A Pathway to Circular Economy-Converting Li-Ion Battery Recycling Waste into Graphite/rGO Composite Electrocatalysts for Zinc–Air Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Catalyst Materials
2.2. Physicochemical Characterisation
2.3. Electrochemical Characterisation
2.4. Zinc-Air Battery Testing
3. Results and Discussion
3.1. Analysis of Materials Physicochemical Properties
3.1.1. Physicochemical Analysis of Precursor Materials
3.1.2. XRD, SEM-EDS, and TEM-EDS Analysis of Composites
3.1.3. Raman and N2 Physisorption Analysis of Studied Materials
3.1.4. Surface Composition and Chemical State Characterization of Materials via XPS
3.2. Evaluation of Bifunctional Oxygen Electrocatalysis
3.3. Performance of Composite Catalysts as Air Electrodes in Zinc–Air Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. Global EV Outlook 2024; IEA: Paris, France, 2024. [Google Scholar]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Yu, Y.X. Can all nitrogen-doped defects improve the performance of graphene anode materials for lithium-ion batteries? Phys. Chem. Chem. Phys. 2013, 15, 16819–16827. [Google Scholar] [CrossRef] [PubMed]
- Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs (European Commission); Grohol, M.; Veeh, C. European Commission: Directorate-General for Internal Market Entrepreneurship and SMEs, I. In Study on the Critical Raw Materials for the EU 2023—Final Report; Grohol, M., Veeh, C., Eds.; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- IEA. Global Critical Minerals Outlook 2024; IEA: Paris, France, 2024. [Google Scholar]
- IEA. World Energy Outlook; IEA: Paris, France, 2024. [Google Scholar]
- IEA. Recycling of Critical Minerals; IEA: Paris, France, 2024. [Google Scholar]
- Natarajan, S.; Divya, M.L.; Aravindan, V. Should we recycle the graphite from spent lithium-ion batteries? The untold story of graphite with the importance of recycling. J. Energy Chem. 2022, 71, 351–369. [Google Scholar] [CrossRef]
- He, S.; Wu, M.; Li, S.; Jiang, Z.; Hong, H.; Cloutier, S.G.; Yang, H.; Omanovic, S.; Sun, S.; Zhang, G. Research Progress on Graphite-Derived Materials for Electrocatalysis in Energy Conversion and Storage. Molecules 2022, 27, 8644. [Google Scholar] [CrossRef] [PubMed]
- Morales-Torres, S.; Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A.; McDonald, M. Fuel Cell Systems Explained; John Wiley & Sons Ltd.: Chichester, UK, 2003. [Google Scholar]
- Yang, D.; Zhang, L.; Yan, X.; Yao, X. Recent Progress in Oxygen Electrocatalysts for Zinc–Air Batteries. Small Methods 2017, 1, 1700209. [Google Scholar] [CrossRef]
- Sun, J.; Wang, N.; Qiu, Z.; Xing, L.; Du, L. Recent Progress of Non-Noble Metal Catalysts for Oxygen Electrode in Zn-Air Batteries: A Mini Review. Catalysts 2022, 12, 843. [Google Scholar] [CrossRef]
- Liu, T.; Cai, S.; Gao, Z.; Liu, S.; Li, H.; Chen, L.; Li, M.; Guo, H. Facile synthesis of the porous FeCo@nitrogen-doped carbon nanosheets as bifunctional oxygen electrocatalysts. Electrochim. Acta 2020, 335, 135647. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, N.; Cai, S.; Wang, R.; Guo, W.; Tang, H. Co-N-doped hierarchically ordered macro/mesoporous carbon as bifunctional electrocatalyst toward oxygen reduction/evolution reactions. Int. J. Energy Res. 2021, 45, 6250–6261. [Google Scholar] [CrossRef]
- Yang, L.; Wang, D.; Lv, Y.; Cao, D. Nitrogen-doped graphitic carbons with encapsulated CoNi bimetallic nanoparticles as bifunctional electrocatalysts for rechargeable Zn–Air batteries. Carbon 2019, 144, 8–14. [Google Scholar] [CrossRef]
