Experimental Study on Thermal Runaway Characteristics of High-Nickel Ternary Lithium-Ion Batteries under Normal and Low Pressures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Battery Samples
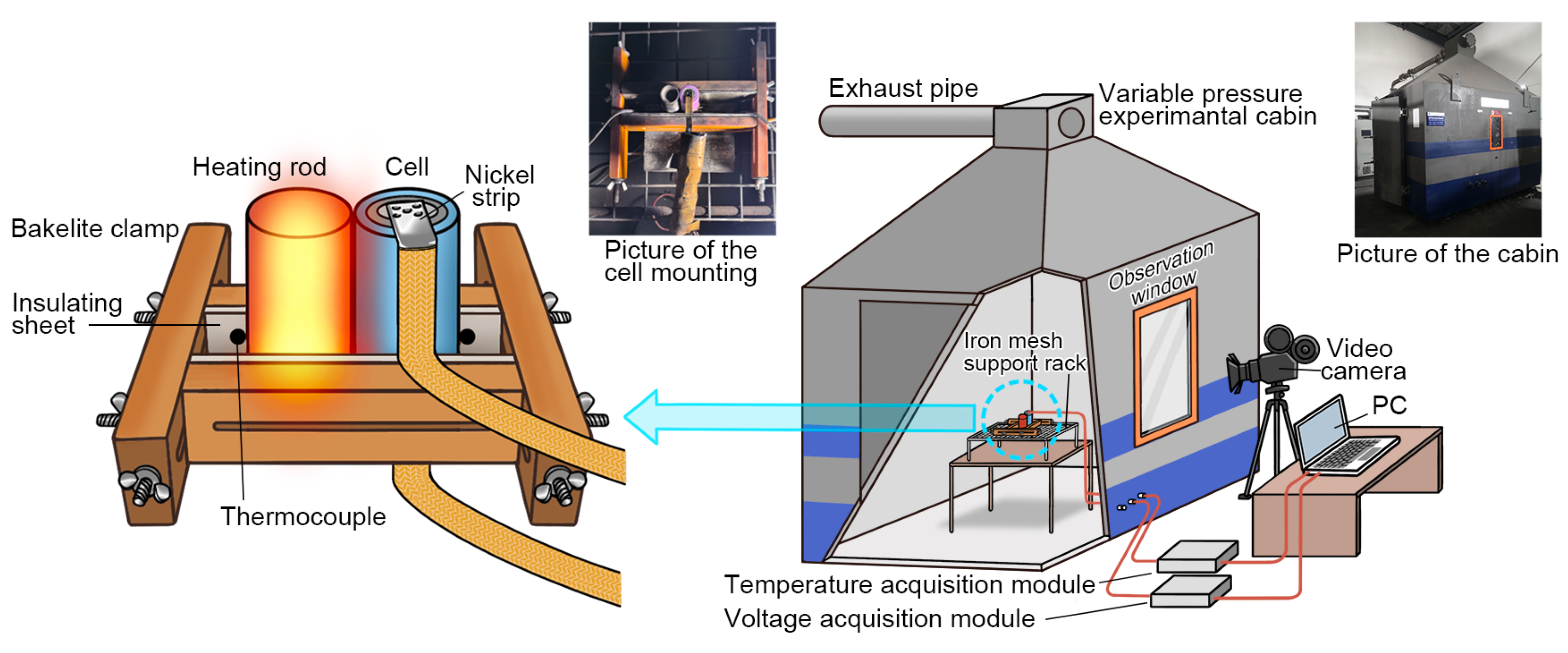
2.2. Experimental Setup
3. Results and Discussion
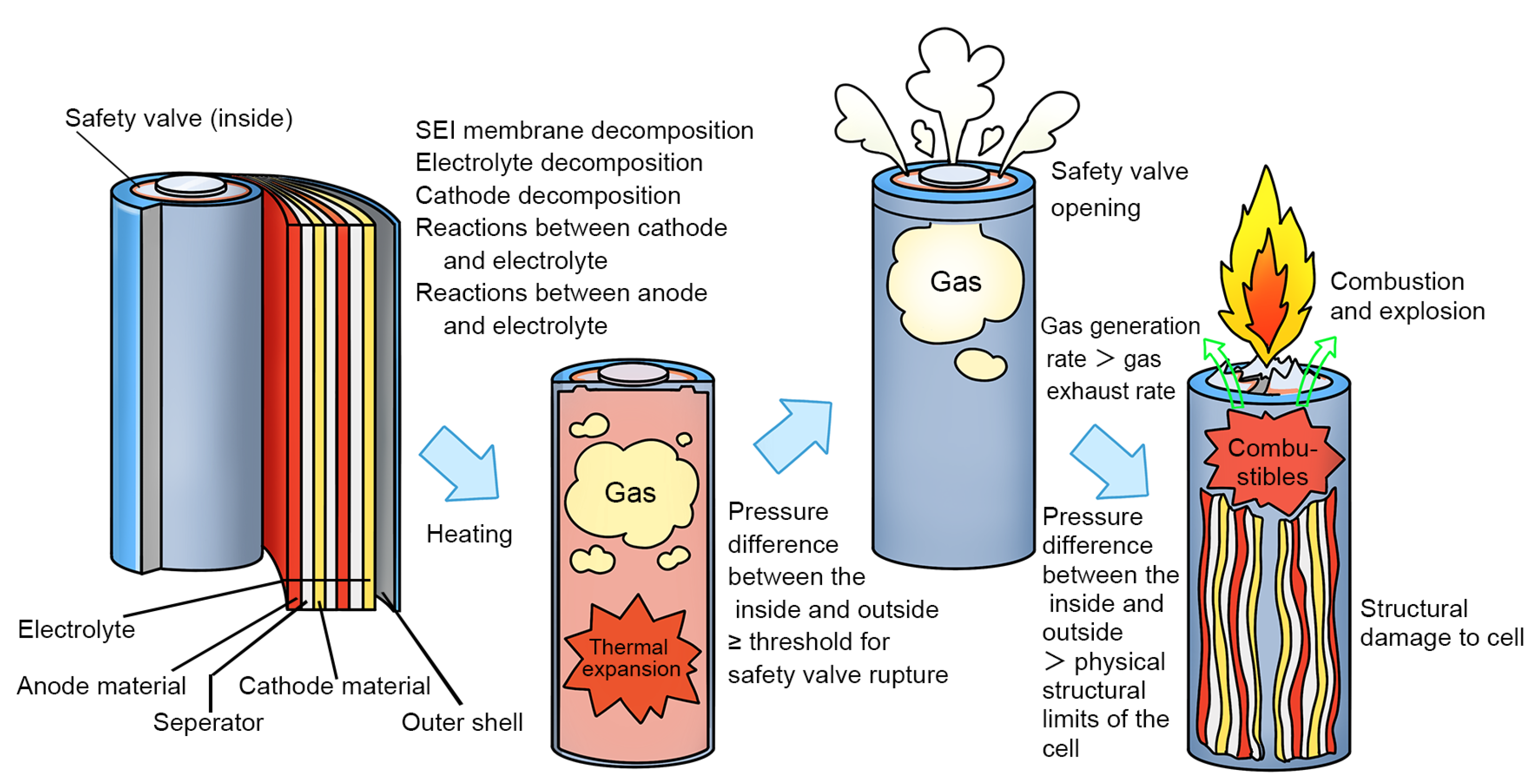
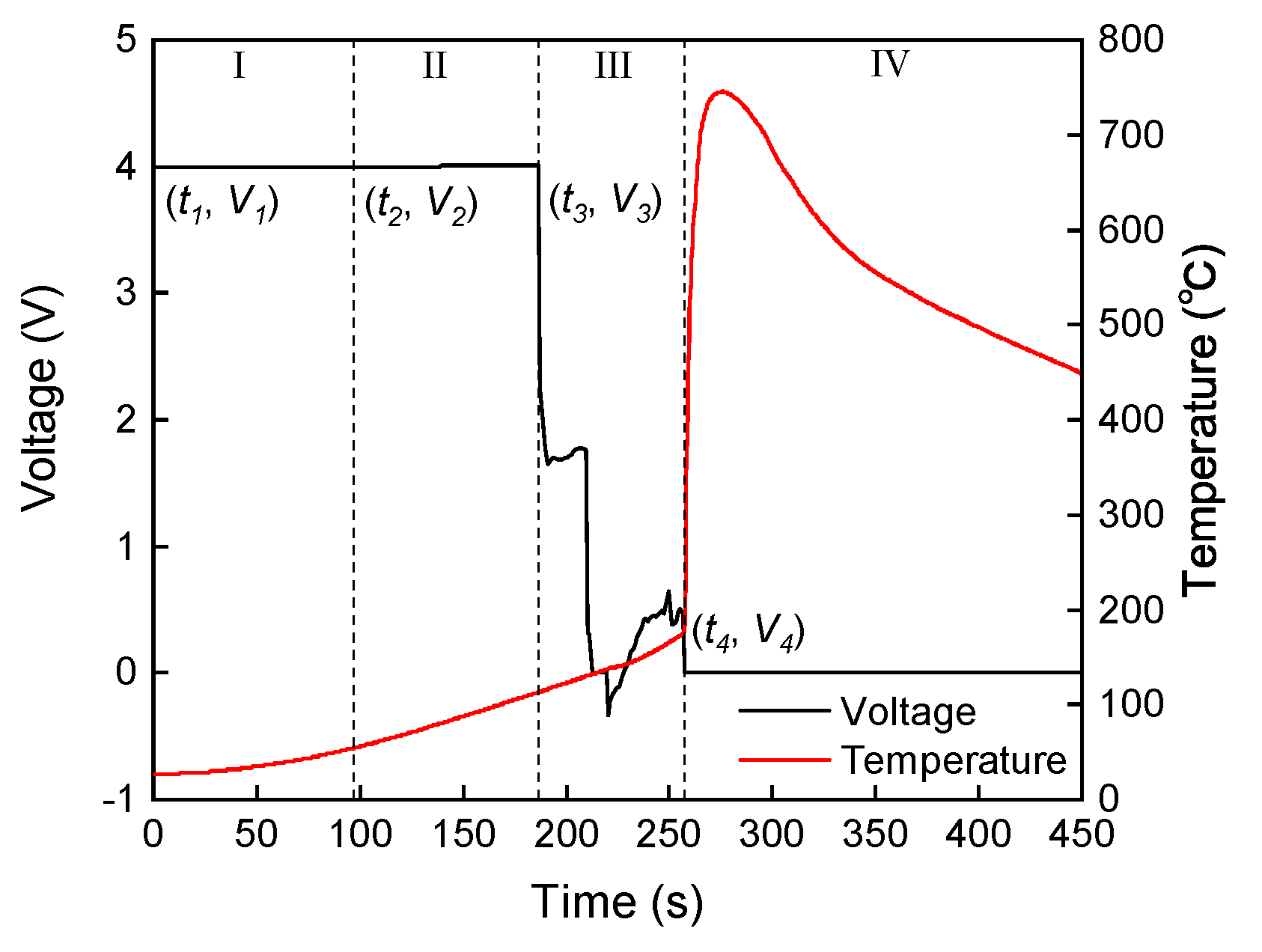
3.1. Criteria for Thermal Runaway and Venting
3.2. Thermal Runaway Behavior
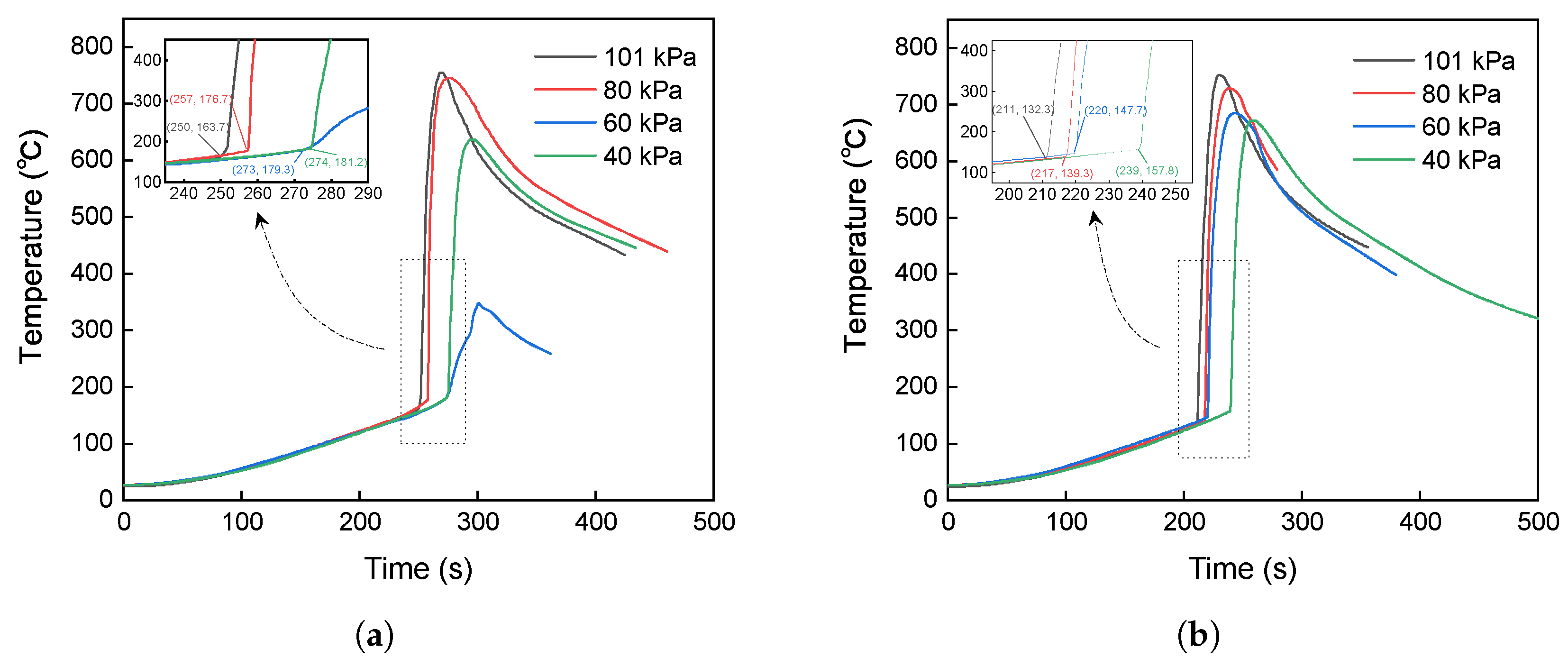
- Stage I (Heating stage): The cell is heated, and the temperature increases with an average temperature rise rate of 0.50 °C/s at 80 kPa and of 0.49 °C/s at 40 kPa. Unstable chemical reactions occur inside the cell, gradually raising the surface temperature while the voltage drops sharply.
- Stage II (Venting stage): When internal reactions accelerate and internal temperature rises to a certain level, the safety valve of the cell breaks, entering stage II, which is marked by a decrease in the temperature rise rate. It should be noted that venting does not always lead to a decrease in temperature [15,20,21,22,23]. A more accurate description is a decrease in the temperature rise rate, which will be further discussed below. The surface temperature of the cell rises relatively quickly during this stage, with an average temperature rise rate of 1.05 °C/s at 80 kPa and of 0.87 °C/s at 40 kPa. This stage is very brief, typically lasting within 1 min.
- Stage III (Thermal runaway occurrence stage): The inside of the cell is entirely out of control, with surface temperature rising rapidly to a maximum rate of 235.44 °C/s at 80 kPa and of 72.88 °C/s at 40 kPa, releasing significant energy, finally causing fire or explosion.
