Binders for Li-Ion Battery Technologies and Beyond: A Comprehensive Review
Abstract
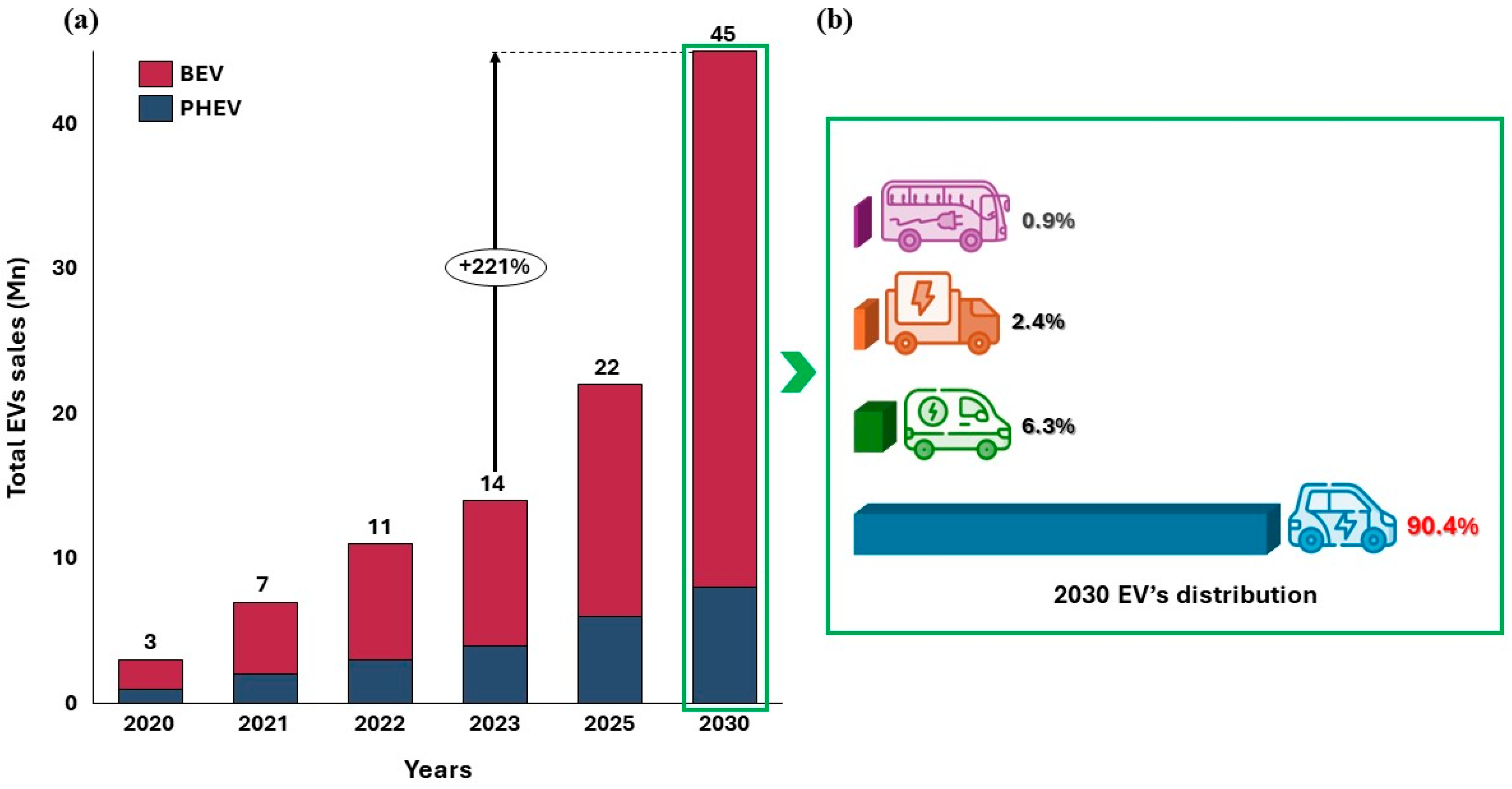
1. Introduction
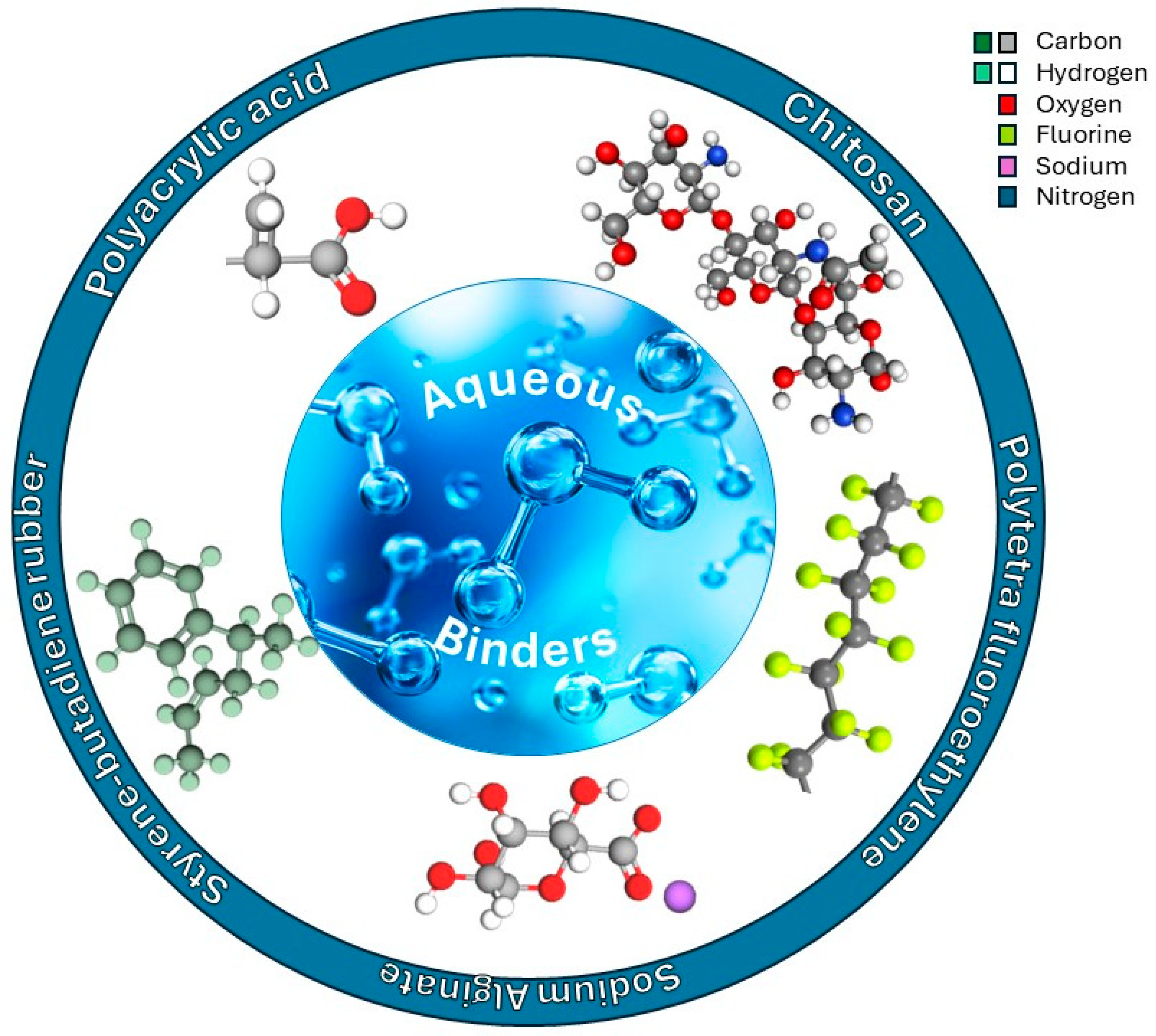
2. Binders for LIBs and SIBs
2.1. Non-Aqueous Binders
2.1.1. Poly (Vinylidene Difluoride) (PVDF)
2.1.2. Polyacrylonitrile (PAN)
2.2. Aqueous Binders
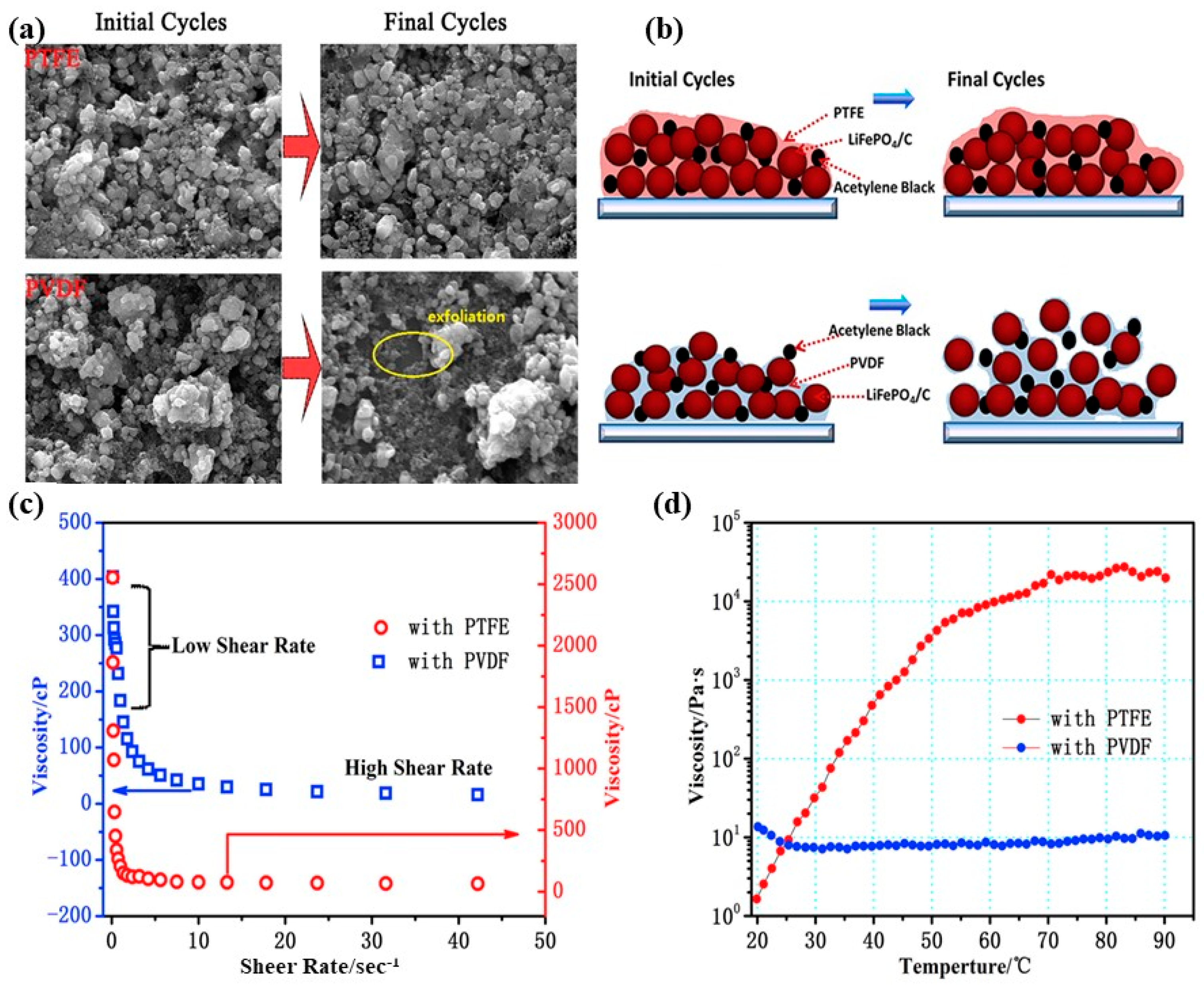
2.2.1. Polytetrafluoroethylene (PTFE)
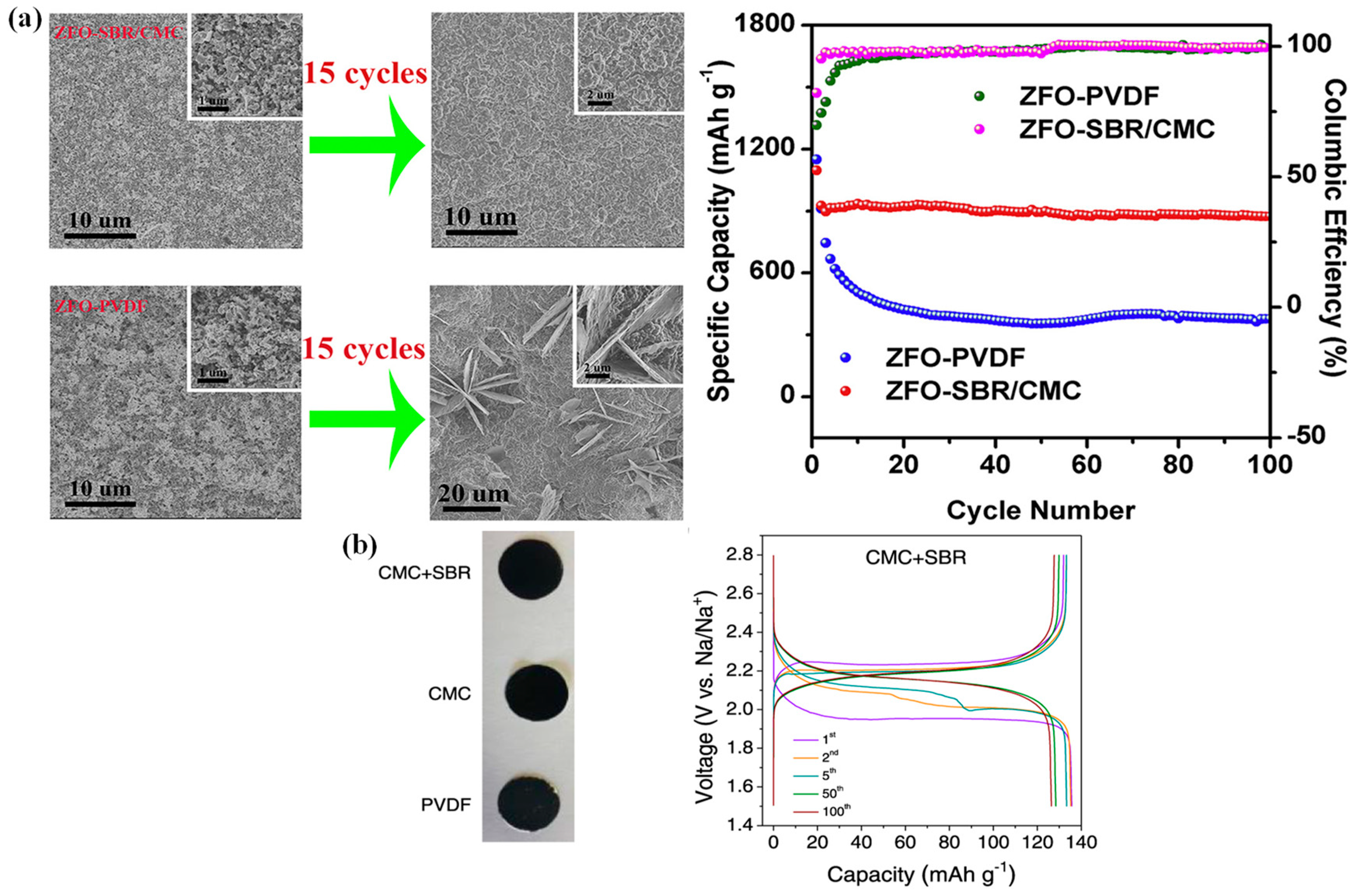
2.2.2. Styrene–Butadiene Rubber (SBR)
2.2.3. Polyacrylic Acid (PAA)
2.2.4. Chitosan
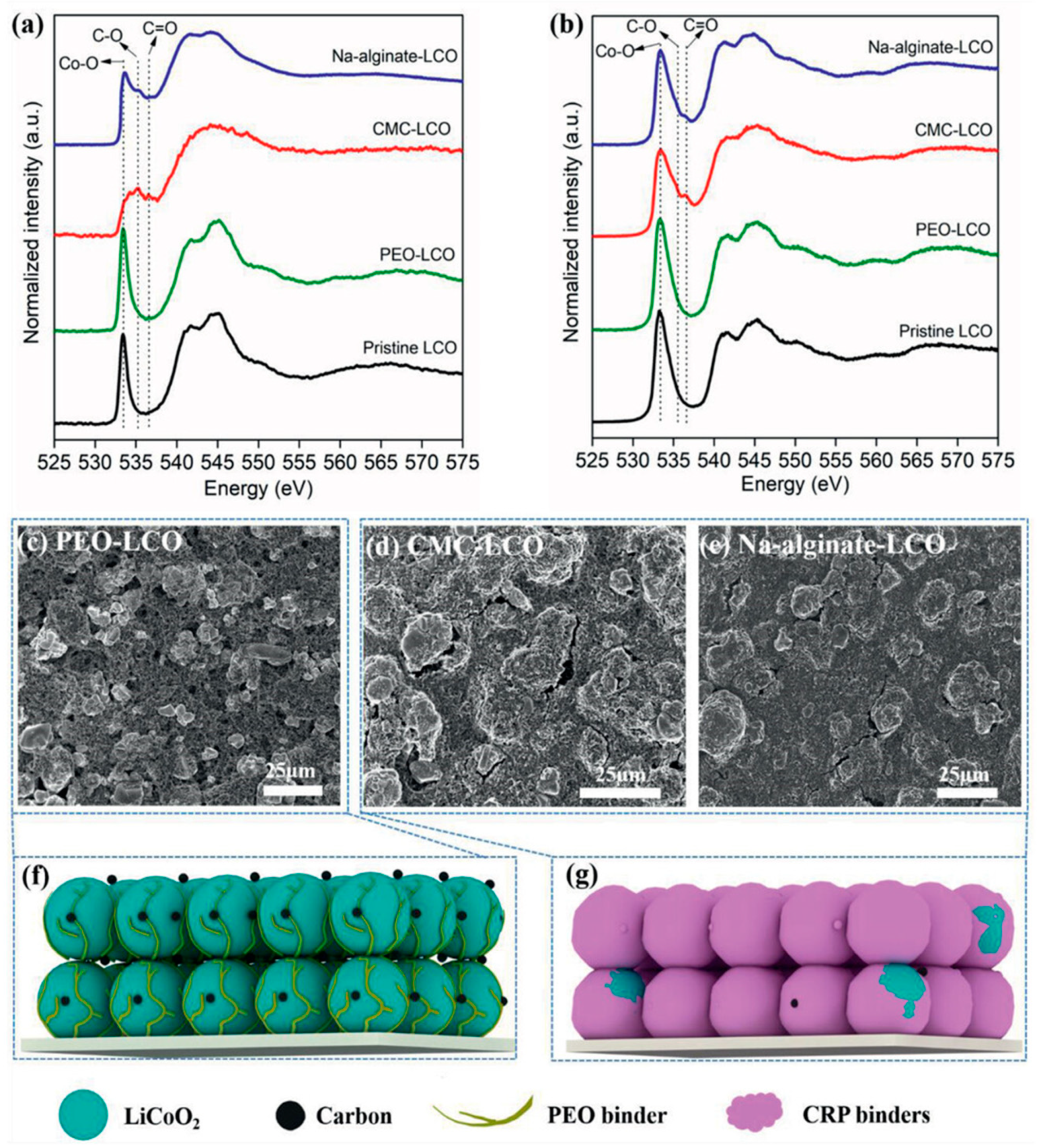
2.2.5. Sodium Alginate (SA)
3. Strategies of Novel Binders
3.1. Conductive Binders
3.2. Composite Binders
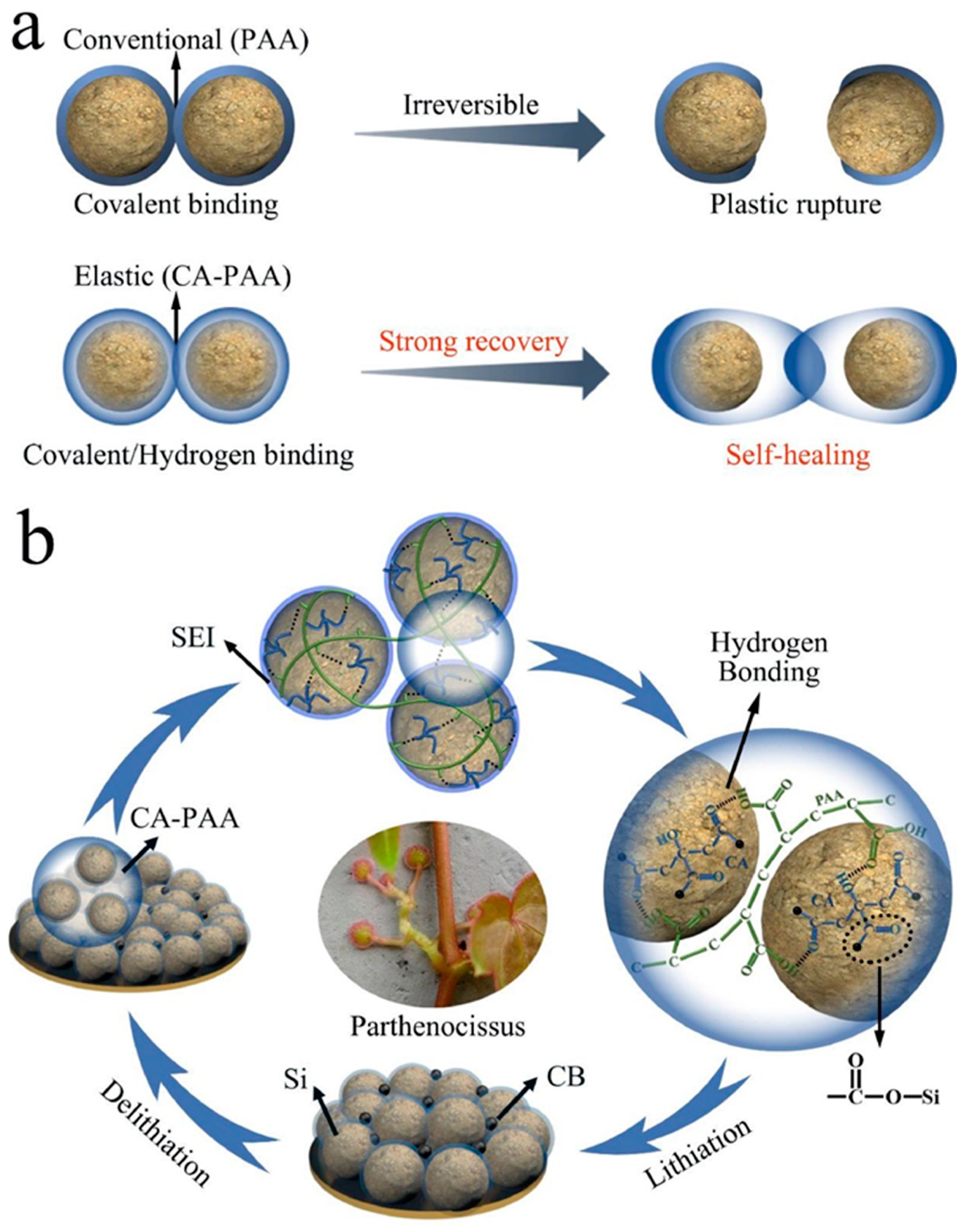
3.3. Self-Healing Binders
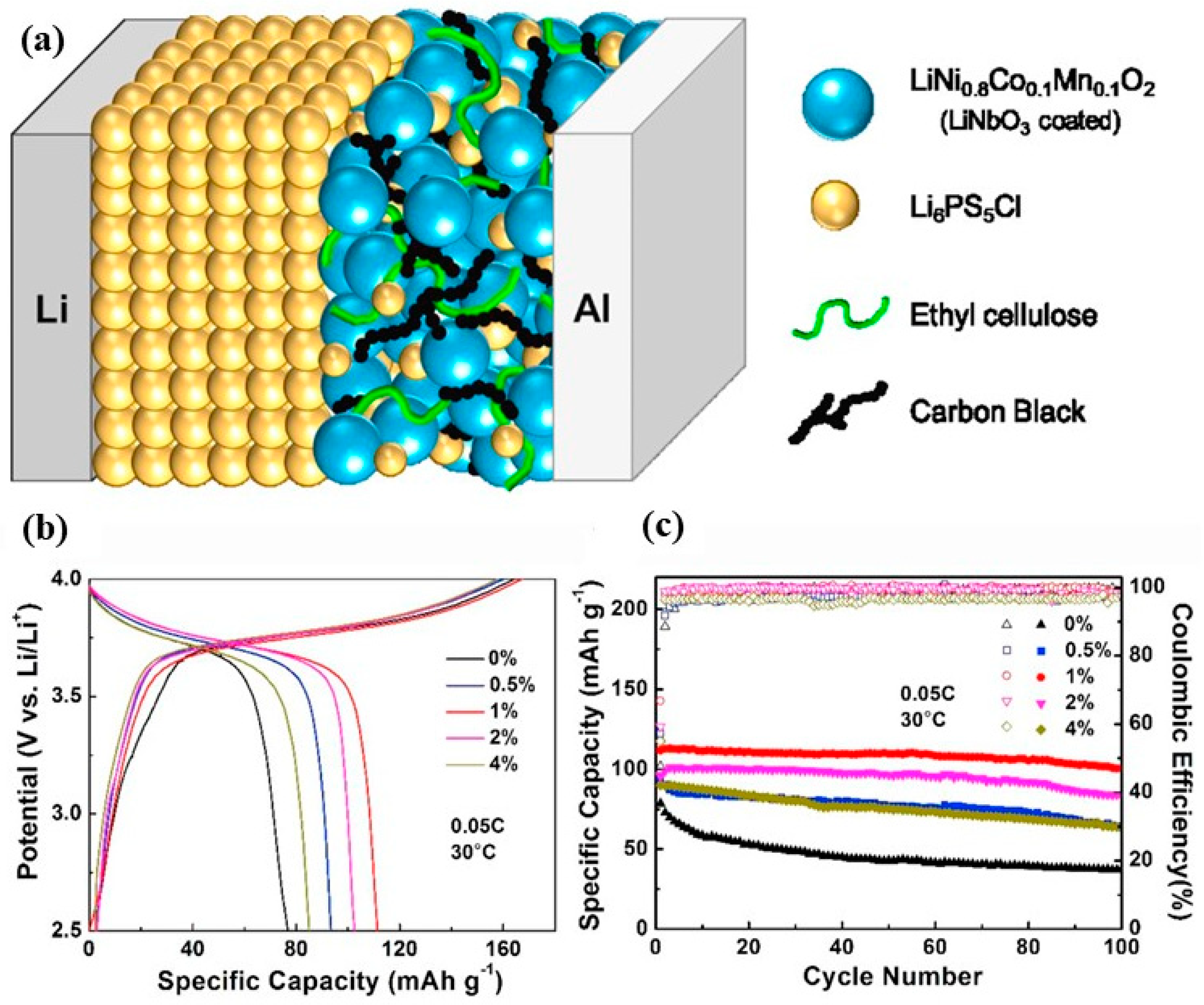
4. Binders for SSBs
5. Properties of Binders
5.1. Mechanical Properties
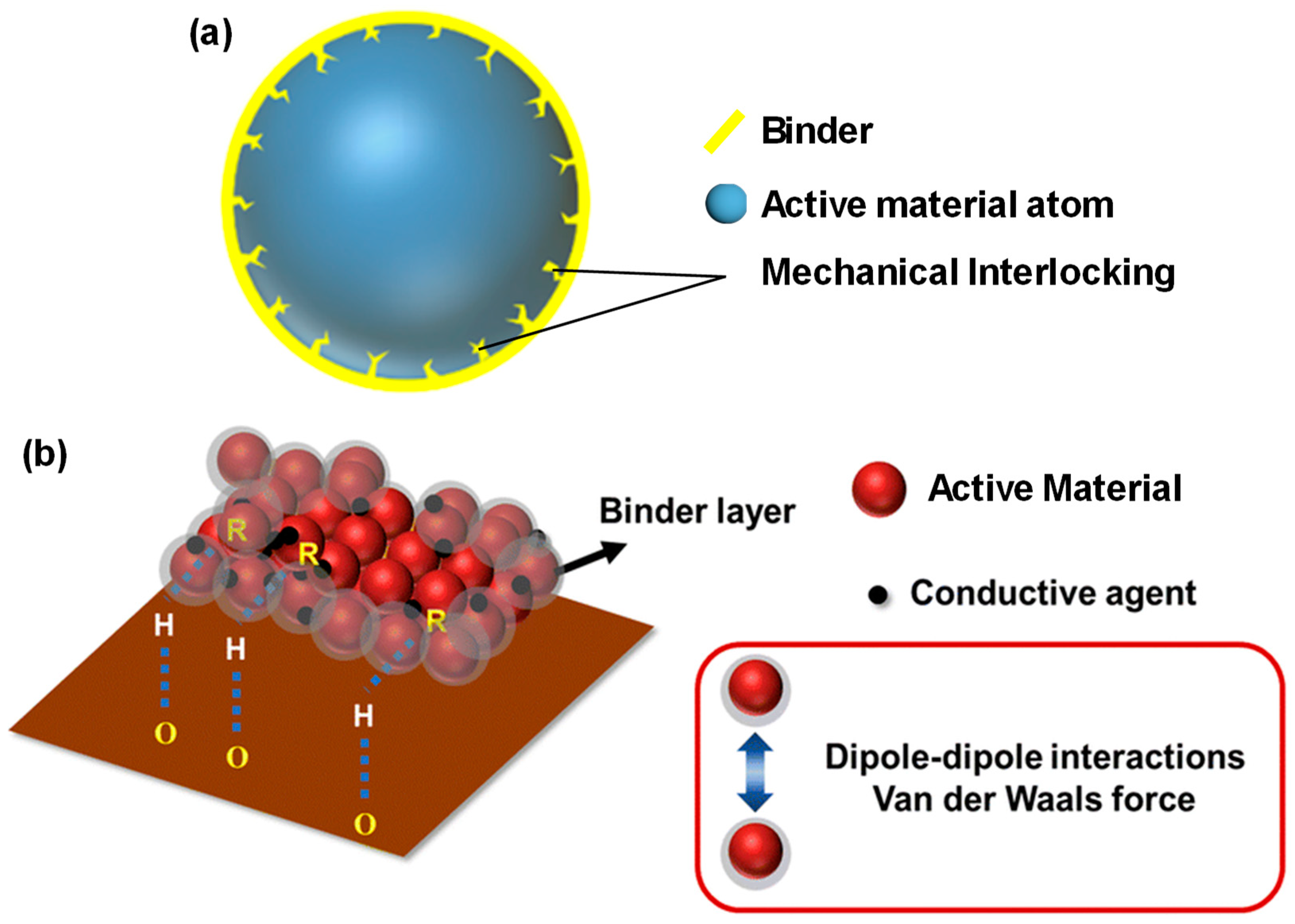
5.1.1. Adhesion
5.1.2. Tensile Strength
5.1.3. Elasticity and Flexibility
5.2. Electrical and Ionic Conductivity
5.3. Electrochemical and Chemical Stability
5.4. Thermal Stability
5.5. Dispersion
6. Binder and Electrolyte
7. Mechanism of Binders
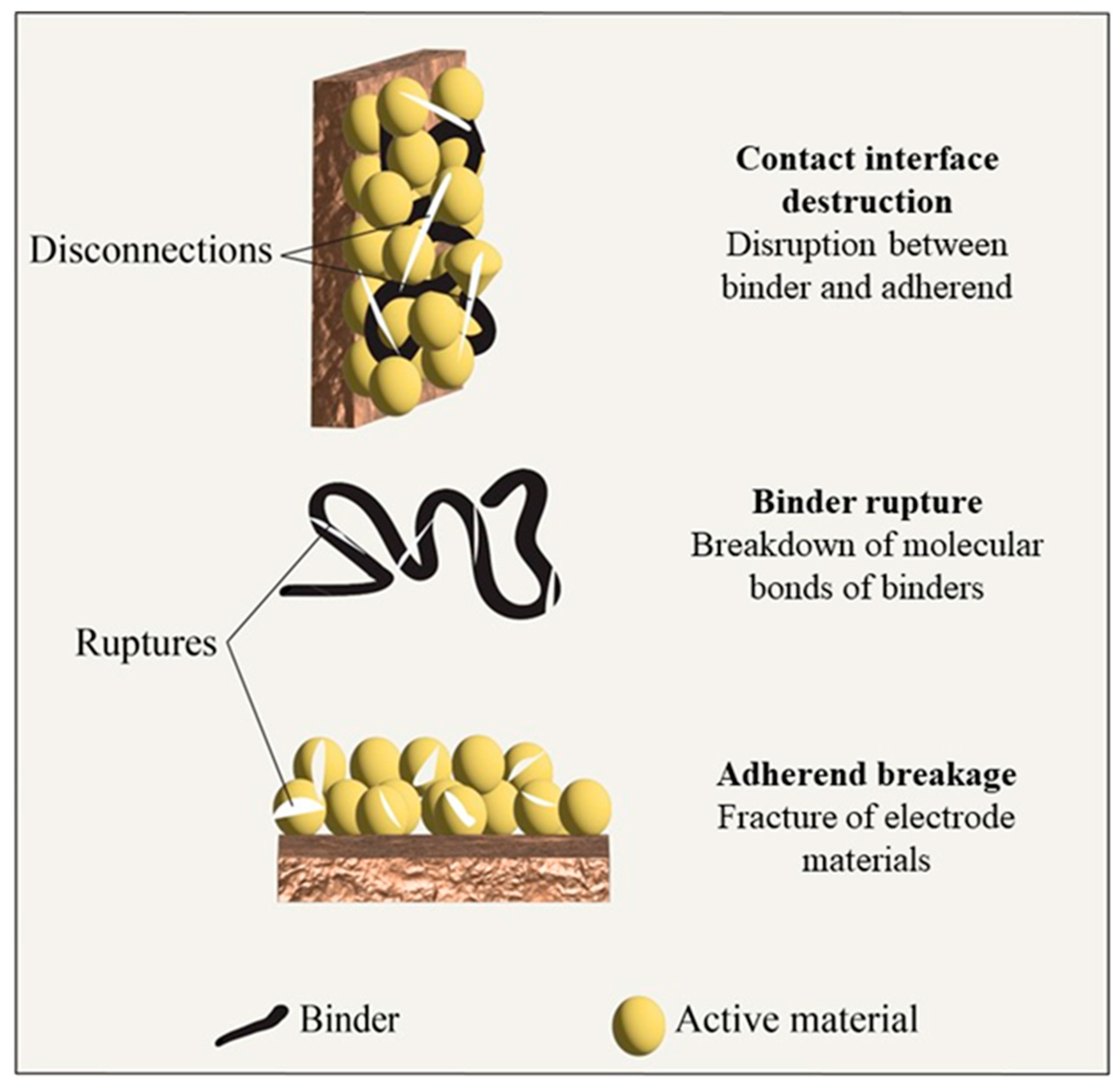
8. Binder Failure Mechanism
9. Commercial Viability of Binders
10. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). “Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change”, Climate Change 2021—The Physical Science Basis. July 2023. Available online: https://www.cambridge.org/core/books/climate-change-2021-the-physical-science-basis/415F29233B8BD19FB55F65E3DC67272B (accessed on 22 January 2024).
- “Greenhouse Gas Concentrations—Canada.ca”. Available online: https://www.canada.ca/en/environment-climate-change/services/environmental-indicators/greenhouse-gas-concentrations.html (accessed on 31 January 2024).
- COP28 UAE. Available online: https://www.cop28.com/en/ (accessed on 8 April 2024).
- Sakunai, T.; Ito, L.; Tokai, A. Environmental impact assessment on production and material supply stages of lithium-ion batteries with increasing demands for electric vehicles. J. Mater. Cycles Waste Manag. 2021, 23, 470–479. [Google Scholar] [CrossRef]
- Transport—Energy System—IEA. Available online: https://www.iea.org/energy-system/transport (accessed on 31 January 2024).
- Liu, W.; Placke, T.; Chau, K.T. Overview of batteries and battery management for electric vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Global EV Data Explorer. Available online: https://www.iea.org/data-and-statistics/data-tools/global-ev-data-explorer (accessed on 14 May 2024).
- Policy Developments—Global EV Outlook 2023—Analysis—IEA. Available online: https://www.iea.org/reports/global-ev-outlook-2023/policy-developments (accessed on 31 January 2024).
