Exogenous Melatonin Protects Lime Plants from Drought Stress-Induced Damage by Maintaining Cell Membrane Structure, Detoxifying ROS and Regulating Antioxidant Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Design and Treatments
2.2. Estimation of Proline Content
2.3. Measurement of Electrolyte Leakage (EL)
2.4. Measurement of Lipid Peroxidation and Hydrogen Peroxide (H2O2) Concentration in Leaves
2.5. Antioxidant Enzymes Activity
2.6. RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
3.1. Proline Content
3.2. Electrolyte Leakage
3.3. Hydrogen Peroxide and Malondialdehyde Contents in Leaf Extracts
3.4. Changes in the Activities of Antioxidant Enzymes
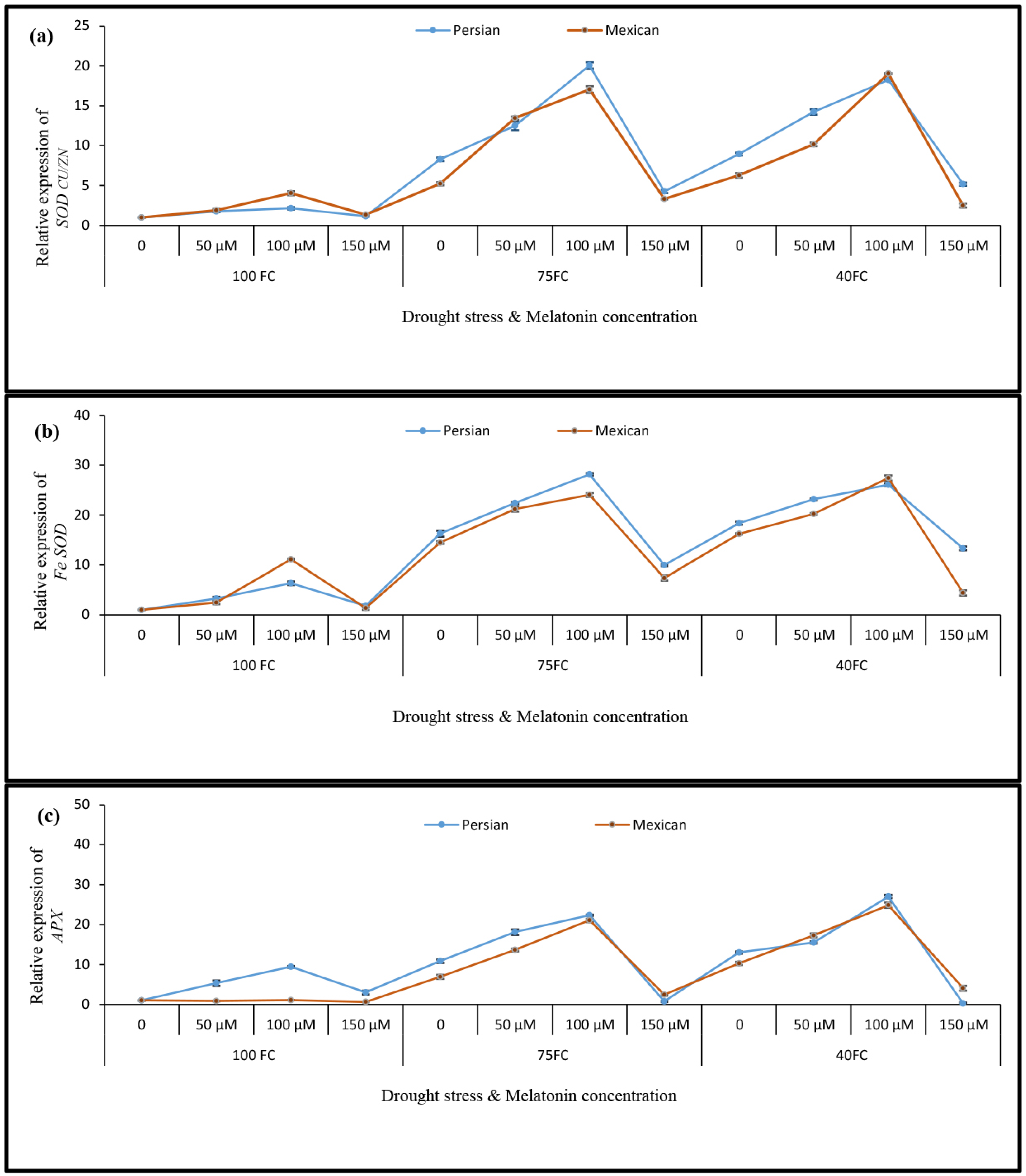
3.5. Relative Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jafari, M.; Shahsavar, A. The effect of foliar application of melatonin on changes in secondary metabolite contents in two Citrus species under drought stress conditions. Front. Plant Sci. 2021, 12, 692735. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh Khankahdani, H.; Rastegar, S.; Golein, B.; Golmohammadi, M.; Aboutalebi Jahromi, A. Relationship among vegetative growth and nutrient elements in the scion of different Persian lime accessions and its effect on WBDL phytoplasma. J. Plant Dis. Prot. 2022, 129, 145–154. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A. The application of artificial neural networks in modeling and predicting the effects of melatonin on morphological responses of citrus to drought stress. PLoS ONE 2020, 15, e0240427. [Google Scholar] [CrossRef] [PubMed]
- Yoosefzadeh Najafabadi, M.; Soltani, F.; Noory, H.; Díaz-Pérez, J.C. Growth, Yield and Enzyme Activity Response of Watermelon Accessions Exposed to Irrigation Water Deficit. Int. J. Veg. Sci. 2018, 24, 323–337. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A.R. Sodium nitroprusside: Its beneficial role in drought stress tolerance of “Mexican lime” (Citrus aurantifolia (Christ.) Swingle) under in vitro conditions. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 155–168. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Y.-L.; Wu, H.-T.; Shalmani, A.; Liu, W.-T.; Li, W.-Q.; Xu, J.-W.; Chen, K.-M. OsRbohB-mediated ROS production plays a crucial role in drought stress tolerance of rice. Plant Cell Rep. 2020, 39, 1767–1784. [Google Scholar] [CrossRef] [PubMed]
- Jothimani, K.; Arulbalachandran, D. Physiological and biochemical studies of black gram (Vigna mungo (L.) Hepper) under polyethylene glycol induced drought stress. Biocatal. Agric. Biotechnol. 2020, 29, 101777. [Google Scholar] [CrossRef]
- Kaczmarek, P.; Rapp, M.; Koroniak, H. Pyrrolidine and oxazolidine ring transformations in proline and serine derivatives of α-hydroxyphosphonates induced by deoxyfluorinating reagents. RSC Adv. 2018, 8, 24444–24457. [Google Scholar] [CrossRef] [Green Version]
- Hesami, M.; Tohidfar, M.; Alizadeh, M.; Daneshvar, M.H. Effects of sodium nitroprusside on callus browning of Ficus religiosa: An important medicinal plant. J. For. Res. 2020, 31, 789–796. [Google Scholar] [CrossRef]
