Magnetic Nanomaterials as Biocatalyst Carriers for Biomass Processing: Immobilization Strategies, Reusability, and Applications
Abstract
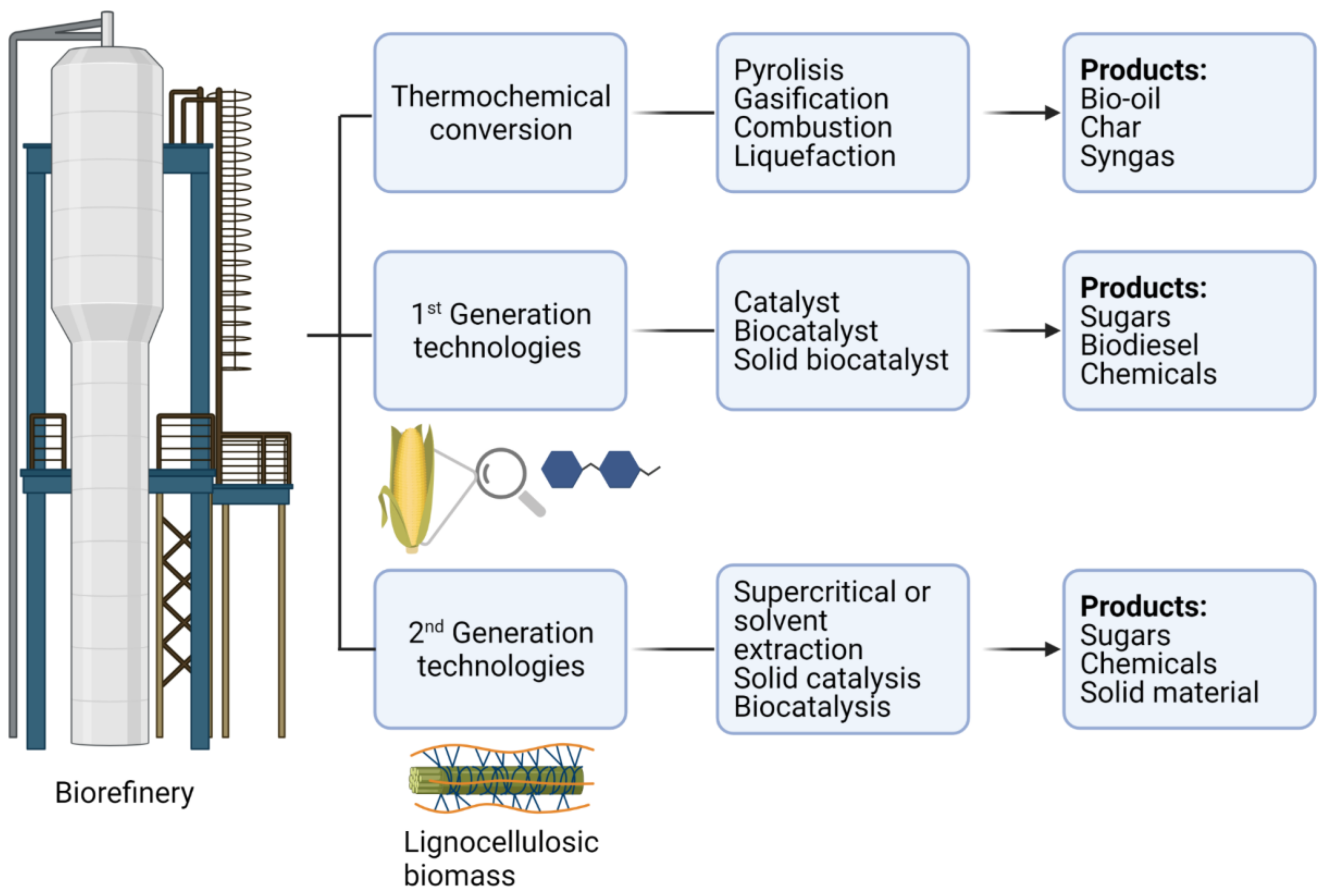
:1. Introduction
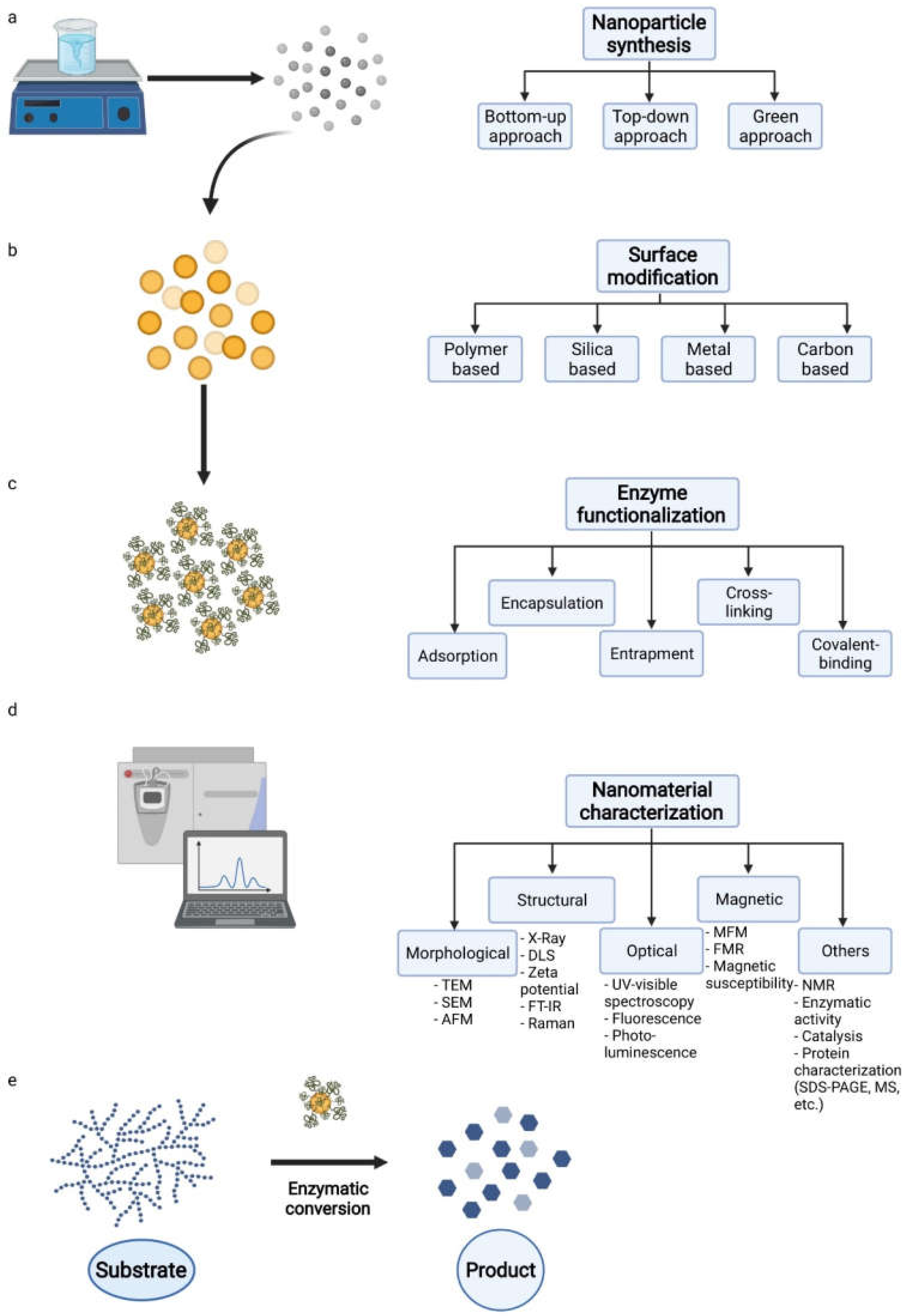
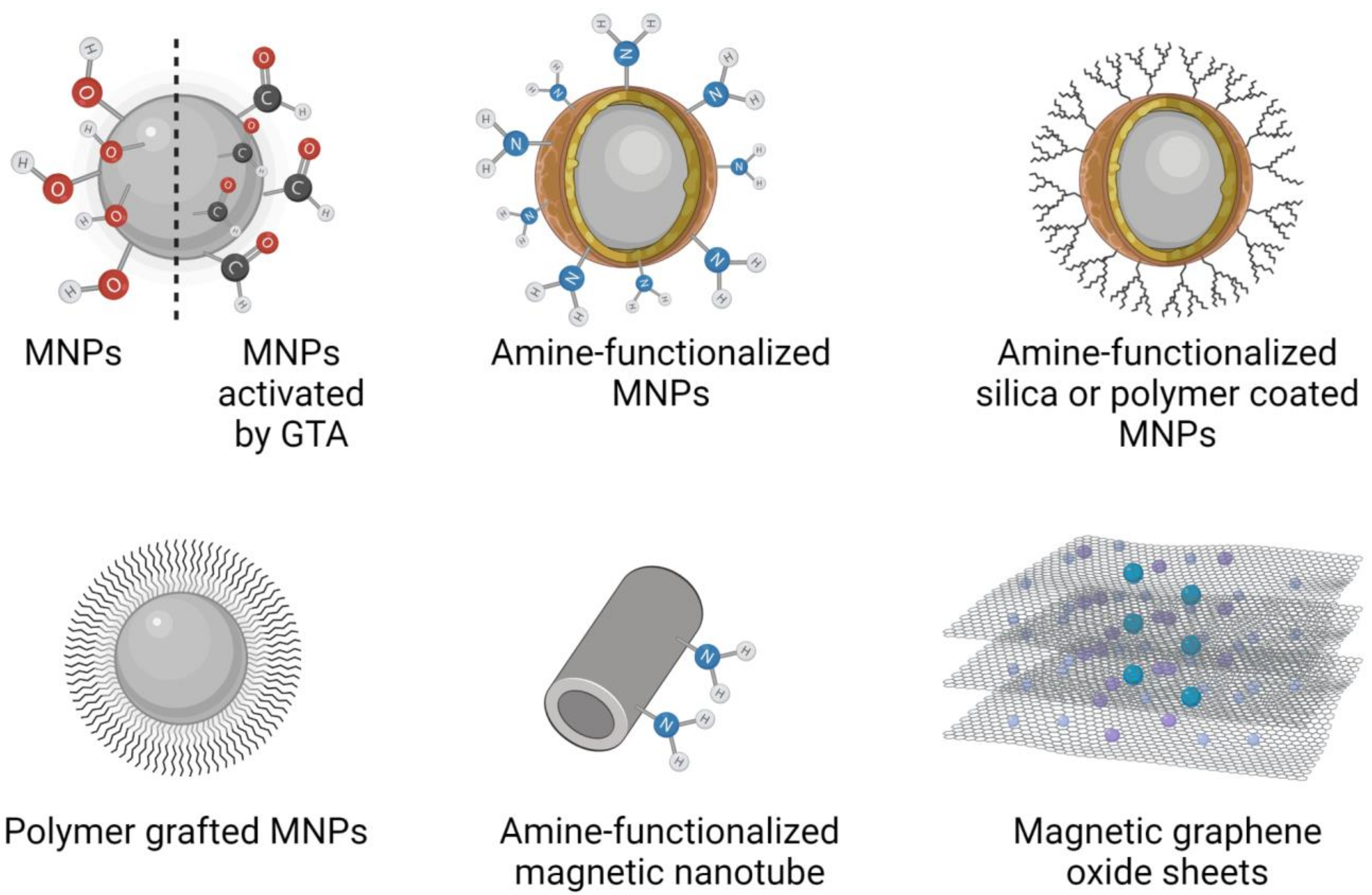
2. Synthesis of Magnetic Nanoparticles Used as Biocatalyst Carriers for Biomass Conversion
Characterization of Magnetic Nanoparticles
3. Enzyme Immobilization Strategies Involving Magnetic Nanocarriers for Biomass Conversion
3.1. Immobilization and Co-Immobilization of Cellulose-Degrading Enzymes Using Silica-Based Carriers
3.2. Immobilization and Co-Immobilization of Cellulose-Degrading Enzymes Polymer-Based
3.3. Immobilization and Co-Immobilization of Hemicellulose-Degrading Enzymes
3.4. Immobilization of Lignin-Degrading Enzymes
4. Key Factors of Magnetic Enzyme Immobilization