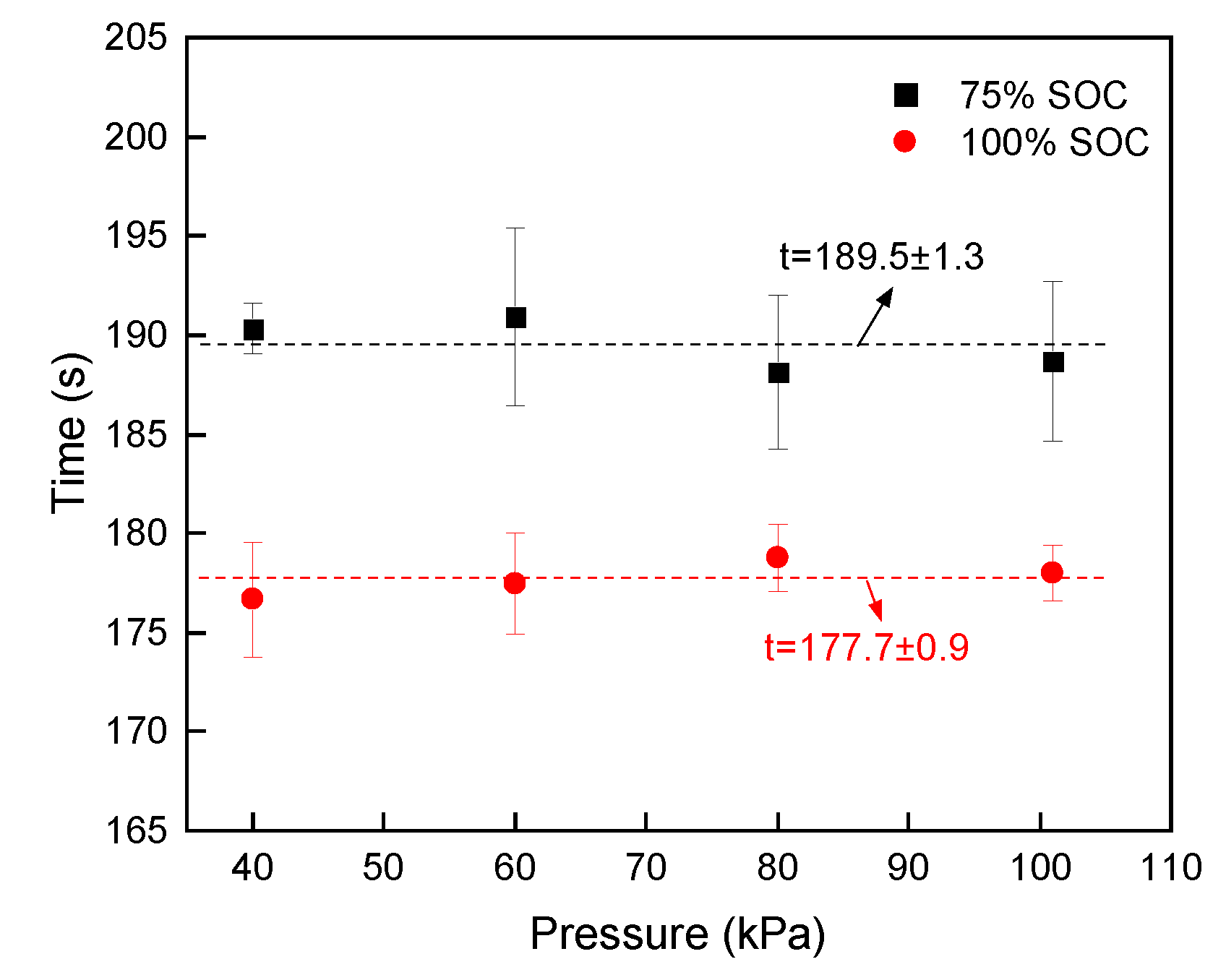
3.3. Venting and Thermal Runaway Events
3.4. Open Circuit Voltage
- Stage I (Voltage stabilization stage): This stage spans from a surface temperature of 28 °C to 55 °C. Before thermal runaway, there is a period during which the voltage remains stable. During this stage, the voltage may exhibit slight fluctuations due to minor heat accumulation, but these changes are not significant. The voltage slightly increases from (3.9973 V) to (3.9998 V).
- Stage II (Voltage rise stage): This stage spans from a surface temperature of 55 °C to 113 °C. During the accelerated heating phase, the voltage rises above its initial value, reaching a peak at (4.0042 V). This may be attributed to the enhanced ionic conductivity of the electrolyte and reduced internal resistance as the temperature increases [40], resulting in a temporary voltage rise.
- Stage III (Voltage decline stage): This stage spans from a surface temperature of 113 °C to 177 °C. After reaching the peak voltage, the voltage begins to sharply decline as severe chemical and structural degradation occurs within the cell. Internal short circuits are caused by the collapse of the separator [41,42]. This sharp voltage drop is an advanced characteristic indicator that the cell is approaching thermal runaway in contrast to the sharp surface temperature rise.
- Stage IV (Voltage collapse stage): At the onset of thermal runaway, the voltage collapses to an extremely low level, reaching (−0.0006 V). This indicates that the internal chemical reactions are almost entirely out of control and the internal structure of the battery is completely destroyed.
