A Biostimulant from Kappaphycus alvarezii Enhances the Growth and Development of Basil (Ocimum basilicum L.) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Biostimulant
2.3. Experiment in Hydroponics
Basil Cultivation
2.4. Morphological Analyses
2.5. Biochemical Analyses
2.5.1. Chlorophyll (Chl) and Total Carotenoid (TCN)
2.5.2. Total Soluble Sugars (TSS)
2.5.3. Total Starch (TS)
2.5.4. Total Carbohydrate (TC)
2.5.5. Total Phenolic Content (TPC)
2.5.6. Total Flavonoid Content (TFC)
2.5.7. DPPH Antioxidant Activity
2.5.8. Total Soluble Protein (TP)
2.5.9. Total Amino Acids (TAAs)
2.6. Profile of Biogenic Amines and Amino Acids
2.7. Essential Oil Extraction
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphological Parameters
3.2. Biochemical Parameters
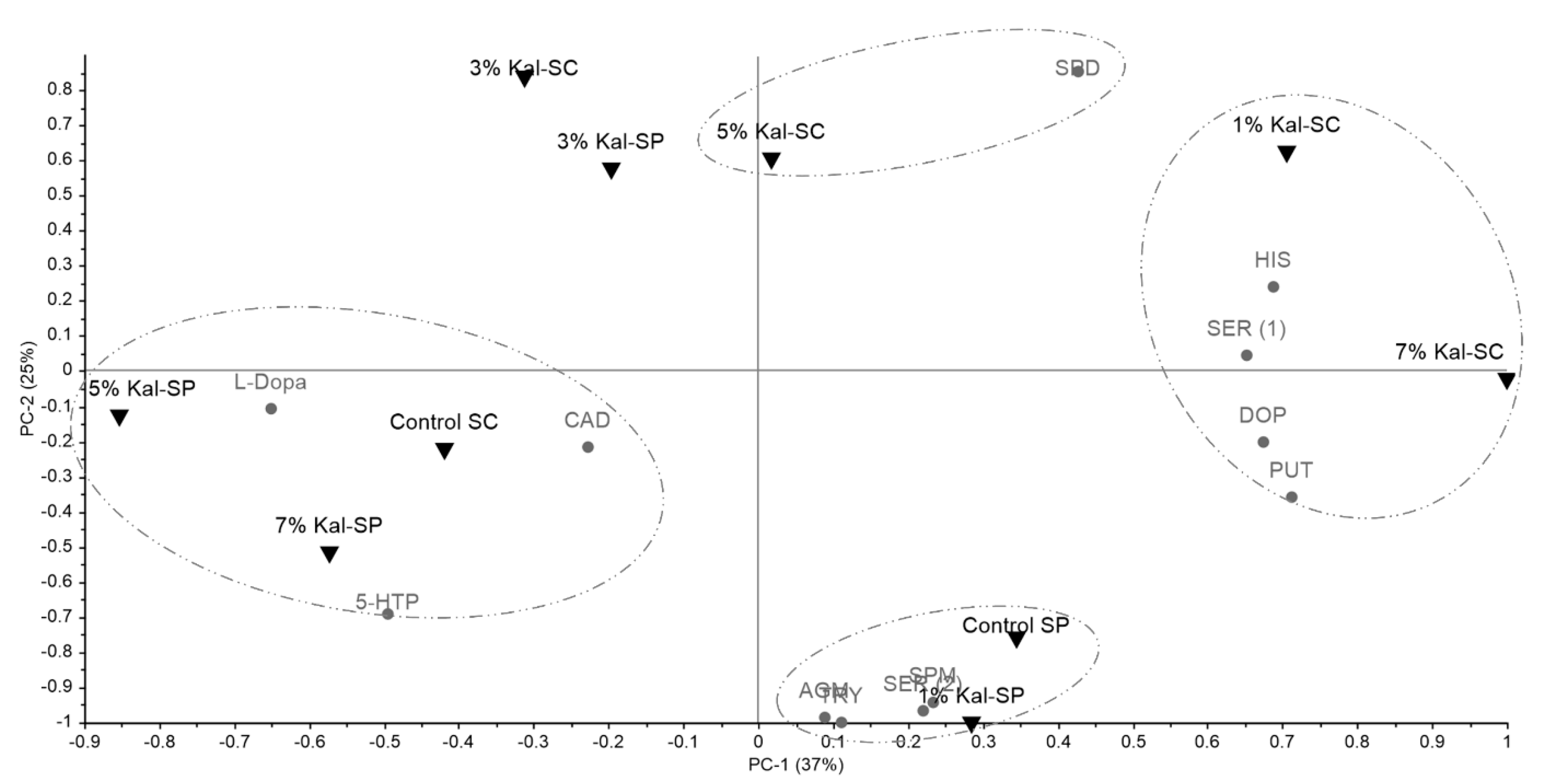
3.3. Biogenic Amines and Precursor Amino Acids
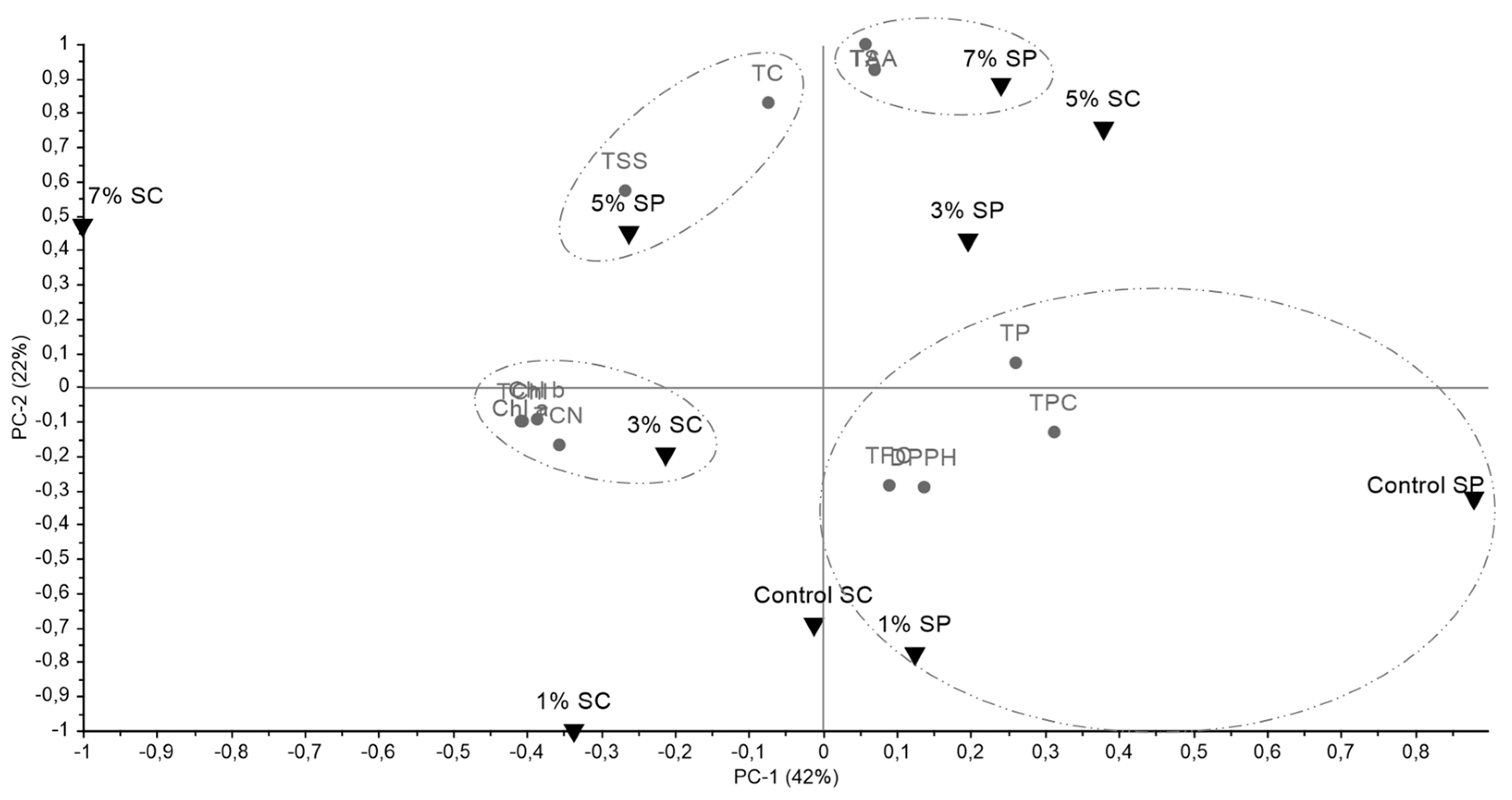
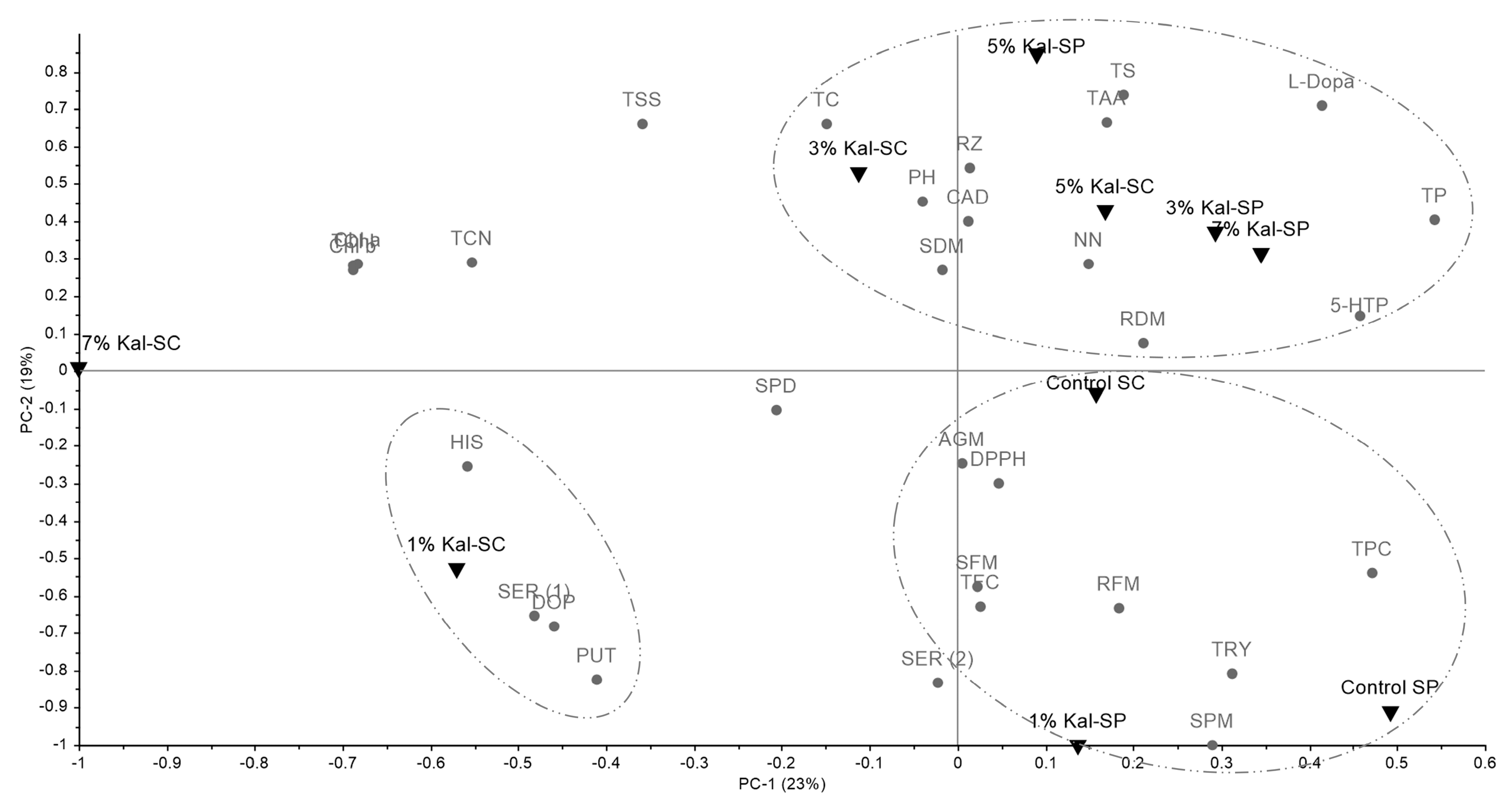
3.4. Complete Analysis of All Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamiołkowska, A. Natural Compounds as Elicitors of Plant Resistance against Diseases and New Biocontrol Strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The Future of Food Security and Food Safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Illera-Vives, M.; Seoane Labandeira, S.; Fernández-Labrada, M.; López-Mosquera, M.E. Agricultural Uses of Seaweed. In Sustainable Seaweed Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 591–612. [Google Scholar] [CrossRef]
- Munaro, D.; Nunes, A.; Schmitz, C.; Bauer, C.; Coelho, D.S.; Oliveira, E.R.; Yunes, R.A.; Moura, S.; Maraschin, M. Metabolites produced by macro- and microalgae as plant biostimulants. Stud. Nat. Prod. Chem. 2021, 71, 87–120. [Google Scholar]
- Rudke, A.R.; de Andrade, C.J.; Ferreira, S.R.S. Kappaphycus alvarezii Macroalgae: An Unexplored and Valuable Biomass for Green Biorefinery Conversion. Trends Food Sci. Technol. 2020, 103, 214–224. [Google Scholar] [CrossRef]
- Shukla, P.S.; Nivetha, N.; Nori, S.S.; Bose, D.; Kumar, S.; Khandelwal, S.; Critchley, A.; Suryanarayan, S. Understanding the mode of action of AgroGain®, a biostimulant derived from the red seaweed Kappaphycus alvarezii in the stimulation of cotyledon expansion and growth of Cucumis sativa (cucumber). Front. Plant Sci. 2023, 14, 1136563. [Google Scholar] [CrossRef] [PubMed]