- Weng, Y.; Ge, L.; Jia, S.; Mao, P.; Ma, X. Cyclophilin AtROC1S58F confers Arabidopsis cold tolerance by modulating jasmonic acid signaling and antioxidant metabolism. Plant Physiol. Biochem. 2020, 152, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jing, D.; Liu, F.; Ma, H.; Liu, X.; Peng, L. Serendipita indica alleviates drought stress responses in walnut (Juglans regia L.) seedlings by stimulating osmotic adjustment and antioxidant defense system. Appl. Microbiol. Biotechnol. 2021, 105, 8951–8968. [Google Scholar] [CrossRef] [PubMed]
- Reyhani Haghighi, S.; Hosseininaveh, V.; Maali-Amiri, R.; Talebi, K.; Irani, S. Improving the drought tolerance in pistachio (Pistacia vera) seedlings by foliar application of salicylic acid. Gesunde Pflanz. 2021, 73, 495–507. [Google Scholar] [CrossRef]
- Aazami, M.A.; Shamsinow, E.; Hasan Poor Aghdam, M.B. Evaluation some of the physiological and biochemical changes in three grapevine cultivars (Vitis vinifera L.) in response to drought stress. Appl. Biol. 2021, 34, 58–81. [Google Scholar] [CrossRef]
- Melaouhi, A.; Baraza, E.; Escalona, J.M.; El-AouOuad, H.; Mahjoub, I.; Bchir, A.; Braham, M.; Bota, J. Physiological and biochemical responses to water deficit and recovery of two olive cultivars (Olea europaea L., Arbequina and Empeltre cvs.) under Mediterranean conditions. Theor. Exp. Plant Physiol. 2021, 33, 369–383. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Song, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic acid and hydrogen peroxide are involved in drought priming-induced drought tolerance in wheat (Triticum aestivum L.). Plant Biol. 2020, 22, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-X.; Xu, N.-W.; Yang, M.; Li, X.-L.; Han, J.-L.; Lin, X.-H.; Yang, Q.; Lv, G.-H.; Wang, J. Responses of photosynthesis, antioxidant enzymes, and related gene expression to nicosulfuron stress in sweet maize (Zea mays L.). Environ. Sci. Pollut. Res. 2022, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Uddin, M.J.; Hossain, M.A.; Henry, R.; Begum, M.K.; Sohel, M.A.T.; Mou, M.A.; Ahn, J.; Cheong, E.J.; Lim, Y.-S. Exogenous putrescine attenuates the negative impact of drought stress by modulating physio-biochemical traits and gene expression in sugar beet (Beta vulgaris L.). PLoS ONE 2022, 17, e0262099. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.; Hu, Y.; Mukherjee, S.; Aftab, T.; Fahad, S.; Raza, A.; Ahmad, M.; Siddiqui, M.H. Silicon-induced postponement of leaf senescence is accompanied by modulation of antioxidative defense and ion homeostasis in mustard (Brassica juncea) seedlings exposed to salinity and drought stress. Plant Physiol. Biochem. 2020, 157, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, J.; Yan, K.; Zhou, Z.; Zhao, W.; Zhang, X.; Pu, Y.; Yu, R. Beneficial effects of abscisic acid and melatonin in overcoming drought stress in cotton (Gossypium hirsutum L.). Physiol. Plant. 2021, 173, 2041–2054. [Google Scholar] [CrossRef]
- Manbir; Singh, P.; Kumari, A.; Gupta, K.J. Alternative oxidase plays a role in minimizing ROS and RNS produced under salinity stress in Arabidopsis thaliana. Physiol. Plant. 2022, 174, e13649. [Google Scholar] [CrossRef]
- Zhanassova, K.; Kurmanbayeva, A.; Gadilgereyeva, B.; Yermukhambetova, R.; Iksat, N.; Amanbayeva, U.; Bekturova, A.; Tleukulova, Z.; Omarov, R.; Masalimov, Z. ROS status and antioxidant enzyme activities in response to combined temperature and drought stresses in barley. Acta Physiol. Plant. 2021, 43, 114. [Google Scholar] [CrossRef]