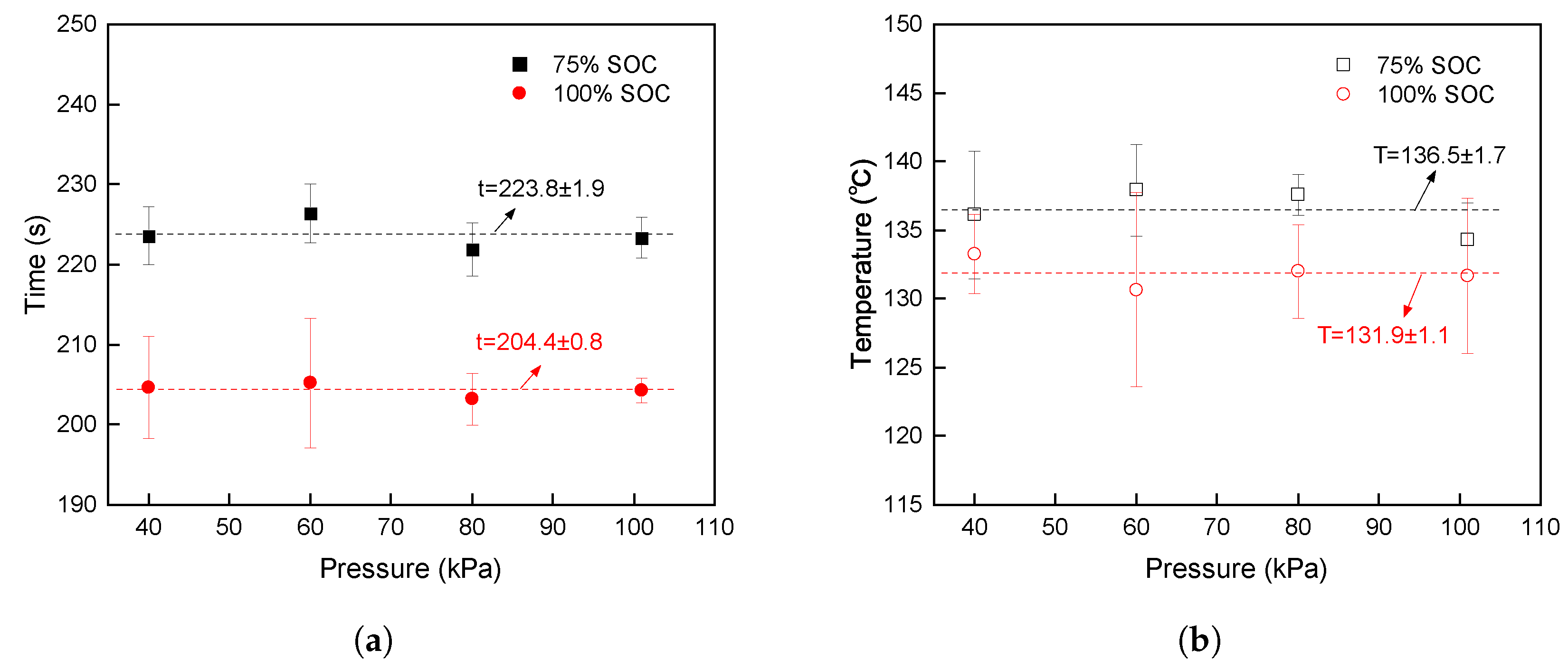
3.5. Comparison of Characteristic Parameters
3.6. Mass Loss
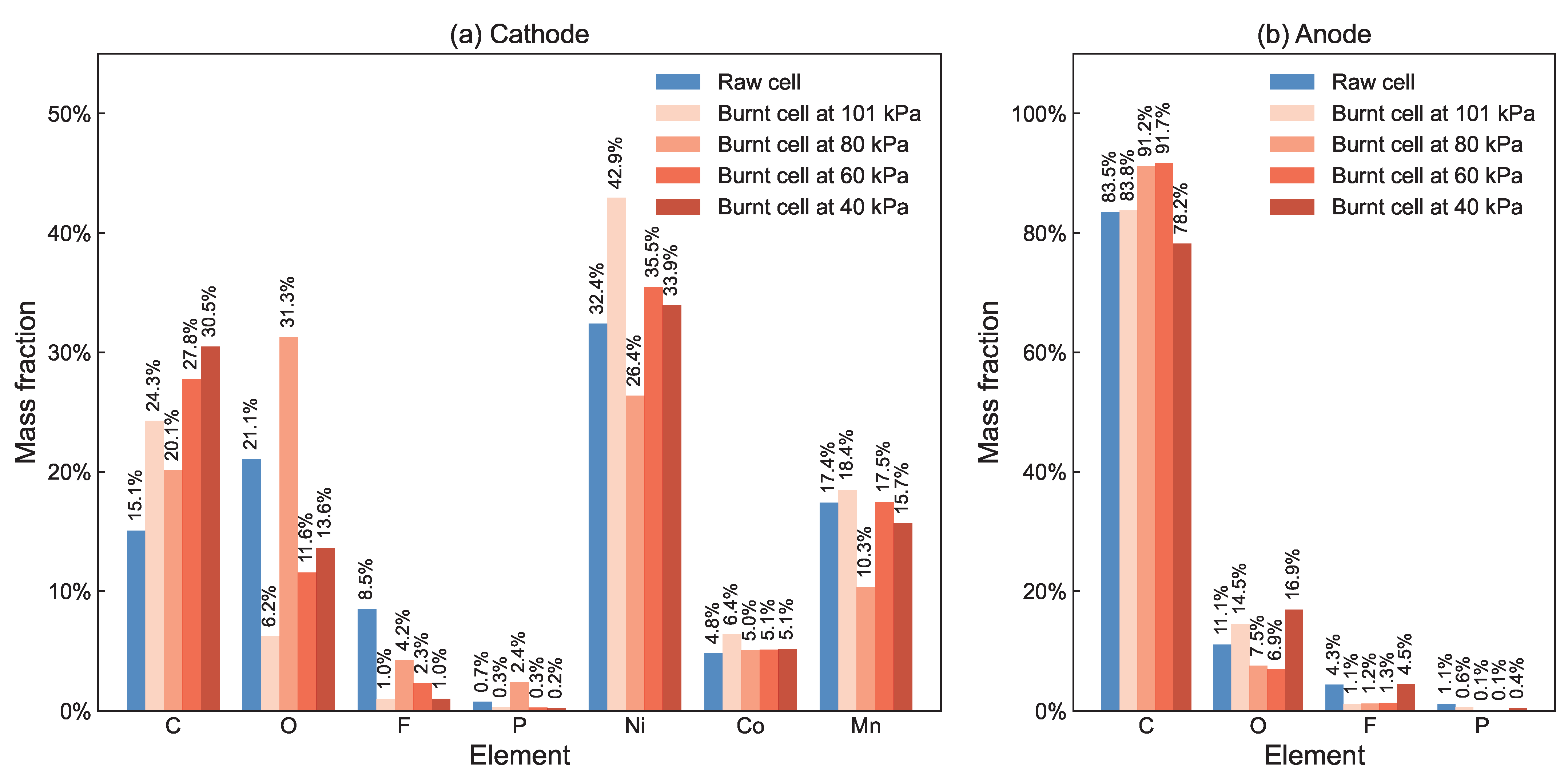
3.7. Post Testing Analysis
3.8. Heat Transfer Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion Battery: State of the Art and Future Perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wang, L.; Feng, X.; Ren, D.; Wu, Y.; Xu, G.; Lu, L.; Hou, J.; Zhang, W.; et al. Thermal Runaway Mechanism of Lithium-Ion Battery with LiNi0.8Mn0.1Co0.1O2 Cathode Materials. Nano Energy 2021, 85, 105878. [Google Scholar] [CrossRef]
- Cui, Z.; Manthiram, A. Thermal Stability and Outgassing Behaviors of High-nickel Cathodes in Lithium-ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202307243. [Google Scholar] [CrossRef]
- Federal Aviation Administration (FAA). Lithium Battery Air Incidents Involving Smoke, Fire, or Extreme Heat. 2024. Available online: https://www.faa.gov/hazmat/resources/lithium_batteries/incidents (accessed on 29 January 2024).
- Chen, M.; Liu, J.; Ouyang, D.; Wang, J. Experimental Investigation on the Effect of Ambient Pressure on Thermal Runaway and Fire Behaviors of Lithium-ion Batteries. Int. J. Energy Res. 2019, 43, 4898–4911. [Google Scholar] [CrossRef]
- Chen, M.; Ouyang, D.; Weng, J.; Liu, J.; Wang, J. Environmental Pressure Effects on Thermal Runaway and Fire Behaviors of Lithium-Ion Battery with Different Cathodes and State of Charge. Process Saf. Environ. Prot. 2019, 130, 250–256. [Google Scholar] [CrossRef]
- Xie, S.; Ren, L.; Yang, X.; Wang, H.; Sun, Q.; Chen, X.; He, Y. Influence of Cycling Aging and Ambient Pressure on the Thermal Safety Features of Lithium-Ion Battery. J. Power Sources 2020, 448, 227425. [Google Scholar] [CrossRef]
- Xie, S.; Ren, L.; Gong, Y.; Li, M.; Chen, X. Effect of Charging/Discharging Rate on the Thermal Runaway Characteristics of Lithium-Ion Batteries in Low Pressure. J. Electrochem. Soc. 2020, 167, 140503. [Google Scholar] [CrossRef]
- Xie, S.; Sun, J.; Chen, X.; He, Y. Thermal Runaway Behavior of Lithium-ion Batteries in Different Charging States under Low Pressure. Int. J. Energy Res. 2021, 45, 5795–5805. [Google Scholar] [CrossRef]
- Xie, S.; Gong, Y.; Li, G.; Ping, X. Effect of Low-Pressure Environment on Thermal Runaway Behavior of NCM523/Graphite Pouch Cells with Different Overcharge Cycles. J. Energy Storage 2022, 55, 105444. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, Q.; Zhu, W.; Yi, X.; Han, X. Thermal Runaway Characteristics of 18650 NCM Lithium-ion Batteries under the Different Initial Pressures. Electrochemistry 2022, 90, 087004. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, F.; Niu, J. Risk analysis method of thermal runaway gas explosion in lithium-ion batteries. Energy Storage Sci. Technol. 2023, 12, 2263. [Google Scholar]