- van Tol de Castro, T.A.; Tavares, O.C.H.; de Oliveira Torchia, D.F.; Pereira, E.G.; Rodrigues, N.F.; Santos, L.A.; do Amaral Vendramini, A.L.; da Mata, M.R., Jr.; Viêgas, C.V.; Berbara, R.L.L.; et al. Regulation of Growth and Stress Metabolism in Rice Plants through Foliar and Root Application of Seaweed Extract from Kappaphycus alvarezii (Rhodophyta). J. Appl. Phycol. 2024, 36, 2295–2310. [Google Scholar] [CrossRef]
- Nivetha, N.; Shukla, P.S.; Nori, S.S.; Kumar, S.; Suryanarayan, S. A red seaweed Kappaphycus alvarezii-based biostimulant (AgroGain®) improves the growth of Zea mays and impacts agricultural sustainability by beneficially priming rhizosphere soil microbial community. Front. Microbiol. 2024, 15, 1330237. [Google Scholar] [CrossRef]
- Shukla, P.S.; Nivetha, N.; Nori, S.S.; Kumar, S.; Critchley, A.T.; Suryanarayan, S. A biostimulant prepared from red seaweed Kappaphycus alvarezii induces flowering and improves the growth of Pisum sativum grown under optimum and nitrogen-limited conditions. Front. Plant Sci. 2024, 14, 1265432. [Google Scholar] [CrossRef]
- Nunes, A.; Azevedo, G.Z.; de Souza Dutra, F.; dos Santos, B.R.; Schneider, A.R.; Oliveira, E.R.; Moura, S.; Vianello, F.; Maraschin, M.; Lima, G.P.P. Uses and Applications of the Red Seaweed Kappaphycus alvarezii: A Systematic Review. J. Appl. Phycol. 2024, 36, 3409–3450. [Google Scholar] [CrossRef]
- Markets and Markets. Hydroponics Market by Growing System (Aggregate Hydroponic Systems, Liquid Hydroponic Systems, Hybrid Hydroponic Systems), Equipment, Crop Type, and Region—Global Forecast to 2027. 2024. Available online: https://www.marketsandmarkets.com/Market-Reports/hydroponics-market-140124230.html (accessed on 3 November 2024).
- Song, J.; Yang, J.; Jeong, B.R. Characterization of physiology, photosynthesis, and nutrition based on induced deficiencies of macro- and micronutrients in basil (Ocimum basilicum L.). Agronomy 2024, 14, 208. [Google Scholar] [CrossRef]
- Meselmani, M.A.A. Nutrient solution for hydroponics. In Recent Research and Advances in Soilless Culture; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Umbreit, W.W.; Burris, R.H. Method for glucose and others sugars. In Manometric Techniques: A Manual Describing Methods Applicable to the Study of Tissue Metabolism, 4th ed.; Umbreit, W.W., Burris, R.H., Stauffer, J.P., Eds.; Burgess: Minneapolis, MN, USA, 1964. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of Propolis: Some Parameters and Procedures for Chemical Quality Control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Da Silveira, C.M.; Furlong, E.B. Caracterização de Compostos Nitrogenados Presentes Em Farelos Fermentados Em Estado Sólido. Food Sci. Technol. 2007, 27, 805–811. [Google Scholar] [CrossRef]