- Shin, J.; Choi, J.W.; Shin, J.; Choi, J.W. Opportunities and Reality of Aqueous Rechargeable Batteries. Adv. Energy Mater. 2020, 10, 2001386. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, X.; Qian, W.; Pan, K.; Zhang, X.; Li, L.; Jia, M.; Zhang, S. Exploring More Functions in Binders for Lithium Batteries. Electrochem. Energy Rev. 2023, 6, 36. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Qi, Y.; Sun, T.; Li, X. Unveiling the Roles of Binder in the Mechanical Integrity of Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2013, 160, A1502–A1509. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A. Rechargeable batteries: Technological advancement, challenges, current and emerging applications. Energy 2023, 266, 126408. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Yang, Q.; Guo, X.; Yang, S.; Chen, A.; Liang, G.-J.; Zhi, C.-Y. Strategies of binder design for high-performance lithium-ion batteries: A mini review. Rare Met. 2021, 41, 745–761. [Google Scholar] [CrossRef]
- Nekahi, A.; MR, A.K.; Li, X.; Deng, S.; Zaghib, K. Sustainable LiFePO4 and LiMnxFe1-xPO4 (x = 0.1–1) cathode materials for lithium-ion batteries: A systematic review from mine to chassis. Mater. Sci. Eng. R Rep. 2024, 159, 100797. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Whittingham, M.S. Materials challenges facing electrical energy storage. MRS Bull 2008, 33, 411–419. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interfaces. Chem. Rev. 2020, 120, 6820–6877. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, N.; Kong, L.; Pecht, M. The significance of aqueous binders in lithium-ion batteries. Renew. Sustain. Energy Rev. 2021, 147, 111227. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent progress of advanced anode materials of lithium-ion batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Mohamed, N.; Allam, N.K. Recent advances in the design of cathode materials for Li-ion batteries. RSC Adv. 2020, 10, 21662–21685. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Muhammad, S.; Chernov, S.; Lee, H.; Yoon, J.; Kang, Y.-M.; Yoon, W.-S. Advances in the Cathode Materials for Lithium Rechargeable Batteries. Angew. Chem. Int. Ed. 2020, 59, 2578–2605. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Prakash, A.S. Advancement of technology towards developing Na-ion batteries. J. Power Sources 2018, 378, 268–300. [Google Scholar] [CrossRef]
- Thakur, A.K.; Ahmed, M.S.; Oh, G.; Kang, H.; Jeong, Y.; Prabakaran, R.; Vikram, M.P.; Sharshir, S.W.; Kim, J.; Hwang, J.-Y. Advancement in graphene-based nanocomposites as high capacity anode materials for sodium-ion batteries. J. Mater. Chem. A 2021, 9, 2628–2661. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring Chemical, Mechanical, and Electrical Functionalities of Binders for Advanced Energy-Storage Devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef] [PubMed]
- Jeschull, F.; Maibach, J.; Edström, K.; Brandell, D. On the Electrochemical Properties and Interphase Composition of Graphite: PVdF-HFP Electrodes in Dependence of Binder Content. J. Electrochem. Soc. 2017, 164, A1765–A1772. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, J.; Cui, G. Small things make big deal: Powerful binders of lithium batteries and post-lithium batteries. Energy Storage Mater. 2019, 20, 146–175. [Google Scholar] [CrossRef]
- Saal, A.; Hagemann, T.; Schubert, U.S. Polymers for Battery Applications—Active Materials, Membranes, and Binders. Adv. Energy Mater. 2021, 11, 2001984. [Google Scholar] [CrossRef]