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shuttleworth, P.S.; De Bruyn, M.; Parker, H.L.; Hunt, A.J.; Budarin, V.L.; Matharu, A.S.; Clark, J.H. Applications of nanoparticles in biomass conversion to chemicals and fuels. Green Chem. 2014, 16, 573–584. [Google Scholar] [CrossRef]
- Jankowska, K.; Zdarta, J.; Grzywaczyk, A.; Kijeńska-Gawrońska, E.; Biadasz, A.; Jesionowski, T. Electrospun poly(methyl methacrylate)/polyaniline fibres as a support for laccase immobilisation and use in dye decolorisation. Environ. Res. 2020, 184, 109332. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Machałowski, T.; Degórska, O.; Bachosz, K.; Fursov, A.; Ehrlich, H.; Ivanenko, V.N.; Jesionowski, T. 3D Chitin scaffolds from the marine demosponge Aplysina archeri as a support for laccase immobilization and its use in the removal of pharmaceuticals. Biomolecules 2020, 10, 646. [Google Scholar] [CrossRef] [PubMed]
- Misson, M.; Zhang, H.; Jin, B. Nanobiocatalyst advancements and bioprocessing applications. J. R. Soc. Interface 2015, 12, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meryam Sardar, R.A. Enzyme immobilization: An overview on nanoparticles as an immobilization matrix. Biochem. Anal. Biochem. 2015, 4, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Roth, H.C.; Schwaminger, S.P.; Peng, F.; Berensmeier, S. Immobilization of cellulase on magnetic nanocarriers. Chem. Open 2016, 5, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, Q. Nanomaterials immobilized cellulolytic enzymes and their industrial applications: A literature review. JSM Biochem. Mol. Biol. 2017, 4, 1029–1045. Available online: https://www.jscimedcentral.com/Biochemistry/biochemistry-4-1029.pdf (accessed on 20 September 2021).