- Borges, C.V.; Belin, M.A.F.; Amorim, E.P.; Minatel, I.O.; Monteiro, G.C.; Gomez Gomez, H.A.; Monar, G.R.S.; Lima, G.P.P. Bioactive Amines Changes during the Ripening and Thermal Processes of Bananas and Plantains. Food Chem. 2019, 298, 125020. [Google Scholar] [CrossRef]
- Diamante, M.S.; Borges, C.V.; da Silva, M.B.; Minatel, I.O.; Corrêa, C.R.; Gomez Gomez, H.A.; Lima, G.P.P. Bioactive Amines Screening in Four Genotypes of Thermally Processed Cauliflower. Antioxidants 2019, 8, 311. [Google Scholar] [CrossRef]
- van Tol de Castro, T.A.; Tavares, O.C.H.; de Oliveira Torchia, D.F.; Oliveira da Silva, H.F.; de Moura, O.V.T.; Cantarino, R.E.; de Abreu Lopes, S.; Viêgas, C.V.; do Amaral Vendramini, A.L.; Santos, L.A.; et al. Organic Fragments of K-Carrageenan, Lipids and Peptides plus K-Rich Inorganic Fraction in Kappaphycus alvarezii Biomass Are Responsible for Growth Stimulus in Rice Plant When Applied Both Foliar and Root Pathway. Algal Res. 2023, 71, 103040. [Google Scholar] [CrossRef]
- Pramanick, B.; Brahmachari, K.; Mahapatra, B.S.; Ghosh, A.; Ghosh, D.; Kar, S. Growth, Yield and Quality Improvement of Potato Tubers through the Application of Seaweed Sap Derived from the Marine Alga Kappaphycus alvarezii. J. Appl. Phycol. 2017, 29, 3253–3260. [Google Scholar] [CrossRef]
- Singh, I.; Anand, K.G.V.; Solomon, S.; Shukla, S.K.; Rai, R.; Zodape, S.T.; Ghosh, A. Can We Not Mitigate Climate Change Using Seaweed Based Biostimulant: A Case Study with Sugarcane Cultivation in India. J. Clean. Prod. 2018, 204, 992–1003. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanaka, R. Chlorophyll Metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll Hormesis: Are Chlorophylls Major Components of Stress Biology in Higher Plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef]
- Winterhalter, P.; Rouseff, R. Carotenoid-derived aroma compounds: An introduction. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; pp. 1–17. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant Carotenoids: Recent Advances and Future Perspectives. Mol Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of Soluble Sugars in Reactive Oxygen Species Balance and Responses to Oxidative Stress in Plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a Determinant of Plant Fitness under Abiotic Stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Zodapea, S.T.; Mukherjee, S.; Reddy, M.P.; Chaudhary, D.R. Effect of Kappaphycus alvarezii (Doty) Doty ex Silva. extract on grain quality, yield and some yield components of wheat (Triticum aestivum L.). Int. J. Plant Prod. 2009, 3, 97–101. [Google Scholar]
