Layered Iron Vanadate as a High-Capacity Cathode Material for Nonaqueous Calcium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis and Materials Characterization
2.2. Electrochemical Characterization
2.3. Structural Analysis
3. Results and Discussion
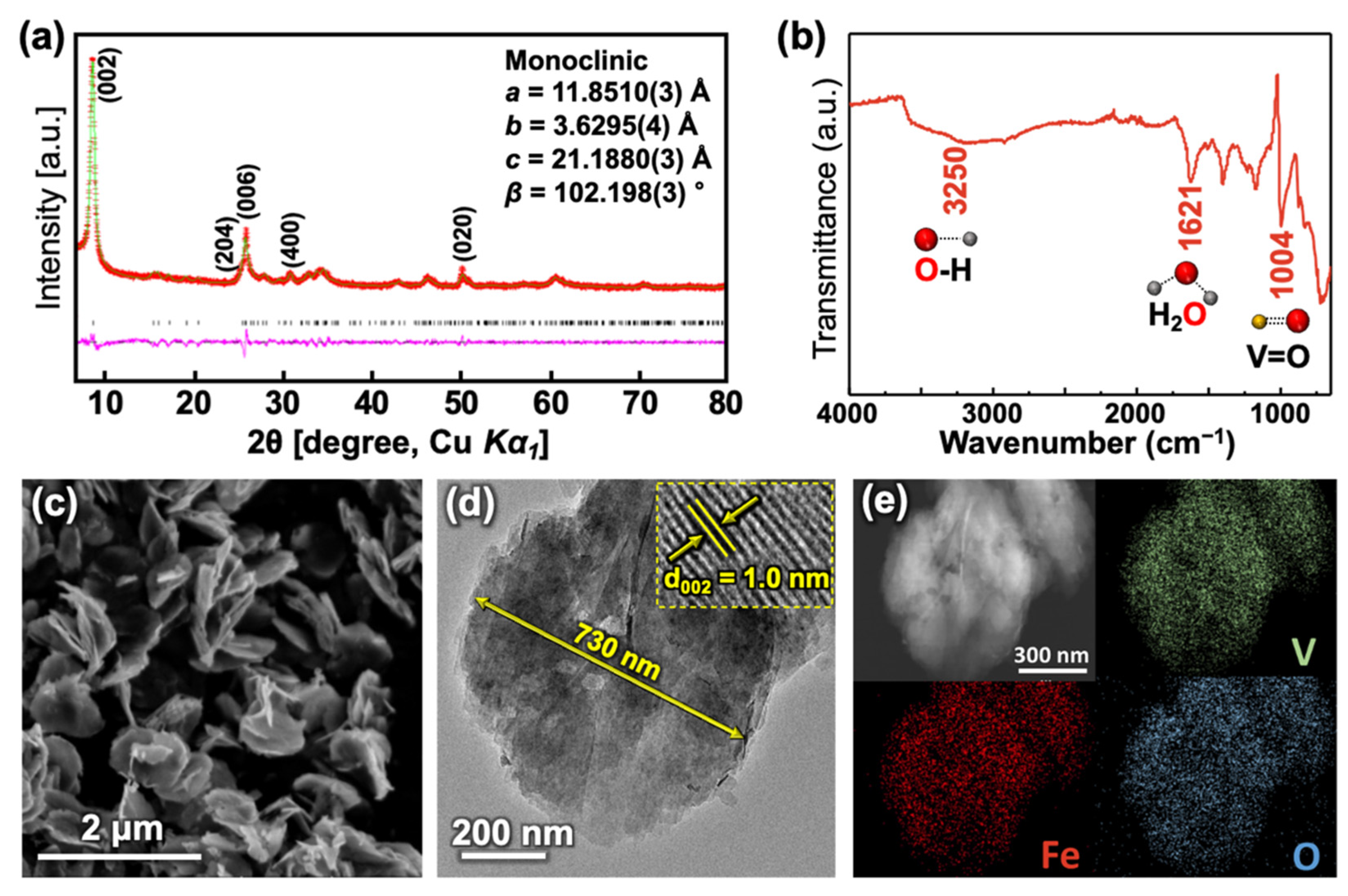
3.1. Characterization of the Synthesized Materials
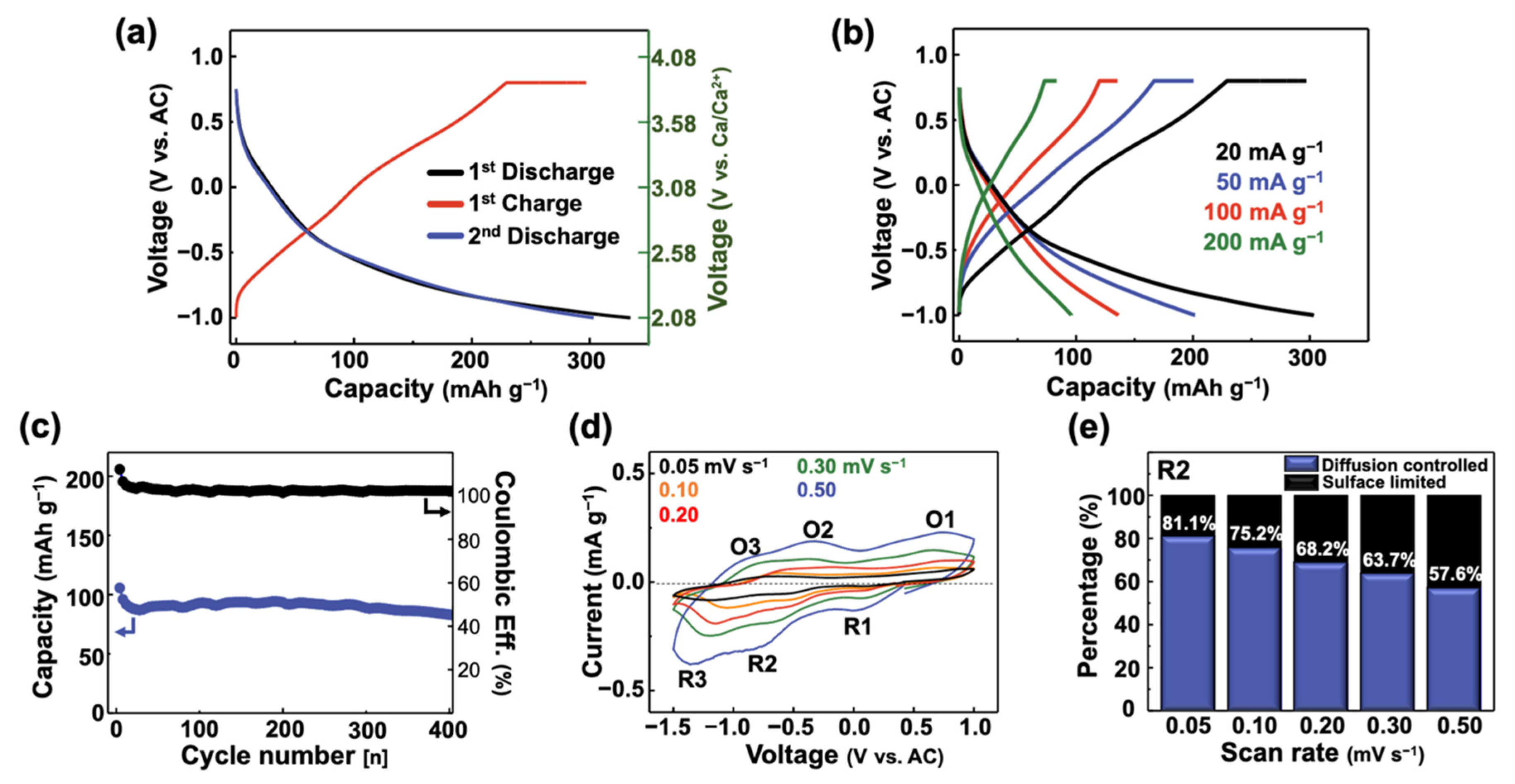
3.2. Electrochemical Performance of FeV3O9∙1.2H2O
3.3. Elemental Analysis of FeV3O9∙1.2H2O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 1603. [Google Scholar] [CrossRef]
- Ponrouch, A.; Bitenc, J.; Dominko, R.; Lindahl, N.; Johansson, P.; Palacin, M.R. Multivalent rechargeable batteries. Energy Storage Mater. 2019, 20, 253–262. [Google Scholar] [CrossRef]
- Arroyo-de Dompablo, M.E.P.; Ponrouch, A.; Johansson, P.; Palacín, M. Rosa Achievements, Challenges, and Prospects of Calcium Batteries. Chem. Rev. 2020, 120, 6331–6357. [Google Scholar] [CrossRef]
- Gummow, R.J.; Vamvounis, G.; Kannan, M.B.; He, Y. Calcium-ion batteries: Current state-of-the-art and future perspectives. Adv. Mater. 2018, 30, 1801702. [Google Scholar] [CrossRef] [PubMed]
- Maroni, F.; Dongmo, S.; Gauckler, C.; Marinaro, M.; Wohlfahrt-Mehrens, M. Through the Maze of Multivalent-Ion Batteries: A Critical Review on the Status of the Research on Cathode Materials for Mg2+ and Ca2+ Ions Insertion. Batter. Supercaps 2021. [Google Scholar] [CrossRef]
- Ponrouch, A.; Frontera, C.; Bardé, F.; Palacín, M.R. Towards a calcium-based rechargeable battery. Nat. Mater. 2016, 15, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Gao, X.; Chen, Y.; Jin, L.; Kuss, C.; Bruce, P.G. Plating and stripping calcium in an organic electrolyte. Nat. Mater. 2018, 17, 16. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H.-M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667. [Google Scholar] [CrossRef]
