.2 mm | 0.2–0.02 mm | 0.02–0.002 mm | <0.002 mm | >0.02 mm | <0.02 mm | ||||
| 0–15 cm | |||||||||
| JakPL | 1.449 | 2.702 | 46.36 | 6.5 | 54.6 | 15.4 | 23.5 | 61.1 | 38.9 |
| JakCON | 1.449 | 2.609 | 44.46 | 5.0 | 51.9 | 17.3 | 25.8 | 56.9 | 43.1 |
| SmePL | 1.480 | 2.648 | 44.12 | 8.3 | 38.8 | 26.1 | 26.8 | 47.1 | 52.9 |
| SmeCON | 1.480 | 2.573 | 42.48 | 4.5 | 28.0 | 37.2 | 30.3 | 32.5 | 67.5 |
| LesPL | n.d | 2.740 | n.d | 27.1 | 48.1 | 11.9 | 12.9 | 75.2 | 24.8 |
| LesCON | n.d | 2.699 | n.d | 27.4 | 45.7 | 13.3 | 13.6 | 73.1 | 26.9 |
| LSD | * | ** | ** | ||||||
| Site | Respiration, CO2, mg/kg | Cmin, mg/kg | Rate Constant, k | Rsqr | P | SEE |
|---|---|---|---|---|---|---|
| Jak PL | 403.33 | 1865.67 | 0.030 | 0.948 | <0.0001 | 113.25 |
| JakCON | 891.41 | 2012.59 | 0.021 | 0.981 | <0.0001 | 76.68 |
| SmePL | 400.01 | 1807.13 | 0.016 | 0.976 | <0.0001 | 70.51 |
| SmeCON | 366.90 | 2756.10 | 0.016 | 0.948 | <0.0001 | 158.39 |
| LesPL | 419.853 | 1707.98 | 0.008 | 0.966 | <0.0001 | 58.55 |
| LesCON | 969.61 | 1594.87 | 0.012 | 0.979 | <0.0001 | 52.84 |
| LSD | ** |
| MP22 | Sig. (1-Tailed) | MP23 | Sig. (1-Tailed) | |
|---|---|---|---|---|
| MP22 | 1 | |||
| MP23 | 0.681 | 0.068 | 1 | |
| Cmin | −0.425 | 0.2 | −0.794 * | 0.03 |
| porosity | 0.907 * | 0.047 | 0.702 | 0.149 |
| sp.mass | 0.738 * | 0.047 | 0.908 ** | 0.006 |
| totN | −0.726 | 0.051 | −0.853 * | 0.015 |
| totC | −0.625 | 0.092 | −0.919 ** | 0.005 |
| P2O5 | −0.865 * | 0.013 | −0.876 * | 0.011 |
| K2O | −0.856 * | 0.015 | −0.836 * | 0.019 |
| CEC | −0.686 | 0.066 | −0.860 * | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saljnikov, E.; Grujić, T.; Jovković, M.; Perović, V.; Čakmak, D.; Zhapparova, A.; Radović, V.; Stefanović, S.; Miladinović, V.; Stanković, S.; et al. Changes in Soil Properties Under the Influence of Microplastics in Plastic and Open Field Production in Three Serbian Valleys. Horticulturae 2025, 11, 305. https://doi.org/10.3390/horticulturae11030305
Saljnikov E, Grujić T, Jovković M, Perović V, Čakmak D, Zhapparova A, Radović V, Stefanović S, Miladinović V, Stanković S, et al. Changes in Soil Properties Under the Influence of Microplastics in Plastic and Open Field Production in Three Serbian Valleys. Horticulturae. 2025; 11(3):305. https://doi.org/10.3390/horticulturae11030305
Chicago/Turabian StyleSaljnikov, Elmira, Tara Grujić, Marina Jovković, Veljko Perović, Dragan Čakmak, Aigul Zhapparova, Vesela Radović, Slobodan Stefanović, Vladimir Miladinović, Slađan Stanković, and et al. 2025. "Changes in Soil Properties Under the Influence of Microplastics in Plastic and Open Field Production in Three Serbian Valleys" Horticulturae 11, no. 3: 305. https://doi.org/10.3390/horticulturae11030305
APA StyleSaljnikov, E., Grujić, T., Jovković, M., Perović, V., Čakmak, D., Zhapparova, A., Radović, V., Stefanović, S., Miladinović, V., Stanković, S., Marjanović, Ž., Kenzhegulova, S., Tleppayeva, A., Kunypiyaeva, G., & Krnjajić, S. (2025). Changes in Soil Properties Under the Influence of Microplastics in Plastic and Open Field Production in Three Serbian Valleys. Horticulturae, 11(3), 305. https://doi.org/10.3390/horticulturae11030305