- Ghollobi, A.; Meshkat, Z.; Abnous, K.; Ghayour-Mobarhan, M.; Ramezani, M.; Shandiz, F.H.; Verma, K.D.; Darroudi, M. Biopolymer-mediate synthesis of Fe3O4 nanoparticles and investigation of their in vitro cytotoxicity effects. J. Mol. Struct. 2017, 5, 594–599. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on recent progress in magnetic nanoparticles: Synthesis, characterization, and diverse applications. Front. Chem. 2021, 9, 2296–2646. [Google Scholar] [CrossRef]
- Indira, T.K.; Lakshmi, P.K. Magnetic nanoparticles-A review. Int. J. Pharm. Sci. Nanotech. 2010, 3, 1035–1042. [Google Scholar]
- Verma, M.L.; Chaudhary, R.; Tsuzuki, T.; Barrow, C.J.; Puri, M. Immobilization of β-glucosidase on a magnetic nanoparticle improves thermostability: Application in cellobiose hydrolysis. Bioresour. Technol. 2013, 135, 2–6. [Google Scholar] [CrossRef]
- Abraham, R.E.; Verma, M.L.; Barrow, C.J.; Puri, M. Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol. Biofuels 2014, 7, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.; Pathak, S.; Kumar, S.A.; Prawer, S.; Tomljenovic-Hanic, S. ZnO nanomaterials: Green synthesis, toxicity evaluation and new insights in biomedical applications. J. Alloy. Compd. 2021, 876, 160175. [Google Scholar] [CrossRef]
- Priya; Naveen; Kamaljit, K.; Amanpreet, S.K. Green synthesis: An eco-friendly route for the synthesis of iron oxide nanoparticles. Front. Nanotechnol. 2021, 3, 47. [Google Scholar] [CrossRef]
- Muthuvelu, K.S.; Rajarathinam, R.; Selvaraj, R.N.; Rajendren, V.B. A Novel method for improving laccase activity by immobilization onto copper ferrite nanoparticles for lignin degradation. Int. J. Biol. Macromol. 2020, 152, 1098–1107. [Google Scholar] [CrossRef]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of light scattering techniques to nanoparticle characterization and development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Jung, S.; Kim, H.J.; Lee, Y.G.; Nam, K.C.; Lee, H.J.; Bae, H.J. Co-immobilization of three cellulases on Au-doped magnetic silica nanoparticles for the degradation of cellulose. Chem. Commun. 2012, 48, 886–888. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, J.; Yang, B.; Zhao, L.; Hou, Z.; Tang, B. Immobilized cellulase on Fe3O4 nanoparticles as a magnetically recoverable biocatalyst for the decomposition of corncob. Chin. J. Catal. 2016, 37, 389–397. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Immobilization of cellulase on functionalized cobalt ferrite nanoparticles. Korean J. Chem. Eng. 2016, 33, 216–222. [Google Scholar] [CrossRef]
- Han, J.; Luo, P.; Wang, Y.; Wang, L.; Li, C.; Zhang, W.; Dong, J.; Ni, L. The development of nanobiocatalysis via the immobilization of cellulase on composite magnetic nanomaterial for enhanced loading capacity and catalytic activity. Int. J. Biol. Macromol. 2018, 119, 692–700. [Google Scholar] [CrossRef]
- Koo, N.K.; Ismail, A.F.; Dzarfan, M.H.; Rahman, M.A.; Sheng, T.Z. Preparation and characterization of superparamagnetic magnetite (Fe3O4) nanoparticles: A short review. Mal. J. Fund. Appl. Sci. 2019, 15, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Netto, C.G.C.M.; Toma, H.E.; Andrade, L.H. Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J. Mol. Catal. B Enzym. 2013, 85, 71–92. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.S.; Liman, R.; Ruallo, J.; Villaflores, O.B.; Ger, T.R.; Hsiao, C.D. Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818. [Google Scholar] [CrossRef] [PubMed]
- Sandler, S.E.; Fellows, B.; Mefford, T. Best practical for characterization of magnetic nanoparticles for biomedical applications. Anal. Chem. 2019, 91, 14159–14169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mika, N.; Zorn, H.; Rühl, M. Insect-derived enzymes: A treasure for industrial biotechnology and food biotechnology. Adv. Biochem. Eng. 2013, 136, 1–17. [Google Scholar] [CrossRef]
- Lima, J.S.; Araújo, P.H.H.; Sayer, C.; Souza, A.A.U.; Viegas, A.C.; de Oliveira, D. Cellulase immobilization on magnetic nanoparticles encapsulated in polymer nanospheres. Bioproc. Biosyst. Eng. 2017, 40, 511–518. [Google Scholar] [CrossRef]
- Shinkai, M.; Honda, H.; Kobayashit, T. Preparation of fine magnetic particles and application for enzyme immobilization. Biocatalysis 1991, 5, 61–69. [Google Scholar] [CrossRef]
- Valenzuela, R.; Castro, J.F.; Parra, C.; Baeza, J.; Durán, N.; Freer, J. β-Glucosidase immobilisation on synthetic superparamagnetic magnetite nanoparticles and their application in saccharification of wheat straw and Eucalyptus globulus pulps. J. Exp. Nanosci. 2014, 9, 177–185. [Google Scholar] [CrossRef]