- Boy, R.; Indradewa, D.; Putra, E.T.S.; Kurniasih, B. Drought-induced production of reactive oxygen species and antioxidants activity of four local upland rice cultivars in Central Sulawesi, Indonesia. Biodivers. J. Biol. Divers. 2020, 21, 2555–2565. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Pan, Y.; Yun, Z.; Zhang, Z.; Jiang, G.; Jiang, Y. Endogenous melatonin generation plays a positive role in chilling tolerance in relation to redox homeostasis in litchi fruit during refrigeration. Postharvest Biol. Technol. 2021, 178, 111554. [Google Scholar] [CrossRef]
- Liu, L.; Li, D.; Ma, Y.; Shen, H.; Zhao, S.; Wang, Y. Combined application of arbuscular mycorrhizal fungi and exogenous melatonin alleviates drought stress and improves plant growth in tobacco seedlings. J. Plant Growth Regul. 2021, 40, 1074–1087. [Google Scholar] [CrossRef]
- Azmat, A.; Yasmin, H.; Hassan, M.N.; Nosheen, A.; Naz, R.; Sajjad, M.; Ilyas, N.; Akhtar, M.N. Co-application of bio-fertilizer and salicylic acid improves growth, photosynthetic pigments and stress tolerance in wheat under drought stress. PeerJ 2020, 8, e9960. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Shazad, R.; Bilal, S.; Imran, Q.M.; Khan, M.; Kang, S.-M.; Khan, A.L.; Yun, B.-W.; Lee, I.-J. Exogenous Melatonin mediates the regulation of endogenous nitric oxide in Glycine max L. to reduce effects of drought stress. Environ. Exp. Bot. 2021, 188, 104511. [Google Scholar] [CrossRef]
- Ren, J.; Yang, X.; Ma, C.; Wang, Y.; Zhao, J. Melatonin enhances drought stress tolerance in maize through coordinated regulation of carbon and nitrogen assimilation. Plant Physiol. Biochem. 2021, 167, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.; Niu, J.; Irshad, A.; Kareem, H.A.; Hassan, M.U.; Xu, N.; Sui, X.; Guo, Z.; Amo, A.; Wang, Q. Exogenous melatonin protects alfalfa (Medicago sativa L.) seedlings from drought-induced damage by modulating reactive oxygen species metabolism, mineral balance and photosynthetic efficiency. Plant Stress 2021, 2, 100044. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Hu, L.; Zhang, J.; Yue, H.; Yang, S.; Liu, Y.; Gong, X.; Ma, F. Overexpression of MdASMT9, an N-acetylserotonin methyltransferase gene, increases melatonin biosynthesis and improves water-use efficiency in transgenic apple. Tree Physiol. 2021, 157, 1–13. [Google Scholar] [CrossRef]
- Zou, J.-N.; Yu, Q.; Jin, X.-J.; Wang, M.-Y.; Qin, B.; Ren, C.-Y.; Wang, M.-X.; Zhang, Y.-X. Effects of exogenous melatonin on physiology and yield of soybean during seed filling stage under drought stress. Acta Agron. Sin. 2020, 46, 745–758. [Google Scholar] [CrossRef]
- Cao, L.; Qin, B.; Zhang, Y.X. Exogenous application of melatonin may contribute to enhancement of soybean drought tolerance via its effects on glucose metabolism. Biotechnol. Biotechnol. Equip. 2021, 35, 964–976. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Mukherjee, S.; Flores, F.B.; Arnao, M.B.; Luo, Z.; Corpas, F.J. Functions of melatonin during postharvest of horticultural crops. Plant Cell Physiol. 2021, pcab175, 1–23. [Google Scholar] [CrossRef]