- Shukla, P.S.; Prithiviraj, B. Ascophyllum nodosum Biostimulant Improves the Growth of Zea mays Grown Under Phosphorus Impoverished Conditions. Front. Plant Sci. 2021, 11, 601843. [Google Scholar] [CrossRef] [PubMed]
- Seğmen, E.; Özdamar Ünlü, H. Effects of Foliar Applications of Commercial Seaweed and Spirulina Platensis Extracts on Yield and Fruit Quality in Pepper (Capsicum annuum L.). Cogent Food Agric. 2023, 9, 2233733. [Google Scholar] [CrossRef]
- Ashour, M.; Hassan, S.M.; Elshobary, M.E.; Ammar, G.A.G.; Gaber, A.; Alsanie, W.F.; Mansour, A.T.; El-Shenody, R. Impact of Commercial Seaweed Liquid Extract (TAM®) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum). Plants 2021, 10, 1045. [Google Scholar] [CrossRef]
- Mannino, G.; Ricciardi, M.; Gatti, N.; Serio, G.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. Changes in the Phytochemical Profile and Antioxidant Properties of Prunus Persica Fruits after the Application of a Commercial Biostimulant Based on Seaweed and Yeast Extract. Int. J. Mol. Sci. 2022, 23, 15911. [Google Scholar] [CrossRef]
- Kulbat, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. In Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; pp. 517–532. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections between amino acid metabolisms in plants: Lysine as an example. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Chang, F.; Guo, C.; Sun, F.; Zhang, J.; Wang, Z.; Kong, J.; He, Q.; Sharmin, R.A.; Zhao, T. Genome-wide association studies for dynamic plant height and number of nodes on the main stem in summer sowing soybeans. Front. Plant Sci. 2018, 9, 1184. [Google Scholar] [CrossRef]
- Sulistyo, A.; Purwantoro; Sari, K.P. Correlation, path analysis and heritability estimation for agronomic traits contribute to yield on soybean. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012034. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Wang, C.; Yang, X.; Li, D.; Zhang, X.; Xu, C.; Zhang, Y.; Li, W.; Zhao, L. Identification of traits contributing to high and stable yields in different soybean varieties across three Chinese latitudes. Front. Plant Sci. 2020, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11. [Google Scholar] [CrossRef]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The Ever-Changing Roles of Serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef]