- Alahakoon, T.; Koh, J.W.; Chong, X.W.C.; Lim, W.T.L. Immobilization of cellulases on amine and aldehyde functionalized Fe2O3 magnetic nanoparticles. Prep. Biochem. Biotechnol. 2012, 42, 234–248. [Google Scholar] [CrossRef]
- Alftrén, J.; Hobley, T.J. Immobilization of cellulase mixtures on magnetic particles for hydrolysis of lignocellulose and ease of recycling. Biomass Bioenergy 2014, 65, 72–78. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, J.; Feng, H.; Zang, L.; Sakai, E. Increase in stability of cellulase immobilized on functionalized magnetic nanospheres. J. Magn. Magn. Mater. 2015, 375, 117–123. [Google Scholar] [CrossRef]
- Baskar, G.; Naveen Kumar, R.; Heronimus Melvin, X.; Aiswarya, R.; Soumya, S. Sesbania aculeata biomass hydrolysis using magnetic nanobiocomposite of cellulase for bioethanol production. Renew. Energy 2016, 98, 23–28. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, W.; Yang, Z.; Yang, X.; Wang, N.; Yu, X. Novel magnetic cross-linked cellulase aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules 2017, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, D.; Wu, C.; Yao, K.; Li, Z.; Shi, N.; Wen, F.; Gates, I.D. Co-immobilization of cellulase and lysozyme on amino-functionalized magnetic nanoparticles: An activity-tunable biocatalyst for extraction of lipids from microalgae. Bioresour. Technol. 2018, 263, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Sillu, D.; Agnihotri, S. Cellulase immobilization onto magnetic halloysite nanotubes: Enhanced enzyme activity and stability with high cellulose saccharification. ACS Sustain. Chem. Eng. 2020, 8, 900–913. [Google Scholar] [CrossRef]
- Xu, C.; Chen, X.; Zheng, R.; Zheng, Y. Immobilization of multi-enzymes on supports materials for efficient biocatalysis. Front. Bioeng. Biotechnol. 2020, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Chen, D.; Yuan, L.; Zheng, M.; Zhu, Y.; Liu, X. Immobilized cellulase by polyvinyl alcohol/Fe2O3 magnetic nanoparticle to degrade microcrystalline cellulose. Carbohydr. Polym. 2010, 82, 600–604. [Google Scholar] [CrossRef]
- Gokhale, A.A.; Lu, J.; Lee, I. Immobilization of cellulase on magneto responsive graphene nano-supports. J. Mol. Catal. B Enzym. 2013, 90, 76–86. [Google Scholar] [CrossRef]
- Kudina, O.; Zakharchenko, A.; Trotsenko, O.; Tokarev, A.; Ionov, L.; Stoychev, G.; Puretskiy, N.; Pryor, S.W.; Voronov, A.; Minko, S. Highly efficient phase boundary biocatalysis with enzymogel nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Qiu, J.; Wu, X.; Zhang, W.; Sakai, E.; Wei, Y. Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind. Eng. Chem. R. 2014, 53, 3448–3454. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, J.; Martínez-Hernández, J.L.; Segura-Ceniceros, P.; López, G.; Saade, H.; Medina-Morales, M.A.; Ramos-González, R.; Aguilar, C.N.; Ilyina, A. Cellulases immobilization on chitosan-coated magnetic nanoparticles: Application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioproc. Biosyst. Eng. 2017, 40, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Qiu, J. Preparation of chitosan/magnetic porous biochar as support for cellulase immobilization by Using glutaraldehyde. Polymers 2020, 12, 2672. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Luo, J.; Wan, Y. Immobilization of cellulase on a core-shell structured metal-organic framework composites: Better inhibitors tolerance and easier recycling. Bioresour. Technol. 2018, 268, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Ulaganathan, K.; Goud, B.S.; Reddy, M.M.; Kumar, V.P.; Balsingh, J.; Radhakrishna, S. Proteins for breaking barriers in lignocellulosic bioethanol production. Curr. Protein Pept. Sc. 2015, 16, 100–134. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Pletschke, B.I. Magnetic cross-linked enzyme aggregates (CLEAs): A novel concept towards carrier free immobilization of lignocellulolytic enzymes. Enzym. Microb. Technol. 2014, 61, 17–27. [Google Scholar] [CrossRef]
- Soozanipour, A.; Taheri-Kafrani, A.; Landarani Isfahani, A. Covalent attachment of xylanase on functionalized magnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2015, 270, 235–243. [Google Scholar] [CrossRef]
- Landarani-Isfahani, A.; Taheri-Kafrani, A.; Amini, M.; Mirkhani, V.; Moghadam, M.; Soozanipour, A.; Razmjou, A.; Taheri-Kafrani, A. Xylanase immobilized on novel multifunctional hyperbranched polyglycerol-grafted magnetic nanoparticles: An efficient and robust biocatalyst. Langmuir 2015, 31, 9219–9227. [Google Scholar] [CrossRef]