- Ohashi, K.; Miyaki, Y.; Goto, K. Binders for Electrodes and Their Production Method. WO1997032347A1, 4 September 1997. [Google Scholar]
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Chemistry of intercalation compounds: Metal guests in chalcogenide hosts. Prog. Solid State Chem. 1978, 12, 41–99. [Google Scholar] [CrossRef]
- Armand, M.B.; Whittingham, M.S.; Huggins, R.A. The iron cyanide bronzes. Mater. Res. Bull. 1972, 7, 101–107. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x~l): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar]
- Liu, Q.; Su, X.; Lei, D.; Qin, Y.; Wen, J.; Guo, F.; Wu, Y.A.; Rong, Y.; Kou, R.; Xiao, X.; et al. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Akira, Y.; Kenichi, S.; Takayuki, N. Secondary battery. US4668595A, 26 May 1987. [Google Scholar]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Ozawa, K. Lithium-ion rechargeable batteries with LiCoO2 and carbon electrodes: The LiCoO2/C system. Solid State Ion 1994, 69, 212–221. [Google Scholar] [CrossRef]
- Megahed, S.; Scrosati, B. Lithium-ion rechargeable batteries. J. Power Sources 1994, 51, 79–104. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From lithium to sodium: Cell chemistry of room temperature sodium–air and sodium–sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef] [PubMed]
- Newman, G.H.; Klemann, L.P. Ambient Temperature Cycling of an Na-TiS2 Cell. J. Electrochem. Soc. 1980, 127, 2097–2099. [Google Scholar] [CrossRef]
- Delmas, C.; Braconnier, J.; Fouassier, C.; Hagenmuller, P. Electrochemical intercalation of sodium in NaxCoO2 bronzes. Solid State Ion 1981, 3–4, 165–169. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Takahashi, Y.; Kiyabu, T.; Doi, T.; Yamaki, J.-I.; Nishida, T. Layered Transition Metal Oxides as Cathodes for Sodium Secondary Battery. ECS Meet. Abstr. 2006, MA2006-02, 201. [Google Scholar] [CrossRef]
- Li, J.-T.; Wu, Z.-Y.; Lu, Y.-Q.; Zhou, Y.; Huang, Q.-S.; Huang, L.; Sun, S.-G. Water Soluble Binder, an Electrochemical Performance Booster for Electrode Materials with High Energy Density. Adv. Energy Mater. 2017, 7, 1701185. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Higher, Stronger, Better… A Review of 5 Volt Cathode Materials for Advanced Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 922–939. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Memb. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, T.; Lai, Y.; Jia, M.; Li, J. A comparative study of different binders and their effects on electrochemical properties of LiMn 2 O 4 cathode in lithium ion batteries. J. Power Sources 2014, 247, 1–8. [Google Scholar] [CrossRef]
- Marshall, J.E.; Zhenova, A.; Roberts, S.; Petchey, T.; Zhu, P.; Dancer, C.E.; McElroy, C.R.; Kendrick, E. On the Solubility and Stability of Polyvinylidene Fluoride. Polymers 2021, 13, 1354. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Muralidharan, N.; Li, J.; Essehli, R.; Belharouak, I. Sustainable Direct Recycling of Lithium-Ion Batteries via Solvent Recovery of Electrode Materials. Available online: http://energy.gov/downloads/doe-public-access-plan (accessed on 22 March 2024).
