Abstract
Two one-dimensional oxalate-bridged Cu(II) ammonium salts, [(CH3)3NH]2[Cu(μ-C2O4)(C2O4)]·2.5H2O (I) and [(C2H5)3NH]2[Cu(μ-C2O4)(C2O4)]·H2O (II) were obtained and characterized. They were composed of ammonium: (CH3)3NH+ in (I), (C2H5)3NH+ in (II), [Cu(μ-C2O4)(C2O4)2−]n and H2O. The Jahn–Teller-distorted Cu(II) is octahedrally coordinated by six O atoms from three oxalates and forms a one-dimensional zigzag chain. The hydrogen bonds between ammonium, the anion and H2O form a three-dimensional network. There is no hydrogen bond between the anion chains. They were insulated at 20 °C with a relative humidity of 40%. Ferromagnetic and weak-ferromagnetic behaviors were observed in I and II, separately. No long-range ordering was observed above 2 K.
1. Introduction
The Jahn–Teller effect plays an important role in inorganic superconductors and colossal magneto-resistance materials [1,2,3,4,5,6,7,8,9]. Interesting conductivity and magnetic behaviors are expected when the Jahn–Teller effect exists in molecular crystals, such as metal–organic frameworks. Oxalate (C2O42−), as one of the most commonly used short connectors, plays a key role in molecular-based magnets [10,11,12]. Long-range ordering has been reported in metal–oxalate framework compounds from one-dimensional zigzag chains (K2Fe(μ-C2O4)(C2O4), K2Co(μ-C2O4)(C2O4), Co(μ-C2O4)(μ-HOC3H6OH), TTF[Fe(μ-C2O4)Cl2] and κ-BETS2[Fe(μ-C2O4)Cl2]) to two-dimensional honeycomb lattices ([(C4H9)4N][CrMn(μ-C2O4)3], A[MIIFeIII(μ-C2O4)3] (A = ammonium; M = Mn, Fe), [C5H10N3O]2[Fe2(μ-C2O4)3], Fe2(μ-C2O4)3·4H2O) and square lattices ([Fe(μ-C2O4)(CH3OH)]n) to three-dimensional metal–oxalate framework compounds ([Co(bpy)3][Co2(μ-C2O4)3]ClO4, Mn(μ-C2O4)(H2O)0.25, [ZII(bpy)3][MIICrIII(μ-C2O4)3][ClO4] (M = Mn, Fe, Co, Ni) and (Me4N)6[Mn3Cr4(μ-C2O4)12]·6H2O), while a single chain magnet [C12H24O6K]0.5[(C12H24O6)(FC6H4NH3)]0.5[Co(H2O)2Cr(μ-C2O4)(C2O4)2] has been reported [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29].
Quantum spin liquid is an intriguing magnetic state, where spin ordering or freezing prevents spin frustration in a resonating valence bond (RVB) state. In 1979, P. W. Anderson proposed the RVB state in S = 1/2, a two-dimensional triangular lattice [30]. In 1987, he proposed that La2CuO4 is a parent compound of cuprate superconductors. The antiferromagnetic insulator La2CuO4 turns into a diamagnetic superconductor after hole doping, and a quantum spin liquid with Jahn–Teller distortion on Cu(II) is an indispensable magnetic state [31]. The spin-frustrated copper-oxalate framework with Jahn–Teller distortion supports a platform for molecular-based quantum spin liquids [32,33]. Strong antiferromagnetic interactions without a long-range ordering above 2 K with spin frustration were observed in two-dimensional honeycomb lattices: θ21-(BEDT-TTF)3[Cu2(μ-C2O4)3]·2CH3OH, θ21-(BETS-TTF)3[Cu2(μ-C2O4)3]·2CH3OH, [(C3H7)3NH]2[Cu2(μ-C2O4)3]·2.2H2O, hydrogen-bonded square lattice β″-(BEDT-TTF)3[Cu2(μ-C2O4)(C2O4)2(CH3OH)(H2O)], and three-dimensional hyperhoneycomb lattice [(C2H5)3NH]2[Cu2(μ-C2O4)3] [34,35,36,37,38]. [(C2H5)3NH]2[Cu2(μ-C2O4)3], which is a quantum spin liquid with no long-range ordering was observed until 60 mK [39]. In these compounds, the antiferromagnetic behavior depends on the antiferromagnetic interaction between the ferromagnetic couple. The magnetic structure of [(C2H5)3NH]2[Cu2(μ-C2O4)3] is lower than the three-dimensional, with the coexistence of ferromagnetic and antiferromagnetic interactions between Jahn–Teller distorted Cu(II) [37,39]. Researching the magnetic properties of Jahn–Teller distorted one-dimensional copper-oxalate frameworks [Cu(μ-C2O4)(C2O4)22−]n without the hydrogen bond between anions will help us to quantitatively analyze the magnetic interaction in Jahn–Teller-distorted two-dimensional and three-dimensional copper-oxalate frameworks and design new candidate quantum spin liquids. Two one-dimensional copper-oxalate framework compounds, [(CH3)3NH]2[Cu(μ-C2O4)(C2O4)]·2.5H2O (I) and [(C2H5)3NH]2[Cu(μ-C2O4)(C2O4)]·H2O (II), have been obtained and characterized. The related work is presented here.
