The Role of Protective Surface Coatings on the Thermal Stability of Delithiated Ni-Rich Layered Oxide Cathode Materials
Abstract
1. Introduction
2. Results
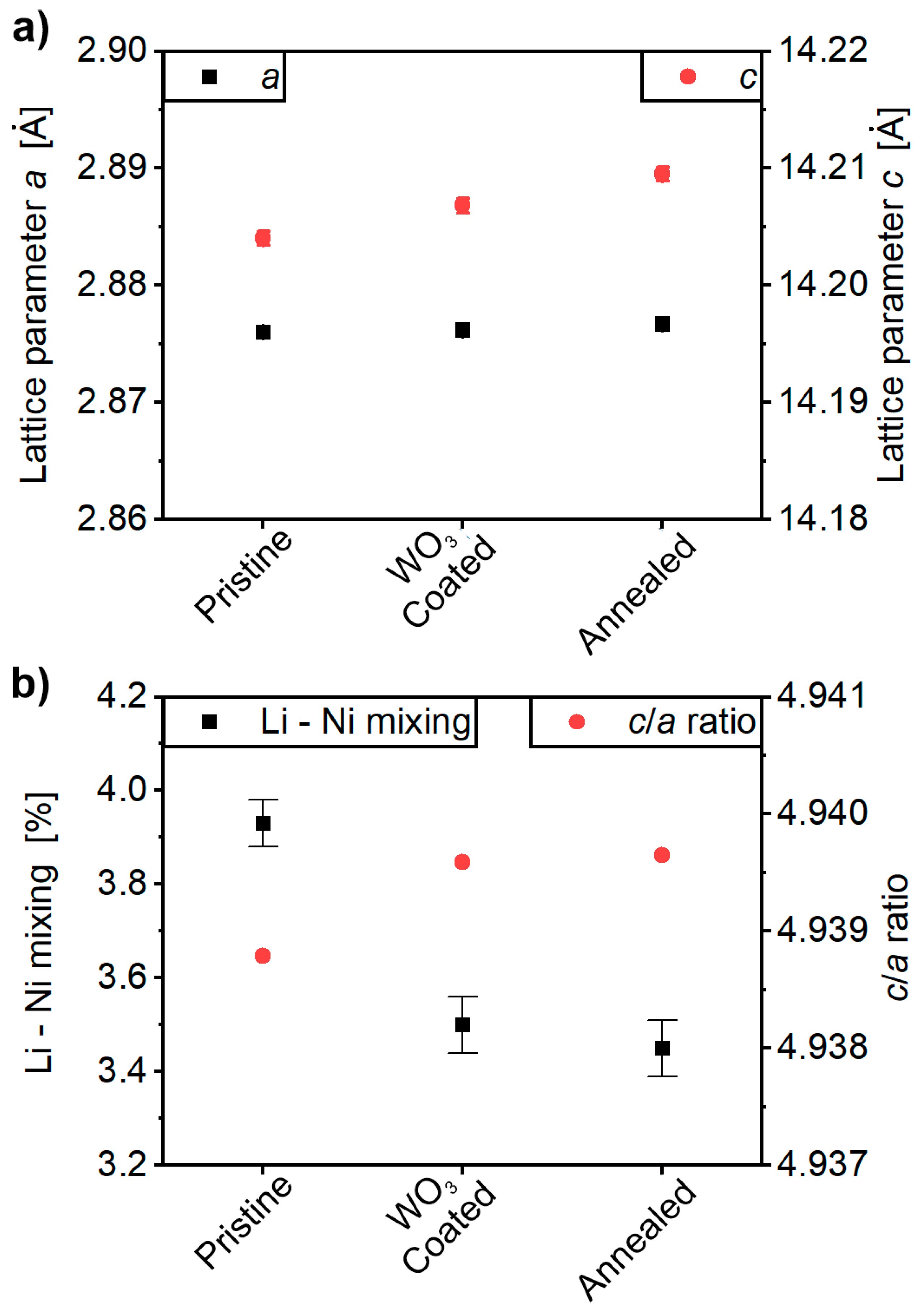
2.1. Characterization of the Cathode-Active Materials (CAMs)
2.2. Characterization of the Coating Surface Coverage on the CAM