- Rogosic, J. Towards the Development of Calcium Ion Batteries. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2014. [Google Scholar]
- Kuperman, N.; Padigi, P.; Goncher, G.; Evans, D.; Thiebes, J.; Solanki, R. High performance Prussian Blue cathode for nonaqueous Ca-ion intercalation battery. J. Power Sources 2017, 342, 414–418. [Google Scholar] [CrossRef]
- Tojo, T.; Sugiura, Y.; Inada, R.; Sakurai, Y. Reversible calcium ion batteries using a dehydrated prussian blue analogue cathode. Electrochim. Acta 2016, 207, 22–27. [Google Scholar] [CrossRef]
- Lipson, A.L.; Han, S.-D.; Kim, S.; Pan, B.; Sa, N.; Liao, C.; Fister, T.T.; Burrell, A.K.; Vaughey, J.T.; Ingram, B.J. Nickel hexacyanoferrate, a versatile intercalation host for divalent ions from nonaqueous electrolytes. J. Power Sources 2016, 325, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Padigi, P.; Goncher, G.; Evans, D.; Solanki, R. Potassium barium hexacyanoferrate—A potential cathode material for rechargeable calcium ion batteries. J. Power Sources 2015, 273, 460–464. [Google Scholar] [CrossRef]
- Lipson, A.L.; Pan, B.F.; Lapidus, S.H.; Liao, C.; Vaughey, J.T.; Ingram, B.J. Rechargeable Ca-Ion Batteries: A New Energy Storage System. Chem. Mater. 2015, 27, 8442–8447. [Google Scholar] [CrossRef]
- Cabello, M.; Nacimiento, F.; González, J.R.; Ortiz, G.; Alcántara, R.; Lavela, P.; Pérez-Vicente, C.; Tirado, J.L. Advancing towards a veritable calcium-ion battery: CaCo2O4 positive electrode material. Electrochem. Commun. 2016, 67, 59–64. [Google Scholar] [CrossRef]
- Lipson, A.L.; Kim, S.; Pan, B.; Liao, C.; Fister, T.T.; Ingram, B.J. Calcium intercalation into layered fluorinated sodium iron phosphate. J. Power Sources 2017, 369, 133–137. [Google Scholar] [CrossRef]
- Vo, T.N.; Kim, H.; Hur, J.; Choi, W.; Kim, I.T. Surfactant-assisted ammonium vanadium oxide as superior cathode for calcium-ion batteries. J. Mater. Chem. A 2018, 6, 22645–22654. [Google Scholar] [CrossRef]