2. Experiment
[(CH3)3NH]2[Cu(μ-C2O4)(C2O4)]·2.5H2O (I) and [(C2H5)3NH]2[Cu(μ-C2O4)(C2O4)]·H2O (II) were obtained from a methanol solution of Cu(NO3)2·3H2O and H2C2O4·2H2O with (CH3)3N for I and (C2H5)3N for II in a 1:3:5 ratio at room temperature. Bulk blue plateful crystals of I and II were obtained after four weeks. The crystal was washed with CH3COOC2H5 and dried. Elemental analysis calculated (%) for C10H25CuN2O10.50 (I): C 29.67, H 6.22 and N 6.92 and found C 29.87, H, 6.08 and N 6.97. For C16H34CuN2O9 (II): C 41.60, H 7.42, N 6.06 and found C 42.03, H 7.46 and N 6.11.
Elemental analyses of carbon, hydrogen and nitrogen were performed using the Flash EA 1112 elemental analyzer. The IR spectra were recorded on a Bio-rad FTS6000/UMA500 spectrometer (Figure S1). Thermogravimeter analysis was carried out on a Shimadzu DTG-60 analyzer at a 10 °C/min heating rate from room temperature to 550 °C under N2 gas with an Al bag. I remains stable until 40 °C, and II remains stable until 80 °C.
X-ray powder diffraction was carried out using a Rigaku RINT2000 diffractometer at room temperature with Cu Kα radiation (λ = 1.54056 Å) in a flat-plate geometry (Figures S2 and S3).
Single-crystal X-ray diffraction was carried out on an Enraf-Nonius KappaCCD diffractometer at room temperature. The crystal structure was solved using the direct method and refined using the full-matrix least square on F2 using the SHELX program, with anisotropic thermal parameters for all non-hydrogen atoms [40]. The hydrogen atoms on C and N were located through calculation, and on H2O they were located through a difference Fourier map. All of the H were refined isotropically. The crystallographic data are listed in Table S1.
The resistance measurement was performed on a single crystal at Tonghui TH2828. Gold wires were attached to the best developed surfaces of a single crystal with a size of 0.40 ∗ 0.30 ∗ 0.11 mm (I) and 0.71 ∗ 0.60 ∗ 0.17 mm (II) using gold paste. The two-probe conductivity was measured at 20 °C and a relative humidity (RH) of 40%.
Magnetization measurements were performed on a polycrystalline sample tightly packed into a capsule on a Quantum Design MPMS 7XL SQUID system above 2 K. Susceptibility data were corrected for the diamagnetism of the sample by Pascal constants and background by experimental measurement of the sample holder [41]. Temperature-dependent magnetization was performed under an applied field of 1000 G. Isothermal magnetization was measured at 2 K from 0 to 65 kG.
3. Result and Discussion
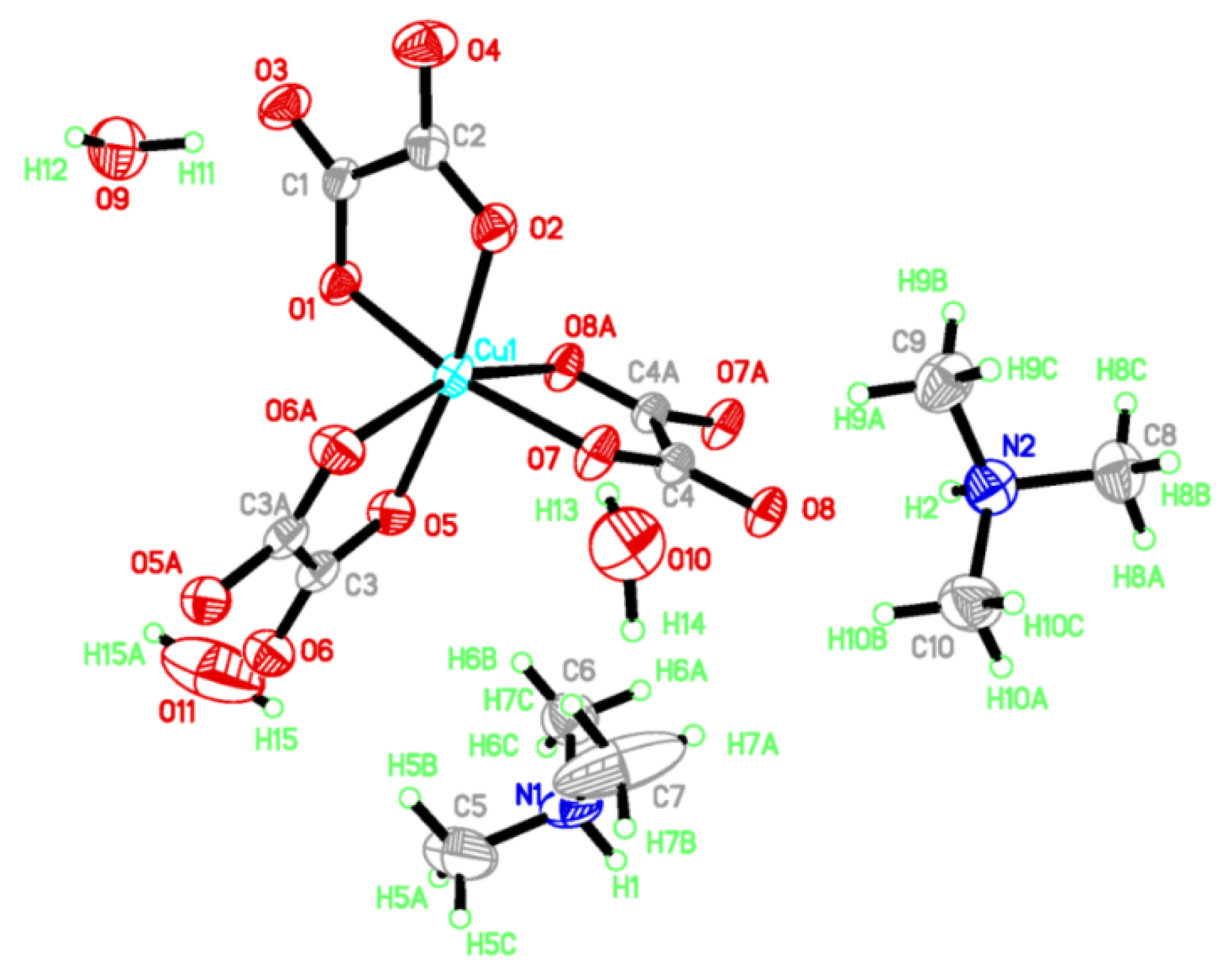
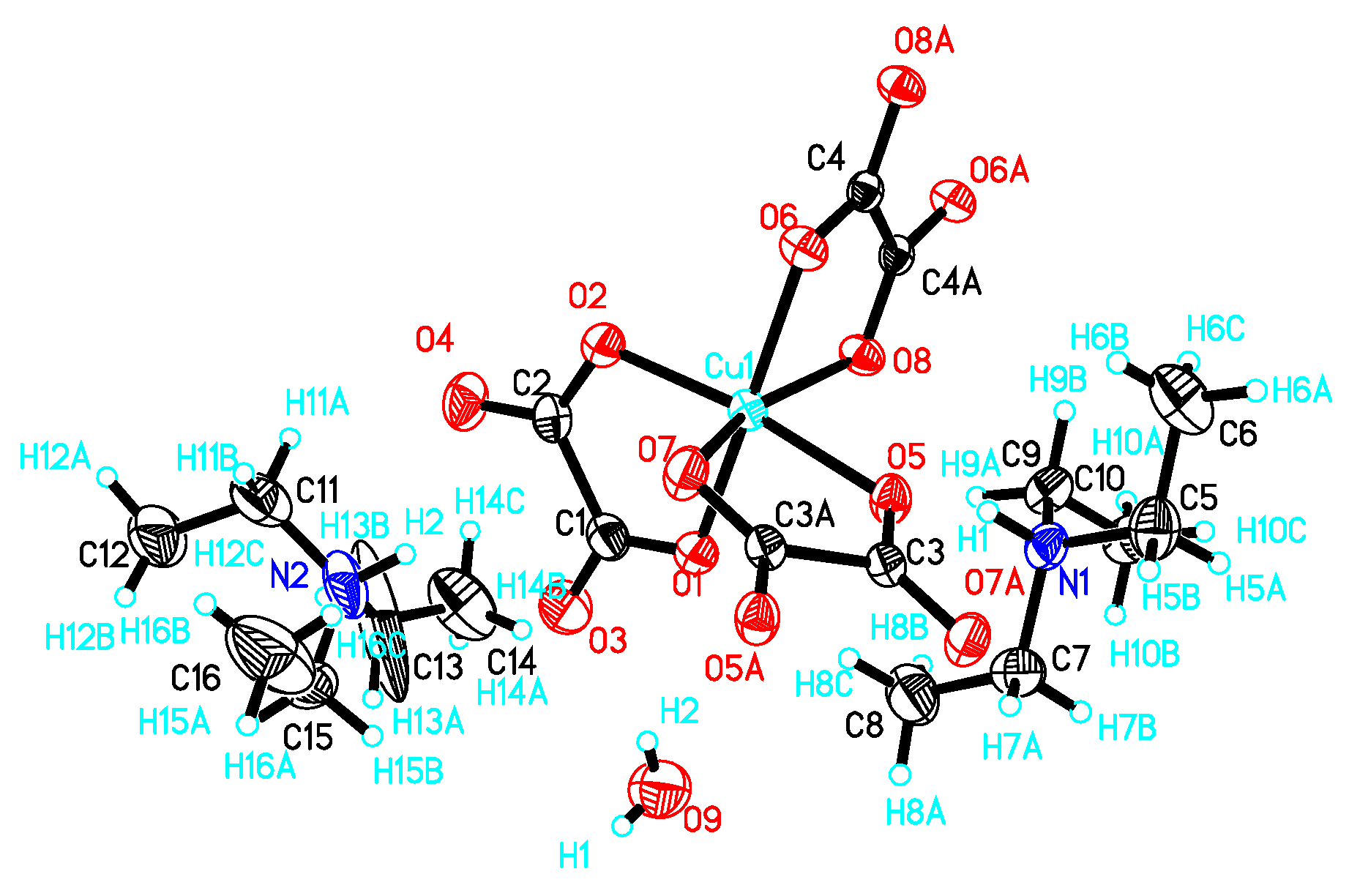
I crystallizes in a triclinic system with space group P 1. There are two (CH3)3NH+, one Cu2+, one oxalate anion, two half-oxalate anions and one-and-a-half H2O coexisting in an independent unit (Figure 1). Cu2+ is coordinated to two O atoms from one bidentate oxalate (O1 and O2) and four O atoms from two disbidentate oxalates in the Q3 Jahn–Teller distortion mode of a CuO6 octahedron [8].
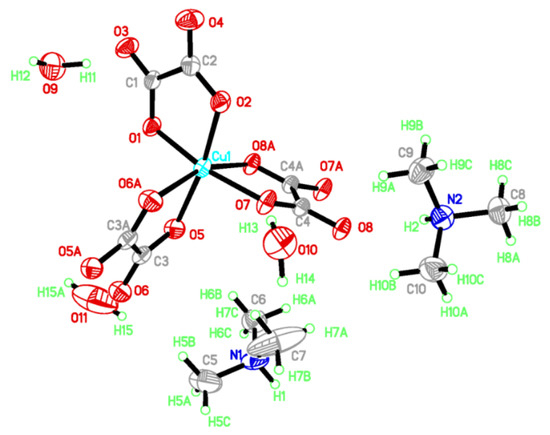
Figure 1.
Atomic structure of I in an independent unit with scheme label and 50% ellipsoids. Asymmetry code for C3A, O5A, O6A: 2 − x, 2 − y, −z; C4A, O7A, O8A: 1 − x, 1 − y, −z; H15A: −x, 2 − y, −z.
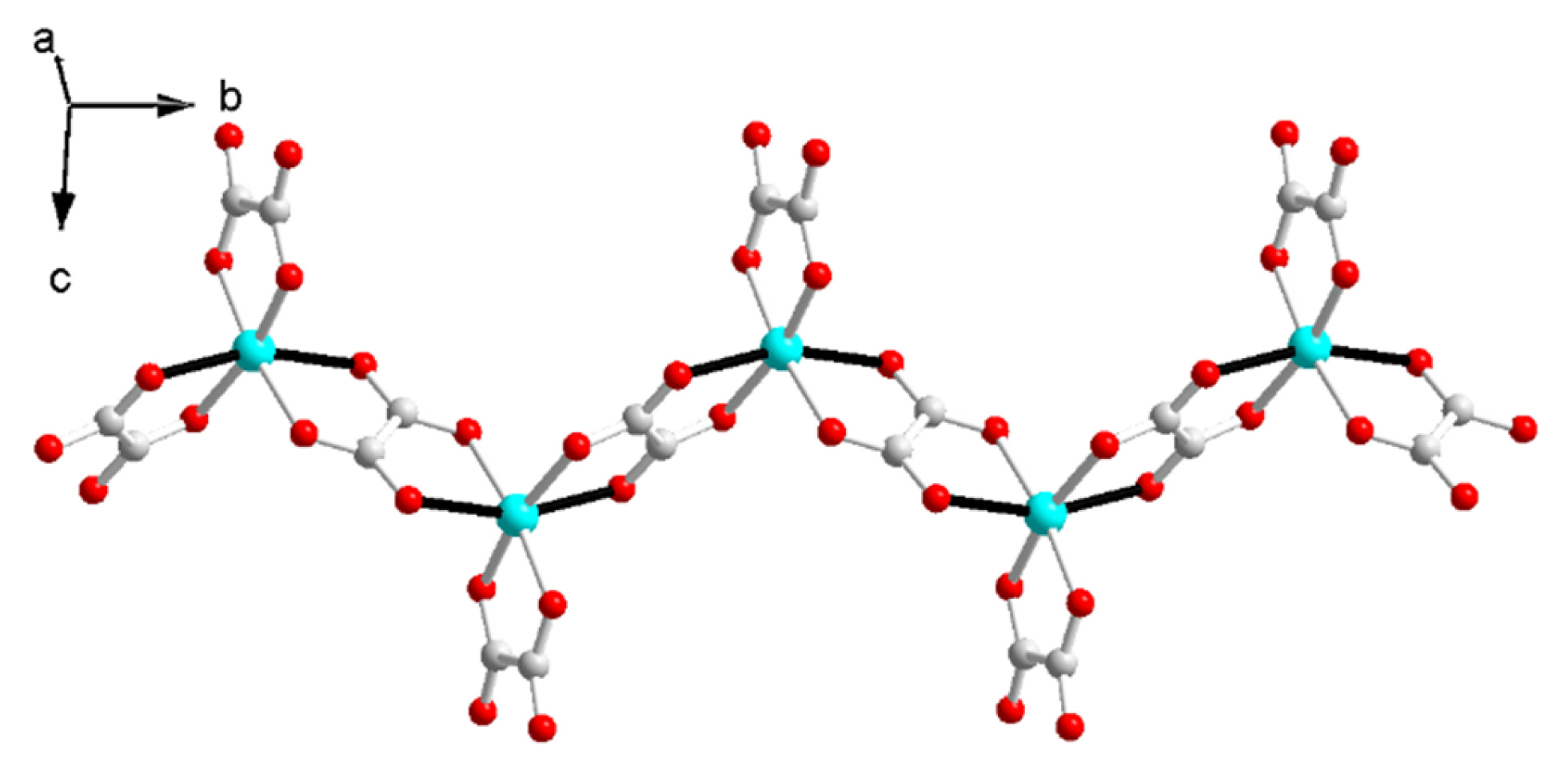
The Cu–O distances are 1.954(2)~1.992(2) Å on the equatorial plane and 2.292(2) Å, 2.329(2) Å from the apex as a result of the Jahn–Teller distortion. The elongated Cu–O bonds (Cu1–O6, Cu1–O8) on the Jahn–Teller-distorted octahedron around Cu2+ are highlighted with solid black lines (Figure 2). The cis O–Cu–O angles are 77.73° and 77.12° for the bridged oxalate, 83.56° for the terminal oxalate, and 90.70°, 98.89°, 90.31°, 96.85°, 93.12° and 100.68° among the terminal and bridged oxalate. The trans O–Cu–O angles are in the range of 161.01(6)~171.46(6)°. The axial Cu to oxalate-oxygen angles are 109.17° (Cu1–O6–C3) and 108.27° (Cu1–O8–C4). A one-dimensional zigzag [Cu(μ-C2O4)(C2O4)2−]n is formed along the b axis.
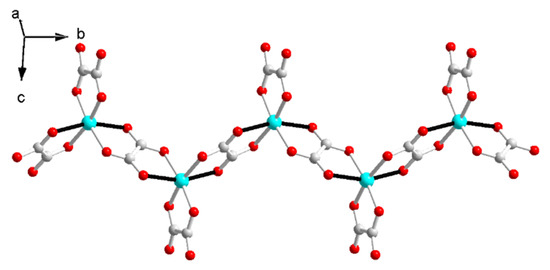
Figure 2.
The zigzag anionic chain in I.