- Ganesan, P.; Ishihara, A.; Staykov, A.; Nakashima, N. Recent Advances in Nanocarbon-Based Nonprecious Metal Catalysts for Oxygen/Hydrogen Reduction/Evolution Reactions and Zn-Air Battery. Bull. Chem. Soc. Jpn. 2023, 96, 429–443. [Google Scholar] [CrossRef]
- Xu, H.M.; Zhu, H.R.; Huang, C.J.; Zhang, Z.J.; Shuai, T.Y.; Zhan, Q.N.; Li, G.R. Recent advances in Fe-N-C- and Co-N-C-based materials as bifunctional electrocatalysts for oxygen reduction and oxygen evolution. Sci. China Chem. 2023, 67, 1137–1160. [Google Scholar] [CrossRef]
- Zou, X.; Tang, M.; Lu, Q.; Wang, Y.; Shao, Z.; An, L. Carbon-based electrocatalysts for rechargeable Zn–air batteries: Design concepts, recent progress and future perspectives. Energy Environ. Sci. 2024, 17, 386–424. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Z.; Wu, D.; Yang, J. Optimizing the electronic structure of cobalt via synergized oxygen vacancy and Co-N-C to boost reversible oxygen electrocatalysis for rechargeable Zn-air batteries. Appl. Catal. B 2020, 278, 119300. [Google Scholar] [CrossRef]
- Li, C.; Zhou, E.; Yu, Z.; Liu, H.; Xiong, M. Tailor-made open porous 2D CoFe/SN-carbon with slightly weakened adsorption strength of ORR/OER intermediates as remarkable electrocatalysts toward zinc-air batteries. Appl. Catal. B 2020, 269, 118771. [Google Scholar] [CrossRef]
- Liivand, K.; Sainio, J.; Wilson, B.P.; Kruusenberg, I.; Lundström, M. Overlooked residue of Li-ion battery recycling waste as high-value bifunctional oxygen electrocatalyst for Zn-air batteries. Appl. Catal. B Environ. 2023, 332, 122767. [Google Scholar] [CrossRef]
- Liivand, K.; Kazemi, M.; Walke, P.; Mikli, V.; Uibu, M.; Macdonald, D.D.; Kruusenberg, I. Spent Li-Ion Battery Graphite Turned Into Valuable and Active Catalyst for Electrochemical Oxygen Reduction. ChemSusChem 2021, 14, 1103–1111. [Google Scholar] [CrossRef]
- Ruan, D.; Zou, K.; Du, K.; Wang, F.; Wu, L.; Zhang, Z.; Wu, X.; Hu, G. Recycling of Graphite Anode from Spent Lithium-ion Batteries for Preparing Fe-N-doped Carbon ORR Catalyst. ChemCatChem 2021, 13, 2025–2033. [Google Scholar] [CrossRef]
- Bian, H.; Wu, W.; Zhu, Y.; Tsang, C.H.; Cao, Y.; Xu, J.; Liao, X.; Lu, Z.; Lu, X.Y.; Liu, C.; et al. Waste to Treasure: Regeneration of Porous Co-Based Catalysts from Spent LiCoO2 Cathode Materials for an Efficient Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2023, 11, 670–678. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, K.; Xu, R.; Chen, Z.; Shi, K.; Li, W.; Li, J. Efficient bifunctional 3D porous Co–N–C catalyst from spent Li–ion batteries and biomass for Zinc–Air batteries. Chem. Eng. Sci. 2023, 268, 118433. [Google Scholar] [CrossRef]
- Kotkar, A.; Dash, S.; Bhanja, P.; Sahu, S.; Verma, A.; Mukherjee, A.; Mohapatra, M.; Basu, S. Microwave assisted recycling of spent Li-ion battery electrode material into efficient oxygen evolution reaction catalyst. Electrochim. Acta 2023, 442, 141842. [Google Scholar] [CrossRef]
- Balqis, F.; Irmawati, Y.; Geng, D.; Nugroho, F.A.A.; Sumboja, A. Nanostructured Ball-Milled Ni-Co-Mn Oxides from Spent Li-Ion Batteries as Electrocatalysts for Oxygen Evolution Reaction. ACS Appl. Nano Mater. 2024, 7, 18138–18145. [Google Scholar] [CrossRef]
- Chernyaev, A.; Partinen, J.; Klemettinen, L.; Wilson, B.P.; Jokilaakso, A.; Lundström, M. The efficiency of scrap Cu and Al current collector materials as reductants in LIB waste leaching. Hydrometallurgy 2021, 203, 105608. [Google Scholar] [CrossRef]