- Tojo, T.; Tawa, H.; Oshida, N.; Inada, R.; Sakurai, Y. Electrochemical characterization of a layered α-MoO3 as a new cathode material for calcium ion batteries. J. Electroanal. Chem. 2018, 825, 51–56. [Google Scholar] [CrossRef]
- Cabello, M.; Nacimiento, F.; Alcantara, R.; Lavela, P.; Vicente, C.P.; Tirado, J.L. Applicability of Molybdite as an Electrode Material in Calcium Batteries: A Structural Study of Layer-type CaxMoO3. Chem. Mater. 2018, 30, 5853–5861. [Google Scholar] [CrossRef]
- Tchitchekova, D.S.; Ponrouch, A.; Verrelli, R.; Broux, T.; Frontera, C.; Sorrentino, A.; Barde, F.; Biskup, N.; Arroyo-de Dompablo, M.E.; Palacín, M.R. Electrochemical Intercalation of Calcium and Magnesium in TiS2: Fundamental Studies Related to Multivalent Battery Applications. Chem. Mater. 2018, 30, 847–856. [Google Scholar] [CrossRef]
- Murata, Y.; Takada, S.; Obata, T.; Tojo, T.; Inada, R.; Sakurai, Y. Effect of water in electrolyte on the Ca2+ insertion/extraction properties of V2O5. Electrochim. Acta 2019, 294, 210–216. [Google Scholar] [CrossRef]
- Pathreeker, S.; Reed, S.; Chando, P.; Hosein, I.D. A study of calcium ion intercalation in perovskite calcium manganese oxide. J. Electroanal. Chem. 2020, 874, 114453. [Google Scholar] [CrossRef]
- Xu, X.; Duan, M.; Yue, Y.; Li, Q.; Zhang, X.; Wu, L.; Wu, P.; Song, B.; Mai, L. Bilayered Mg0.25V2O5·H2O as a Stable Cathode for Rechargeable Ca-Ion Batteries. ACS Energy Lett. 2019, 4, 1328–1335. [Google Scholar] [CrossRef]
- Wang, J.; Tan, S.; Xiong, F.; Yu, R.; Wu, P.; Cui, L.; An, Q. VOPO4.2H2O as a new cathode material for rechargeable Ca-ion batteries. Chem. Commun. 2020, 56, 3805–3808. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.; Heo, J.W.; Hyoung, J.; Kwak, H.H.; Lee, D.M.; Hong, S.-T. Reversible Calcium-Ion Insertion in NASICON-Type NaV2(PO4)3. Chem. Mater. 2020, 32, 8772–8780. [Google Scholar] [CrossRef]