- Biensan, P.; Simon, B.; Peres, J.P.; De Guibert, A.; Broussely, M.; Bodet, J.M.; Perton, F. On safety of lithium-ion cells. J. Power Sources 1999, 81–82, 906–912. [Google Scholar] [CrossRef]
- Lux, S.F.; Schappacher, F.; Balducci, A.; Passerini, S.; Winter, M. Low Cost, Environmentally Benign Binders for Lithium-Ion Batteries. J. Electrochem. Soc. 2010, 157, A320. [Google Scholar] [CrossRef]
- Xu, J.; Chou, S.L.; Gu, Q.F.; Liu, H.K.; Dou, S.X. The effect of different binders on electrochemical properties of LiNi1/3Mn1/3Co1/3O2 cathode material in lithium ion batteries. J. Power Sources 2013, 225, 172–178. [Google Scholar] [CrossRef]
- Lee, J.-H.; Paik, U.; Hackley, V.A.; Choi, Y.-M. Effect of Carboxymethyl Cellulose on Aqueous Processing of Natural Graphite Negative Electrodes and their Electrochemical Performance for Lithium Batteries. J. Electrochem. Soc. 2005, 152, A1763. [Google Scholar] [CrossRef]
- Bouguern, M.D.; Reddy, A.K.M.R.; Li, X.; Deng, S.; Laryea, H.; Zaghib, K. Engineering Dry Electrode Manufacturing for Sustainable Lithium-Ion Batteries. Batteries 2024, 10, 39. [Google Scholar] [CrossRef]
- Bai, Y.; Hawley, W.B.; Jafta, C.J.; Muralidharan, N.; Polzin, B.J.; Belharouak, I. Sustainable recycling of cathode scraps via Cyrene-based separation. Sustain. Mater. Technol. 2020, 25, e00202. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xu, Y.; Zhang, H.; Han, X.; Dong, H.; Xu, X.; Chen, C.; Zhang, Y.; Lin, J. Comprehensive Understanding of High Polar Polyacrylonitrile as an Effective Binder for Li-Ion Battery Nano-Si Anodes. ACS Appl. Mater. Interfaces 2016, 8, 8154–8161. [Google Scholar] [CrossRef]
- Dou, W.; Zheng, M.; Zhang, W.; Liu, T.; Wang, F.; Wan, G.; Liu, Y.; Tao, X. Review on the Binders for Sustainable High-Energy-Density Lithium Ion Batteries: Status, Solutions, and Prospects. Adv. Funct. Mater. 2023, 33, 2305161. [Google Scholar] [CrossRef]
- Tron, A.; Park, Y.D.; Mun, J. AlF3-coated LiMn2O4 as cathode material for aqueous rechargeable lithium battery with improved cycling stability. J. Power Sources 2016, 325, 360–364. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, T.; Qu, C.; Lu, H.; Jia, M.; Lai, Y.; Li, J. Cycle performance improvement of LiFePO4 cathode with polyacrylic acid as binder. Electrochim. Acta 2012, 80, 440–444. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Evaluation on a water-based binder for the graphite anode of Li-ion batteries. J. Power Sources 2004, 138, 226–231. [Google Scholar] [CrossRef]
- Yen, J.-P.; Chang, C.-C.; Lin, Y.-R.; Shen, S.-T.; Hong, J.-L. Effects of Styrene-Butadiene Rubber/Carboxymethylcellulose (SBR/CMC) and Polyvinylidene Difluoride (PVDF) Binders on Low Temperature Lithium Ion Batteries. J. Electrochem. Soc. 2013, 160, A1811–A1818. [Google Scholar] [CrossRef]
- Mazouzi, D.; Karkar, Z.; Hernandez, C.R.; Manero, P.J.; Guyomard, D.; Roué, L.; Lestriez, B. Critical roles of binders and formulation at multiscales of silicon-based composite electrodes. J. Power Sources 2015, 280, 533–549. [Google Scholar] [CrossRef]
- Chen, Z.; Christensen, L.; Dahn, J.R. Large-volume-change electrodes for Li-ion batteries of amorphous alloy particles held by elastomeric tethers. Electrochem. Commun. 2003, 5, 919–923. [Google Scholar] [CrossRef]
- Vogt, L.O.; El Kazzi, M.; Berg, E.J.; Villar, S.P.; Novák, P.; Villevieille, C. Understanding the Interaction of the Carbonates and Binder in Na-Ion Batteries: A Combined Bulk and Surface Study. Chem. Mater. 2015, 27, 1210–1216. [Google Scholar] [CrossRef]
- Ostrovskii, D.; Jacobsson, P. Concentrational changes in PAN-based polymer gel electrolyte under current flow: In situ micro-Raman investigation. J. Power Sources 2001, 97–98, 667–670. [Google Scholar] [CrossRef]
- Peramunage, D.; Pasquariello, D.M.; Abraham, K.M. Polyacrylonitrile-Based Electrolytes with Ternary Solvent Mixtures as Plasticizers. J. Electrochem. Soc. 1995, 142, 1789–1798. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li + -conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Gong, L.; Nguyen, M.H.T.; Oh, E.-S. High polar polyacrylonitrile as a potential binder for negative electrodes in lithium ion batteries. Electrochem. Commun. 2013, 29, 45–47. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Lee, B.-R.; Yun, G.; Oh, E.-S.; Lee, H. Effects of binder content on manganese dissolution and electrochemical performances of spinel lithium manganese oxide cathodes for lithium ion batteries. Curr. Appl. Phys. 2015, 15, 429–434. [Google Scholar] [CrossRef]
- Li, G.; Liao, Y.; He, Z.; Zhou, H.; Xu, N.; Lu, Y.; Sun, G.; Li, W. A new strategy to improve the cyclic stability of high voltage lithium nickel manganese oxide cathode by poly(butyl methacrylate-acrylonitrile-styrene) terpolymer as co-binder in lithium ion batteries. Electrochim. Acta 2019, 319, 527–540. [Google Scholar] [CrossRef]
- Yang, X.; Shen, L.; Wu, B.; Zuo, Z.; Mu, D.; Wu, B.; Zhou, H. Improvement of the cycling performance of LiCoO2 with assistance of cross-linked PAN for lithium ion batteries. J. Alloys Compd. 2015, 639, 458–464. [Google Scholar] [CrossRef]
- Tsao, C.-H.; Hsu, C.-H.; Kuo, P.-L. Ionic Conducting and Surface Active Binder of Poly (ethylene oxide)-block-poly(acrylonitrile) for High Power Lithium-ion Battery. Electrochim. Acta 2016, 196, 41–47. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Cheng, S.; Gao, S.; Chai, J.; Jiang, Q.; Liu, Z.; Liu, X.; Liu, J.; Xie, M.; et al. Insight into Superior Electrochemical Performance of 4.5 V High-Voltage LiCoO2 Using a Robust Polyacrylonitrile Binder. ACS Appl. Energy Mater. 2022, 5, 3072–3080. [Google Scholar] [CrossRef]
- Umirov, N.; Moon, S.; Park, G.; Kim, H.-Y.; Lee, K.J.; Kim, S.-S. Novel silane-treated polyacrylonitrile as a promising negative electrode binder for LIBs. J. Alloys Compd. 2020, 815, 152481. [Google Scholar] [CrossRef]
- Jin, J.; Yu, B.; Shi, Z.; Wang, C.; Chong, C. Lignin-based electrospun carbon nanofibrous webs as free-standing and binder-free electrodes for sodium ion batteries. J. Power Sources 2014, 272, 800–807. [Google Scholar] [CrossRef]
- Campéon, B.D.L.; Umezawa, R.; Pandey, A.K.; Ishikawa, T.; Tsuchiya, Y.; Ishigaki, Y.; Kanto, R.; Yabuuchi, N. Efficient Surface Passivation of Ti-Based Layered Materials by a Nonfluorine Branched Copolymer for Durable and High-Power Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 3396–3405. [Google Scholar] [CrossRef] [PubMed]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage—The transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef]
- Priyono, S.; Lubis, B.M.; Humaidi, S.; Prihandoko, B. Heating Effect on Manufacturing Li 4 Ti 5 O 12 Electrode Sheet with PTFE Binder on Battery Cell Performance. IOP Conf. Ser. Mater. Sci. Eng. 2018, 367, 012007. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, X.; Shen, Z.; Ma, H.; Wang, J.; Wang, S.; Liu, L.; Liu, B.; Liu, L.; Zhao, Y. Green water-based binders for LiFePO 4/C cathodes in Li-ion batteries: A comparative study. New J. Chem. 2021, 45, 9846–9855. [Google Scholar] [CrossRef]
- Rae, P.J.; Brown, E.N. The properties of poly(tetrafluoroethylene) (PTFE) in tension. Polymer (Guildf) 2005, 46, 8128–8140. [Google Scholar] [CrossRef]
- Pruitt, L.A. Deformation, yielding, fracture and fatigue behavior of conventional and highly cross-linked ultra high molecular weight polyethylene. Biomaterials 2005, 26, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mays, S.; Lam, D. Material and finite element analysis of poly(tetrafluoroethylene) rotary seals. Plast. Rubber Compos. 2002, 31, 359–363. [Google Scholar] [CrossRef]
- Brown, E.N.; Rae, P.J.; Orler, E.B.; Gray, G.T.; Dattelbaum, D.M. The effect of crystallinity on the fracture of polytetrafluoroethylene (PTFE). Mater. Sci.Eng. C 2006, 26, 1338–1343. [Google Scholar] [CrossRef]
- Brown, E.N.; Dattelbaum, D.M. The role of crystalline phase on fracture and microstructure evolution of polytetrafluoroethylene (PTFE). Polymer (Guildf) 2005, 46, 3056–3068. [Google Scholar] [CrossRef]
- Joyce, J.A. Fracture toughness evaluation of polytetrafluoroethylene. Polym. Eng. Sci. 2003, 43, 1702–1714. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Zhang, K.; Huang, L.; Shen, H.; Chen, Z.; Rong, C.; Wang, G.; Jiang, Z. A Polytetrafluoroethylene-Based Solvent-Free Procedure for the Manufacturing of Lithium-Ion Batteries. Materials 2023, 16, 7232. [Google Scholar] [CrossRef]
- Gao, S.; Su, Y.; Bao, L.; Li, N.; Chen, L.; Zheng, Y.; Tian, J.; Li, J.; Chen, S.; Wu, F. High-performance LiFePO4/C electrode with polytetrafluoroethylene as an aqueous-based binder. J. Power Sources 2015, 298, 292–298. [Google Scholar] [CrossRef]
- Sisanth, K.S.; Thomas, M.G.; Abraham, J.; Thomas, S. General introduction to rubber compounding. In Progress in Rubber Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–39. [Google Scholar] [CrossRef]
- Yoshio, M.; Kugino, S.; Dimov, N. Electrochemical behaviors of silicon based anode material. J. Power Sources 2006, 153, 375–379. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novák, P. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, X.; Zhang, D.; Qiu, H.; Fu, Q.; Na, H.; Guo, Z.; Du, F.; Chen, G.; Wei, Y. Water soluble styrene butadiene rubber and sodium carboxyl methyl cellulose binder for ZnFe2O4 anode electrodes in lithium ion batteries. J. Power Sources 2015, 285, 227–234. [Google Scholar] [CrossRef]
- Li, J.; Le, D.-B.; Ferguson, P.P.; Dahn, J.R. Lithium polyacrylate as a binder for tin–cobalt–carbon negative electrodes in lithium-ion batteries. Electrochim. Acta 2010, 55, 2991–2995. [Google Scholar] [CrossRef]
- Park, K.; Yoo, H.E.; Jung, Y.; Ryu, M.; Myeong, S.; Lee, D.; Kim, S.C.; Kim, C.; Kim, J.; Kwon, J.; et al. Styrene-butadiene rubber patterned current collector for improved Li-ion kinetics of the anode for high energy density lithium-ion batteries. J. Power Sources 2023, 577, 233238. [Google Scholar] [CrossRef]
- Eom, J.-Y.; Kim, S.-I.; Ri, V.; Kim, C. The effect of polymeric binders in the sulfur cathode on the cycling performance for lithium–sulfur batteries. Chem. Commun. 2019, 55, 14609–14612. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Yue, F.-S.; Li, S.-C.; Zhang, Y.; Tian, Z.-R.; Xu, Q.; Xin, S.; Guo, Y.-G. Advances of polymer binders for silicon-based anodes in high energy density lithium-ion batteries. InfoMat 2021, 3, 460–501. [Google Scholar] [CrossRef]
- Zhang, Y.; Grant, A.; Carroll, A.; Gulzar, U.; Ferguson, M.; Roy, A.; Nicolosi, V.; O’Dwyer, C. Water-Soluble Binders That Improve Electrochemical Sodium-Ion Storage Properties in a NaTi2(PO4)3 Anode. J. Electrochem. Soc. 2023, 170, 050529. [Google Scholar] [CrossRef]
- Isozumi, H.; Horiba, T.; Kubota, K.; Hida, K.; Matsuyama, T.; Yasuno, S.; Komaba, S. Application of modified styrene-butadiene-rubber-based latex binder to high-voltage operating LiCoO2 composite electrodes for lithium-ion batteries. J. Power Sources 2020, 468, 228332. [Google Scholar] [CrossRef]
- Jolley, M.J.; Pathan, T.S.; Jenkins, C.; Loveridge, M.J. Investigating the effect of the degree of cross-linking in styrene butadiene rubber on the performance of graphite anodes for the use in lithium-ion batteries. J. Appl. Polym. Sci. 2024, 141, e55135. [Google Scholar] [CrossRef]
- Ma, Z.; Lyu, Y.; Yang, H.; Li, Q.; Guo, B.; Nie, A. Systematic investigation of the Binder’s role in the electrochemical performance of tin sulfide electrodes in SIBs. J. Power Sources 2018, 401, 195–203. [Google Scholar] [CrossRef]
- Parikh, P.; Sina, M.; Banerjee, A.; Wang, X.; D’Souza, M.S.; Doux, J.M.; Wu, E.A.; Trieu, O.Y.; Gong, Y.; Zhou, Q.; et al. Role of Polyacrylic Acid (PAA) Binder on the Solid Electrolyte Interphase in Silicon Anodes. Chem. Mater. 2019, 31, 2535–2544. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, W.; Duan, J.; Hong, K.; Xue, L.; Huang, Y. Effects of binders on electrochemical performance of nitrogen-doped carbon nanotube anode in sodium-ion battery. Electrochim. Acta 2015, 174, 970–977. [Google Scholar] [CrossRef]
- Fan, X.; Hsieh, Y.; Krochta, J.M.; Kurth, M.J. Study on molecular interaction behavior, and thermal and mechanical properties of polyacrylic acid and lactose blends. J. Appl. Polym. Sci. 2001, 82, 1921–1927. [Google Scholar] [CrossRef]
- Hu, B.; Shkrob, I.A.; Zhang, S.; Zhang, L.; Zhang, J.; Li, Y.; Liao, C.; Zhang, Z.; Lu, W.; Zhang, L. The existence of optimal molecular weight for poly(acrylic acid) binders in silicon/graphite composite anode for lithium-ion batteries. J. Power Sources 2018, 378, 671–676. [Google Scholar] [CrossRef]
- Sun, F.; Wheeler, D.R. The Effects of Lithium Ions and pH on the Function of Polyacrylic Acid Binder for Silicon Anodes. J. Electrochem. Soc. 2023, 170, 080502. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward Efficient Binders for Li-Ion Battery Si-Based Anodes: Polyacrylic Acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef]