- Praats, R.; Sainio, J.; Vikberg, M.; Klemettinen, L.; Wilson, B.P.; Lundström, M.; Kruusenberg, I.; Liivand, K. Utilizing waste lithium-ion batteries for the production of graphite-carbon nanotube composites as oxygen electrocatalysts in zinc–air batteries. RSC Sustain. 2025, 3, 546–556. [Google Scholar] [CrossRef]
- Praats, R.; Chernyaev, A.; Sainio, J.; Lundström, M.; Kruusenberg, I.; Liivand, K. Supporting critical raw material circularity—Upcycling graphite from waste LIBs to Zn–air batteries. Green Chem. 2024, 26, 2874–2883. [Google Scholar] [CrossRef]
- Regulation (EU) 2023/1542 of the European Parliament and of the Council of 12 July 2023 Concerning Batteries and Waste Batteries, Amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and Repealing Directive 2006/66/EC (Text. with EEA Relevance); European Union: Brussels, Belgium, 2023. [Google Scholar]
- Kazemi, M.; Liivand, K.; Prato, M.; Vacek, P.; Walmsley, J.; Dante, S.; Divitini, G.; Kruusenberg, I. Graphite and Cobalt Recycled from Li-Ion Batteries: A Valuable Raw Material for Oxygen Reduction Reaction Electrocatalysts. Energy Fuels 2023, 38, 659–670. [Google Scholar] [CrossRef]
- Balachandran, S.; Forsberg, K.; Lemaître, T.; Vieceli, N.; Lombardo, G.; Petranikova, M. Comparative Study for Selective Lithium Recovery via Chemical Transformations during Incineration and Dynamic Pyrolysis of EV Li-Ion Batteries. Metals 2021, 11, 1240. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, X.; Xu, S. A simplified process for recovery of Li and Co from spent LiCoO2 cathode using Al foil as the in situ reductant. ACS Sustain. Chem. Eng. 2019, 7, 12222–12230. [Google Scholar] [CrossRef]
- Zhai, L.F.; Kong, S.Y.; Zhang, H.; Tian, W.; Sun, M.; Sun, H.; Wang, S. Facile synthesis of Co-N-rGO composites as an excellent electrocatalyst for oxygen reduction reaction. Chem. Eng. Sci. 2019, 194, 45–53. [Google Scholar] [CrossRef]
- Kang, J.; Kim, Y.; Kim, H.M.; Hu, X.; Saito, N.; Choi, J.H.; Lee, M.H. In-situ one-step synthesis of carbon-encapsulated naked magnetic metal nanoparticles conducted without additional reductants and agents. Sci. Rep. 2016, 6, 38652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, K.; Zhang, W.; Zhang, X.; Wang, L.; An, Y.; Sun, X.; Li, C.; Wang, K.; Ma, Y. Carbon Nano-Onion-Encapsulated Ni Nanoparticles for High-Performance Lithium-Ion Capacitors. Batteries 2023, 9, 102. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 1999, 61, 14095–14107. [Google Scholar] [CrossRef]
- Perumbilavil, S.; Sankar, P.; Priya Rose, T.; Philip, R. White light Z-scan measurements of ultrafast optical nonlinearity in reduced graphene oxide nanosheets in the 400–700 nm region. Appl. Phys. Lett. 2015, 107, 051104. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Wu, B.; Mi, J.L.; Hu, H.; Zu, Z.Y.; Liu, L. Fe-, S-, and N-Doped Carbon Nanotube Networks as Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2020, 3, 12297–12307. [Google Scholar] [CrossRef]
- Lu, A.H.; Li, W.C.; Salabas, E.L.; Spliethoff, B.; Schüth, F. Low temperature catalytic pyrolysis for the synthesis of high surface area, nanostructured graphitic carbon. Chem. Mater. 2006, 18, 2086–2094. [Google Scholar] [CrossRef]
- Okpalugo, T.I.T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N.M.D. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- NIST X-Ray Photoelectron Spectroscopy (XPS) Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000.