- Kim, S.; Yin, L.; Lee, M.H.; Parajuli, P.; Blanc, L.; Fister, T.T.; Park, H.; Kwon, B.J.; Ingram, B.J.; Zapol, P.; et al. High-Voltage Phosphate Cathodes for Rechargeable Ca-Ion Batteries. ACS Energy Lett. 2020, 5, 3203–3211. [Google Scholar] [CrossRef]
- Chae, M.S.; Kwak, H.H.; Hong, S.-T. Calcium Molybdenum Bronze as a Stable High-Capacity Cathode Material for Calcium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 5107–5112. [Google Scholar] [CrossRef]
- Hess, F.L.; Henderson, E.P. Fervanite, a hydrous ferric vanadate. Am. Mineral. 1931, 16, 273–277. [Google Scholar]
- Weeks, A.D.; Thompson, M.E.; Sherwood, A.M. Navajoite, a new vanadium oxide from Arizona. Am. Mineral. 1955, 40, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankinovitch, E.A.; Bekenova, G.K.; Podlipaeva, N.I. Fe3+5V4+3V5+12O39(OH)9·8.55H2O, a new hydrous ferrovanadian mineral from carbonaceous-siliceous formation, the Nw Karatau (South Kazakhstan). Proc. Russ. Mineral. Soc. 1989, 118, 95–100. [Google Scholar]
- Poizot, P.; Laruelle, S.; Touboul, M.; Tarascon, J.-M. Wet-chemical synthesis of various iron (III) vanadates (V) by co-precipitation route. Comptes Rendus Chim. 2003, 6, 125–134. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Q.; Li, Q.; An, Q.; Zhao, Y.; Peng, Z.; Jiang, Y.; Tan, S.; Yan, M.; Mai, L. Pseudocapacitive layered iron vanadate nanosheets cathode for ultrahigh-rate lithium ion storage. Nano Energy 2018, 47, 294–300. [Google Scholar] [CrossRef]
- Peng, Z.; Wei, Q.; Tan, S.; He, P.; Luo, W.; An, Q.; Mai, L. Novel layered iron vanadate cathode for high-capacity aqueous rechargeable zinc batteries. Chem. Commun. 2018, 54, 4041–4044. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Bu, H.; Kim, D.; Hyoung, J.; Hong, S.-T. Crystal structure of calcium perchlorate anhydrate, Ca (ClO4)2, from laboratory powder X-ray diffraction data. Acta Cryst. 2018, E74, 514–517. [Google Scholar] [CrossRef] [Green Version]