- Ishihara, A.; Hashimoto, Y.; Tanaka, C.; Dubouzet, J.G.; Nakao, T.; Matsuda, F.; Nishioka, T.; Miyagawa, H.; Wakasa, K. The Tryptophan Pathway Is Involved in the Defense Responses of Rice against Pathogenic Infection via Serotonin Production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Back, K.; Tan, D.-X.; Reiter, R.J. Melatonin Biosynthesis in Plants: Multiple Pathways Catalyze Tryptophan to Melatonin in the Cytoplasm or Chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Vitalini, S.; Dei Cas, M.; Rubino, F.M.; Vigentini, I.; Foschino, R.; Iriti, M.; Paroni, R. LC-MS/MS-Based Profiling of Tryptophan-Related Metabolites in Healthy Plant Foods. Molecules 2020, 25, 311. [Google Scholar] [CrossRef]
- He, H.; Lei, Y.; Yi, Z.; Raza, A.; Zeng, L.; Yan, L.; Xiaoyu, D.; Yong, C.; Xiling, Z. Study on the Mechanism of Exogenous Serotonin Improving Cold Tolerance of Rapeseed (Brassica napus L.) Seedlings. Plant Growth Regul. 2021, 94, 161–170. [Google Scholar] [CrossRef]
- Sadak, M.S.; Ramadan, A.A.E.-M. Impact of Melatonin and Tryptophan on Water Stress Tolerance in White Lupine (Lupinus termis L.). Physiol. Mol. Biol. Plants 2021, 27, 469–481. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, Y.; Wang, Y.; Zhang, L.; Li, L.; Looi, L.J.; Zhang, Z. The potential of melatonin and its crosstalk with other hormones in the fight against stress. Front. Plant Sci. 2024, 15. [Google Scholar] [CrossRef]




| Treatment | Plant Height | Length of the Roots | Number of Nodes | Root Fresh Mass | Root Dry Mass | Shoot Fresh Mass | Shoot Dry Mass |
|---|---|---|---|---|---|---|---|
| São Paulo | |||||||
| Control | 48.17 ± 3.62 b | 29.30 ± 4.11 b | 32.40 ± 4.01 a | 72.40 ± 7.30 a | 2.27 ± 0.70 a | 135.40 ± 16.0 a | 11.67 ± 2.31 a |
| 1% | 45.31 ± 1.12 b | 30.24 ± 6.69 b | 34.73 ± 1.12 a | 78.27 ± 8.62 a | 2.47 ± 0.64 a | 143.27 ± 14.6 a | 18.00 ± 6.00 a |
| 3% | 56.40 ± 2.43 a | 45.35 ± 7.50 a | 34.50 ± 2.43 a | 80.60 ± 6.43 a | 2.53 ± 0.99 a | 149.47 ± 11.7 a | 21.00 ± 8.66 a |
| 5% | 49.33 ± 1.28 b | 29.15 ± 0.62 b | 37.93 ± 1.28 a | 60.27 ± 11.8 a | 2.13 ± 0.52 a | 119.27 ± 6.50 a | 14.67 ± 4.51 a |
| 7% | 49.57 ± 5.75 b | 28.40 ± 7.22 b | 29.67 ± 5.75 a | 74.40 ± 4.66 a | 2.20 ± 0.41 a | 132.13 ± 7.51 a | 15.67 ± 6.51 a |