- Kousar, A.; Pande, I.; Pascual, L.F.; Peltola, E.; Sainio, J.; Laurila, T. Modulating the Geometry of the Carbon Nanofiber Electrodes Provides Control over Dopamine Sensor Performance. Anal. Chem. 2023, 95, 2983–2991. [Google Scholar] [CrossRef]
- Susi, T.; Pichler, T.; Ayala, P. X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with heteroatoms. Beilstein J. Nanotechnol. 2015, 6, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bi, J.; Zhao, Y.; Yang, L.; Zhang, C.; Ma, Y.; Wu, Q.; Wang, X.; Hu, Z. Nitrogen-doped carbon nanocages as efficient metal-free electrocatalysts for oxygen reduction reaction. Adv. Mater. 2012, 24, 5593–5597. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Wang, D.; Zhang, J.; Cao, C.; Li, Y. Bamboo-Like Nitrogen-Doped Carbon Nanotubes with Co Nanoparticles Encapsulated at the Tips: Uniform and Large-Scale Synthesis and High-Performance Electrocatalysts for Oxygen Reduction. Chem.—Eur. J. 2015, 21, 14022–14029. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, Y.; Ding, W.; Chen, S.G.; Xiong, K.; Qi, X.Q.; Zhang, Y.; Wang, J.; Wei, Z.D. Unification of catalytic oxygen reduction and hydrogen evolution reactions: Highly dispersive Co nanoparticles encapsulated inside Co and nitrogen co-doped carbon. Chem. Commun. 2015, 51, 8942–8945. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Lian, C.; Wei, F.; Zhang, D.; Wu, G.; Chen, B.; Wang, S. Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as Fenton-like catalysts for organic pollutant removal. J. Hazard. Mater. 2016, 314, 129–139. [Google Scholar] [CrossRef]
- Xue, H.; Meng, A.; Lian, T.; Yang, T.; Gao, J.; Singh, C.V.; Geng, Z.; Chen, L.; Li, Z. Co-Pyridinic-N Bond Constructed at the Interface of CoxP and N-Doped Carbon to Effectively Facilitate Oxygen Reduction. ACS Sustain. Chem. Eng. 2023, 11, 5238–5249. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.K.; Oh, Y.J.; Kang, Y.C. Hierarchical hollow microspheres grafted with Co nanoparticle-embedded bamboo-like N-doped carbon nanotube bundles as ultrahigh rate and long-life cathodes for rechargeable lithium-oxygen batteries. Chem. Eng. J. 2018, 334, 2500–2510. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Ma, J.; Wang, Z.; Liu, J.; Gong, X. ORR and OER of Co–N codoped carbon-based electrocatalysts enhanced by boundary layer oxygen molecules transfer. Carbon 2021, 172, 556–568. [Google Scholar] [CrossRef]
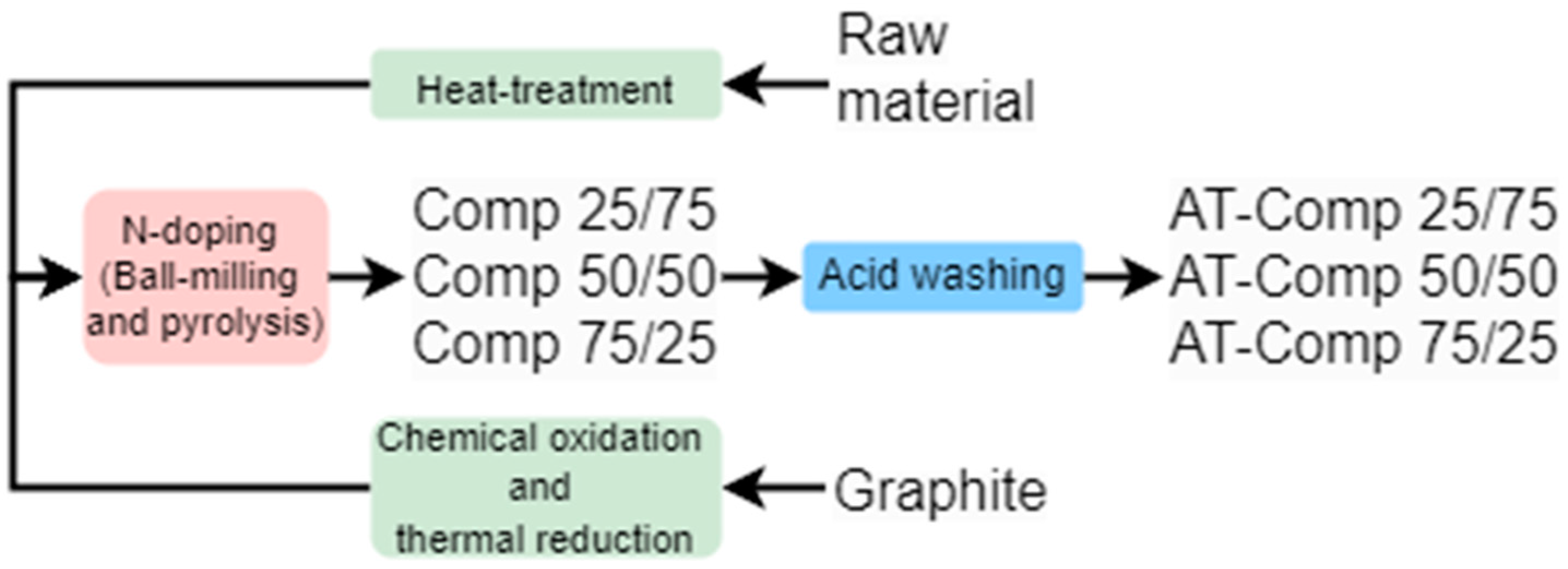
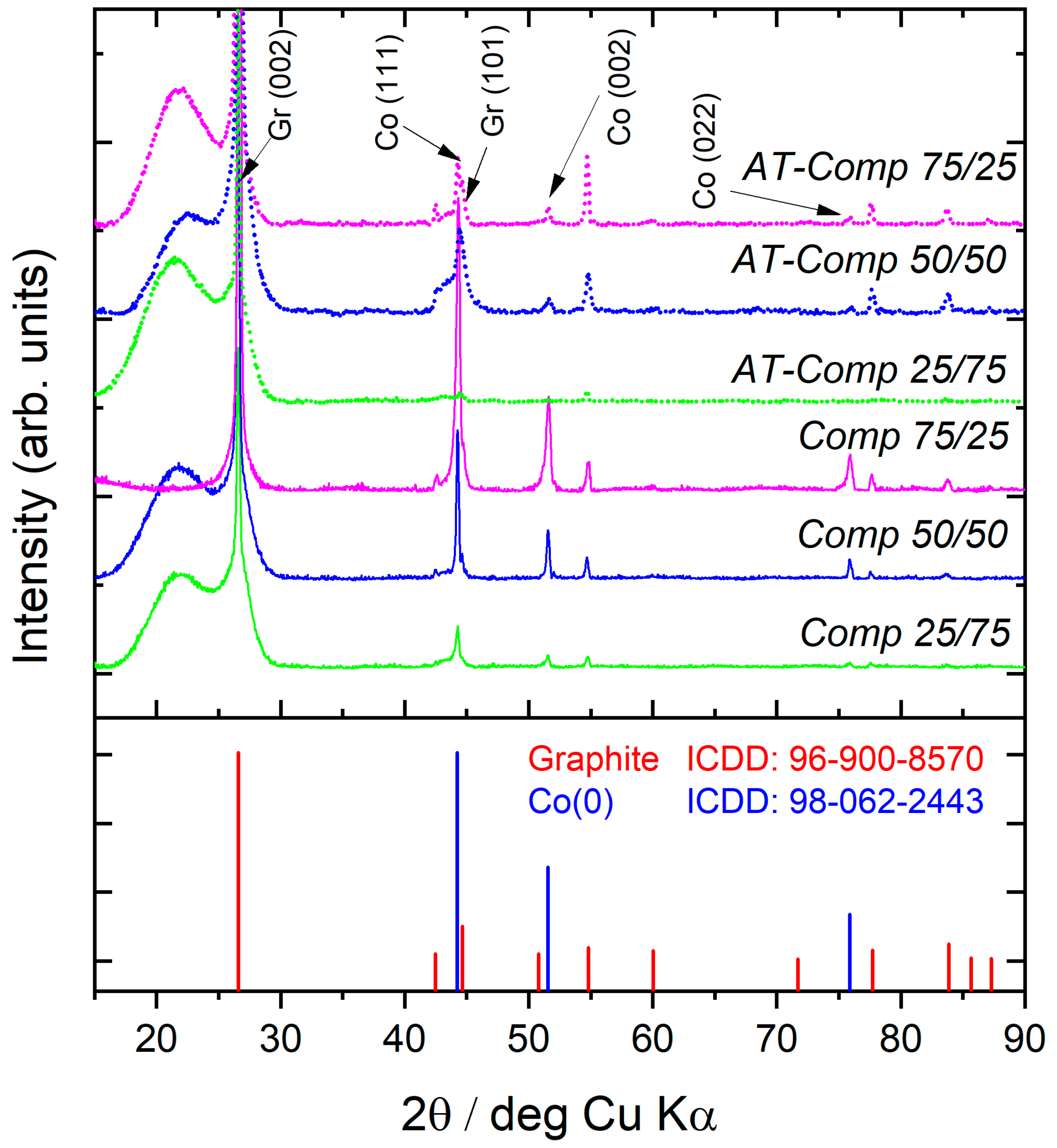

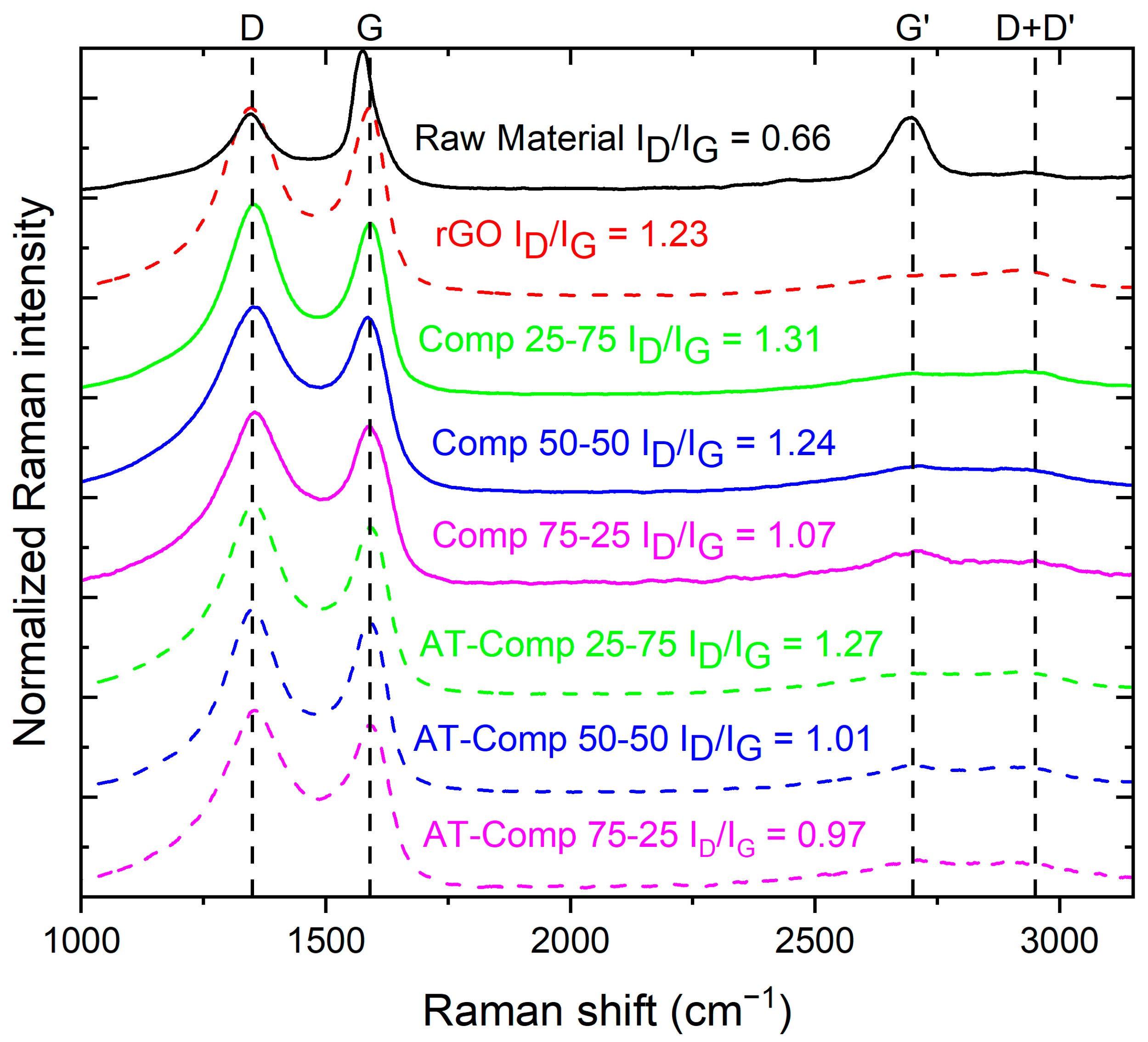

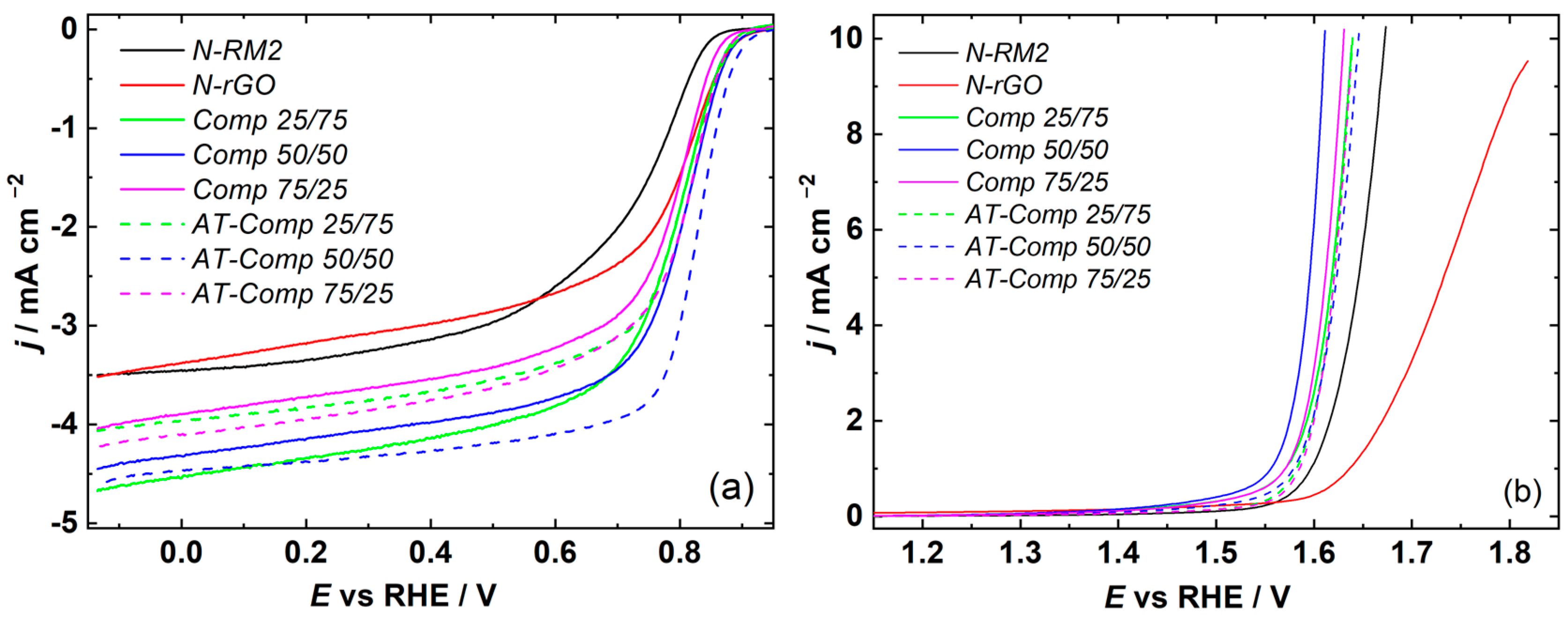

| Element | Li | Co | Mn | Ni | Cu | Fe |
|---|---|---|---|---|---|---|
| Concentration in Raw Material (wt%) | 0.9 | 18.6 | 1.3 | 0.8 | 0.2 | 0.1 |
| Standard error of mean (+/− wt%) | ±0.02 | ±1.13 | ±0.03 | ±0.02 | ±0.01 | ±0.01 |
| Sample Name | Raw Material | N-RM | rGO | N-rGO | Comp 25/75 | Comp 50/50 | Comp 75/25 | AT-Comp 25/75 | AT-Comp 50/50 | AT-Comp 75/25 |
|---|---|---|---|---|---|---|---|---|---|---|
| SBET (m2/g) | 1.9 | 51.3 | 280.5 | 211.7 | 149.0 | 77.7 | 63.5 | 183.3 | 124.6 | 90.1 |