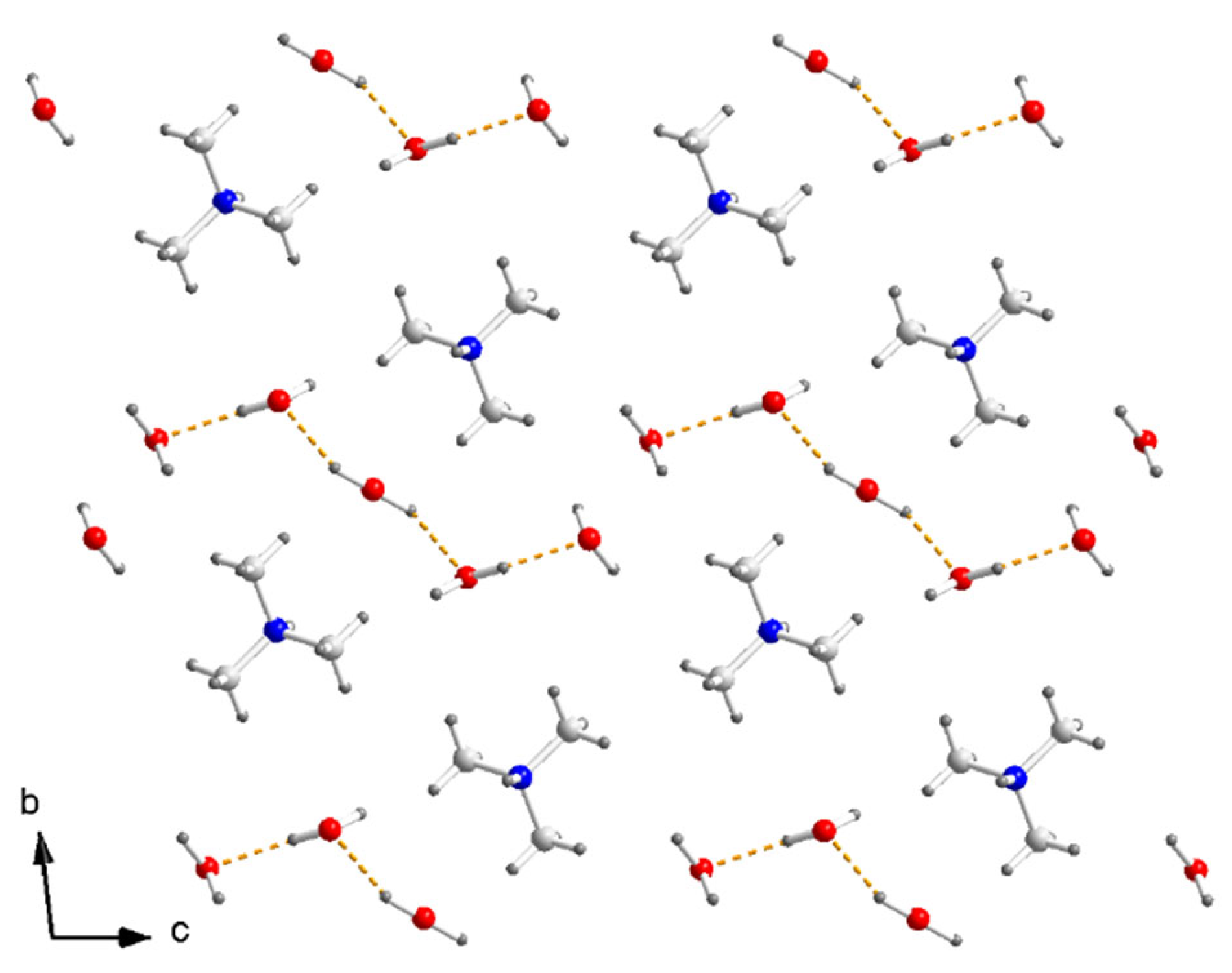
The one-dimensional [Cu(μ-C2O4)(C2O4)2−]n zigzag chain running along the b axis is separated by (CH3)3NH+ (N1) along the c axis, and there are hydrogen bonds N–H⋯O, C–H⋯O between the cation and the out O atom in the oxalate. The anionic sheets composed of a [Cu(μ-C2O4)(C2O4)2−]n chain and (CH3)3NH+ in a 1:1 ratio are separated by a cation layer composed of a zigzag (CH3)3NH+ (N2) chain and a zigzag H2O chain along the c axis. There are hydrogen bonds between neighboring H2O molecules. Five H2O molecules formed a hydrogen-bond [H2O]5 linear cluster along the c axis (Figure 3).
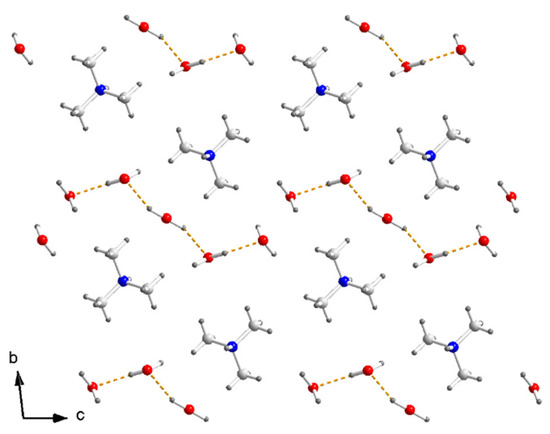
Figure 3.
Arrangement of (CH3)3NH+ and H2O in cation layer of I. Dashed yellow lines are hydrogen bonds between H2O in [H2O]5.
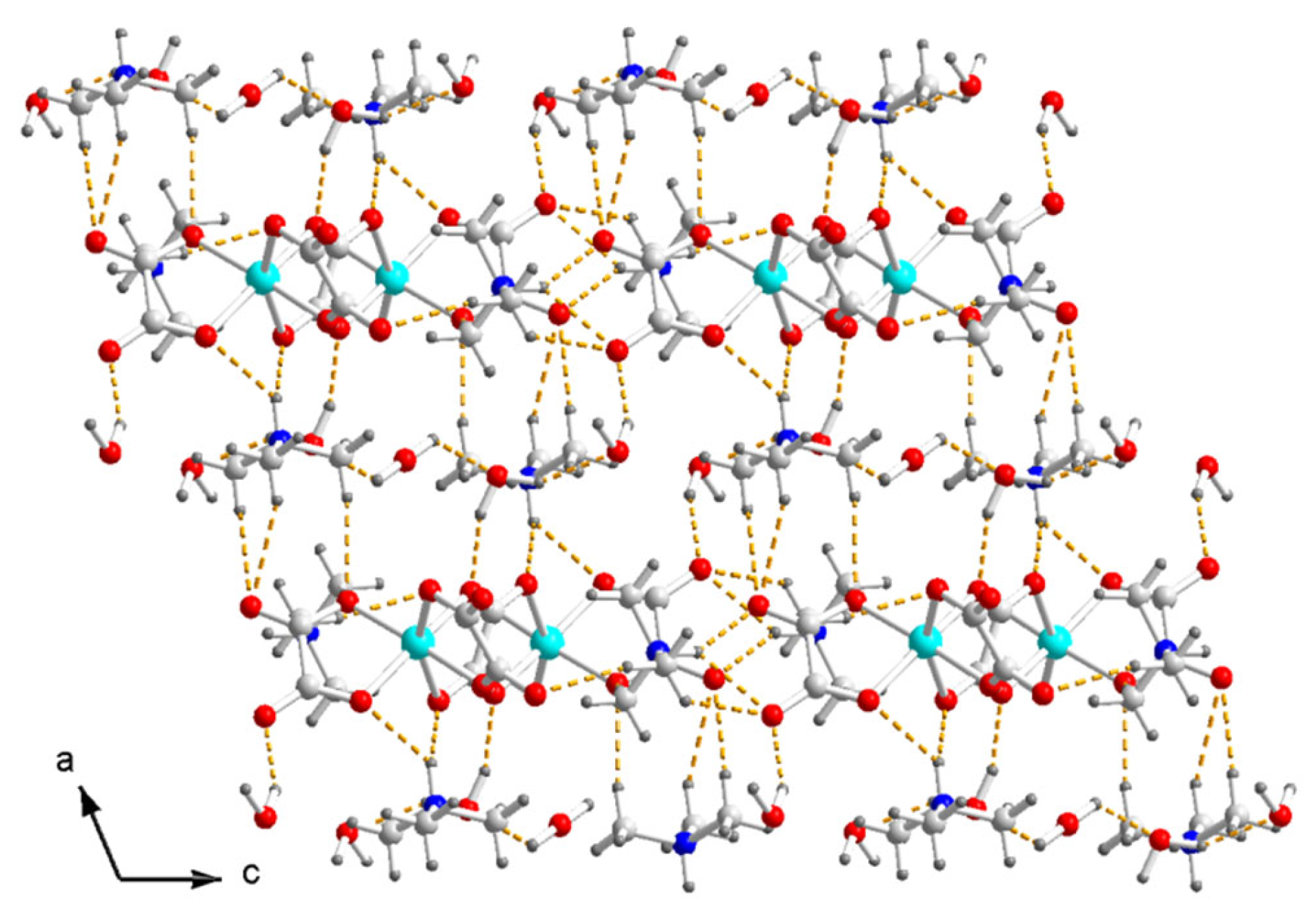
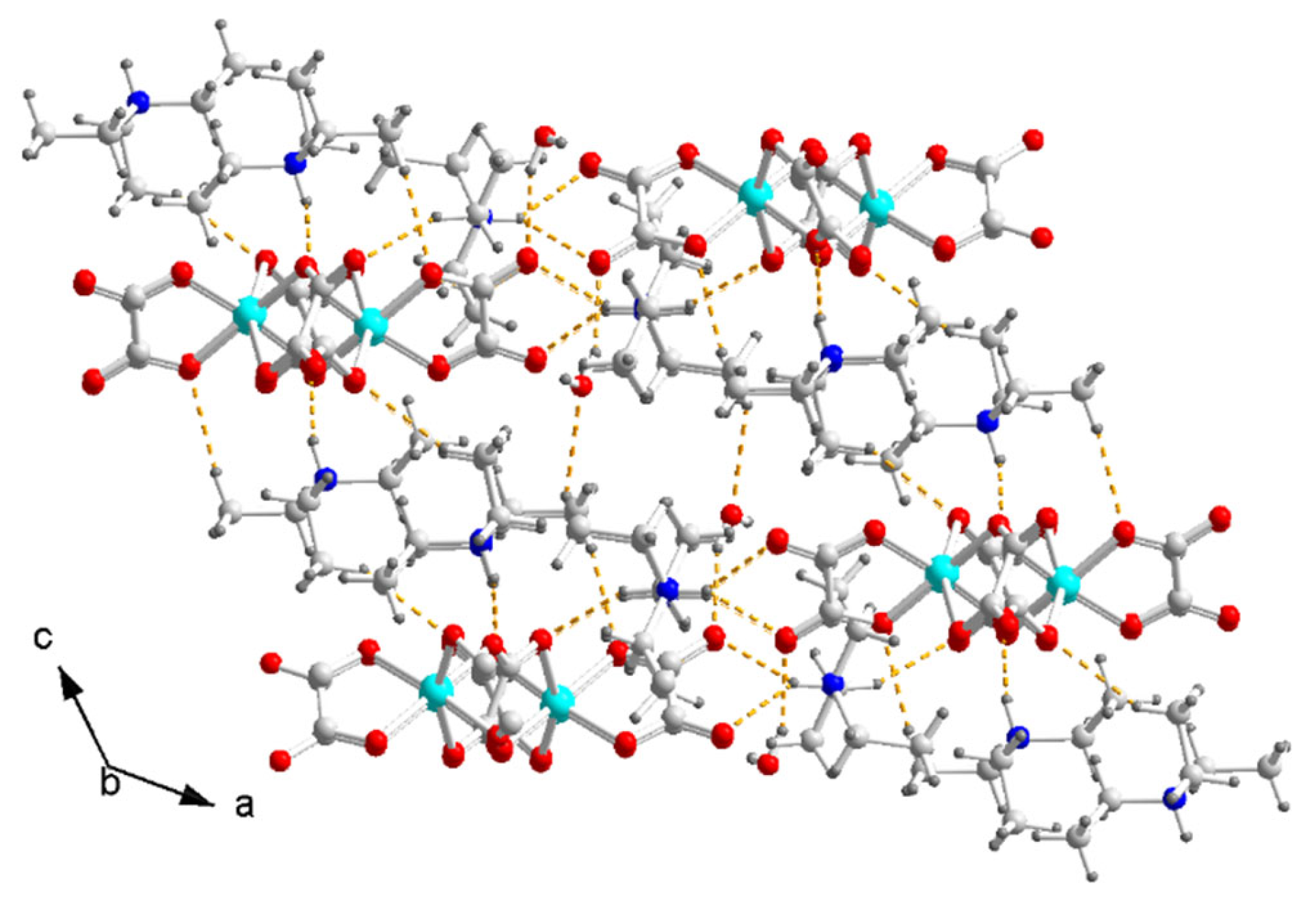
At last, the hydrogen bonds N–H⋯O and C–H⋯O between the ammonium and the inner O of the terminal and bridged oxalate, the O–H⋯O between H2O and the oxalate, and the O–H⋯O between the H2O molecules form a three-dimensional hydrogen-bonded network in crystal (Figure 4). There is no hydrogen bond between the anions.
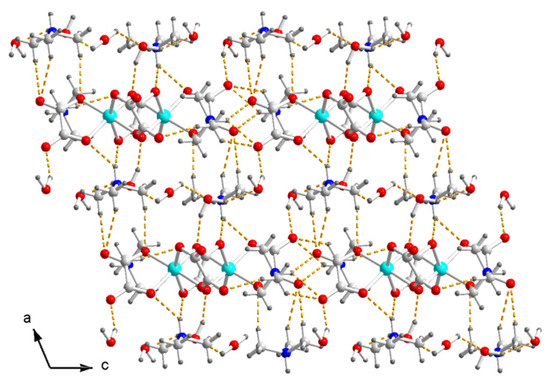
Figure 4.