- Chen, X.; Zhang, X.; Wang, H.; Jia, J.; Xie, S.; Zhi, M.; Fu, J.; Sun, Q. Influence of Ambient Pressure and Heating Power on the Thermal Runaway Features of Lithium-Ion Battery. J. Electrochem. Energy Convers. Storage 2021, 18, 021014. [Google Scholar] [CrossRef]
- Fang, J.; Cai, J.; He, X. Experimental Study on the Vertical Thermal Runaway Propagation in Cylindrical Lithium-ion Batteries: Effects of Spacing and State of Charge. Appl. Therm. Eng. 2021, 197, 117399. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, H.; Liu, J.; Huang, X. Layer-to-Layer Thermal Runaway Propagation of Open-Circuit Cylindrical Li-Ion Batteries: Effect of Ambient Pressure. J. Energy Storage 2022, 55, 105709. [Google Scholar] [CrossRef]
- Incropera, F.P.; DeWitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; Wiley: New York, NY, USA, 1996; Volume 6. [Google Scholar]
- Ping, P. Lithium Ion Battery Thermal Runaway and Fire Risk Analysis and the Development on the Safer Battery System. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2014. [Google Scholar]
- Sommer, L.W.; Raghavan, A.; Kiesel, P.; Saha, B.; Schwartz, J.; Lochbaum, A.; Ganguli, A.; Bae, C.J.; Alamgir, M. Monitoring of Intercalation Stages in Lithium-Ion Cells over Charge-Discharge Cycles with Fiber Optic Sensors. J. Electrochem. Soc. 2015, 162, A2664–A2669. [Google Scholar] [CrossRef]
- Kim, H.; Sung, N. The Effect of Ambient Pressure on the Evaporation of a Single Droplet and a Spray. Combust. Flame 2003, 135, 261–270. [Google Scholar] [CrossRef]
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; Hunt, I.; Mason, T.J.; Millichamp, J.; Di Michiel, M.; Offer, G.J.; Hinds, G.; et al. In-Operando High-Speed Tomography of Lithium-Ion Batteries during Thermal Runaway. Nat. Commun. 2015, 6, 6924. [Google Scholar] [CrossRef] [PubMed]
- Jindal, P.; Bhattacharya, J. Review—Understanding the Thermal Runaway Behavior of Li-Ion Batteries through Experimental Techniques. J. Electrochem. Soc. 2019, 166, A2165–A2193. [Google Scholar] [CrossRef]
- Mei, W.; Liu, Z.; Wang, C.; Wu, C.; Liu, Y.; Liu, P.; Xia, X.; Xue, X.; Han, X.; Sun, J.; et al. Operando Monitoring of Thermal Runaway in Commercial Lithium-Ion Cells via Advanced Lab-on-Fiber Technologies. Nat. Commun. 2023, 14, 5251. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-Runaway Experiments on Consumer Li-ion Batteries with Metal-Oxide and Olivin-Type Cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, Z. Thermology Thermodynamics and Statistical Physics; Science Press: Beijing, China, 2015. [Google Scholar]
- Zou, K.; He, K.; Lu, S. Venting Composition and Rate of Large-Format LiNi0.8Co0.1Mn0.1O2 Pouch Power Battery during Thermal Runaway. Int. J. Heat Mass Transf. 2022, 195, 123133. [Google Scholar] [CrossRef]