- Perwez, M.; Ahmad, R.; Sardar, M. A reusable multipurpose magnetic nanobiocatalyst for industrial applications. Int. J. Biol. Macromol. 2017, 103, 16–24. [Google Scholar] [CrossRef]
- Kumari, A.; Kaila, P.; Tiwari, P.; Singh, V.; Kaul, S.; Singhal, N.; Guptasarma, P. Multiple thermostable enzyme hydrolases on magnetic nanoparticles: An immobilized enzyme-mediated approach to saccharification through simultaneous xylanase, cellulase and amylolytic glucanotransferase action. Int. J. Biol. Macromol. 2018, 120, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, K.; Santhalembi, L.; Mortha, G.; Aurousseau, M.; Boyer, A.; Subramanian, S. Bioconversion of lignocellulosic biomass to fermentable sugars by immobilized magnetic cellulolytic enzyme cocktails. Langmuir 2018, 34, 6546–6555. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, M.; Tran, J.L.; Chu, K.H. Effective one-step saccharification of lignocellulosic biomass using magnetite-biocatalysts containing saccharifying enzymes. Sci. Total Environ. 2019, 647, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Mehnati-Najafabadi, V.; Taheri-Kafrani, A.; Bordbar, A.K. Xylanase immobilization on modified superparamagnetic graphene oxide nanocomposite: Effect of PEGylation on activity and stability. Int. J. Biol. Macromol. 2018, 107, 418–425. [Google Scholar] [CrossRef]
- Paz-Cedeno, F.R.; Carceller, J.M.; Iborra, S.; Donato, R.K.; Godoy, A.P.; Veloso de Paula, A.; Monti, R.; Corma, A.; Masarin, F. Magnetic graphene oxide as a platform for the immobilization of cellulases and xylanases: Ultrastructural characterization and assessment of lignocellulosic biomass hydrolysis. Renew. Energy 2021, 164, 491–501. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Motamedi, E.; Hosseini Salekdeh, G. Immobilization of enzyme cocktails on dopamine functionalized magnetic cellulose nanocrystals to enhance sugar bioconversion: A biomass reusing loop. Carbohydr. Polym. 2021, 256, 117511. [Google Scholar] [CrossRef]
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-degrading enzymes. FEBS J. 2015, 282, 1190–1213. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Colpa, D.I.; Habib, M.H.M.; Fraaije, M.W. Bacterial enzymes involved in lignin degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Voběrková, S.; Solčány, V.; Vršanská, M.; Adam, V. Immobilization of ligninolytic enzymes from white-rot fungi in cross-linked aggregates. Chemosphere 2018, 202, 694–707. [Google Scholar] [CrossRef]
- Hu, J.; Yuan, B.; Zhang, Y.; Guo, M. Immobilization of laccase on magnetic silica nanoparticles and its application in the oxidation of guaiacol, a phenolic lignin model compound. RSC Adv. 2015, 5, 99439–99447. [Google Scholar] [CrossRef]
- Amin, R.; Khorshidi, A.; Shojaei, A.F.; Rezaei, S.; Faramarzi, M.A. Immobilization of laccase on modified Fe3O4@SiO2@Kit-6 magnetite nanoparticles for enhanced delignification of olive pomace bio-waste. Int. J. Biol. Macromol. 2018, 114, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, D.; Neeraj, G.; Swaroopini, R.; Shobana, R.; Kumar, V.V.; Cabana, H. Synergetic integration of laccase and versatile peroxidase with magnetic silica microspheres towards remediation of biorefinery wastewater. Environ. Sci. Pollut. R. 2017, 24, 17993–18009. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, B.; Feng, M.; Zhao, D.; Sun, J. Immobilized laccase on magnetic nanoparticles for enhanced lignin model compounds degradation. Chin. J. Chem. Eng. 2020, 28, 2152–2159. [Google Scholar] [CrossRef]
- Gou, Z.; Ma, N.L.; Zhang, W.; Lei, Z.; Su, Y.; Sun, C.; Wang, G.; Chen, H.; Zhang, S.; Chen, G.; et al. Innovative hydrolysis of corn stover biowaste by modified magnetite laccase immobilized nanoparticles. Environ. Res. 2020, 188, 109829. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Krishnaswamy, S.; Chandrababu, R.; Veerabagu, U.; Pugazhendhi, A.; Mathimani, T. Optimal mmobilization of Trichoderma asperellum laccase on polymer coated Fe3O4@SiO2 nanoparticles for enhanced biohydrogen production from delignified lignocellulosic biomass. Fuel 2020, 273, 117777. [Google Scholar] [CrossRef]
- Mariño, M.; Moretti, P.; Tasic, L. Immobilized commercial cellulases onto amino-functionalized magnetic beads for biomass hydrolysis: Enhanced stability by non-polar silanization. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]

| Enzyme | Magnetic Carrier | Carrier Size/MS | Results | Reference |
|---|---|---|---|---|
| β-Glucosidase from almond | Amine-functionalized Fe3O4NPs | 4–70 nm MS: ND | 70% of activity remained after 5 months at 4°C. Protein loading: 168 mg g−1 | Shinkai et al. (1991) [29] |