- Shafi, A.; Singh, A.K.; Zahoor, I. Melatonin: Role in abiotic stress resistance and tolerance. In Plant Growth Regulators; Aftab, T., KR, H., Eds.; Springer: Cham, Switzerland, 2021; pp. 239–273. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gulen, H.; Eris, A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004, 166, 739–744. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Hemeda, H.M.; Klein, B.P. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tavanti, T.R.; Melo, A.A.R.d.; Moreira, L.D.K.; Sanchez, D.E.J.; Silva, R.d.S.; Silva, R.M.d.; Reis, A.R.d. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Gholamin, R.; Khayatnezhad, M. Study of bread wheat genotype physiological and biochemical responses to drought stress. Helix 2020, 10, 87–92. [Google Scholar] [CrossRef]
- Hesami, M.; Daneshvar, M.H.; Yoosefzadeh-Najafabadi, M. An efficient in vitro shoot regeneration through direct organogenesis from seedling-derived petiole and leaf segments and acclimatization of Ficus religiosa. J. For. Res. 2019, 30, 807–815. [Google Scholar] [CrossRef]
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J.; et al. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 2017, 7, 41236. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadak, M.S.; Bakry, B.A. Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol. Mol. Biol. Plants 2020, 26, 907–919. [Google Scholar] [CrossRef]
- Madebo, M.P.; Luo, S.-m.; Wang, L.; Zheng, Y.-h.; Jin, P. Melatonin treatment induces chilling tolerance by regulating the contents of polyamine, γ-aminobutyric acid, and proline in cucumber fruit. J. Integr. Agric. 2021, 20, 3060–3074. [Google Scholar] [CrossRef]
- Silalert, P.; Pattanagul, W. Foliar application of melatonin alleviates the effects of drought stress in rice (Oryza sativa L.) seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12417. [Google Scholar] [CrossRef]
- Balci, G. Effects of melatonin applications on certain biochemical characteristics of strawberry seedlings in lime stress conditions. Turk. J. Agric. For. 2021, 45, 285–289. [Google Scholar] [CrossRef]
- Alyammahi, O.; Gururani, M.A. Chlorophyll-a fluorescence analysis reveals differential response of photosynthetic machinery in melatonin-treated oat plants exposed to osmotic stress. Agronomy 2020, 10, 1520. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [Green Version]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Chattopadhyay, A.; Bandyopadhyay, D. Melatonin and biological membrane bilayers: A never ending amity. Melatonin Res. 2021, 4, 232–252. [Google Scholar] [CrossRef]
- Malmir, M.; Naderi Noreini, S.; Ghafarizadeh, A.; Faraji, T.; Asali, Z. Ameliorative effect of melatonin on apoptosis, DNA fragmentation, membrane integrity and lipid peroxidation of spermatozoa in the idiopathic asthenoteratospermic men: In vitro. Andrologia 2021, 53, e13944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.M.; Tian, G.; Li, X.H.; Zhang, Z.Z.; Liu, J.; Li, Y.H.; Xie, J.F.; Wang, P.F. ROS Produced via BsRBOHD Plays an Important Role in Low Temperature-Induced Anthocyanin Biosynthesis in Begonia semperflorens. Russ. J. Plant Physiol. 2020, 67, 250–258. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin Inhibits Cadmium Translocation and Enhances Plant Tolerance by Regulating Sulfur Uptake and Assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Mao, Q.; Yan, Y.; Wu, M.; Wang, Y.; Ren, J.; Guo, P.; Liu, A.; Chen, S. Role of Melatonin in Arbuscular Mycorrhizal Fungi-Induced Resistance to Fusarium Wilt in Cucumber. Phytopathology 2020, 110, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.-F.; Xu, T.-F.; Wang, Z.-Z.; Fang, Y.-L.; Xi, Z.-M.; Zhang, Z.-W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, Y.; Rui, C.; Zhang, H.; Xu, N.; Dai, M.; Chen, X.; Lu, X.; Wang, D.; Wang, J.; et al. Melatonin Improves Cotton Salt Tolerance by Regulating ROS Scavenging System and Ca2+ Signal Transduction. Front. Plant Sci. 2021, 12, 693690. [Google Scholar] [CrossRef]
- Maleki, M.; Shojaeiyan, A.; Mokhtassi-Bidgoli, A. Genotypic variation in biochemical and physiological responses of fenugreek (Trigonella foenum-graecum L.) landraces to prolonged drought stress and subsequent rewatering. Sci. Hortic. 2021, 287, 110224. [Google Scholar] [CrossRef]
- Ahmad, S.; Su, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Javed, T.; Han, Q. Foliar application of melatonin delay leaf senescence in maize by improving the antioxidant defense system and enhancing photosynthetic capacity under semi-arid regions. Protoplasma 2020, 257, 1079–1092. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Fahadi Hoveizeh, N.; Gholami, R.; Abdelrahman, M.; Tran, L.-S.P. Exogenous melatonin mitigates salinity-induced damage in olive seedlings by modulating ion homeostasis, antioxidant defense, and phytohormone balance. Physiol. Plant. 2021, 173, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Altaf, M.M.; Khan, L.U.; Shahid, S.; Jahan, M.S. Melatonin alleviates salt damage in tomato seedling: A root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci. Hortic. 2021, 285, 110145. [Google Scholar] [CrossRef]
- Gantait, S.; Mukherjee, E. Hairy root culture technology: Applications, constraints and prospect. Appl. Microbiol. Biotechnol. 2021, 105, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Gao, T.; Liang, B.; Zhao, Q.; Ma, F.; Li, C. Effects of exogenous melatonin on methyl viologen-mediated oxidative stress in apple leaf. Int. J. Mol. Sci. 2018, 19, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; Ni, Z.; Hu, R.; Lin, L.; Deng, H.; Wang, J.; Tang, Y.; Sun, G.; Wang, X.; Li, H.; et al. Melatonin alleviates drought stress by a non-enzymatic and enzymatic antioxidative system in kiwifruit seedlings. Int. J. Mol. Sci. 2020, 21, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Omran, V.O.; Ghorbani, A.; Sajjadi-Otaghsara, S.A. Melatonin alleviates NaCl-induced damage by regulating ionic homeostasis, antioxidant system, redox homeostasis, and expression of steviol glycosides-related biosynthetic genes in in vitro cultured Stevia rebaudiana Bertoni. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 319–331. [Google Scholar] [CrossRef]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin Improves Drought Resistance in Maize Seedlings by Enhancing the Antioxidant System and Regulating Abscisic Acid Metabolism to Maintain Stomatal Opening under PEG-Induced Drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Effects of melatonin on antioxidant capacity in naked oat seedlings under drought stress. Molecules 2018, 23, 1580. [Google Scholar] [CrossRef] [Green Version]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Río, L.A.d.