| SDFT (m2/g) | 2.7 | 37.9 | 239.8 | 188.5 | 126.5 | 64.5 | 52.9 | 156.0 | 105.6 | 75.0 |
| Catalyst | ORR | OER | |||
|---|---|---|---|---|---|
| Eonset (V vs. RHE) | E1/2 (V vs. RHE) | j (mA cm−2) | E (V) @ 10 mA cm−2 | ΔE | |
| N-RM | 0.852 | 0.727 | 4.25 | 1.674 | 0.885 |
| N-rGO | 0.897 | 0.780 | 3.52 | - | - |
| Comp 25/75 | 0.887 | 0.777 | 4.67 | 1.631 | 0.853 |
| Comp 50/50 | 0.895 | 0.792 | 4.45 | 1.621 | 0.828 |
| Comp 75/25 | 0.875 | 0.777 | 4.05 | 1.624 | 0.846 |
| AT-Comp 25/75 | 0.895 | 0.790 | 4.06 | 1.634 | 0.842 |
| AT-Comp 50/50 | 0.912 | 0.820 | 4.63 | 1.639 | 0.818 |
| AT-Comp 75/25 | 0.887 | 0.797 | 4.22 | 1.632 | 0.834 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praats, R.; Sainio, J.; Vikberg, M.; Klemettinen, L.; Wilson, B.P.; Lundström, M.; Kruusenberg, I.; Liivand, K. A Pathway to Circular Economy-Converting Li-Ion Battery Recycling Waste into Graphite/rGO Composite Electrocatalysts for Zinc–Air Batteries. Batteries 2025, 11, 165. https://doi.org/10.3390/batteries11040165