- Ui, K.; Kikuchi, S.; Mikami, F.; Kadoma, Y.; Kumagai, N. Improvement of electrochemical characteristics of natural graphite negative electrode coated with polyacrylic acid in pure propylene carbonate electrolyte. J. Power Sources 2007, 173, 518–521. [Google Scholar] [CrossRef][Green Version]
- Nguyen, V.H.; Wang, W.L.; Jin, E.M.; Gu, H.-B. Impacts of different polymer binders on electrochemical properties of LiFePO4 cathode. Appl. Surf. Sci. 2013, 282, 444–449. [Google Scholar] [CrossRef]
- Cai, Z.P.; Liang, Y.; Li, W.S.; Xing, L.D.; Liao, Y.H. Preparation and performances of LiFePO4 cathode in aqueous solvent with polyacrylic acid as a binder. J. Power Sources 2009, 189, 547–551. [Google Scholar] [CrossRef]
- Mathew, A.; van Ekeren, W.; Andersson, R.; Lacey, M.J.; Heiskanen, S.K.; Younesi, R.; Brandell, D. Limitations of Polyacrylic Acid Binders When Employed in Thick LNMO Li-ion Battery Electrodes. J. Electrochem. Soc. 2024, 171, 020531. [Google Scholar] [CrossRef]
- Nagulapati, V.M.; Kim, D.S.; Oh, J.; Lee, J.H.; Hur, J.; Kim, I.T.; Lee, S.G. Enhancing the Electrochemical Performance of SbTe Bimetallic Anodes for High-Performance Sodium-Ion Batteries: Roles of the Binder and Carbon Support Matrix. Nanomaterials 2019, 9, 1134. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Qu, Q.; Zhang, L.; Shen, M.; Zhang, L.; Zheng, H. Chitosan, a new and environmental benign electrode binder for use with graphite anode in lithium-ion batteries. Electrochim. Acta 2013, 105, 378–383. [Google Scholar] [CrossRef]
- Hamzelui, N.; Linhorst, M.; Nyenhuis, G.M.; Haneke, L.; Eshetu, G.G.; Placke, T.; Winter, M.; Moerschbacher, B.M.; Figgemeier, E. Chitosan as Enabling Polymeric Binder Material for Silicon-Graphite-Based Anodes in Lithium-Ion Batteries. Energy Technol. 2023, 11, 2201239. [Google Scholar] [CrossRef]
- Gopakumar, D.A.; Pai, A.R.; Pasquini, D.; Shao-Yuan, L.; HPS, A.K.; Thomas, S. Nanomaterials—State of Art, New Challenges, and Opportunities. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Cord-Landwehr, S.; Moerschbacher, B.M. Deciphering the ChitoCode: Fungal chitins and chitosans as functional biopolymers. Fungal. Biol. Biotechnol. 2021, 8, 19. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, L.; Zhong, H. Carboxymethyl chitosan: A new water soluble binder for Si anode of Li-ion batteries. J. Power Sources 2014, 247, 327–331. [Google Scholar] [CrossRef]
- Shih, F.-Y.; Fung, K.-Z.; Wang, J.-W. Fabrication of LiMn2O4 thin film cathode from chitosan-containing solution. J. Alloys Compd. 2006, 407, 282–288. [Google Scholar] [CrossRef]
- Prasanna, K.; Subburaj, T.; Jo, Y.N.; Lee, W.J.; Lee, C.W. Environment-Friendly Cathodes Using Biopolymer Chitosan with Enhanced Electrochemical Behavior for Use in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 7884–7890. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yu, Z.; Lau, D. Effect of Acetyl Group on Mechanical Properties of Chitin/Chitosan Nanocrystal: A Molecular Dynamics Study. Int. J. Mol. Sci. 2016, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhou, W.; Jang, J.; Goodenough, J.B. Cross-Linked Chitosan as a Polymer Network Binder for an Antimony Anode in Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502130. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of Cross-Linking with Calcium Ions on the Physical Properties of Alginate Films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Smidsrød, O.; Skjakbrk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Oh, D.X.; Jo, C.; Lee, J.; Hwang, D.S. Improvement of desolvation and resilience of alginate binders for Si-based anodes in a lithium ion battery by calcium-mediated cross-linking. Phys. Chem. Chem. Phys. 2014, 16, 25628–25635. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhou, M.; Yi, R.; Xu, T.; Gordin, M.L.; Tang, D.; Yu, Z.; Regula, M.; Wang, D. Interpenetrated Gel Polymer Binder for High-Performance Silicon Anodes in Lithium-ion Batteries. Adv. Funct. Mater. 2014, 24, 5904–5910. [Google Scholar] [CrossRef]
- Deng, S.; Wang, H.; Liu, H.; Liu, J.; Yan, H. Research Progress in Improving the Rate Performance of LiFePO4 Cathode Materials. Nanomicro Lett. 2014, 6, 209–226. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, S.; Han, D.; Xiao, M.; Wang, S.; Meng, Y. Aqueous sodium alginate as binder: Dramatically improving the performance of dilithium terephthalate-based organic lithium ion batteries. J. Power Sources 2019, 438, 227007. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Wang, N.; Ding, Y.; Guan, L. Enhanced performance of a MnO2–graphene sheet cathode for lithium ion batteries using sodium alginate as a binder. J. Mater. Chem. 2012, 22, 13002. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Chai, L.; Xue, P.; Hao, W.; Zheng, H. A coordinatively cross-linked polymeric network as a functional binder for high-performance silicon submicro-particle anodes in lithium-ion batteries. J. Mater. Chem. A 2014, 2, 19036–19045. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Wu, Z.Y.; Wu, J.H.; Li, J.T.; Huang, L.; Sun, S.G. A high-performance alginate hydrogel binder for the Si/C anode of a Li-ion battery. Chem. Commun. 2014, 50, 6386. [Google Scholar] [CrossRef] [PubMed]
- Ryou, M.H.; Kim, J.; Lee, I.; Kim, S.; Jeong, Y.K.; Hong, S.; Ryu, J.H.; Kim, T.S.; Park, J.K.; Lee, H.; et al. Mussel-Inspired Adhesive Binders for High-Performance Silicon Nanoparticle Anodes in Lithium-Ion Batteries. Adv. Mater. 2013, 25, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, R.; Yang, S.; Zhang, W.; Fang, Y.; Li, L.; Liu, Y.; Huang, J. High-performance Si flexible anode with rGO substrate and Ca2+ crosslinked sodium alginate binder for lithium ion battery. Synth. Met. 2019, 247, 212–218. [Google Scholar] [CrossRef]
- Ling, L.; Bai, Y.; Wang, Z.; Ni, Q.; Chen, G.; Zhou, Z.; Wu, C. Remarkable Effect of Sodium Alginate Aqueous Binder on Anatase TiO 2 as High-Performance Anode in Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 5560–5568. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, K.; Zhang, X.; Zhang, X.; Guo, S.; Zhou, H. Sodium Alginate Enabled Advanced Layered Manganese-Based Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 26817–26823. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.A.; Kuss, C. Review—Conducting Polymer-Based Binders for Lithium-Ion Batteries and Beyond. J. Electrochem. Soc. 2020, 167, 065501. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Lu, P.; Jiang, M.; Shi, F.; Song, X.; Zheng, Z.; Zhou, X.; Fu, Y.; Abdelbast, G.; et al. Toward Practical Application of Functional Conductive Polymer Binder for a High-Energy Lithium-Ion Battery Design. Nano Lett. 2014, 14, 6704–6710. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, M.H.; Choi, S.Y.; Lee, J.G.; Jang, J.; Lee, J.B.; Ryu, J.H.; Hwang, S.S.; Park, J.H.; Shin, K.; et al. Poly(phenanthrenequinone) as a conductive binder for nano-sized silicon negative electrodes. Energy Environ. Sci. 2015, 8, 1538–1543. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, T.; de la Fuente, M.S.; Liu, G. Aqueous emulsion of conductive polymer binders for Si anode materials in lithium ion batteries. Eur. Polym. J. 2019, 114, 265–270. [Google Scholar] [CrossRef]
- Higgins, T.M.; Park, S.H.; King, P.J.; Zhang, C.; McEvoy, N.; Berner, N.C.; Daly, D.; Shmeliov, A.; Khan, U.; Duesberg, G.; et al. A Commercial Conducting Polymer as Both Binder and Conductive Additive for Silicon Nanoparticle-Based Lithium-Ion Battery Negative Electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, Y.; Tan, R.; Tian, L.-L.; Liu, Y.; Chen, H.; Pan, F. Novel conductive binder for high-performance silicon anodes in lithium ion batteries. Nano Energy 2017, 36, 206–212. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, J.; Li, Y.; Xu, Q.; Jiang, Y.; Yang, C.; Shi, L.; Chen, L.; Liu, P.; Zhang, J.; et al. Mixed ion-electron conductive binders coupling superior stiffness and toughness establish dual crosslinking stable silicon anodes. Chem. Eng. J. 2024, 479, 147807. [Google Scholar] [CrossRef]
- Zhao, H.; Du, A.; Ling, M.; Battaglia, V.; Liu, G. Conductive polymer binder for nano-silicon/graphite composite electrode in lithium-ion batteries towards a practical application. Electrochim. Acta 2016, 209, 159–162. [Google Scholar] [CrossRef]
- Lyness, C. Lithium-secondary cell: Sources of risks and their effects. In Electrochemical Power Sources: Fundamentals, Systems, and Applications Li-Battery Safety; Elsevier: Amsterdam, The Netherlands, 2018; pp. 143–266. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, K.; He, J.; Chu, X.; He, Y.-B.; Wu, M.; Li, B.; Kang, F. In-situ polymerized lithium polyacrylate (PAALi) as dual-functional lithium source for high-performance layered oxide cathodes. Electrochim. Acta 2017, 249, 43–51. [Google Scholar] [CrossRef]
- Virya, A.; Lian, K. Lithium polyacrylate-polyacrylamide blend as polymer electrolytes for solid-state electrochemical capacitors. Electrochem. Commun. 2018, 97, 77–81. [Google Scholar] [CrossRef]
- Rao, L.; Jiao, X.; Yu, C.-Y.; Schmidt, A.; O’Meara, C.; Seidt, J.; Sayre, J.R.; Khalifa, Y.M.; Kim, J.-H. Multifunctional Composite Binder for Thick High-Voltage Cathodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 861–872. [Google Scholar] [CrossRef]
- Zhong, H.; Lu, J.; He, A.; Sun, M.; He, J.; Zhang, L. Carboxymethyl chitosan/poly(ethylene oxide) water soluble binder: Challenging application for 5 V LiNi0.5Mn1.5O4 cathode. J. Mater. Sci. Technol. 2017, 33, 763–767. [Google Scholar] [CrossRef]
- Fu, Z.; Feng, H.L.; Xiang, X.D.; Rao, M.M.; Wu, W.; Luo, J.C.; Chen, T.T.; Hu, Q.P.; Feng, A.B.; Li, W.S. A novel polymer composite as cathode binder of lithium ion batteries with improved rate capability and cyclic stability. J. Power Sources 2014, 261, 170–174. [Google Scholar] [CrossRef]
- Hapuarachchi, S.N.S.; Wasalathilake, K.C.; Nerkar, J.Y.; Jaatinen, E.; O’Mullane, A.P.; Yan, C. Mechanically Robust Tapioca Starch Composite Binder with Improved Ionic Conductivity for Sustainable Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 9857–9865. [Google Scholar] [CrossRef]
- Shao, D.; Zhong, H.; Zhang, L. Water-Soluble Conductive Composite Binder Containing PEDOT:PSS as Conduction Promoting Agent for Si Anode of Lithium-Ion Batteries. ChemElectroChem 2014, 1, 1679–1687. [Google Scholar] [CrossRef]
- Zhong, H.; He, A.; Lu, J.; Sun, M.; He, J.; Zhang, L. Carboxymethyl chitosan/conducting polymer as water-soluble composite binder for LiFePO4 cathode in lithium ion batteries. J. Power Sources 2016, 336, 107–114. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, C.; Jeong, J.; Song, W.-J.; Park, S.; Song, H.-K. Bipolymer-Cross-Linked Binder to Improve the Reversibility and Kinetics of Sodiation and Desodiation of Antimony for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 43039–43045. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Di, F.; Zheng, J.G.; Zhao, H.W.; Zhang, H.; Li, L.X.; Geng, X.; Sun, C.G.; Yang, H.M.; Zhou, W.M.; et al. Self-healing polymer binders for the Si and Si/carbon anodes of lithium-ion batteries. New Carbon Mater. 2022, 37, 802–826. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Han, Z.; Li, Q.; He, J.; Wang, Q. Self-Healing Polymers for Electronics and Energy Devices. Chem. Rev. 2023, 123, 558–612. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.A.; Santana, M.H. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review. Mater. Horiz 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Lim, T.; Yoon, J.; Lee, C.; Baek, K.-Y.; Jeon, J.-W.; Cho, S. Self-Healable and Degradable Polycaprolactone-Based Polymeric Binders for Lithium-Ion Batteries. ACS Appl. Polym. Mater. 2024, 6, 4050–4059. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-Healing of Polymers via Supramolecular Chemistry. Adv. Mater. Interfaces 2018, 5, 1800384. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Guo, Z.; Hu, Y. Double dynamic bonds tough hydrogel with high self-healing properties based on acylhydrazone bonds and borate bonds. Polym. Adv. Technol. 2022, 33, 2528–2541. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Xie, X.; Kong, Z.; Tong, Y.; Xu, H.; Xu, H.; Jin, H. A novel multi-functional binder based on double dynamic bonds for silicon anode of lithium-ion batteries. Electrochim. Acta 2022, 425, 140620. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.; Zhang, X.; Liu, Y.; Wu, S.; Guo, B. Covalently Cross-Linked Elastomers with Self-Healing and Malleable Abilities Enabled by Boronic Ester Bonds. ACS Appl. Mater. Interfaces 2018, 10, 24224–24231. [Google Scholar] [CrossRef]