Packing diagram of I viewed along the b axis. Dash yellow lines are hydrogen bonds. Color code: Cu, cyan; O, red; C, white; N, blue; H, light grey.
II crystallizes in a monoclinic system with the space group P21/c. There are two (C2H5)3NH+, one Cu2+, one and two half oxalates, and one H2O in an independent unit (Figure 5). The Cu2+ is octahedrally coordinated by six O atoms from two bisbidentate oxalates and one bidentate oxalate, as in I, with Cu–O distances of 1.960(2)~1.999(2) Å on the equatorial plane, and Cu1–O8: 2.314(2) Å and Cu1–O7: 2.368(2) Å from the apex. The Cu–O distances of II in the equatorial plane are shorter than the direction of the apex as a result of the Q3 Jahn–Teller distortion mode, as in I. The cis–O–Cu–O angles are 75.93° and 77.79° for the bridged oxalate, 88.31° for the terminal oxalate and 92.34°, 93.72°, 95.98°, 89.66°, 95.48° and 102.48° between the terminal and bridged oxalate. The trans-O–Cu–O angles are in the range of 160.21(7)~172.64(7)°. The axial Cu to oxalate-oxygen angles are 108.09° (Cu1–O1–C3) and 108.53° (Cu1–O8–C4). The Cu–O distances and O–Cu–O angles in I and II are in the same range of the Cu–oxalate coordination polymer [29,30,34,35,37,38]. A one-dimensional oxalate-bridged zigzag [Cu(μ-C2O4)(C2O4)2−]n chain is formed along the b axis.
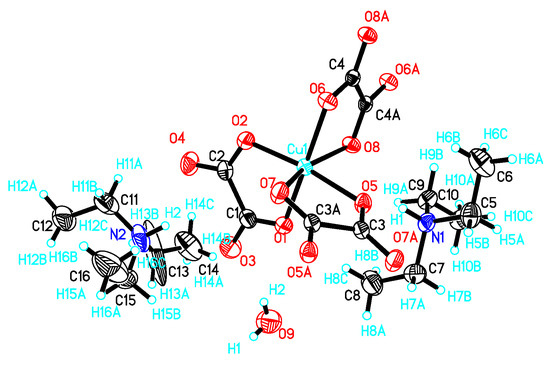
Figure 5.
ORTEP drawing of II in an independent unit with scheme label and 50% ellipsoids. Asymmetry code for C3A, O5, O7: −x, −y, 1 − z; C4A, C6A, C8A: −x, 1 − y, 1 − z.
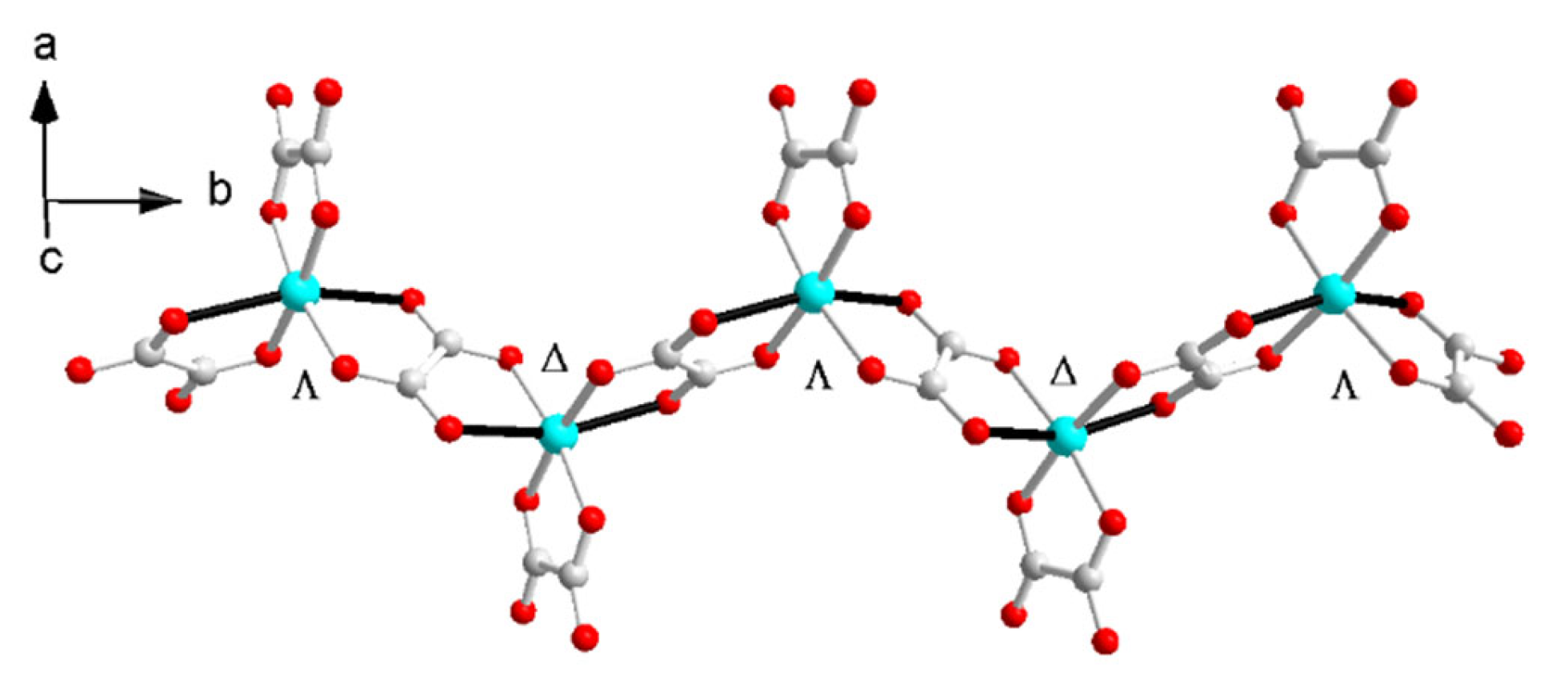
The zigzag chain in I and II is centrosymmetric with the inversion center located at the middle point of the oxalate bridge; thus, the metal sites have a ΔΛΔΛ configuration along the b axis in I and II (Figure 6). It is similar to [(CH3)4N]2Cu(C2O4)2(H2O) [42,43]. The hydrogen bonds between ammonium and the anion and H2O and the anion influence the bond length of the CuO6 octahedron due to the Jahn–Teller distortion. Due to the magnetic orbitals of dx2 − y2 on Cu(II) with the unpaired electrons parallel to each other and the axial Cu to oxalate-oxygen angles, which are sensitive to magnetic interaction and smaller than 109.5°, a ferromagnetic interaction was expected [43,44].
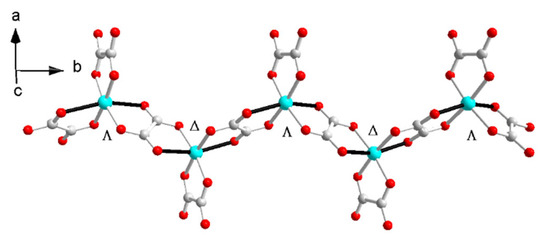
Figure 6.
The zigzag anionic chain in II.
In II, the [Cu(μ-C2O4)(C2O4)2−]n chain is surrounded by zigzag chains of (C2H5)3NH+ and H2O. A pair (C2H5)3NH+ column separates two zigzag chains along the c axis. There are hydrogen bonds between the N of the ammonium and the O on the bridged oxalate: N1–H1⋯O5 2.10 Å/146.9°, N1–H1⋯O8 2.44 Å/131.7°. There are hydrogen bonds between the N of the ammonium and the O on the terminal oxalate: N2–H2⋯O3 2.20 Å/139°, N2–H2⋯O4 2.21 Å/143.1°. There are hydrogen bonds between H2O and the terminal oxalate: O9–H4⋯O3 2.11 Å/159°, O9–H3⋯O4 2.20 Å/164°. There are hydrogen bonds between ammonium and H2O: C8–H8C⋯O1 2.45 Å/174°; C15–H15A⋯O8 2.40 Å/155°. The hydrogen bond forms a two-dimensional (2D) network on the (201) plane (Figure 7). There is no hydrogen bond between the one-dimensional [Cu(μ-C2O4)(C2O4)2−)]n chains.
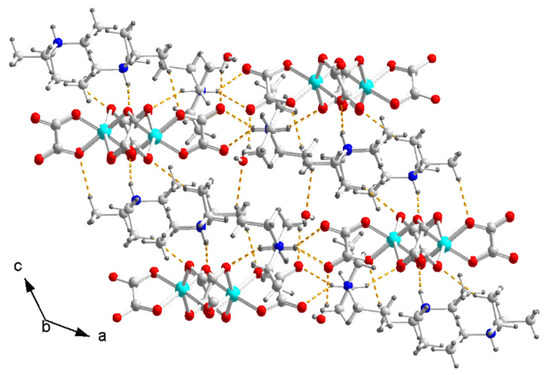
Figure 7.
Arrangement of (C2H5)3NH+, zigzag [Cu(μ-C2O4)(C2O4)2−]n chain and H2O on two-dimensional hydrogen-bond network viewed along the b axis in II. Dashed yellow lines are hydrogen bonds. Color code: Cu, cyan; O, red; C, white; N, blue; H, light grey.
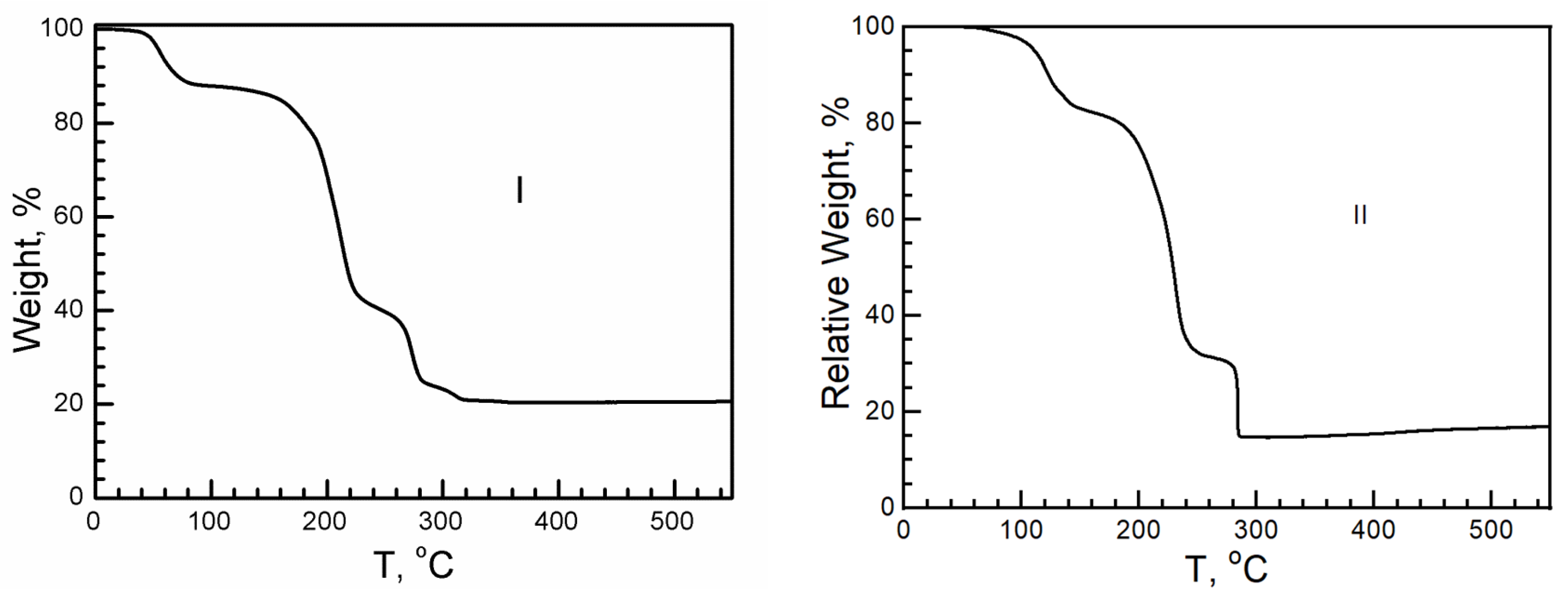
On the basis of the hydrogen bonded cation layer, the resistance, as the proton conductivity under different relative humidities (RH), was measured. Depending on the thermal dynamic analysis, I and II dehydrate at 40 °C (I) and 76 °C (II), losing H2O, with a relative weight of 11.2% in I and 4% in II; therefore, the experiment should be carried out below 40 °C (Figure 8). When the RH increased, the conductivity of I and II increased. Under a relatively high RH, the surfaces of the crystal were covered with debris at first, which was solvable in gel. Although the sample was restored to a solid state when the RH decreased and reached the same value as the beginning, the sample turned out to be in a polycrystalline state but not a single crystal. When a single crystal of I or II was exposed to air under a low relative humidity, such as when the relative humidity was lower than 35%, guest molecules, such as H2O, in I and II would escape from the crystal, leading to crystalline collapse. The crystal surface remained transparent and clear after measurements at 20 °C and an RH of 40%. The resistance comes from the intrinsic behavior of the crystal. The resistance is 1 × 109 Ω·cm in I and 1 × 107 Ω·cm in II. They are insulators.