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- He, J.-D.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci. Hortic. 2020, 262, 108745. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, J.; Achary, V.M.M.; Reddy, M.K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 2015, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Panchuk, I.I.; Zentgraf, U.; Volkov, R.A. Expression of the Apx gene family during leaf senescence of Arabidopsis thaliana. Planta 2005, 222, 926–932. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, A.I.; Rafudeen, M.S.; Gomaa, A.M.; Hasanuzzaman, M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 173, 1369–1381. [Google Scholar] [CrossRef]
- Surender Reddy, P.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kavi Kishor, P.B. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskandar, H.M.; Widyaningrum, D.; Suhandono, S. Cloning and characterization of P5CS1 and P5CS2 genes from Saccharum officinarum L. under drought stress. J. Trop. Crop Sci. 2014, 1, 23–30. [Google Scholar] [CrossRef]
- De Carvalho, K.; de Campos, M.K.F.; Domingues, D.S.; Pereira, L.F.P.; Vieira, L.G.E. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 2013, 40, 3269–3279. [Google Scholar] [CrossRef] [PubMed]
- Funck, D.; Baumgarten, L.; Stift, M.; von Wirén, N.; Schönemann, L. Differential Contribution of P5CS Isoforms to Stress Tolerance in Arabidopsis. Front. Plant Sci. 2020, 11, 565134. [Google Scholar] [CrossRef]
- Ghaderi, A.; Jahanbakhsh Godehkahriz, S.; Raeisi Sadati, S.Y. Study of total protein content, soluble sugar, proline content and P5CS gene expression in leaves of three wheat cultivars under drought stress. Agric. Biotechnol. J. 2021, 12, 122–141. [Google Scholar] [CrossRef]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Metabolic and genes expression analyses involved in proline metabolism of two rose species under drought stress. Plant Physiol. Biochem. 2020, 155, 105–113. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Sheikh-Assadi, M.; Sharafi, Y.; Farmani, B.; Fard, J.R.; Razavi, F. Employing exogenous melatonin applying confers chilling tolerance in tomato fruits by upregulating ZAT2/6/12 giving rise to promoting endogenous polyamines, proline, and nitric oxide accumulation by triggering arginine pathway activity. Food Chem. 2019, 275, 549–556. [Google Scholar] [CrossRef]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Razavi, F.; Fard, J.R.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Wang, J.; Rajakulendran, N.; Amirsadeghi, S.; Vanlerberghe, G.C. Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiol. Plant. 2011, 142, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- Wang, J.; Vanlerberghe, G.C. A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiol. Plant. 2013, 149, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef] [PubMed]



| Row | Primer Name | Primer Sequence | Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| 1 | AOX_F | 5′- GCGTAAGTTCCAGCATAGTG -3′ | 20 | 60 |
| AOX_R | 5′- CCTCCAAGTAGCCAACAAC -3′ | 19 | ||
| 2 | Sod-cu/zn–F | 5′- TGATGACGGGAACTAACGGT -3′ | 19 | 60 |
| Sod-cu/zn–R | 5′- AGTGTGAATAATGAGTGCGTGA -3′ | 22 | ||