| β-Glucosidase from A. niger | Amine-functionalized Fe3O4NPs | 40 nm MS: ND | 20% decreased activity over eight 10 min cycles Protein loading: 93% | Verma et al. (2013) [11] |
| β-Glucosidase from T. reesei | Amine-functionalized Fe3O4NPs | 10 nm MS: 55.9 emu g−1. | 20% decreased activity over three 72 h cycles | Valenzuela et al. (2014) [30] |
| Recombinant cellulases from Themotoga maritima | Thiol-functionalized Fe3O4NPs and gold-doped nanoparticles | 27 nm MS: 33.3 emu g−1 | 40% decreased activity over three cycles | Cho et al. (2012) [18] |
| Cellulases from T. reesei | Amine-functionalized Fe3O4NPs | 23.5 nm MS: ND | 23.5% decreased activity over six 24 h cycles. Protein loading: 0.053 mg g−1 | Alahakoon et al. 2013 [31] |
| Cellulases from T. reesei | Zinc-doped Fe3O4NPs | 40 nm MS: ND | No loss of activity after 45 days at 4 °C/50% decreased activity over five 48 h cycles | Abraham et al. (2014) [12] |
| Cellulases from Humicola insolens | Amine-functionalized Fe3O4NPs | Size: ND MS: 32–39 emu g−1 | 23% decreased activity by adsorption on AEAPTMES-condensed MNP. Protein loading: 112 mg g−1 | Zhang et al. (2014) [33] |
| Cellulases (Novozymes) | Amine-functionalized Fe3O4NPs | Size: ND MS: 54.7 emu g−1 | 35% decreased activity over 100 h Protein loading: 161 mg g−1 | Zhang et al. (2016) [19] |
| Cellulases from T. longibrachiatum | Amine-functionalized Fe3O4NPs | 90–100 nm MS: 400 emu g−1 | 40% decreased activity over 15 h of hydrolysis at 30 °C using pretreated Sesbania aculeate | Baskar et al. (2016) [34] |
| Cellulases from T. reesei | Amine-functionalized CoFe2O4NPs | 8 nm MS: 46.01 emu g−1 | 36% decreased activity after six cycles of cellulose hydrolysis. | Bohara et al. (2015) [20] |
| Cellulases from T. reesei | Amine-functionalized Fe3O4NPs | 60 nm | 62% decreased activity over four 24 h cycles Protein loading: 176 mg g−1 | Jia et al. (2017) [35] |
| Cellulases from A. niger | Amine-functionalized Fe3O4NPs | Size: ND MS: 39.8 emu g−1 | 40% decreased activity over six cycles of hydrolysis for lipids extraction | Chen et al. (2018) [36] |
| Cellulases from A. niger | Amine-functionalized magnetic halloysite nanotubes | Size: ND MS: 32.54 emu g−1 | 31.8% decreased activity after seven 48 h cycles Cellulase activity yield 93.5% Protein loading: 80.48%. | Sillu & Agnihotri (2020) [37] |
| Enzyme | Magnetic Carrier | Carrier Size/MS | Results | Reference |
|---|---|---|---|---|
| Cellulases from T. viride | PVA/Fe2O3NPs | 270 nm MS: ND | 60% decreased activity over four 6 h cycles | Liao et al. (2010) [39] |
| Cellulases from T. reesei | 2D graphene with maghemite-magnetite and PAA brushes | ND | 38% and 45% decreased activity over three and four 1 h cycles, respectively. | Gokhale et al. (2013) [40] |
| Cellulases from T. reesei | Silica-coated Fe3O4NPs grafted with PAA brushes | 200 nm MS: ND | About 50% of protein release by pH-triggered over one cycle | Kudina et al. (2014) [41] |
| Cellusoft CR | Poly(methylmethacrylate)-encapsulated Fe3O4NPs | 150 nm MS: 44.6 emu g−1 | 31% decreased activity over eight cycles | Lima et al. (2016) [28] |
| Cellulases from Humicola insolens | Chitosan-coated Fe3O4NPs | 18 nm MS: 46.6 emu g−1 | 50% decreased activity over five 48 h cycles Protein loading: 112.3 mg.g−1 | Zang et al. (2014) [44] |
| Cellulases from T. reesei | Chitosan-coated Fe3O4NPs | 10 nm MS: 45.09 emu g1 | 50% decreased activity over five 20 h cycles | Sánchez-Ramírez et al. (2017) [43] |
| Cellulases from Humicola insolens | Chitosan-coated γ-Fe2O3 magnetized porous biochar | Pore: 3.8 nm MS: 0.67 emu g−1 | 24% of decreased glucose yield over five 48 h cycles. Protein loading: 80.5 mg g−1 | Mo & Qiu, (2020) [44] |
| Cellulases from SunSon group | Poly (sodium 4-styrenesulfonate)-modified Fe3O4NPs and UIO-66-NH2 MOF | 174.5 nm | 30% decreased activity over five 24 h cycles of MCC hydrolysis. Protein loading: 126.2 mg g−1 | Qi et al. (2018) [45] |
| Cellulases from A. niger | Graphene oxide (GO@Fe3O4) coated by 4arm-PEG-NH2 (10 K) | Size: ND MS: 15.8 emu g−1 | 55% decreased activity over eight 2.5 h cycles of a paper filter. Protein loading: 570 mg g−1 | Han et al. (2018) [21] |
| Enzyme | Magnetic Carrier | Carrier Size/MS | Results | Reference |
|---|---|---|---|---|
| Xylanases from Bacillus gelatini ABBP-1 | CLEAs with amine-functionalized Fe3O4NPs, | ND | 78% decreased activity after three 72 h cycles of pretreated sugarcane bagasse | Bhattacharya & Pletschke (2014) [47] |