| Santa Catarina | |||||||
| Control | 50.53 ± 4.31 a | 32.33 ± 3.49 a | 23.40 ± 6.61 b | 73.20 ± 8.28 a | 2.33 ± 0.90 b | 130.13 ± 21.0 a | 15.33 ± 8.08 a |
| 1% | 48.57 ± 1.31 a | 29.49 ± 3.19 a | 28.93 ± 3.36 b | 74.67 ± 8.38 a | 2.20 ± 0.77 b | 131.87 ± 9.91 a | 10.67 ± 1.53 a |
| 3% | 46.87 ± 3.70 a | 30.27 ± 3.80 a | 45.67 ± 8.80 a | 71.13 ± 10.0 a | 2.60 ± 0.63 b | 123.87 ± 9.93 a | 10.33 ± 2.52 a |
| 5% | 47.83 ± 5.91 a | 32.87 ± 4.79 a | 51.53 ± 1.10 a | 67.47 ± 6.35 a | 3.20 ± 1.26 a | 132.20 ± 10.7 a | 13.00 ± 2.52 a |
| 7% | 51.67 ± 1.36 a | 30.70 ± 1.54 a | 34.53 ± 6.23 b | 67.60 ± 9.55 a | 2.53 ± 0.74 b | 138.93 ± 12.2 a | 14.67 ± 5.69 a |
| Treatment | Chlorophyll-a | Chlorophyll-b | Total Chlorophyll | Total Carotenoid |
|---|---|---|---|---|
| São Paulo | ||||
| Control | 0.91 ± 0.07 b | 0.34 ± 0.04 b | 1.25 ± 0.10 b | 0.1480 ± 0.01 c |
| 1% | 0.96 ± 0.05 b | 0.34 ± 0.01 b | 1.30 ± 0.06 b | 0.1646 ± 0.01 b |
| 3% | 0.95 ± 0.05 b | 0.35 ± 0.02 b | 1.30 ± 0.07 b | 0.1581 ± 0.01 c |
| 5% | 1.03 ± 0.06 a | 0.36 ± 0.03 a | 1.39 ± 0.09 a | 0.1787 ± 0.02 a |
| 7% | 0.96 ± 0.05 b | 0.35 ± 0.02 b | 1.32 ± 0.07 b | 0.1581 ± 0.01 c |
| Santa Catarina | ||||
| Control | 1.01 ± 0.07 b | 0.37 ± 0.02 a | 1.38 ± 0.08 b | 0.1537 ± 0.02 b |
| 1% | 1.05 ± 0.08 a | 0.38 ± 0.04 a | 1.42 ± 0.11 a | 0.1742 ± 0.01 a |
| 3% | 1.01 ± 0.13 b | 0.37 ± 0.05 a | 1.38 ± 0.19 b | 0.1701 ± 0.03 a |
| 5% | 0.94 ± 0.04 b | 0.35 ± 0.03 a | 1.29 ± 0.07 b | 0.1593 ± 0.00 b |
| 7% | 1.10 ± 0.09 a | 0.40 ± 0.03 a | 1.50 ± 0.12 a | 0.1748 ± 0.01 a |
| Treatment | Total Soluble Sugars | Total Starch | Total Carbohydrate |
|---|---|---|---|
| São Paulo | |||
| Control | 10.28 ± 1.17 c | 5.64 ± 0.95 c | 36.66 ± 3.95 b |
| 1% | 20.92 ± 1.93 b | 2.28 ± 0.25 c | 28.16 ± 6.56 c |
| 3% | 18.46 ± 1.76 b | 6.69 ± 4.77 b | 46.72 ± 6.49 a |
| 5% | 29.39 ± 14.22 a | 6.30 ± 2.40 b | 48.39 ± 2.43 a |
| 7% | 22.14 ± 8.54 b | 9.28 ± 3.82 a | 38.19 ± 3.06 b |
| Santa Catarina | |||
| Control | 14.01 ± 0.38 b | 3.08 ± 0.61 b | 34.49 ± 7.29 c |
| 1% | 19.61 ± 4.35 b | 2.49 ± 0.63 b | 31.56 ± 5.74 c |
| 3% | 18.09 ± 5.63 b | 5.05 ± 1.73 a | 32.16 ± 0.14 c |
| 5% | 22.85 ± 1.61 a | 6.12 ± 5.93 a | 63.79 ± 7.39 a |
| 7% | 27.33 ± 8.99 a | 6.15 ± 1.18 a | 57.07 ± 8.18 b |
| Treatment | Total Phenolic | Total Flavonoid | DPPH |
|---|---|---|---|
| São Paulo | |||
| Control | 13.41 ± 3.69 a | 0.78 ± 0.06 a | 42.84 ± 10.04 a |
| 1% | 11.10 ± 0.55 b | 0.73 ± 0.20 a | 31.93 ± 8.44 b |