| 3 | Sod-fe_F | 5′- CGTAAGGAGCGGCGAGTA -3′ | 18 | 55 |
| Sod-fe_R | 5′- GTGGCTAATGCGGTGAAT -3′ | 18 | ||
| 4 | APX_F | 5′- AGCAGTTCCCTACCATCTCC -3′ | 20 | 58 |
| APX_R | 5′- TTCAGCCTTGTCATCTCTTCC -3′ | 21 | ||
| 5 | P5CS_F | 5′- TGGACTAGGTGCTGAGGTTG -3′ | 20 | 55 |
| P5CS_R | 5′- ACCCGCTTCCTTTGAGAATC -3′ | 20 | ||
| 6 | P5CR-F | 5′- TCTGCTGTAGGTGAGGCTGC - | 20 | 58 |
| P5CR-R | 5′- ATATGCTGGACCGCTGCCAC -3′ | 20 | ||
| 7 | NADH–F | 5′- CTTCATGCCCAAGGTGTCTGAT -3′ | 22 | 60 |
| NADH–R | 5′- ATCAAGCAGCCCTCCAACAA -3′ | 20 | ||
| 8 | ACT-F | 5′- CCAGGCTGTTCAGTCTCTGTAT -3′ | 22 | 55 |
| ACT-R | 5′- CGCTCGGTAAGGATCTTCATCA -3′ | 22 |
| Lime Species | Melatonin (μM) | % Drought Stress | Proline (μmol g−1 FW) | %EL | H2O2 (μmol g−1 FW) | MDA (μmol g−1 FW) |
|---|---|---|---|---|---|---|
| Persian | 0 | 100 | 65.5 n | 14 kl | 0.5 j | 0.16 k |
| 75 | 116.3 gh | 29.2 e | 3.5 e | 0.36 f | ||
| 40 | 117.8 fg | 33.4 d | 4.5 d | 0.39 e | ||
| 50 | 100 | 70.2 m | 18 hij | 0.9 hij | 0.25 i | |
| 75 | 179.1 b | 23.2 fg | 2.7 f | 0.33 f | ||
| 40 | 180.1 b | 28.5 e | 3.3 e | 0.35 f | ||
| 100 | 100 | 100.5 j | 12 m | 0.3 l | 0.11 m | |
| 75 | 202.5 a | 14.9 jkl | 0.7 ij | 0.18 k | ||
| 40 | 204.3 a | 20.7 gh | 1.7 g | 0.30 g | ||
| 150 | 100 | 83.6 k | 19.3 hi | 1.1 hi | 0.28 h | |
| 75 | 156.2 c | 21 gh | 1.5 g | 0.30 g | ||
| 40 | 75.6 l | 25 f | 2.7 f | 0.34 f | ||
| Mexican | 0 | 100 | 55.5 p | 16.8 jkl | 0.7 ij | 0.21 j |
| 75 | 110.7 i | 48.4 b | 7.8 a | 0.60 b | ||
| 40 | 112.7 i | 59.4 a | 7.8 a | 0.64 a | ||
| 50 | 100 | 60.5 o | 17.7 hij | 0.8 hij | 0.25 i | |
| 75 | 125.2 e | 34.1 d | 5.3 c | 0.45 d | ||
| 40 | 139.9 d | 38.7 c | 6.7 b | 0.52 c | ||
| 100 | 100 | 97.8 j | 13.1 l | 0.5 k | 0.14 l | |
| 75 | 141.1 d | 17.6 hij | 0.7 ij | 0.21 j | ||
| 40 | 155.3 c | 33.6 d | 4.7 d | 0.40 e | ||
| 150 | 100 | 83.1 k | 19.9 ghi | 1.2 h | 0.28 h | |
| 75 | 122.3 ef | 37.8 c | 5.7 c | 0.46 d | ||
| 40 | 72.5 l | 46.1 b | 6.9 b | 0.53 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, M.; Shahsavar, A.R.; Talebi, M.; Hesami, M. Exogenous Melatonin Protects Lime Plants from Drought Stress-Induced Damage by Maintaining Cell Membrane Structure, Detoxifying ROS and Regulating Antioxidant Systems. Horticulturae 2022, 8, 257. https://doi.org/10.3390/horticulturae8030257
Jafari M, Shahsavar AR, Talebi M, Hesami M. Exogenous Melatonin Protects Lime Plants from Drought Stress-Induced Damage by Maintaining Cell Membrane Structure, Detoxifying ROS and Regulating Antioxidant Systems. Horticulturae. 2022; 8(3):257. https://doi.org/10.3390/horticulturae8030257
Chicago/Turabian StyleJafari, Marziyeh, Ali Reza Shahsavar, Majid Talebi, and Mohsen Hesami. 2022. "Exogenous Melatonin Protects Lime Plants from Drought Stress-Induced Damage by Maintaining Cell Membrane Structure, Detoxifying ROS and Regulating Antioxidant Systems" Horticulturae 8, no. 3: 257. https://doi.org/10.3390/horticulturae8030257
APA StyleJafari, M., Shahsavar, A. R., Talebi, M., & Hesami, M. (2022). Exogenous Melatonin Protects Lime Plants from Drought Stress-Induced Damage by Maintaining Cell Membrane Structure, Detoxifying ROS and Regulating Antioxidant Systems. Horticulturae, 8(3), 257. https://doi.org/10.3390/horticulturae8030257