- Taynton, P.; Ni, H.; Zhu, C.; Yu, K.; Loob, S.; Jin, Y.; Qi, H.J.; Zhang, W. Repairable Woven Carbon Fiber Composites with Full Recyclability Enabled by Malleable Polyimine Networks. Adv. Mater. 2016, 28, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, P.; Zhao, Y.; Liu, M.; Xiao, Z. High-Performance Self-Healing Polyurethane Binder Based on Aromatic Disulfide Bonds and Hydrogen Bonds for the Sulfur Cathode of Lithium–Sulfur Batteries. Ind. Eng. Chem. Res. 2021, 60, 12011–12020. [Google Scholar] [CrossRef]
- Kwon, T.; Jeong, Y.K.; Lee, I.; Kim, T.; Choi, J.W.; Coskun, A. Systematic Molecular-Level Design of Binders Incorporating Meldrum’s Acid for Silicon Anodes in Lithium Rechargeable Batteries. Adv. Mater. 2014, 26, 7979–7985. [Google Scholar] [CrossRef]
- Kuo, T.-C.; Chiou, C.-Y.; Li, C.-C.; Lee, J.-T. In situ cross-linked poly(ether urethane) elastomer as a binder for high-performance Si anodes of lithium-ion batteries. Electrochim. Acta 2019, 327, 135011. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Kwon, T.; Lee, I.; Kim, T.-S.; Coskun, A.; Choi, J.W. Millipede-inspired structural design principle for high performance polysaccharide binders in silicon anodes. Energy Environ. Sci. 2015, 8, 1224–1230. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.; Park, K.; Kim, S.; Nam, K.W.; Char, K.; Choi, J.W. Host–Guest Interlocked Complex Binder for Silicon–Graphite Composite Electrodes in Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103718. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Cho, Y.; Shin, H.; Kim, S.; Char, K.; Choi, J.W. Zn2+–Imidazole Coordination Crosslinks for Elastic Polymeric Binders in High-Capacity Silicon Electrodes. Adv. Sci. 2021, 8, 2004290. [Google Scholar] [CrossRef]
- Luo, P.; Lai, P.; Huang, Y.; Yuan, Y.; Wen, J.; Xie, C.; Li, J.; Liu, L. A Highly Stretchable and Self-Healing Composite Binder Based on the Hydrogen-Bond Network for Silicon Anodes in High-Energy-Density Lithium-Ion Batteries. ChemElectroChem 2022, 9, e202200155. [Google Scholar] [CrossRef]
- Liu, M.; Chen, P.; Pan, X.; Pan, S.; Zhang, X.; Zhou, Y.; Bi, M.; Sun, J.; Yang, S.; Vasiliev, A.L.; et al. Synergism of Flame-Retardant, Self-Healing, High-Conductive and Polar to a Multi-Functional Binder for Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2205031. [Google Scholar] [CrossRef]
- Jiao, X.; Yin, J.; Xu, X.; Wang, J.; Liu, Y.; Xiong, S.; Zhang, Q.; Song, J. Highly Energy-Dissipative, Fast Self-Healing Binder for Stable Si Anode in Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2005699. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Q.; Guan, X.; Liu, M.; Wang, F.; Li, R.; Xu, J. Ionically Conductive Self-Healing Polymer Binders with Poly(ether-thioureas) Segments for High-Performance Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 4934–4944. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Z.; Su, Z.; Chen, S.; Yan, C.; Al-Mamun, M.; Tang, Y.; Zhang, S. A mechanically robust self-healing binder for silicon anode in lithium ion batteries. Nano Energy 2021, 81, 105654. [Google Scholar] [CrossRef]
- Munaoka, T.; Yan, X.; Lopez, J.; To, J.W.F.; Park, J.; Tok, J.B.-H.; Cui, Y.; Bao, Z. Ionically Conductive Self-Healing Binder for Low Cost Si Microparticles Anodes in Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703138. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Chen, X.; Jin, H.; Wang, J. Novel constructive self-healing binder for silicon anodes with high mass loading in lithium-ion batteries. Energy Storage Mater. 2021, 38, 121–129. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable Lithium Batteries with Aqueous Electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Koerver, R.; Aygün, I.; Leichtweiß, T.; Dietrich, C.; Zhang, W.; Binder, J.O.; Hartmann, P.; Zeier, W.G.; Janek, J. Capacity Fade in Solid-State Batteries: Interphase Formation and Chemomechanical Processes in Nickel-Rich Layered Oxide Cathodes and Lithium Thiophosphate Solid Electrolytes. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Kim, D.H.; Oh, D.Y.; Park, K.H.; Choi, Y.E.; Nam, Y.J.; Lee, H.A.; Lee, S.-M.; Jung, Y.S. Infiltration of Solution-Processable Solid Electrolytes into Conventional Li-Ion-Battery Electrodes for All-Solid-State Li-Ion Batteries. Nano Lett. 2017, 17, 3013–3020. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Kinoshita, T.; Miura, A.; Higuchi, M.; Tadanaga, K. Effect of the binder content on the electrochemical performance of composite cathode using Li6PS5Cl precursor solution in an all-solid-state lithium battery. Ionics (Kiel) 2017, 23, 1619–1624. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.; Choi, S.; Char, K.; Choi, J.W. Thiol–Ene Click Reaction for Fine Polarity Tuning of Polymeric Binders in Solution-Processed All-Solid-State Batteries. ACS Energy Lett. 2019, 4, 94–101. [Google Scholar] [CrossRef]
- Lee, K.; Kim, S.; Park, J.; Park, S.H.; Coskun, A.; Jung, D.S.; Cho, W.; Choi, J.W. Selection of Binder and Solvent for Solution-Processed All-Solid-State Battery. J. Electrochem. Soc. 2017, 164, A2075–A2081. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, H.; Zheng, C.; Xia, Y.; Liang, C.; Huang, H.; Gan, Y.; Tao, X.; Zhang, W. All-solid-state batteries with slurry coated LiNi0.8Co0.1Mn0.1O2 composite cathode and Li6PS5Cl electrolyte: Effect of binder content. J. Power Sources 2018, 391, 73–79. [Google Scholar] [CrossRef]
- Wan, Z.; Lei, D.; Yang, W.; Liu, C.; Shi, K.; Hao, X.; Shen, L.; Lv, W.; Li, B.; Yang, Q.H.; et al. Low Resistance–Integrated All-Solid-State Battery Achieved by Li7La3Zr2O12 Nanowire Upgrading Polyethylene Oxide (PEO) Composite Electrolyte and PEO Cathode Binder. Adv. Funct. Mater. 2019, 29, 1805301. [Google Scholar] [CrossRef]
- Park, K.H.; Bai, Q.; Kim, D.H.; Oh, D.Y.; Zhu, Y.; Mo, Y.; Jung, Y.S. Design Strategies, Practical Considerations, and New Solution Processes of Sulfide Solid Electrolytes for All-Solid-State Batteries. Adv. Energy Mater. 2018, 8, 1800035. [Google Scholar] [CrossRef]
- Chen, R.-J.; Zhang, Y.-B.; Liu, T.; Xu, B.-Q.; Lin, Y.-H.; Nan, C.-W.; Shen, Y. Addressing the Interface Issues in All-Solid-State Bulk-Type Lithium Ion Battery via an All-Composite Approach. ACS Appl. Mater. Interfaces 2017, 9, 9654–9661. [Google Scholar] [CrossRef]
- Wang, K.; Ye, Q.; Zhang, J.; Huang, H.; Gan, Y.; He, X.; Zhang, W. Halide Electrolyte Li3InCl6-Based All-Solid-State Lithium Batteries with Slurry-Coated LiNi0.8Co0.1Mn0.1O2 Composite Cathode: Effect of Binders. Front. Mater. 2021, 8, 727617. [Google Scholar] [CrossRef]
- Ai, G.; Dai, Y.; Ye, Y.; Mao, W.; Wang, Z.; Zhao, H.; Chen, Y.; Zhu, J.; Fu, Y.; Battaglia, V.; et al. Investigation of surface effects through the application of the functional binders in lithium sulfur batteries. Nano Energy 2015, 16, 28–37. [Google Scholar] [CrossRef]
- Ling, M.; Xu, Y.; Zhao, H.; Gu, X.; Qiu, J.; Li, S.; Wu, M.; Song, X.; Yan, C.; Liu, G.; et al. Dual-functional gum arabic binder for silicon anodes in lithium ion batteries. Nano Energy 2015, 12, 178–185. [Google Scholar] [CrossRef]
- Koo, B.; Kim, H.; Cho, Y.; Lee, K.T.; Choi, N.; Cho, J. A Highly Cross-Linked Polymeric Binder for High-Performance Silicon Negative Electrodes in Lithium Ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 8762–8767. [Google Scholar] [CrossRef]
- Ito, S.; Fujiki, S.; Yamada, T.; Aihara, Y.; Park, Y.; Kim, T.Y.; Baek, S.-W.; Lee, J.-M.; Doo, S.; Machida, N. A rocking chair type all-solid-state lithium ion battery adopting Li2O–ZrO2 coated LiNi0.8Co0.15Al0.05O2 and a sulfide based electrolyte. J. Power Sources 2014, 248, 943–950. [Google Scholar] [CrossRef]
- Inada, T. Fabrications and properties of composite solid-state electrolytes. Solid State Ion 2003, 158, 275–280. [Google Scholar] [CrossRef]
- Banerjee, A.; Park, K.H.; Heo, J.W.; Nam, Y.J.; Moon, C.K.; Oh, S.M.; Hong, S.-T.; Jung, Y.S. Na3SbS4: A Solution Processable Sodium Superionic Conductor for All-Solid-State Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 9634–9638. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Oh, D.Y.; Choi, Y.E.; Nam, Y.J.; Han, L.; Kim, J.-Y.; Xin, H.; Lin, F.; Oh, S.M.; Jung, Y.S. Solution-Processable Glass LiI-Li4SnS4 Superionic Conductors for All-Solid-State Li-Ion Batteries. Adv. Mater. 2016, 28, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.J.; Oh, D.Y.; Jung, S.H.; Jung, Y.S. Toward practical all-solid-state lithium-ion batteries with high energy density and safety: Comparative study for electrodes fabricated by dry- and slurry-mixing processes. J. Power Sources 2018, 375, 93–101. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Otaegui, L.; Singh, G.; Armand, M.; Rodriguez-Martinez, L.M. Estimation of energy density of Li-S batteries with liquid and solid electrolytes. J. Power Sources 2016, 326, 1–5. [Google Scholar] [CrossRef]
- Hippauf, F.; Schumm, B.; Doerfler, S.; Althues, H.; Fujiki, S.; Shiratsuchi, T.; Tsujimura, T.; Aihara, Y.; Kaskel, S. Overcoming binder limitations of sheet-type solid-state cathodes using a solvent-free dry-film approach. Energy Storage Mater. 2019, 21, 390–398. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, L.; Zhou, D.; Weng, W.; Yao, X. Flexible Sulfide Electrolyte Thin Membrane with Ultrahigh Ionic Conductivity for All-Solid-State Lithium Batteries. Nano Lett. 2021, 21, 5233–5239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Ma, T.; Wang, Z.; Gao, Q.; Xu, J.; Chen, L.; Li, H.; Wu, F. Long-Life Sulfide All-Solid-State Battery Enabled by Substrate-Modulated Dry-Process Binder. Adv. Energy Mater. 2022, 12, 2201732. [Google Scholar] [CrossRef]
- Wang, H.; Wu, L.; Xue, B.; Wang, F.; Luo, Z.; Zhang, X.; Calvez, L.; Fan, P.; Fan, B. Improving Cycling Stability of the Lithium Anode by a Spin-Coated High-Purity Li3PS4 Artificial SEI Layer. ACS Appl. Mater. Interfaces 2022, 14, 15214–15224. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, L.; Han, J.; Wen, K.; Guan, S.; Xue, C.; Zhang, Z.; Xu, B.; Lin, Y.; Shen, Y.; et al. Super Long-Cycling All-Solid-State Battery with Thin Li 6 PS 5 Cl-Based Electrolyte. Adv. Energy Mater. 2022, 12, 2200660. [Google Scholar] [CrossRef]
- Inada, T.; Kobayashi, T.; Sonoyama, N.; Yamada, A.; Kondo, S.; Nagao, M.; Kanno, R. All solid-state sheet battery using lithium inorganic solid electrolyte, thio-LISICON. J. Power Sources 2009, 194, 1085–1088. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.T.; Wang, C.; Sun, X. Progress and Prospects of Inorganic Solid-State Electrolyte-Based All-Solid-State Pouch Cells. Adv. Mater. 2023, 35, 2209074. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Fang, Y.; Xue, B.; Wu, Q.; Luo, Z.; Zhang, X.; Calvez, L.; Wu, L. Spray-Printed Flexible Li 2 S Cathode with Inorganic Ion-Conductive Binder Nano-Li 3 PS 4. ACS Appl. Mater. Interfaces 2024, 16, 7182–7188. [Google Scholar] [CrossRef]
- Liang, J.; Chen, D.; Adair, K.; Sun, Q.; Holmes, N.G.; Zhao, Y.; Sun, Y.; Luo, J.; Li, R.; Zhang, L.; et al. Insight into Prolonged Cycling Life of 4 V All-Solid-State Polymer Batteries by a High-Voltage Stable Binder. Adv. Energy Mater. 2021, 11, 2002455. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Q.; Zhao, C.; Gao, X.; Adair, K.R.; Liu, Y.; Luo, J.; Lin, X.; Liang, J.; Huang, H.; et al. High-areal-capacity all-solid-state lithium batteries enabled by rational design of fast ion transport channels in vertically-aligned composite polymer electrodes. Nano Energy 2019, 61, 567–575. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Chen, B.; Wang, L.; Yue, L.; Liu, H.; Zhang, J.; Liu, Z.; Cui, G. A Strategy to Make High Voltage LiCoO2 Compatible with Polyethylene Oxide Electrolyte in All-Solid-State Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A3454–A3461. [Google Scholar] [CrossRef]
- Miyashiro, H.; Kobayashi, Y.; Seki, S.; Mita, Y.; Usami, A.; Nakayama, M.; Wakihara, M. Fabrication of All-Solid-State Lithium Polymer Secondary Batteries Using Al2O3-Coated LiCoO2. Chem. Mater. 2005, 17, 5603–5605. [Google Scholar] [CrossRef]
- Hovington, P.; Lagacé, M.; Guerfi, A.; Bouchard, P.; Mauger, A.; Julien, C.M.; Armand, M.; Zaghib, K. New Lithium Metal Polymer Solid State Battery for an Ultrahigh Energy: Nano C-LiFePO4 versus Nano Li1.2V3O8. Nano Lett. 2015, 15, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Guo, Y.; Bian, H.; Zhang, Q.; Zhang, L.; Zhang, S. An ultra-stable lithium plating process enabled by the nanoscale interphase of a macromolecular additive. J. Mater. Chem. A Mater. 2020, 8, 23844–23850. [Google Scholar] [CrossRef]
- Wang, R.; Feng, L.; Yang, W.; Zhang, Y.; Zhang, Y.; Bai, W.; Liu, B.; Zhang, W.; Chuan, Y.; Zheng, Z.; et al. Effect of Different Binders on the Electrochemical Performance of Metal Oxide Anode for Lithium-Ion Batteries. Nanoscale Res. Lett. 2017, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, W.; Chen, L.; Lu, Y.; Su, Y.; Bao, W.; Wang, J.; Chen, S.; Bao, L. Polyacrylonitrile-polyvinylidene fluoride as high-performance composite binder for layered Li-rich oxides. J. Power Sources 2017, 359, 226–233. [Google Scholar] [CrossRef]