| 3% | 8.19 ± 1.47 c | 0.45 ± 0.12 b | 27.05 ± 4.68 b |
| 5% | 10.97 ± 2.06 b | 0.69 ± 0.11 a | 36.21 ± 11.12 a |
| 7% | 9.67 ± 3.04 c | 0.77 ± 0.17 a | 33.20 ± 7.38 b |
| Santa Catarina | |||
| Control | 8.12 ± 2.59 b | 0.65 ± 0.06 a | 38.98 ± 13.49 a |
| 1% | 9.48 ± 3.32 a | 0.78 ± 0.09 a | 44.25 ± 13.62 a |
| 3% | 7.04 ± 1.29 b | 0.59 ± 0.06 a | 33.12 ± 6.35 b |
| 5% | 10.50 ± 2.95 a | 0.68 ± 0.10 a | 45.34 ± 7.73 a |
| 7% | 6.30 ± 1.86 b | 0.64 ± 0.26 a | 31.36 ± 4.93 b |
| Treatment | Total Protein | Total Amino Acid |
|---|---|---|
| São Paulo | ||
| Control | 0.55 ± 0.11 a | 0.87 ± 0.15 c |
| 1% | 0.39 ± 0.11 b | 0.35 ± 0.04 e |
| 3% | 0.52 ± 0.08 a | 0.59 ± 0.18 d |
| 5% | 0.52 ± 0.14 a | 1.13 ± 0.28 b |
| 7% | 0.49 ± 0.11 a | 1.79 ± 0.27 a |
| Santa Catarina | ||
| Control | 0.55 ± 0.13 a | 0.47 ± 0.09 d |
| 1% | 0.46 ± 0.06 a | 0.38 ± 0.10 d |
| 3% | 0.51 ± 0.08 a | 0.78 ± 0.27 c |
| 5% | 0.51 ± 0.10 a | 1.41 ± 0.65 a |
| 7% | 0.36 ± 0.09 b | 0.95 ± 0.18 b |
| Treatment | Control SP | 1% Kal-SP | 3% Kal-SP | 5% Kal-SP | 7% Kal-SP | Control SC | 1% Kal-SC | 3% Kal-SC | 5% Kal-SC | 7% Kal-SC |
|---|---|---|---|---|---|---|---|---|---|---|
| 5-HTP | 627.33 ± 55.33 a | 575.17 ± 54.88 a | 561.16 ± 57.67 a | 669.53 ± 44.89 a | 583.05 ± 17.92 a | 680.80 ± 56.51 a | 482.66 ± 12.72 b | 522.21 ± 73.74 b | 571.23 ± 1.53 b | 526.14 ± 26.78 b |
| TRY | 433.04 ± 6.99 a | 433.91 ± 22.98 a | 386.97 ± 15.88 b | 396.64 ± 5.20 b | 398.71 ± 15.87 b | 414.90 ± 16.94 a | 395.30 ± 10.29 a | 336.09 ± 12.64 b | 406.48 ± 3.61 a | 382.65 ± 21.84 a |
| SER (s1) | 27.34 ± 0.95 a | 23.61 ± 1.12 a | 23.59 ± 1.17 a | 22.33 ± 0.38 a | 20.76 ± 5.79 a | 20.77 ± 0.69 b | 34.42 ± 2.73 a | 19.47 ± 1.19 b | 20.93 ± 1.51 b | 30.22 ± 5.10 a |
| AGM | 13.02 ± 0.03 a | 14.93 ± 0.57 a | 9.46 ± 1.09 c | 11.47 ± 0.29 b | 14.78 ± 1.86 a | 10.24 ± 0.49 b | 6.58 ± 0.82 c | 10.12 ± 1.62 b | 10.03 ± 0.02 b | 15.67 ± 0.32 a |
| PUT | 6.21 ± 0.02 a | 5.70 ± 0.54 a | 5.26 ± 0.03 b | 3.84 ± 0.31 c | 5.01 ± 0.21 b | 4.77 ± 0.03 c | 6.15 ± 0.15 b | 4.24 ± 0.07 c | 4.33 ± 0.07 c | 7.30 ± 1.10 a |
| CAD | 0.13 ± 0.00 c | 0.15 ± 0.02 c | 0.19 ± 0.01 c | 0.29 ± 0.10 b | 0.39 ± 0.01 a | 0.16 ± 0.01 b | 0.24 ± 0.01 a | 0.08 ± 0.01 c | 0.19 ± 0.02 b | 0.21 ± 0.05 b |
| HIS | 1.90 ± 0.04 a | 1.65 ± 0.49 a | 1.40 ± 0.31 a | 0.78 ± 0.06 b | 1.17 ± 0.09 b | 0.57 ± 0.04 e | 2.40 ± 0.02 c | 1.47 ± 0.07 d | 2.61 ± 0.01 b | 4.18 ± 0.08 a |