| Xylanase from Thermomyces lanuginosus | Amine-functionalizedFe3O4NPs | 9 nm MS: 46.56 emu g−1 | 80% of activity remained after 3 months at 4 °C/35% decreased activity after nine cycles. Protein loading: 280 mg g−1 | Soozanipour et al. (2015) [48] |
| Xylanases, pectinases and cellulases from Pectinex 3XL | Amine-functionalized Fe3O4NPs | 63 nm MS: ND | 20% of decreased activities after six cycles of hydrolysis | Permez et al. (2017) [50] |
| Recombinant xylanases from Bacillus sp. and cellulases from Rhodothermus marinus | Fe3O4NPs | ND | 30% decreased activity after eight 12 h cycles | Kumar et al. (2018) [51] |
| Xylanases, cellulases and β-1.3-gluconase from T. citrinoviride | Amine-functionalized silica -coated Fe3O4NPs | 86.4 nm MS: ND | 10–15% increased hydrolysis yield compared to free enzymes | Periyasamy et al. (2018) [52] |
| Recombinant cellulases and xylanases from genes of Bacillus subtilis and A. fumigatus | Fe3O4NPs-CLEAs | ND | Enhanced yield of reducing sugars during three 48 h cycles of pretreated corn husks hydrolysis | Hwangbo et al. (2019) [53] |
| Xylanases and cellulases from Cellic CTec2 | Graphene oxide nanosheets with Fe3O4NPs | ND | 78% yield for xylose after five 24 h cycles and 34% of glucose from pretreated bagasse. Protein loading: 50mg g−1 | Paz-Cerdeno et al. (2021) [55] |
| Xylanase from T. lanuginosus | Hyperbranched polyglycerol-grafted on silica-coated Fe3O4NPs | Size: ND MS: 46.56 emu g−1 | 34% decreased activity after 10 cycles of hydrolysis Protein loading: 279 mg g−1 | Landarani-Isfahani et al. 2015 [49] |
| Xylanases from T. lanuginosus | Nanocomposite of graphene oxide-nanosheets functionalized by PEG with Fe3O4NPs | 30 nm MS: 34.5 emu g−1 | 50% decreased activity after four cycles of hydrolysis Protein loading: 211 mg g−1 | Mehnati-Najafabadi et al. (2019) [54] |
| Three cellulases and two xylanases purified from rumen microbiota | CNCs functionalized with Fe3O4NPs and dopamine | Diameter: 25 nm Length: 40–150 nm MS: 25.8 emu g−1 | 50% decreased activity after ten 1 h cycles | Ariaeenejad et al. 2021 [56] |
| Enzyme Binding | Magnetic Carrier | Carrier Size/MS | Results | Reference |
|---|---|---|---|---|
| Laccase from Aspergillus | Amine-functionalizedFe3O4NPs | ND | Immobilization efficiency: 53.4% of its initial activity | Hu et al. 2015. [60] |
| Laccase from Trametes versicolor | Amine-functionalized mesoporous material with Fe3O4NPs | ND | 70% of its initial activity after 20 days at 25 °C. Immobilization efficiency: 84.4% | Amin et al. 2018 [61] |
| Laccase from Trametes versicolor | Amine-functionalized CuFe2O4NPs | 50± 20 nm MS: 9.85 emu g−1 | 43.2% of lignin removal of Ipomoea carnea—70% of activity is retained after six 1 h cycles. Protein binding capacity: 118 mg g1 | Muthuvelu et al. 2019 [15] |
| Laccase from Trametes versicolor | Amine-functionalized Fe3O4NPs. | 182 nm | Immobilization efficiency: 64.7%. Activity decreased 30% and 70% after four and eight 30 min cycles | Chen et al. 2020 [63] |
| Laccase from Myrothecium verrucaria 3H6 | Polyethyleneimine (PEI)- functionalized Fe3O4NPs | ND | Immobilization efficiency: 87.4% and 85.3% of adsorbed protein—50% of activity is retained after six 10 min cycles. | Gou et al. 2020 [64] |
| Laccase from Trichoderma asperellum | Chitosan-functionalized silica-coated Fe3O4NPs | 700 nm MS: 27.4 emu g−1 | Immobilization efficiency: 92.4%. Activity decreased 30% after eight 1 h cycles. | Shanmugam et al. 2020 [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariño, M.A.; Fulaz, S.; Tasic, L. Magnetic Nanomaterials as Biocatalyst Carriers for Biomass Processing: Immobilization Strategies, Reusability, and Applications. Magnetochemistry 2021, 7, 133. https://doi.org/10.3390/magnetochemistry7100133
Mariño MA, Fulaz S, Tasic L. Magnetic Nanomaterials as Biocatalyst Carriers for Biomass Processing: Immobilization Strategies, Reusability, and Applications. Magnetochemistry. 2021; 7(10):133. https://doi.org/10.3390/magnetochemistry7100133
Chicago/Turabian StyleMariño, Mayra A., Stephanie Fulaz, and Ljubica Tasic. 2021. "Magnetic Nanomaterials as Biocatalyst Carriers for Biomass Processing: Immobilization Strategies, Reusability, and Applications" Magnetochemistry 7, no. 10: 133. https://doi.org/10.3390/magnetochemistry7100133
APA StyleMariño, M. A., Fulaz, S., & Tasic, L. (2021). Magnetic Nanomaterials as Biocatalyst Carriers for Biomass Processing: Immobilization Strategies, Reusability, and Applications. Magnetochemistry, 7(10), 133. https://doi.org/10.3390/magnetochemistry7100133