- Manickam, M.; Takata, M. Effect of cathode binder on capacity retention and cycle life in transition metal phosphate of a rechargeable lithium battery. Electrochim. Acta 2003, 48, 957–963. [Google Scholar] [CrossRef]
- Zhang, Y.; Huld, F.; Lu, S.; Jektvik, C.; Lou, F.; Yu, Z. Revisiting Polytetrafluorethylene Binder for Solvent-Free Lithium-Ion Battery Anode Fabrication. Batteries 2022, 8, 57. [Google Scholar] [CrossRef]
- Yim, T.; Choi, S.J.; Jo, Y.N.; Kim, T.H.; Kim, K.J.; Jeong, G.; Kim, Y.J. Effect of binder properties on electrochemical performance for silicon-graphite anode: Method and application of binder screening. Electrochim. Acta 2014, 136, 112–120. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Hou, Z.; Wei, H. Poly (acrylic acid sodium) grafted carboxymethyl cellulose as a high performance polymer binder for silicon anode in lithium ion batteries. Sci. Rep. 2016, 6, 19583. [Google Scholar] [CrossRef]
- Rajeev, K.K.; Kim, E.; Nam, J.; Lee, S.; Mun, J.; Kim, T.-H. Chitosan-grafted-polyaniline copolymer as an electrically conductive and mechanically stable binder for high-performance Si anodes in Li-ion batteries. Electrochim. Acta 2020, 333, 135532. [Google Scholar] [CrossRef]
- Nagulapati, V.M.; Lee, J.H.; Kim, H.S.; Oh, J.; Kim, I.T.; Hur, J.; Lee, S.G. Novel hybrid binder mixture tailored to enhance the electrochemical performance of SbTe bi-metallic anode for sodium ion batteries. J. Electroanal. Chem. 2020, 865, 114160. [Google Scholar] [CrossRef]
- Xiao, W.; Sun, Q.; Banis, M.N.; Wang, B.; Li, W.; Li, M.; Lushington, A.; Li, R.; Li, X.; Sham, T.K.; et al. Understanding the Critical Role of Binders in Phosphorus/Carbon Anode for Sodium-Ion Batteries through Unexpected Mechanism. Adv. Funct. Mater. 2020, 30, 2000060. [Google Scholar] [CrossRef]
- Mao, Z.; Wang, R.; He, B.; Jin, J.; Gong, Y.; Wang, H. Cross-Linked Sodium Alginate as A Multifunctional Binder to Achieve High-Rate and Long-Cycle Stability for Sodium-Ion Batteries. Small 2023, 19, 2207224. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Li, D.; Lu, P.; Hou, F.; Liang, J. Enhanced electrochemical stability of carbon-coated antimony nanoparticles with sodium alginate binder for sodium-ion batteries. Prog. Nat. Sci. Mater. Int. 2018, 28, 205–211. [Google Scholar] [CrossRef]
- Yao, Q.; Zhu, Y.; Zheng, C.; Wang, N.; Wang, D.; Tian, F.; Bai, Z.; Yang, J.; Qian, Y.; Dou, S. Intermolecular Cross-Linking Reinforces Polymer Binders for Durable Alloy-Type Anode Materials of Sodium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2202939. [Google Scholar] [CrossRef]
- Ni, D.; Sun, W.; Lu, C.; Wang, Z.; Qiao, J.; Cai, H.; Liu, C.; Sun, K. Improved rate and cycling performance of FeF2-rGO hybrid cathode with poly (acrylic acid) binder for sodium ion batteries. J. Power Sources 2019, 413, 449–458. [Google Scholar] [CrossRef]
- Dai, K.; Zhao, H.; Wang, Z.; Song, X.; Battaglia, V.; Liu, G. Toward high specific capacity and high cycling stability of pure tin nanoparticles with conductive polymer binder for sodium ion batteries. J. Power Sources 2014, 263, 276–279. [Google Scholar] [CrossRef]
- Kim, K.T.; Kwon, T.Y.; Song, Y.B.; Kim, S.M.; Byun, S.C.; Min, H.S.; Kim, S.H.; Jung, Y.S. Wet-slurry fabrication using PVdF-HFP binder with sulfide electrolytes via synergetic cosolvent approach for all-solid-state batteries. Chem. Eng. J. 2022, 450, 138047. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, L.; Zhang, N.; Yang, J.; Liu, G.; Wu, J.; Yao, X. Stable Binder Boosting Sulfide Solid Electrolyte Thin Membrane for All-Solid-State Lithium Batteries. Energy Mater. Adv. 2024, 5, 0074. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, S.; Zhang, Y.; Liu, C.; Wei, X.; Luo, D.; Lin, Z. Towards efficient binders for silicon based lithium-ion battery anodes. Chem. Eng. J. 2021, 406, 126807. [Google Scholar] [CrossRef]
- Jin, H.-J.; Lee, J.; Yoon, J.; Kim, H.; Kim, J. Polymeric Binder Design for Sustainable Lithium-ion Battery Chemistry. Polymers 2024, 16, 254. [Google Scholar] [CrossRef] [PubMed]
- Vogl, U.S.; Das, P.K.; Weber, A.Z.; Winter, M.; Kostecki, R.; Lux, S.F. Mechanism of Interactions between CMC Binder and Si Single Crystal Facets. Langmuir 2014, 30, 10299–10307. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.E. (Ed.) Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Xie, J.; Wang, K.; Yang, J. Binder effect on cycling performance of silicon/carbon composite anodes for lithium ion batteries. J. Appl. Electrochem. 2006, 36, 1099–1104. [Google Scholar] [CrossRef]
- Yao, D.; Feng, J.; Wang, J.; Deng, Y.; Wang, C. Synthesis of silicon anode binders with ultra-high content of catechol groups and the effect of molecular weight on battery performance. J. Power Sources 2020, 463, 228188. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Tan, Q.; Wan, Q.; Han, K.; Liu, Z.; Li, P.; An, F.; Qu, X. A synergetic strategy for an advanced electrode with Fe3O4 embedded in a 3D N-doped porous graphene framework and a strong adhesive binder for lithium/potassium ion batteries with an ultralong cycle lifespan. J. Mater. Chem. A Mater. 2019, 7, 19430–19441. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Gao, Y.; Wang, X.; Zhang, Y.; Zhang, S. A water-soluble, adhesive and 3D cross-linked polyelectrolyte binder for high-performance lithium–sulfur batteries. J. Mater. Chem. A Mater. 2021, 9, 2375–2384. [Google Scholar] [CrossRef]
- Hitomi, S.; Kubota, K.; Horiba, T.; Hida, K.; Matsuyama, T.; Oji, H.; Yasuno, S.; Komaba, S. Application of Acrylic-Rubber-Based Latex Binder to High-Voltage Spinel Electrodes of Lithium-Ion Batteries. ChemElectroChem 2019, 6, 5070–5079. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.; Jeong, J.; Cho, K.Y.; Ryou, M.-H.; Lee, Y.M. Highly Adhesive and Soluble Copolyimide Binder: Improving the Long-Term Cycle Life of Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 14851–14858. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Liu, J.; Deng, Y.; Wu, Z.; Yin, Z.; Guo, D.; Huang, L.; Sun, S. Suppressing the voltage-fading of layered lithium-rich cathode materials via an aqueous binder for Li-ion batteries. Chem. Commun. 2016, 52, 4683–4686. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chu, Q.; Yan, C.; Zhang, S.; Lin, Z.; Lu, J. Interweaving 3D Network Binder for High-Areal-Capacity Si Anode through Combined Hard and Soft Polymers. Adv. Energy Mater. 2019, 9, 1802645. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Choi, J.W. Mussel-Inspired Self-Healing Metallopolymers for Silicon Nanoparticle Anodes. ACS Nano 2019, 13, 8364–8373. [Google Scholar] [CrossRef] [PubMed]
- Yuca, N.; Cetintasoglu, M.E.; Dogdu, M.F.; Akbulut, H.; Tabanli, S.; Colak, U.; Taskin, O.S. Highly efficient poly(fluorene phenylene) copolymer as a new class of binder for high-capacity silicon anode in lithium-ion batteries. Int. J. Energy Res. 2018, 42, 1148–1157. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Jin, B.; Peng, R. A new linear heptafluoro glycidyl ether binder: Synthesis, characterization, and mechanical properties. Macromol. Res. 2023, 31, 699–709. [Google Scholar] [CrossRef]
- Liu, J.; Galpaya, D.G.D.; Yan, L.; Sun, M.; Lin, Z.; Yan, C.; Liang, C.; Zhang, S. Exploiting a robust biopolymer network binder for an ultrahigh-areal-capacity Li–S battery. Energy Environ. Sci. 2017, 10, 750–755. [Google Scholar] [CrossRef]
- Shin, D.; Park, H.; Paik, U. Cross-linked poly(acrylic acid)-carboxymethyl cellulose and styrene-butadiene rubber as an efficient binder system and its physicochemical effects on a high energy density graphite anode for Li-ion batteries. Electrochem. Commun. 2017, 77, 103–106. [Google Scholar] [CrossRef]
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]
- Ahn, J.; Im, H.G.; Lee, Y.; Lee, D.; Jang, H.; Oh, Y.; Chung, K.; Park, T.; Um, M.K.; Yi, J.W.; et al. A novel organosilicon-type binder for LiCoO2 cathode in Li-ion batteries. Energy Storage Mater. 2022, 49, 58–66. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, F.; Xie, Y.; Xu, X.; Li, F.; Han, X.; Yao, X.; Wang, D.; Hou, Y.; Gao, X.; et al. In situ interlocked gradient adaptive network binder with robust adhesion and cycle performance for silicon anodes. J. Power Sources 2023, 580, 233267. [Google Scholar] [CrossRef]
- Wang, Y.; Dang, D.; Li, D.; Hu, J.; Cheng, Y.-T. Influence of polymeric binders on mechanical properties and microstructure evolution of silicon composite electrodes during electrochemical cycling. J. Power Sources 2019, 425, 170–178. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer Binders: Characterization and Development toward Aqueous Electrode Fabrication for Sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Liew, C.; Durairaj, R.; Ramesh, S. Rheological Studies of PMMA–PVC Based Polymer Blend Electrolytes with LiTFSI as Doping Salt. PLoS ONE 2014, 9, e102815. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lehmann, M.L.; Zhu, J.; Liu, T.; Zhou, Z.; Tang, X.; Heish, C.T.; Sokolov, A.P.; Cao, P.; Chen, X.C.; et al. Recent Developments and Challenges in Hybrid Solid Electrolytes for Lithium-Ion Batteries. Front. Energy Res. 2020, 8, 202. [Google Scholar] [CrossRef]
- He, R.; Kyu, T. Effect of Plasticization on Ionic Conductivity Enhancement in Relation to Glass Transition Temperature of Crosslinked Polymer Electrolyte Membranes. Macromolecules 2016, 49, 5637–5648. [Google Scholar] [CrossRef]
- Shetty, S.K.; Ismayil; Noor, I.M. Effect of new crystalline phase on the ionic conduction properties of sodium perchlorate salt doped carboxymethyl cellulose biopolymer electrolyte films. J. Polym. Res. 2021, 28, 415. [Google Scholar] [CrossRef]
- Olmedo-Martínez, J.; Meabe, L.; Basterretxea, A.; Mecerreyes, D.; Müller, A. Effect of Chemical Structure and Salt Concentration on the Crystallization and Ionic Conductivity of Aliphatic Polyethers. Polymers 2019, 11, 452. [Google Scholar] [CrossRef]
- Yim, T.; Choi, S.J.; Park, J.H.; Cho, W.; Jo, Y.N.; Kim, T.H.; Kim, Y.J. The effect of an elastic functional group in a rigid binder framework of silicon–graphite composites on their electrochemical performance. Phys. Chem. Chem. Phys. 2015, 17, 2388–2393. [Google Scholar] [CrossRef]
- Jenkins, C.A.; Coles, S.R.; Loveridge, M.J. Investigation into Durable Polymers with Enhanced Toughness and Elasticity for Application in Flexible Li-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 12494–12505. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, L.; Peng, X.; Liu, T.; Jiang, Y.; Qin, F.; Hu, L.; Chu, P.K.; Huo, K.; Zhou, Y. Enhanced Ion Conductivity in Conducting Polymer Binder for High-Performance Silicon Anodes in Advanced Lithium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702314. [Google Scholar] [CrossRef]
- Qiu, D.; Guan, J.; Li, M.; Kang, C.; Wei, J.; Li, Y.; Xie, Z.; Wang, F.; Yang, R. Kinetics Enhanced Nitrogen-Doped Hierarchical Porous Hollow Carbon Spheres Boosting Advanced Potassium-Ion Hybrid Capacitors. Adv. Funct. Mater. 2019, 29, 1903496. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Vorobeva, K.A.; Eliseeva, S.N.; Apraksin, R.V.; Kamenskii, M.A.; Tolstopjatova, E.G.; Kondratiev, V.V. Improved electrochemical properties of cathode material LiMn2O4 with conducting polymer binder. J. Alloys Compd. 2018, 766, 33–44. [Google Scholar] [CrossRef]
- Eom, J.-Y.; Cao, L. Effect of anode binders on low-temperature performance of automotive lithium-ion batteries. J. Power Sources 2019, 441, 227178. [Google Scholar] [CrossRef]
- Younesi, R.; Hahlin, M.; Treskow, M.; Scheers, J.; Johansson, P.; Edström, K. Ether Based Electrolyte, LiB(CN)4 Salt and Binder Degradation in the Li–O2 Battery Studied by Hard X-ray Photoelectron Spectroscopy (HAXPES). J. Phys. Chem. C 2012, 116, 18597–18604. [Google Scholar] [CrossRef]
- Le, A.V.; Wang, M.; Noelle, D.J.; Shi, Y.; Meng, Y.S.; Wu, D.; Fan, J.; Qiao, Y. Using high-HFP-content cathode binder for mitigation of heat generation of lithium-ion battery. Int. J. Energy Res. 2017, 41, 2430–2438. [Google Scholar] [CrossRef]
- Komoda, Y.; Ishibashi, K.; Kuratani, K.; Suzuki, K.; Ohmura, N.; Kobayashi, H. Effects of drying rate and slurry microstructure on the formation process of LiB cathode and electrochemical properties. J. Power Sources 2023, 568, 232983. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Su, C.; Gao, X. Recent Advances in Battery Binders with an Insight of Predictive Commercial Development. In Electronics, Communications and Networks: Proceedings of the 13th International Conference (CECNet 2023), Macao, China, 17–20 November 2023; IOS Press: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Lim, S.; Chu, H.; Lee, K.; Yim, T.; Kim, Y.J.; Mun, J.; Kim, T.H. Physically Cross-linked Polymer Binder Induced by Reversible Acid–Base Interaction for High-Performance Silicon Composite Anodes. ACS Appl. Mater. Interfaces 2015, 7, 23545–23553. [Google Scholar] [CrossRef]