- Wei, N.; Zhang, F.; Zhang, W.; Li, X. Comparative Study on the Thermal Runaway Characteristics of Li(NixCoyMnz)O2 Batteries. Heliyon 2024, 10, e31203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Niu, J.; Yang, J.; Liu, T.; Bao, F.; Wang, Q. In-Situ Explosion Limit Analysis and Hazards Research of Vent Gas from Lithium-Ion Battery Thermal Runaway. J. Energy Storage 2022, 56, 106146. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhang, Z.; Wang, Q.; Jin, C.; Wu, C.; Xu, C.; Hao, J.; Sun, L.; Du, Z.; et al. Fire and Explosion Characteristics of Vent Gas from Lithium-Ion Batteries after Thermal Runaway: A Comparative Study. eTransportation 2022, 13, 100190. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse Behavior of High-Power, Lithium-Ion Cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Kim, G.H.; Pesaran, A.; Spotnitz, R. A Three-Dimensional Thermal Abuse Model for Lithium-Ion Cells. J. Power Sources 2007, 170, 476–489. [Google Scholar] [CrossRef]
- Mao, B.; Zhao, C.; Chen, H.; Wang, Q.; Sun, J. Experimental and Modeling Analysis of Jet Flow and Fire Dynamics of 18650-Type Lithium-Ion Battery. Appl. Energy 2021, 281, 116054. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Chu, G. Lithium Ion Battery Fire Furthermore, Explosion. Fire Saf. Sci. 2005, 8, 375–382. [Google Scholar] [CrossRef]
- Finegan, D.P.; Darcy, E.; Keyser, M.; Tjaden, B.; Heenan, T.M.M.; Jervis, R.; Bailey, J.J.; Vo, N.T.; Magdysyuk, O.V.; Drakopoulos, M.; et al. Identifying the Cause of Rupture of Li-Ion Batteries during Thermal Runaway. Adv. Sci. 2018, 5, 1700369. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, S.; Shi, L.; Cheng, X.; Zhang, H. Ignition and Combustion Characteristics of Lithium Ion Batteries under Low Atmospheric Pressure. Energy 2018, 161, 38–45. [Google Scholar] [CrossRef]
- Qin, P.; Sun, J.; Wang, Q. A New Method to Explore Thermal and Venting Behavior of Lithium-Ion Battery Thermal Runaway. J. Power Sources 2021, 486, 229357. [Google Scholar] [CrossRef]
- Mao, B.; Fear, C.; Chen, H.; Zhou, H.; Zhao, C.; Mukherjee, P.P.; Sun, J.; Wang, Q. Experimental and Modeling Investigation on the Gas Generation Dynamics of Lithium-Ion Batteries during Thermal Runaway. eTransportation 2023, 15, 100212. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; He, Y.; Yuen, R.; Wang, J. Study of the Fire Hazards of Lithium-Ion Batteries at Different Pressures. Appl. Therm. Eng. 2017, 125, 1061–1074. [Google Scholar] [CrossRef]
- Bak, S.M.; Nam, K.W.; Chang, W.; Yu, X.; Hu, E.; Hwang, S.; Stach, E.A.; Kim, K.B.; Chung, K.Y.; Yang, X.Q. Correlating Structural Changes and Gas Evolution during the Thermal Decomposition of Charged LixNi0.8Co0.15Al0.05O2 Cathode Materials. Chem. Mater. 2013, 25, 337–351. [Google Scholar] [CrossRef]
- Bak, S.M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.J.; Kim, K.B.; Chung, K.Y.; Yang, X.Q.; Nam, K.W. Structural Changes and Thermal Stability of Charged LiNi xMnyCoz O2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef] [PubMed]