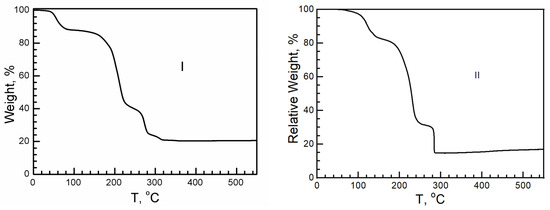
Figure 8.
Schematic TGA curves of I and II. Above 300 °C, the final residue is CuO.
On the basis of the oxalate-bridging and Jahn–Teller distortion of the Cu(II) ion, Cu(II) in an independent unit, and the magnetic properties were studied per Cu2+/mol.
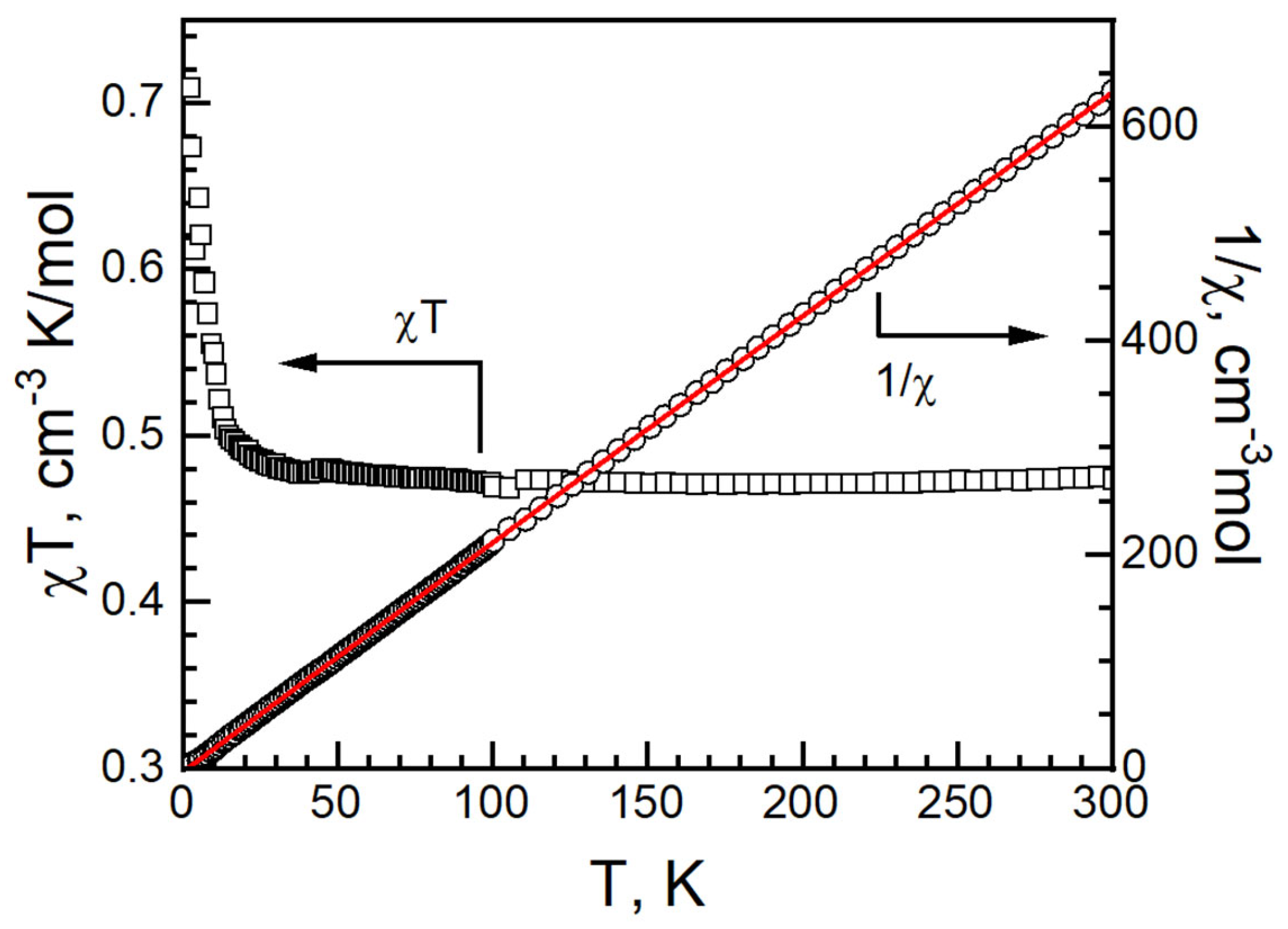
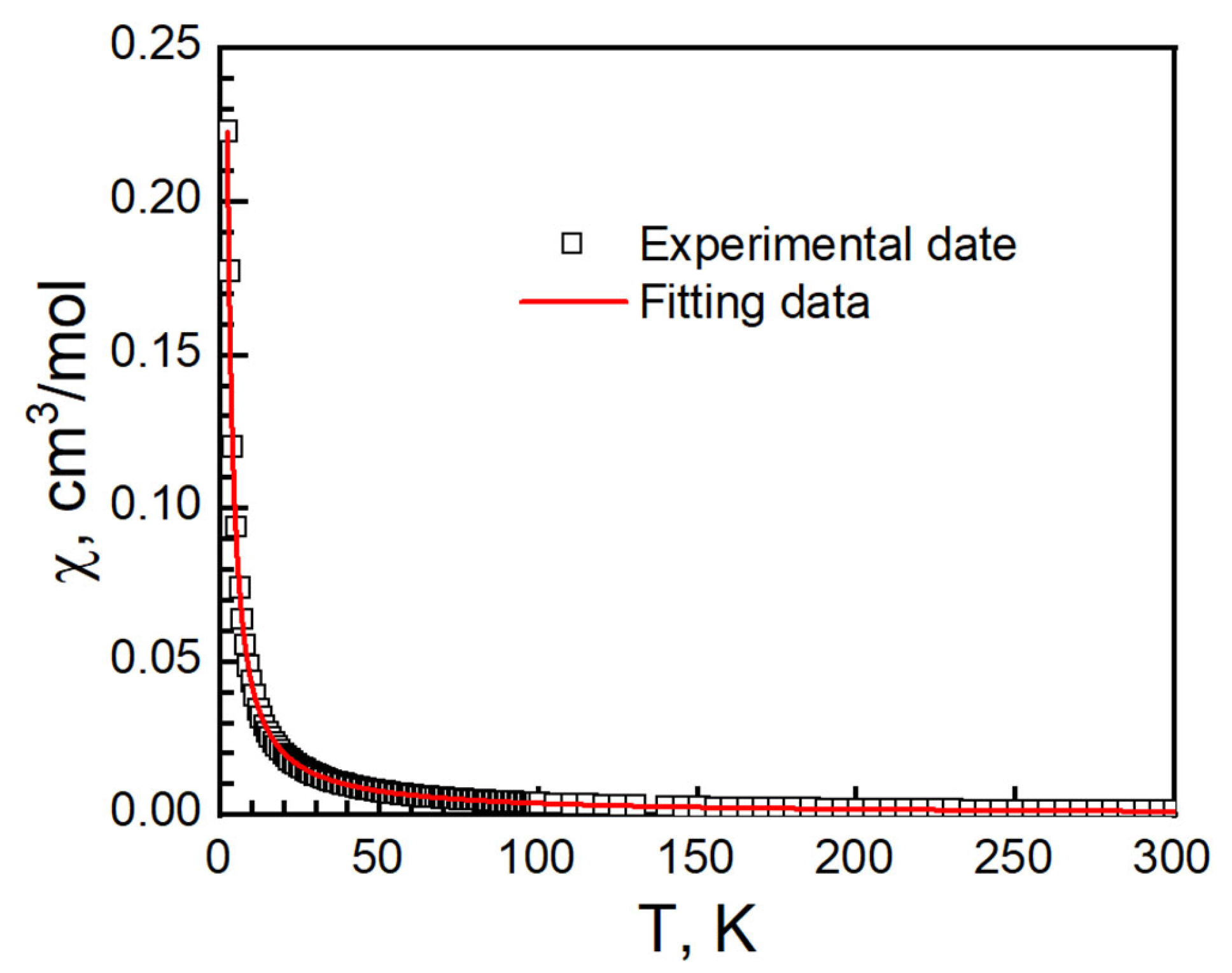
At 300 K, the χT value of I was 0.473 cm3 K mol−1 and g = 2.25. It is higher than 0.375 cm3 K mol−1 for an isolated, spin only Cu(II) ion with S = 1/2, g = 2.00 and in the range of Cu2+ compounds [34,35,36,37,45,46]. The χT value remained stable at 0.478 cm3 K mol−1 at 40 K and increased slowly, reaching 0.71 cm3 K mol−1 at 2 K. No bifurcation is observed from zero-field-cool magnetization and field-cooled magnetization (ZFCM/FCM) measurements from 2 K to 100 K under 100 G (Figure S4). The magnetic data were fitted with the Curie–Weiss law from 2 to 300 K: C = 0.4711(2) cm−1·K/mol, θ = 0.61(6) K and R = 1.37 × 10−5. It suggests a ferromagnetic interaction in I (Figure 9) [47,48].
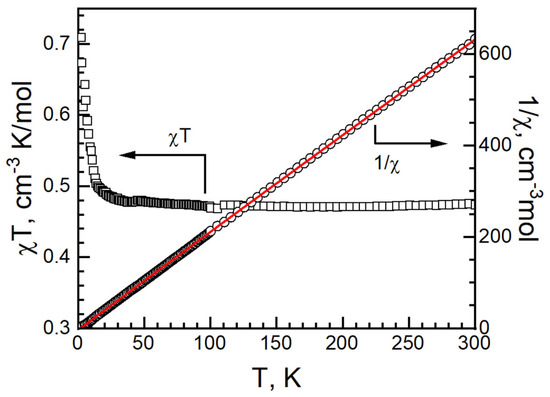
Figure 9.
χT vs. T (left, black empty square), 1/χ vs. T (right, black empty circle) and Curie–Weiss fitting data (red solid) of I.