| SER (s2) | 5.09 ± 0.10 c | 7.88 ± 0.53 a | 4.18 ± 0.00 d | 3.91 ± 0.15 d | 6.45 ± 0.21 b | 5.01 ± 0.22 a | 5.20 ± 0.19 a | 4.30 ± 0.32 b | 3.41 ± 0.00 c | 5.46 ± 0.49 a |
| SPD | 0.10 ± 0.03 a | 0.11 ± 0.04 a | 0.20 ± 0.12 a | 0.03 ± 0.00 a | 0.04 ± 0.01 a | 0.07 ± 0.00 b | 0.20 ± 0.06 a | 0.12 ± 0.00 b | 0.23 ± 0.04 a | 0.14 ± 0.02 b |
| L-Dopa | 0.004 ± 0.00 c | 0.004 ± 0.00 c | 0.008 ± 0.00 b | 0.014 ± 0.00 a | 0.013 ± 0.00 a | 0.004 ± 0.00 a | 0.004 ± 0.00 a | 0.004 ± 0.00 a | 0.006 ± 0.00 a | 0.000 ± 0.00 a |
| DOP | 0.67 ± 0.08 a | 0.63 ± 0.01 a | 0.44 ± 0.01 b | 0.53 ± 0.00 b | 0.47 ± 0.06 b | 0.58 ± 0.03 c | 0.75 ± 0.00 a | 0.43 ± 0.03 d | 0.66 ± 0.01 b | 0.80 ± 0.09 a |
| SPM | 19.78 ± 3.37 a | 15.71 ± 2.61 b | 0.08 ± 0.01 c | 0.11 ± 0.00 c | 0.10 ± 0.02 c | 0.10 ± 0.00 c | 0.17 ± 0.00 b | 0.13 ± 0.00 c | 0.22 ± 0.02 a | 0.25 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.; Dutra, F.d.S.; Santos, L.R.B.; Martins, C.P.; Schneider, A.R.; Oliveira, E.R.; Pereira-Vasques, M.S.; Azevedo, G.Z.; Oliveira, J.L.B.; Gelli, V.C.; et al. A Biostimulant from Kappaphycus alvarezii Enhances the Growth and Development of Basil (Ocimum basilicum L.) Plants. Horticulturae 2025, 11, 424. https://doi.org/10.3390/horticulturae11040424
Nunes A, Dutra FdS, Santos LRB, Martins CP, Schneider AR, Oliveira ER, Pereira-Vasques MS, Azevedo GZ, Oliveira JLB, Gelli VC, et al. A Biostimulant from Kappaphycus alvarezii Enhances the Growth and Development of Basil (Ocimum basilicum L.) Plants. Horticulturae. 2025; 11(4):424. https://doi.org/10.3390/horticulturae11040424
Chicago/Turabian StyleNunes, Aline, Felipe de Souza Dutra, Lohan Rodrigues Brandão Santos, Camila Pimentel Martins, Alex Ricardo Schneider, Eva Regina Oliveira, Milene Stefani Pereira-Vasques, Gadiel Zilto Azevedo, Jorge Luiz Barcelos Oliveira, Valéria Cress Gelli, and et al. 2025. "A Biostimulant from Kappaphycus alvarezii Enhances the Growth and Development of Basil (Ocimum basilicum L.) Plants" Horticulturae 11, no. 4: 424. https://doi.org/10.3390/horticulturae11040424
APA StyleNunes, A., Dutra, F. d. S., Santos, L. R. B., Martins, C. P., Schneider, A. R., Oliveira, E. R., Pereira-Vasques, M. S., Azevedo, G. Z., Oliveira, J. L. B., Gelli, V. C., Santos, A. A. d., Maraschin, M., & Lima, G. P. P. (2025). A Biostimulant from Kappaphycus alvarezii Enhances the Growth and Development of Basil (Ocimum basilicum L.) Plants. Horticulturae, 11(4), 424. https://doi.org/10.3390/horticulturae11040424