- Hossain Ahmed, S.; Kang, X.; Bade Shrestha, S.O. Effects of Temperature on Internal Resistances of Lithium-Ion Batteries. J. Energy Resour. Technol. 2015, 137, 031901. [Google Scholar] [CrossRef]
- Venugopal, G. Characterization of Thermal Cut-off Mechanisms in Prismatic Lithium-Ion Batteries. J. Power Sources 2001, 101, 231–237. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J. Enhancing the Safety of Lithium Ion Batteries by 4-Isopropyl Phenyl Diphenyl Phosphate. Mater. Lett. 2007, 61, 3338–3340. [Google Scholar] [CrossRef]
- Li, W.; Lucht, B.L. Lithium-Ion Batteries: Thermal Reactions of Electrolyte with the Surface of Metal Oxide Cathode Particles. J. Electrochem. Soc. 2006, 153, A1617. [Google Scholar] [CrossRef]
- Saito, Y.; Rahman, M.K. Investigation of Positive Electrodes after Cycle Testing of High-Power Li-ion Battery Cells IV. J. Power Sources 2007, 174, 877–882. [Google Scholar] [CrossRef]
- Weng, J.; Ouyang, D.; Liu, Y.; Chen, M.; Li, Y.; Huang, X.; Wang, J. Alleviation on Battery Thermal Runaway Propagation: Effects of Oxygen Level and Dilution Gas. J. Power Sources 2021, 509, 230340. [Google Scholar] [CrossRef]







| Item | Specification |
|---|---|
| Height | 65.05 ± 0.15 mm |
| Diameter | 18.35 ± 0.15 mm |
| Weight | 46 ± 2 g |
| Anode material | Graphite |
| Cathode material | LiNi0.8Co0.1Mn0.1O2 |
| Nominal capacity | 2600 mAh |
| Nominal voltage | 3.6 V |
| Charge cut-off voltage | 4.2 V |
| Discharge cut-off voltage | 2.75 V |
| Charging cut-off current | 0.02 C (52 mA) |
| Testing Group | Testing No. | SOC | Ambient Pressure (kPa) | Heating Power (W) |
|---|---|---|---|---|
| 1 | 1/2/3/4 | 75% | 101/80/60/40 | 150 |
| 2 | 5/6/7/8 | 100% | 101/80/60/40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Meng, D.; Zhao, C.-X.; Yu, J.-L.; Wang, X.-H.; Wang, J. Experimental Study on Thermal Runaway Characteristics of High-Nickel Ternary Lithium-Ion Batteries under Normal and Low Pressures. Batteries 2024, 10, 287. https://doi.org/10.3390/batteries10080287
Jin Y, Meng D, Zhao C-X, Yu J-L, Wang X-H, Wang J. Experimental Study on Thermal Runaway Characteristics of High-Nickel Ternary Lithium-Ion Batteries under Normal and Low Pressures. Batteries. 2024; 10(8):287. https://doi.org/10.3390/batteries10080287
Chicago/Turabian StyleJin, Ye, Di Meng, Chen-Xi Zhao, Jia-Ling Yu, Xue-Hui Wang, and Jian Wang. 2024. "Experimental Study on Thermal Runaway Characteristics of High-Nickel Ternary Lithium-Ion Batteries under Normal and Low Pressures" Batteries 10, no. 8: 287. https://doi.org/10.3390/batteries10080287
APA StyleJin, Y., Meng, D., Zhao, C.-X., Yu, J.-L., Wang, X.-H., & Wang, J. (2024). Experimental Study on Thermal Runaway Characteristics of High-Nickel Ternary Lithium-Ion Batteries under Normal and Low Pressures. Batteries, 10(8), 287. https://doi.org/10.3390/batteries10080287