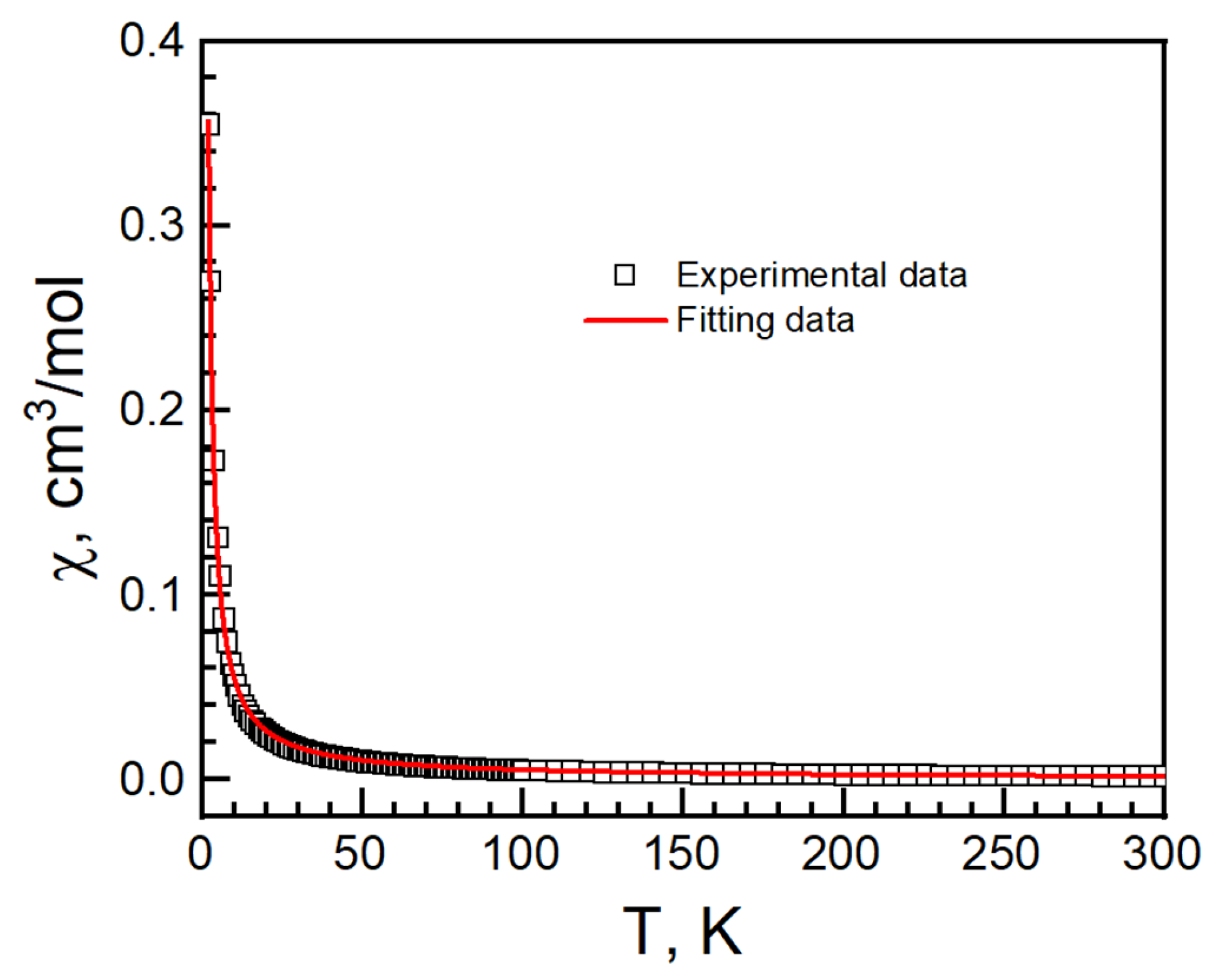
A one-dimensional Baker–Rushbrooke–Gilbert model was used to fit the temperature-dependent magnetization above 2 K, yielding J = 0.60(2) cm−1, g = 2.31(1) and R = 9.2 × 10−4 (Figure 10) [49]. It shows an intrachain ferromagnetic interaction and corresponds with the Curie–Weiss fitting.
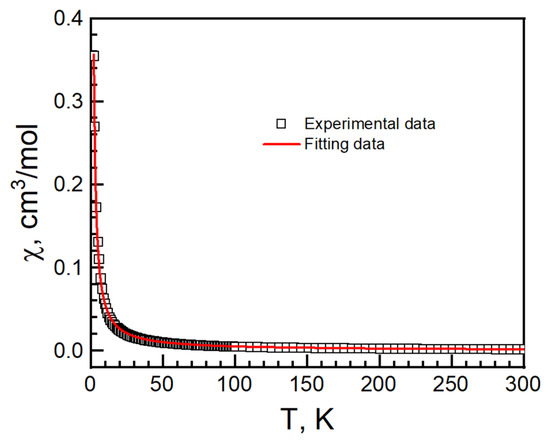
Figure 10.
Temperature-dependent susceptibility of I. Black empty square is experimental data. Red solid curve is the best fit from the Baker–Rushbrooke–Gilbert model.
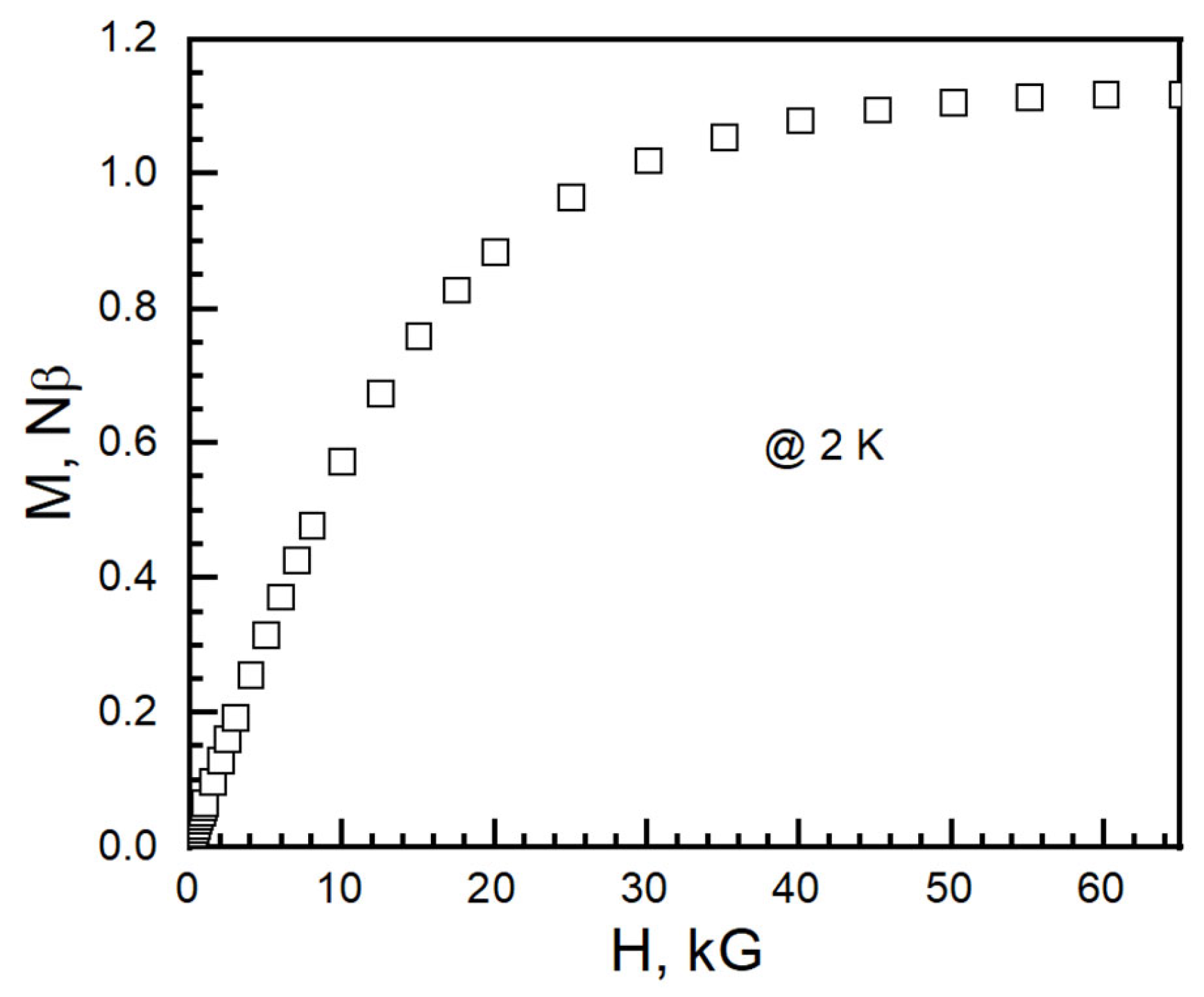
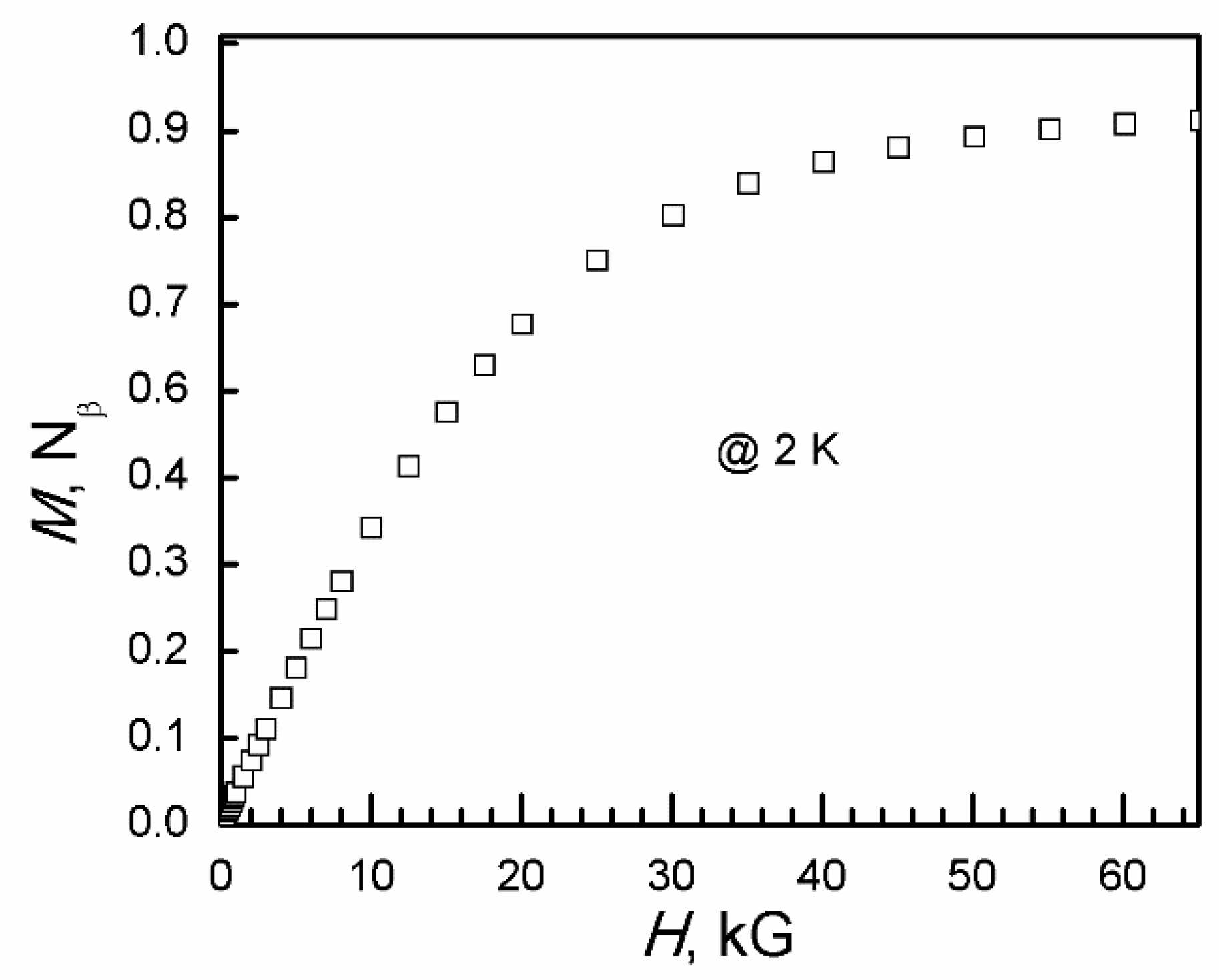
At 2 K, the isothermal magnetization (M) saturated at 1.11 Nβ (N is Avogadro’s number and β is the Bohn magneton, 1 Nβ = 5585 cm−1 G mol−1) at 65 kG (Figure 11). The average anisotropic g-factor calculated from isothermal magnetization at 2 K is 2.22. It is in the range of 2.25 from χT at 300 K and 2.31 from Baker–Rushbrooke–Gilbert model fitting.