Praats R, Sainio J, Vikberg M, Klemettinen L, Wilson BP, Lundström M, Kruusenberg I, Liivand K. A Pathway to Circular Economy-Converting Li-Ion Battery Recycling Waste into Graphite/rGO Composite Electrocatalysts for Zinc–Air Batteries. Batteries. 2025; 11(4):165. https://doi.org/10.3390/batteries11040165
Chicago/Turabian StylePraats, Reio, Jani Sainio, Milla Vikberg, Lassi Klemettinen, Benjamin P. Wilson, Mari Lundström, Ivar Kruusenberg, and Kerli Liivand. 2025. "A Pathway to Circular Economy-Converting Li-Ion Battery Recycling Waste into Graphite/rGO Composite Electrocatalysts for Zinc–Air Batteries" Batteries 11, no. 4: 165. https://doi.org/10.3390/batteries11040165
APA StylePraats, R., Sainio, J., Vikberg, M., Klemettinen, L., Wilson, B. P., Lundström, M., Kruusenberg, I., & Liivand, K. (2025). A Pathway to Circular Economy-Converting Li-Ion Battery Recycling Waste into Graphite/rGO Composite Electrocatalysts for Zinc–Air Batteries. Batteries, 11(4), 165. https://doi.org/10.3390/batteries11040165