- Kim, H.-M.; Yoo, B.-I.; Yi, J.-W.; Choi, M.-J.; Yoo, J.-K. Solvent-Free Fabrication of Thick Electrodes in Thermoplastic Binders for High Energy Density Lithium-Ion Batteries. Nanomaterials 2022, 12, 3320. [Google Scholar] [CrossRef] [PubMed]
- Ruschhaupt, P.; Pohlmann, S.; Varzi, A.; Passerini, S. Determining Realistic Electrochemical Stability Windows of Electrolytes for Electrical Double-Layer Capacitors. Batter. Supercaps 2020, 3, 698–707. [Google Scholar] [CrossRef]
- Liang, X.; Ahmad, N.; Zhang, B.; Zeng, C.; Cao, X.; Dong, Q.; Yang, W. Research progress of robust binders with superior mechanical properties for high-performance silicon-based lithium-ion batteries. Mater. Chem. Front. 2024, 8, 1480–1512. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y.; Zhu, K.; Gao, Y.; Zhang, D.; Chen, G.; Wang, C.; Wei, Y. Enhanced electrochemical properties of TiO2(B) nanoribbons using the styrene butadiene rubber and sodium carboxyl methyl cellulose water binder. J. Power Sources 2014, 246, 95–102. [Google Scholar] [CrossRef]
- Hong, X.; Jin, J.; Wen, Z.; Zhang, S.; Wang, Q.; Shen, C.; Rui, K. On the dispersion of lithium-sulfur battery cathode materials effected by electrostatic and stereo-chemical factors of binders. J. Power Sources 2016, 324, 455–461. [Google Scholar] [CrossRef]
- Nakazawa, T.; Ikoma, A.; Kido, R.; Ueno, K.; Dokko, K.; Watanabe, M. Effects of compatibility of polymer binders with solvate ionic liquid electrolytes on discharge and charge reactions of lithium-sulfur batteries. J. Power Sources 2016, 307, 746–752. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, M.; Meng, P.; Jiang, M.; Qiu, X.; Zhang, J.; Fu, C. Aqueous Binders Compatible with Ionic Liquid Electrolyte for High-Performance Aluminum-Ion Batteries. Chem. Eur. J. 2023, 29, e202203546. [Google Scholar] [CrossRef]
- Xing, J.; Bliznakov, S.; Bonville, L.; Oljaca, M.; Maric, R. A Review of Nonaqueous Electrolytes, Binders, and Separators for Lithium-Ion Batteries. Electrochem. Energy Rev. 2022, 5, 14. [Google Scholar] [CrossRef]
- Tron, A.; Hamid, R.; Zhang, N.; Paolella, A.; Wulfert-Holzmann, P.; Kolotygin, V.; López-Aranguren, P.; Beutl, A. Film processing of Li6PS5Cl electrolyte using different binders and their combinations. J. Energy Storage 2023, 66, 107480. [Google Scholar] [CrossRef]
- Drago, R.S.; Vogel, G.C.; Needham, T.E. Four-parameter equation for predicting enthalpies of adduct formation. J. Am. Chem. Soc. 1971, 93, 6014–6026. [Google Scholar] [CrossRef]
- Pizzi, A.; Mtsweni, B.; Parsons, W. Wood-induced catalytic activation of PF adhesives autopolymerization vs. PF/wood covalent bonding. J. Appl. Polym. Sci. 1994, 52, 1847–1856. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Aleinikova, I.N.; Toporov, Y.P. On the role of electrostatic forces in the adhesion of polymer particles to solid surfaces. Powder Technol. 1969, 2, 154–158. [Google Scholar] [CrossRef]
- McBain, J.W.; Hopkins, D.G. On Adhesives and Adhesive Action. J. Phys. Chem. 1925, 29, 188–204. [Google Scholar] [CrossRef]
- Gardner, D.J.; Blumentritt, M.; Wang, L.; Yildirim, N. Adhesion Theories in Wood Adhesive Bonding. In Progress in Adhesion and Adhesives; Wiley: Hoboken, NJ, USA, 2015; pp. 125–168. [Google Scholar] [CrossRef]
- Shi, Q.; Wong, S.-C.; Ye, W.; Hou, J.; Zhao, J.; Yin, J. Mechanism of Adhesion between Polymer Fibers at Nanoscale Contacts. Langmuir 2012, 28, 4663–4671. [Google Scholar] [CrossRef]
- Léger, L.; Creton, C. Adhesion mechanisms at soft polymer interfaces. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2008, 366, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Fourche, G. An overview of the basic aspects of polymer adhesion. Part I: Fundamentals. Polym. Eng. Sci. 1995, 35, 957–967. [Google Scholar] [CrossRef]
- Licari, J.J.; Swanson, D.W. Functions and theory of adhesives. In Adhesives Technology for Electronic Applications; Elsevier: Amsterdam, The Netherlands, 2011; pp. 35–74. [Google Scholar] [CrossRef]
- Qin, T.; Yang, H.; Li, Q.; Yu, X.; Li, H. Design of functional binders for high-specific-energy lithium-ion batteries: From molecular structure to electrode properties. Ind. Chem. Mater. 2024, 2, 191–225. [Google Scholar] [CrossRef]
- Xiao, X.; Wu, W.; Huang, X. A multi-scale approach for the stress analysis of polymeric separators in a lithium-ion battery. J. Power Sources 2010, 195, 7649–7660. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Park, H.-K.; Kong, B.-S.; Oh, E.-S. Effect of high adhesive polyvinyl alcohol binder on the anodes of lithium ion batteries. Electrochem. Commun. 2011, 13, 1051–1053. [Google Scholar] [CrossRef]
- Guerfi, A.; Kaneko, M.; Petitclerc, M.; Mori, M.; Zaghib, K. LiFePO4 water-soluble binder electrode for Li-ion batteries. J. Power Sources 2007, 163, 1047–1052. [Google Scholar] [CrossRef]
- Chou, S.-L.; Pan, Y.; Wang, J.-Z.; Liu, H.-K.; Dou, S.-X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Huang, X.; Jiang, M.; Chapman, S.J.; Protas, B.; Richardson, G. Causes of binder damage in porous battery electrodes and strategies to prevent it. J. Power Sources 2017, 350, 140–151. [Google Scholar] [CrossRef]
- Han, D.; Han, I.K.; Son, H.B.; Kim, Y.S.; Ryu, J.; Park, S. Layering Charged Polymers Enable Highly Integrated High-Capacity Battery Anodes (Adv. Funct. Mater. 17/2023). Adv. Funct. Mater. 2023, 33, 2213458. [Google Scholar] [CrossRef]
- Li, H.; Peng, L.; Wu, D.; Wu, J.; Zhu, Y.; Hu, X. Ultrahigh-Capacity and Fire-Resistant LiFePO4-Based Composite Cathodes for Advanced Lithium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1802930. [Google Scholar] [CrossRef]
- Hu, L.; Jin, M.; Zhang, Z.; Chen, H.; Ajdari, F.B.; Song, J. Interface-Adaptive Binder Enabled by Supramolecular Interactions for High-Capacity Si/C Composite Anodes in Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2111560. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, J.; Elabd, A.; Choi, S.; Park, K.; Kwon, T.-W.; Lee, J.; Char, K.; Coskun, A.; Choi, J.W. A Pyrene–Poly(acrylic acid)–Polyrotaxane Supramolecular Binder Network for High-Performance Silicon Negative Electrodes. Adv. Mater. 2019, 31, 1905048. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shi, Z.; Wang, C. Electrochemical Performance of Electrospun carbon nanofibers as free-standing and binder-free anodes for Sodium-Ion and Lithium-Ion Batteries. Electrochim. Acta 2014, 141, 302–310. [Google Scholar] [CrossRef]
- Kang, Y.; Deng, C.; Chen, Y.; Liu, X.; Liang, Z.; Li, T.; Hu, Q.; Zhao, Y. Binder-Free Electrodes and Their Application for Li-Ion Batteries. Nanoscale Res. Lett. 2020, 15, 112. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, J.; Zhao, Q.; Wang, Z.; Zhu, Y.; Ma, X.; Cao, C. Supported SnS2 nanosheet array as binder-free anode for sodium ion batteries. Electrochim. Acta 2019, 308, 174–184. [Google Scholar] [CrossRef]
- Checko, S.; Ju, Z.; Zhang, B.; Zheng, T.; Takeuchi, E.S.; Marschilok, A.C.; Takeuchi, K.J.; Yu, G. Fast-Charging, Binder-Free Lithium Battery Cathodes Enabled via Multidimensional Conductive Networks. Nano Lett. 2024, 24, 1695–1702. [Google Scholar] [CrossRef]
- Xia, J.; Yuan, Y.; Yan, H.; Liu, J.; Zhang, Y.; Liu, L.; Zhang, S.; Li, W.; Yang, X.; Shu, H.; et al. Electrospun SnSe/C nanofibers as binder-free anode for lithium–ion and sodium-ion batteries. J. Power Sources 2020, 449, 227559. [Google Scholar] [CrossRef]
- Gao, X.; An, Y.; Zhang, W.; Yu, M.; Ci, L.; Feng, J. Self-supporting soft carbon fibers as binder-free and flexible anodes for high-performance sodium-ion batteries. Mater. Technol. 2018, 33, 810–814. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.; Byeon, H.; Kim, J.; Yang, J.W.; Kim, Y.; Kim, J.-K. Binder-free organic cathode based on nitroxide radical polymer-functionalized carbon nanotubes and gel polymer electrolyte for high-performance sodium organic polymer batteries. J. Mater. Chem. A Mater. 2020, 8, 17980–17986. [Google Scholar] [CrossRef]
- Fu, S.; Ni, J.; Xu, Y.; Zhang, Q.; Li, L. Hydrogenation Driven Conductive Na2Ti3O7 Nanoarrays as Robust Binder-Free Anodes for Sodium-Ion Batteries. Nano Lett. 2016, 16, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Zhai, S.; Wang, S.; Ru, Q.; Hou, X.; Hui, K.S.; Hui, K.N.; Chen, F. Recent Progress in Binder-Free Electrodes Synthesis for Electrochemical Energy Storage Application. Batter. Supercaps 2021, 4, 860–880. [Google Scholar] [CrossRef]
- Lithium-ion Battery Binders Market. Available online: https://www.marketsandmarkets.com/Market-Reports/lithium-ion-battery-binders-market-143858620.html#:~:text=The%20global%20lithium%2Dion%20battery,cagr%20from%202022%20to%202027 (accessed on 13 July 2024).
- Battery Binders Market. Available online: https://www.precedenceresearch.com/battery-binders-market (accessed on 13 July 2024).
- Wood, D.L.; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Duffner, F.; Mauler, L.; Wentker, M.; Leker, J.; Winter, M. Large-scale automotive battery cell manufacturing: Analyzing strategic and operational effects on manufacturing costs. Int. J. Prod. Econ. 2021, 232, 107982. [Google Scholar] [CrossRef]





| Binder | Electrode | Battery Technology | Electrode Loading (mg cm−1) | Capacity Retention (%) | Number of Cycles | C-Rate | Specific Capacity (mAhg−1) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Material | Type | ||||||||
| PVDF | LCO | Cathode | LIB | - | 84.5 | 300 | - | - | [152] |
| PVDF | NMC (111) | Cathode | LIB | 6 | 86.3 | 200 | 0.5 | 111.7 | [56] |
| PVDF Solvay5130 | CuO | Anode | LIB | - | 30 | 50 | 0.2 | 158.4 | [218] |
| Alginate | NMC (111) | Cathode | LIB | 6 | 89.2 | 200 | 0.5 | 126 | [56] |
| SA | LFP | Cathode | LIB | - | - | 50 | 0.1 | 165 | [84] |
| SBR/CMC | CuO | Anode | LIB | - | 86.85 | 50 | 0.2 | 461.3 | [218] |
| SBR/CMC | ZnFeO4 | Anode | LIB | 3 | - | 100 | 0.1 | 873.8 | [96] |
| SBR/CMC | LFP | Cathode | LIB | - | - | 50 | 0.1 | 153 | [84] |
| PEO-b-PAN | LFP | Cathode | LIB | - | - | 200 | 0.5 | 141 | [77] |
| PAN | LNMO | Cathode | LIB | 2 | 85.1 | 40 | 0.1 | 228.2 | [219] |
| PAN-PVDF | LNMO | Cathode | LIB | 2 | 77.5 | 40 | 0.1 | 202.7 | [219] |
| PTFE | Cr0.5Nb1.5(PO4)3 | Cathode | LIB | - | 73 | 20 | - | 145 | [220] |
| PTFE | Hard Carbon | Anode | LIB | - | 80 | 100 | 0.05 | 172 | [221] |
| CMC/PTFE | LFP | Cathode | LIB | - | - | 50 | 0.1 | 166 | [84] |
| PAA | Si-graphite composite | Anode | LIB | 1.5 | 38.2 | 300 | 5 | 372.3 | [222] |
| PAA | LFP | Cathode | LIB | - | - | 50 | 0.1 | 146.4 | [84] |
| NaPAA-g-CMC | Si | Anode | LIB | 0.45 | 79.3 | 100 | 0.2 | 1816 | [223] |
| CS-g-PANI | Si | Anode | LIB | 0.8–0.9 | - | 200 | - | 1091 | [224] |
| PVDF | SbTe | Anode | SIB | 1.5–2.2 | 17.9 | 500 | - | 49 | [225] |
| PVDF | P/C | Anode | SIB | 1.5 | - | 100 | 1 | 30 | [226] |
| SA/graphene oxide | MoS2 | Anode | SIB | - | 93.1 | 200 | - | 336.5 | [227] |
| SA/graphene oxide | Na3(VO)2(PO4)2F | Cathode | SIB | - | 95 | 300 | 0.5 | 117.9 | [227] |
| SA | Sb-C NP | Anode | SIB | 0.6–0.8 | - | 50 | - | 553 | [228] |
| SBR/CMC | NaTi2(PO4)3 | Anode | SIB | - | 90.5 | 500 | 0.2 | 120 | [101] |
| PAA-GLY | Sn | Anode | SIB | 1.2–1.4 | 68.4 | 300 | - | 377.5 | [229] |
| PAA | FeF2-rGO | Cathode | SIB | 1.1 | - | 200 | - | 120 | [230] |
| PAA | SbTe | Anode | SIB | 1.5–2.2 | 55.2 | 500 | 144 | [225] | |
| Poly (9,9-dioctylfluorene-co-fluorenone-co-methylbenzoic ester) | Sn | Anode | SIB | 1.5 | - | 10 | 0.1 | 610 | [231] |
| Ethyl cellulose | NMC | Cathode | SSB | 4 | 89.7 | 100 | 0.05 | 100 | [188] |
| SBR | NMC | Cathode | SSB | 3.5 | 78.7 | 45 | - | 102.5 | [186] |
| PVDF-HFP | NMC | Cathode | SSB | 4 | - | - | 0.2 | 160 | [232] |
| NBR | NMC | Cathode | SSB | 4 | - | - | 0.2 | 149 | [232] |
| p(DMAEMA) | LCO | Cathode | SSB | - | 89.93 | 200 | 0.1 | 110.6 | [233] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, M.; M. R., A.K.; Zaghib, K. Binders for Li-Ion Battery Technologies and Beyond: A Comprehensive Review. Batteries 2024, 10, 268. https://doi.org/10.3390/batteries10080268
Srivastava M, M. R. AK, Zaghib K. Binders for Li-Ion Battery Technologies and Beyond: A Comprehensive Review. Batteries. 2024; 10(8):268. https://doi.org/10.3390/batteries10080268
Chicago/Turabian StyleSrivastava, Muskan, Anil Kumar M. R., and Karim Zaghib. 2024. "Binders for Li-Ion Battery Technologies and Beyond: A Comprehensive Review" Batteries 10, no. 8: 268. https://doi.org/10.3390/batteries10080268
APA StyleSrivastava, M., M. R., A. K., & Zaghib, K. (2024). Binders for Li-Ion Battery Technologies and Beyond: A Comprehensive Review. Batteries, 10(8), 268. https://doi.org/10.3390/batteries10080268