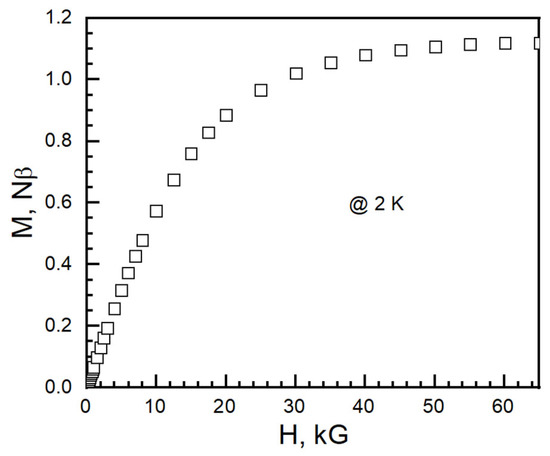
Figure 11.
Isothermal magnetization of I at 2 K.
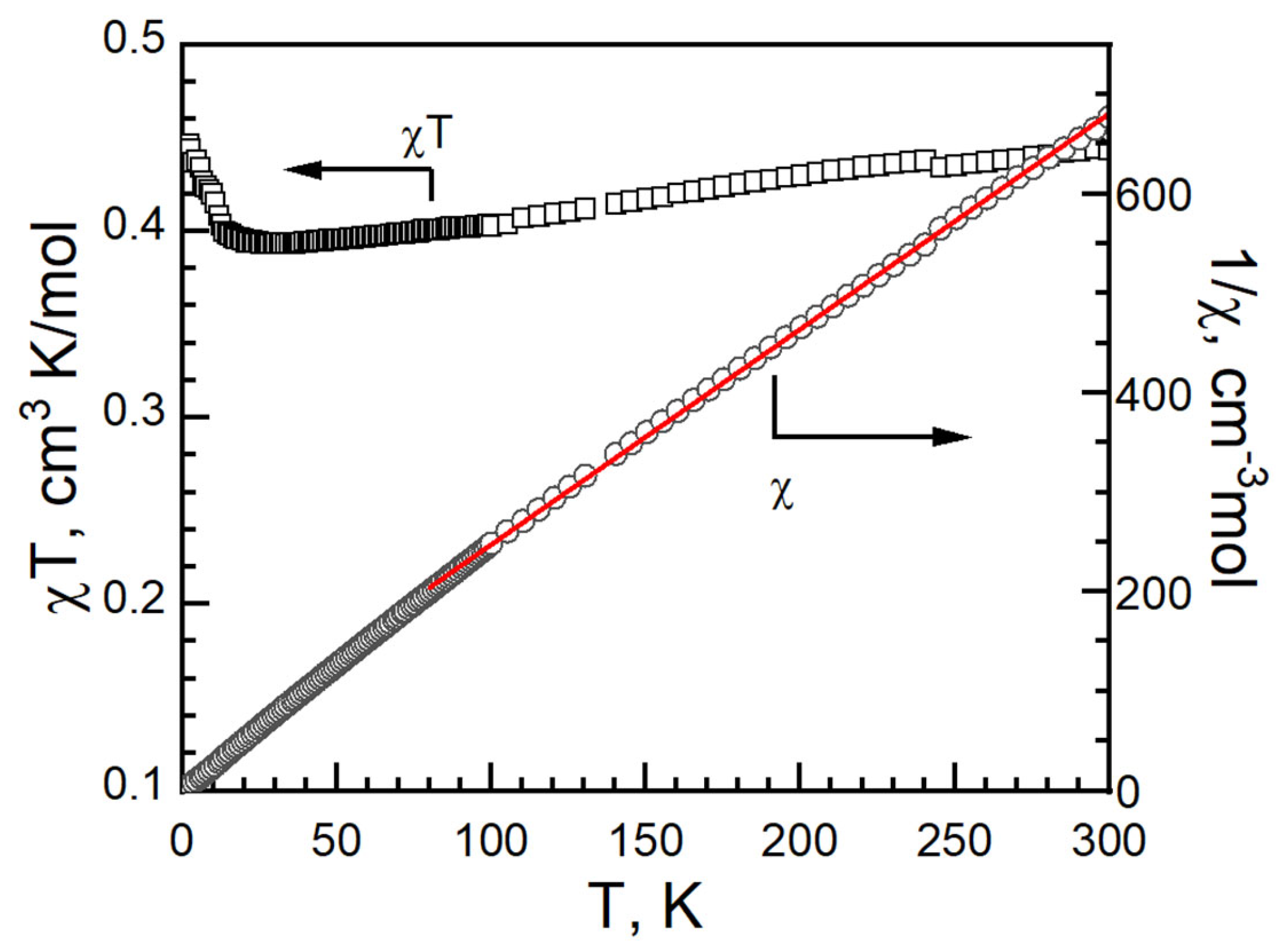
In II, the χT value was 0.443 cm3 K mol−1 at 300 K with a g-factor of 2.13. It is higher than the 0.375 cm3 K mol−1 of an isolated, spin only Cu(II) ion with S = 1/2, g = 2.00. It is in the range of Cu2+ compounds, as in I [34,35,36,37,45,46]. As the temperature decreased, the χT value decreased slowly to 0.393 cm3 K mol−1 around 30 K, and then increased, reaching 0.446 cm3 K mol−1 at 2 K (Figure 12). No bifurcation was observed in the ZFCM/FCM measurement from 2 K to 100 K under 100 G (Figure S5). The magnetic data were fitted with Curie–Weiss law from 80 to 300 K with C = 0.462(1)) cm−1·K/mol, g = −14.2(4) K and R = 3.8 × 10−5 (Figure 12).
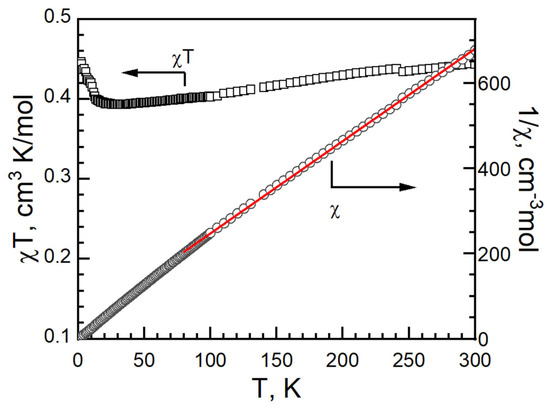
Figure 12.
cT vs. T (left, black empty square), 1/c vs. T (right, black empty circle) and Curie–Weiss fitting data (red solid) of II.
A one-dimensional Baker–Rushbrooke–Gilbert model combined with exchange coupling was used to fit the temperature-dependent magnetization from 2 to 300 K with J = 0.87(2) cm−1, g = 2.035(3), zJ = −0.65(2) cm−1 and R = 6.76 × 10−5 (Figure 13) [50]. It shows that intrachain ferromagnetic interaction is stronger than intrachain antiferromagnetic interaction. The g-factor is in the range of 2.13 from χT at 300 K.
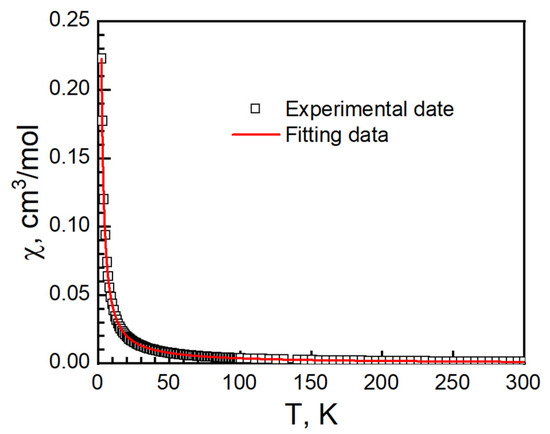
Figure 13.
Temperature-dependent susceptibility of II. Empty solid square, is experimental data. Red solid curve is best fit from the Baker–Rushbrooke–Gilbert model with exchange coupling.
At 2 K, the magnetization increases with increasing field and is saturated at 0.89 Nβ at 65 kG (Figure 14). The average anisotropic g-factor calculated from isothermal magnetization at 2 K is 1.78. Its magnetic behavior is not the same as expected. This means the Jahn–Teller effect is important to the magnetic property of the copper-oxalate framework. This is different from the compounds [CrMn(C2O4)3−]n, where in the ferromagnetic order, temperature and isothermal magnetization at 2 K are the same as those taken from ammonium salts to charge-transfer salts [19,51,52]. Depending on the difference in magnetic behaviors between I and II, the Jahn–Teller effect will help us to obtain molecular-based candidate quantum spin liquid and to look for a new superconductor and colossal magnetoresistance material from copper-oxalate frameworks as cuprate superconductors and colossal magnetoresistance material.
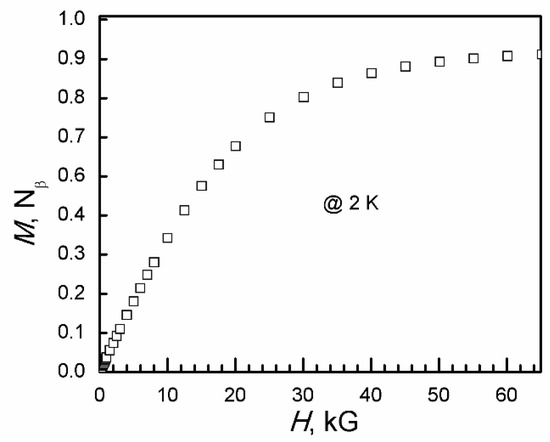
Figure 14.
Isothermal magnetization of II at 2 K.
4. Conclusions
Two one-dimensional copper-oxalate framework compounds were obtained and characterized. The hydrogen bonds among ammonium, H2O and the copper-oxalate framework form a three-dimensional hydrogen-bond network, and there is no hydrogen bond between the one-dimensional [Cu(μ-C2O4)(C2O4)2−]n chains. The Q3-mode Jahn–Teller distortion of elongated CuO6 octahedrons is observed. They are insulators. The Jahn–Teller effect results the ferromagnetic and weak-ferromagnetic interaction between Cu(II) in I and II. No long-range ordering is observed above 2 K.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/magnetochemistry9050120/s1, Table S1. Crystallographic data of I and II; Figure S1. IR spectra on crystalline I (top) and II (bottom); Figure S2. Experimental X-ray powder diffraction pattern of crystalline sample and simulated one based on single crystal structure of I. I shows preferred orientation; Figure S3. Experimental X-ray powder diffraction pattern of crystalline sample and simulated one based on single crystal structure of II; Figure S4. ZFCM/FCM of polycrystal of I under 100 G; Figure S5. ZFCM/FCM of polycrystal of II under 100 G.