- Gershinsky, G.; Yoo, H.D.; Gofer, Y.; Aurbach, D. Electrochemical and spectroscopic analysis of Mg2+ intercalation into thin film electrodes of layered oxides: V2O5 and MoO3. Langmuir 2013, 29, 10964–10972. [Google Scholar] [CrossRef]
- Ruch, P.W.; Cericola, D.; Hahn, M.; Kötz, R.; Wokaun, A. On the use of activated carbon as a quasi-reference electrode in non-aqueous electrolyte solutions. J. Electroanal. Chem. 2009, 636, 128–131. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Yin, H.; Song, C.; Wang, Y.; Li, S.; Zeng, M.; Zhang, Z.; Zhu, Z.; Yu, K. Influence of morphologies and pseudocapacitive contributions for charge storage in V2O5 micro/nano-structures. Electrochim. Acta 2013, 111, 762–770. [Google Scholar] [CrossRef]
- Hyoung, J.; Heo, J.W.; Chae, M.S.; Hong, S.T. Electrochemical Exchange Reaction Mechanism and the Role of Additive Water to Stabilize the Structure of VOPO4⋅2H2O as a Cathode Material for Potassium-Ion Batteries. ChemSusChem 2019, 12, 1069–1075. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, M.S.; Setiawan, D.; Kim, H.J.; Hong, S.-T. Layered Iron Vanadate as a High-Capacity Cathode Material for Nonaqueous Calcium-Ion Batteries. Batteries 2021, 7, 54. https://doi.org/10.3390/batteries7030054
Chae MS, Setiawan D, Kim HJ, Hong S-T. Layered Iron Vanadate as a High-Capacity Cathode Material for Nonaqueous Calcium-Ion Batteries. Batteries. 2021; 7(3):54. https://doi.org/10.3390/batteries7030054
Chicago/Turabian StyleChae, Munseok S., Dedy Setiawan, Hyojeong J. Kim, and Seung-Tae Hong. 2021. "Layered Iron Vanadate as a High-Capacity Cathode Material for Nonaqueous Calcium-Ion Batteries" Batteries 7, no. 3: 54. https://doi.org/10.3390/batteries7030054
APA StyleChae, M. S., Setiawan, D., Kim, H. J., & Hong, S.-T. (2021). Layered Iron Vanadate as a High-Capacity Cathode Material for Nonaqueous Calcium-Ion Batteries. Batteries, 7(3), 54. https://doi.org/10.3390/batteries7030054