Author Contributions
The manuscript was written with the contributions of all authors. Funding acquisition: B.Z., D.Z. and Y.Z. Synthesized the sample: Y.Z. Performed the magnetic measurements: Z.W. Performed the single-crystal X-ray diffraction experiments: T.L., Y.S. and M.L. Performed the X-ray experiments and data analysis: B.Z. Conducted experiments: B.Z. and Z.W. Analyzed the data: B.Z., Y.Z. and Z.W. Wrote the main manuscript text. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China: 22273109, 22073106, 21573242 and 21173230 and the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (Grant No. XDB12030100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The IR spectra, powder X-ray diffraction pattern and ZFCM/FCM of I and II are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states-I—Orbital degeneracy. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1937, 161, 220–235. [Google Scholar]
- Opik, U.; Pryce, M.H.L. Studies of the Jahn-Teller effect. I. A survey of the static problem. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1957, 238, 425–427. [Google Scholar]
- Longuet-Higgins, H.C.; Opik, U.; Pryce, H.I.; Sack, R.A. Studies of the Jahn-Teller effect. II. The dynamical problem. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1958, 244, 1–16. [Google Scholar]
- Bednorz, J.G.; Muller, K.A. Possible high Tc superconductivity in the Ba−La−Cu−O system. Z. Für Phys. B Condens. Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Radaelli, P.G.; Cox, D.E.; Marezio, M.; Cheong, S.W.; Schiffer, P.E.; Ramirez, A.P. Simultaneous Structural, Magnetic, and Electronic Transitions in La 1−x Cax MnO3 with x= 0.25 and 0.50. Phys. Rev. Lett. 1995, 75, 4488–4491. [Google Scholar] [CrossRef]
- Dagotto, E.; Hotta, T.; Moreo, A. Colossal magnetoresistant materials: The key role of phase separation. Phys. Rep. 2001, 344, 1–153. [Google Scholar] [CrossRef]
- Dagotto, E. Complexity in strongly correlated electronic systems. Science 2005, 309, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B. Jahn-Teller phenomena in solids. Annu. Rev. Mater. Sci. 1998, 28, 1–27. [Google Scholar] [CrossRef]
- Bersuker, I.B. The Jahn-Teller Effect; Cambridge University Press, Edinburgh Building: Cambridge, UK, 2006. [Google Scholar]
- Staub, U.; Scagnoli, V.; Muleders, A.M.; Janousch, M.; Honda, Z.; Tonnerre, J.M. Charge/orbital ordering vs. Jahn-Teller distortion in La0. 5Sr1. 5MnO4. Europhys. Lett. 2006, 76, 926–932. [Google Scholar] [CrossRef]
- Clement, R.; Decurtins, S.; Gruselle, M.; Train, C. Polyfunctional two-(2D) and three-(3D) dimensional oxalate bridged bimetallic magnets. Mon. Chem. 2003, 134, 117–135. [Google Scholar] [CrossRef]
- Daze, C.L.F.; Noa, M.A.; Nenwa, J.; Ohrstrom, L. Natural and synthetic metal oxalates–a topology approach. CrystEngComm 2019, 21, 6156–6164. [Google Scholar] [CrossRef]
- Hursthouse, M.B.; Light, M.E.; Price, D.J. One-Dimensional Magnetism in Anhydrous Iron and Cobalt Ternary Oxalates with Rare Trigonal-Prismatic Metal Coordination Environment. Angew. Chem. Int. Ed. 2004, 43, 472–475. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Chang, G.; Wang, Z.; Zhu, D. Crystal-to-Crystal Transformation from K2 [Co (C2O4)2(H2O)2]·4H2O to K2 [Co (μ-C2O4)(C2O4)]. Magnetochemistry 2021, 7, 77. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, Y.; Zhang, B.; Zhu, D. Co(C2O4)(HO(CH2)3OH): An antiferromagnetic neutral zigzag chain compound showing long-range ordering of spin canting. Inorg. Chem. 2008, 47, 9152–9154. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Fujiwara, H.; Kabayashi, H.; Kurmoo, M.; Inoue, K.; Mori, T.; Gao, S.; Zhang, Y.; Zhu, D. Tetrathiafulvalene [FeIII (C2O4)Cl2]: An Organic–Inorganic Hybrid Exhibiting Canted Antiferromagnetism. Adv. Mater. 2005, 17, 1988–1991. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Zhang, Y.; Takahashi, K.; Okano, Y.; Cui, H.; Kobayashi, H.; Inoue, K.; Kurmoo, M.; Pratt, F.; et al. Hybrid Organic—Inorganic Conductor with a Magnetic Chain Anion: κ-BETS2 [FeIII(C2O4)Cl2][BETS = Bis (ethylenedithio) tetraselenafulvalene]. Inorg. Chem. 2006, 45, 3275–3280. [Google Scholar] [CrossRef]
- Tamaki, H.; Zhong, Z.J.; Matsumoto, N.; Kida, S.; Koikawa, M.; Achiwa, N.; Hashimoto, Y.; Okawa, H. Design of metal-complex magnets. Syntheses and magnetic properties of mixed-metal assemblies {NBu4[MCr (ox)3]}x (NBu4+ = tetra (n-butyl) ammonium ion; ox2− = oxalate ion; M = Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+). J. Am. Chem. Soc. 1992, 114, 6974–6979. [Google Scholar] [CrossRef]
- Mathoniere, C.; Nuttall, C.J.; Carling, S.G.; Day, P. Ferrimagnetic mixed-valency and mixed-metal tris (oxalato) iron (III) compounds: Synthesis, structure, and magnetism. Inorg. Chem. 1996, 35, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhang, Y.; Zhang, B.; Zhu, D. Two Homometallic Antiferromagnets Based on Oxalato-Bridged Honeycomb Assemblies:(A)2[MII(C2O4)3](A= Ammonium Salt Derived from Diethylenetriamine; MII= Fe2+, Co2+). Inorg. Chem. 2009, 48, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Rousse, G.; Rodriguez-Carvajal, J. Oxalate-mediated long-range antiferromagnetism order in Fe2(C2O4)3 4H2O. Dalton Trans. 2016, 45, 14311–14316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhang, J.; Li, J.; Zhu, D. A neutral molecular-based layered magnet [Fe (C2O4)(CH3OH)]n exhibiting magnetic ordering at TN≈ 23 K. Dalton Trans. 2008, 5037–5040. [Google Scholar] [CrossRef] [PubMed]
- Decurtins, S.; Schmalle, H.W.; Schneuwly, P.; Esling, J.; Gutlich, P. A concept for the synthesis of 3-dimensional homo-and bimetallic oxalate-bridged networks [M2(ox)3]n. Structural, moessbauer, and magnetic studies in the field of molecular-based magnets. J. Am. Chem. Soc. 1994, 116, 9521–9528. [Google Scholar] [CrossRef]
- Hernandez-Molina, M.; Lloret, F.; Ruiz-Perez, C.; Julve, M. Weak Ferromagnetism in chiral 3-dimensional oxalato-bridged cobalt (II) compounds. Crystal structure of [Co(bpy)3][Co2(ox)3]ClO4. Inorg. Chem. 1998, 37, 4131–4135. [Google Scholar] [CrossRef]
- Coronado, E.; Galan-Mascaros, J.R.; Gomez-Garzia, C.J.; Martinez-Agudo, J.M. Molecule-based magnets formed by bimetallic three-dimensional oxalate networks and chiral tris (bipyridyl) complex cations. The series [ZII (bpy)3][ClO4][MIICrIII (ox)3](ZII= Ru, Fe, Co, and Ni; MII= Mn, Fe, Co, Ni, Cu, and Zn; ox= oxalate dianion). Inorg. Chem. 2001, 40, 113–120. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Zhang, J.; Hao, X.; Zhu, D. Mn (C2O4)(H2O)0.25: An antiferromagnetic oxalato-based cage compound. Dalton Trans. 2011, 40, 5430–5432. [Google Scholar] [CrossRef] [PubMed]
- Mon, M.; Grancha, T.; Verdaguer, M.; Train, C.; Armentano, D.; Pardo, E. Solvent-Dependent Self-Assembly of an Oxalato-Based Three-Dimensional Magnet Exhibiting a Novel Architecture. Inorg. Chem. 2016, 55, 6845–6847. [Google Scholar] [CrossRef] [PubMed]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Coronado, E.; Galan-Mascaros, J.R.; Marti-Gastaldo, C. Single chain magnets based on the oxalate ligand. J. Am. Chem. Soc. 2008, 130, 14987–14989. [Google Scholar] [CrossRef]
- Anderson, P.W. Resonating valence bonds: A new kind of insulator? Mater. Res. Bull. 1973, 8, 153–160. [Google Scholar] [CrossRef]
- Anderson, P.W. The resonating valence bond state in La2CuO4 and superconductivity. Science 1987, 235, 1196–1198. [Google Scholar] [CrossRef]
- Ramirez, A.P. Strongly geometrically frustrated magnets. Annu. Rev. Mater. Sci. 1994, 24, 453–480. [Google Scholar] [CrossRef]
- Balent, I. Spin liquids in frustrated magnets. Nature 2010, 464, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhu, D. (BEDT-TTF)3Cu2(C2O4)3(CH3OH)2: An organic–inorganic hybrid antiferromagnetic semiconductor. Chem. Commun. 2012, 48, 197–198. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Gao, S.; Guo, Y.; Liu, F.; Zhu, D. BETS3[Cu2(C2O4)3](CH3OH)2: An organic–inorganic hybrid antiferromagnetic metal (BETS = bisethylene(tetraselenfulvalene)). CrystEngComm 2013, 15, 3529–3535. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Wang, D.; Yang, D.; Gao, Z.; Chang, G.; Guo, Y.; Mori, T.; Zhao, Z.; et al. Organic–inorganic hybrid metallic conductors based on bis (ethylenedithio) tetrathiafulvalene cations and antiferromagnetic oxalate-bridged copper (ii) dinuclear anions. J. Mater. Chem. C 2022, 10, 2845–2852. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Wang, D.; Baker, P.; Pratt, F.; Zhu, D. Candidate quantum spin liquid due to dimensional reduction of a two-dimensional honeycomb lattice. Sci. Rep. 2014, 4, 6451. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Zhu, D. [(C2H5)3NH]2Cu2(C2O4)3: A three-dimensional metal–oxalato framework showing structurally related dielectric and magnetic transitions at around 165 K. Dalton Trans. 2012, 14, 8509–8511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Baker, P.; Zhang, Y.; Wang, D.; Wang, Z.; Su, S.; Zhu, D.; Pratt, F.L. Quantum spin liquid from a three-dimensional copper-oxalate framework. J. Am. Chem. Soc. 2018, 140, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Shelx-97; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Bain, A.B.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Zhang, B. CCDC843075. Available online: http://www.ccdc.cam.ac.uk (accessed on 5 September 2011).
- Vilela, R.S.; Oliveira, T.I.; Martins, F.T.; Ellena, J.A.; Lloret, F.; Julve, M.; Cangussu, D. Synthesis, crystal structure and magnetic properties of the helical oxalate-bridged copper(II) chain {[(CH3)4N]2[Cu(C2O4)2]·H2O}n. Comptes Rendus Chim. 2012, 15, 856–865. [Google Scholar] [CrossRef]
- Cano, J.; Alemany, P.; Alvarez, M.; Verdaguer, M.; Ruiz, E. Exchange Coupling in Oxalato-Bridged Copper (ii) Binuclear Compounds: A Density Functional Study. Chem. A Eur. J. 1998, 4, 476–484. [Google Scholar] [CrossRef]
- Carlin, R.; van Duyneveldt, A. Magnetic Properties of Transition Metal Compounds; Springer: New York, NY, USA, 1977; p. 69. [Google Scholar]
- Zhang, B.; Zhang, Y.; Zhang, J.; Yan, X.; Zhu, D. Step by step crystal-to-crystal transformation from 1D K2Cu(C2O4)2(H2O)4 (1) to 1D K2Cu(C2O4)2(H2O)2 (2) and then 1D K2Cu(C2O4)2 (3) by dehydration. CrystEngComm 2016, 18, 5062–5065. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publisher Inc.: New York, NY, USA, 1993; pp. 10–29. [Google Scholar]
- Mugiraneza, S.; Hallas, A.M. Tutorial: A beginner’s guide to interpreting magnetic susceptibility data with the Curie-Weiss law. Commun. Phys. 2022, 5, 95. [Google Scholar] [CrossRef]
- Baker, G.A.; Rushbrooke, G.S.; Gilbert, H.E. High-temperature series expansions for the spin-½ Heisenberg model by the method of irreducible representations of the symmetric group. Phys. Rev. 1964, 135, A1272–A1277. [Google Scholar] [CrossRef]
- Carling, R.L. Magnetochemistry; Springer: Berlin/Heidelberg, Germany, 1986; pp. 132–133. [Google Scholar]
- Coronado, E.; Galan-Mascaros, J.R.; Gomez-Gracia, C.J.; Laukhin, V. Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Alberola, A.; Coronado, E.; Galan-Mascaros, J.R.; Gimenez-Saiz, C.; Gomez-Garcia, C.J. A molecular metal ferromagnet from the organic donor bis (ethylenedithio) tetraselenafulvalene and bimetallic oxalate complexes. J. Am. Chem. Soc. 2003, 125, 10774–10775. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).