2.3. Electrochemical Charge/Discharge Cycling in Lithium Battery Cells
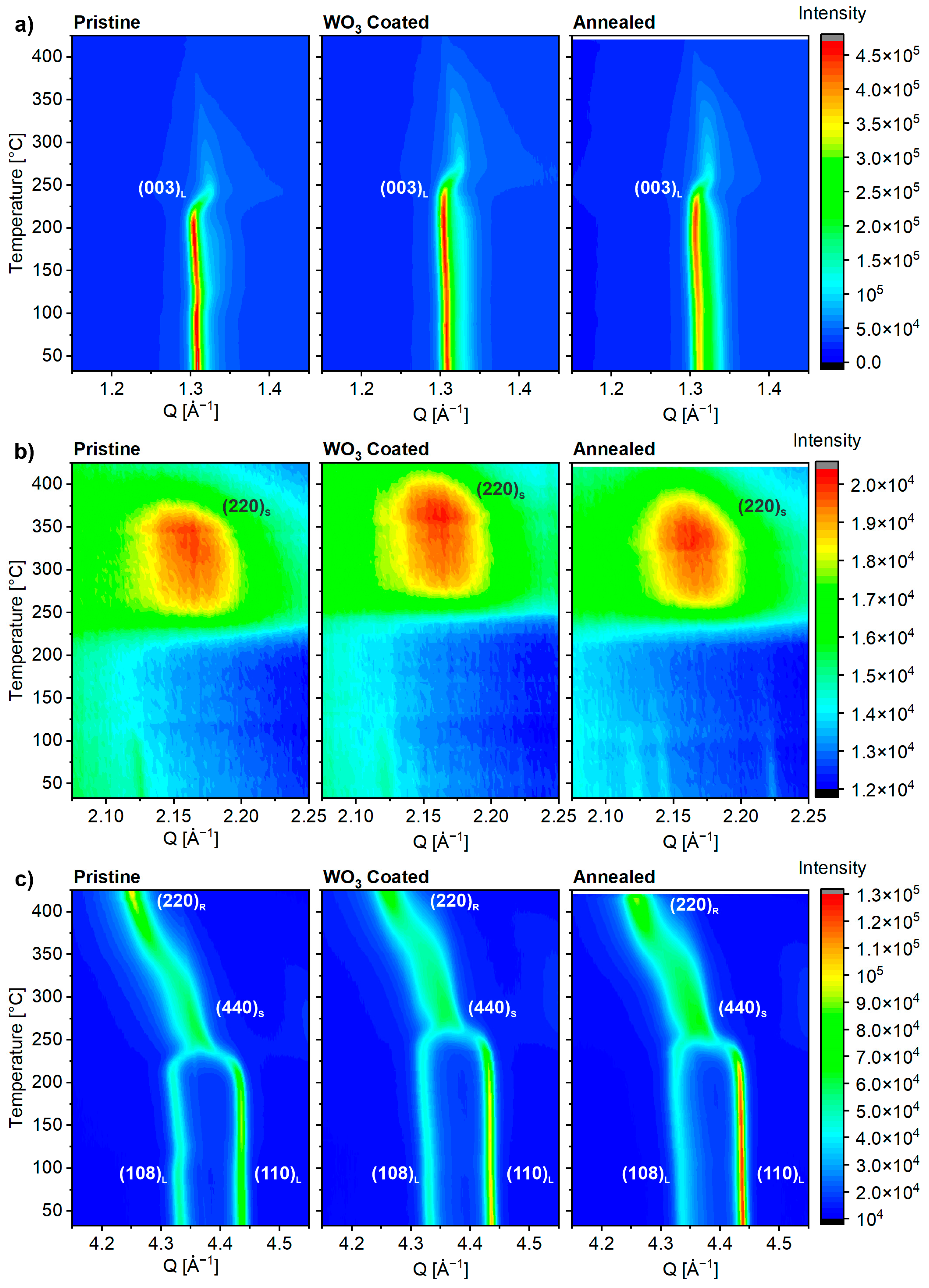
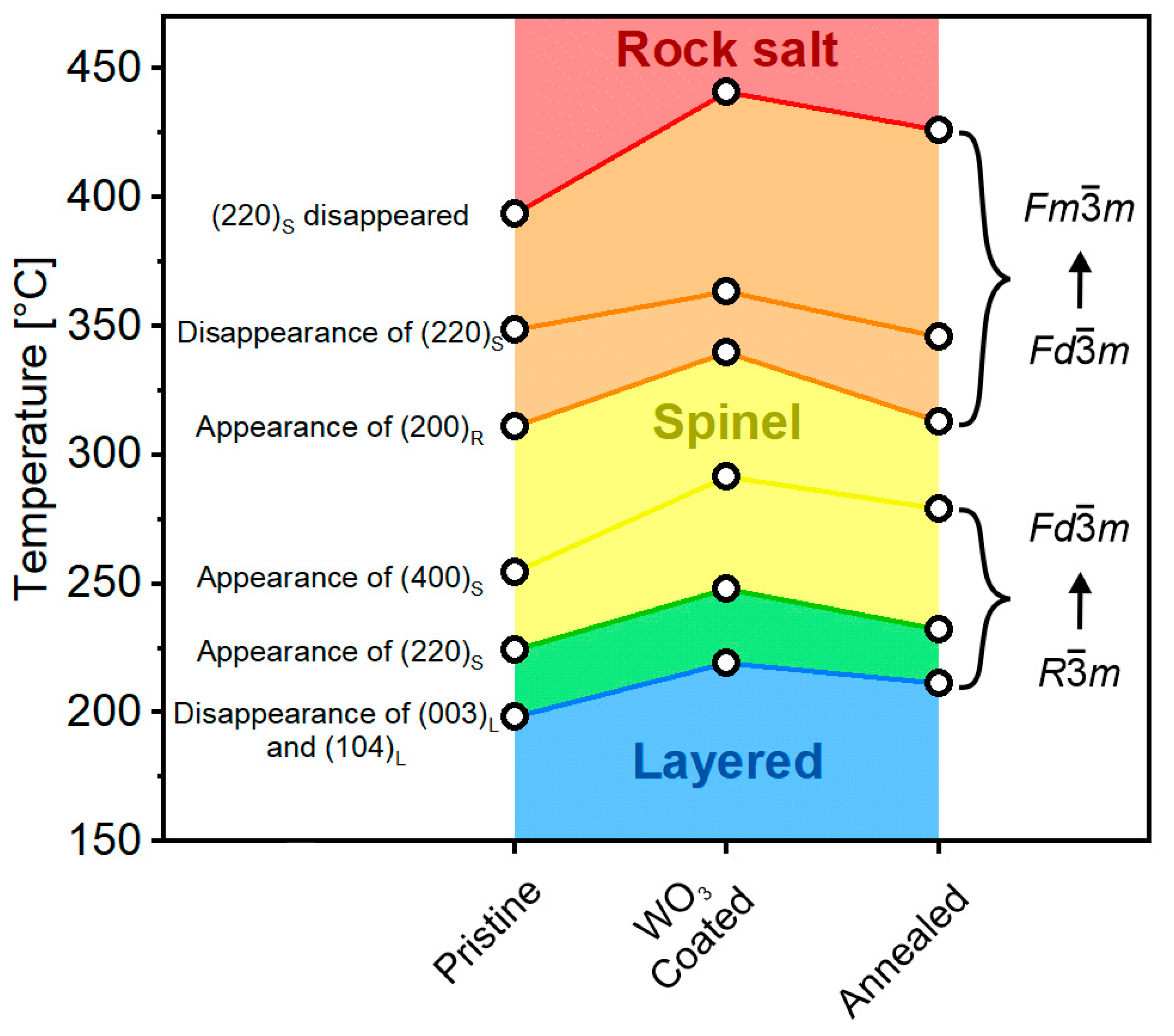
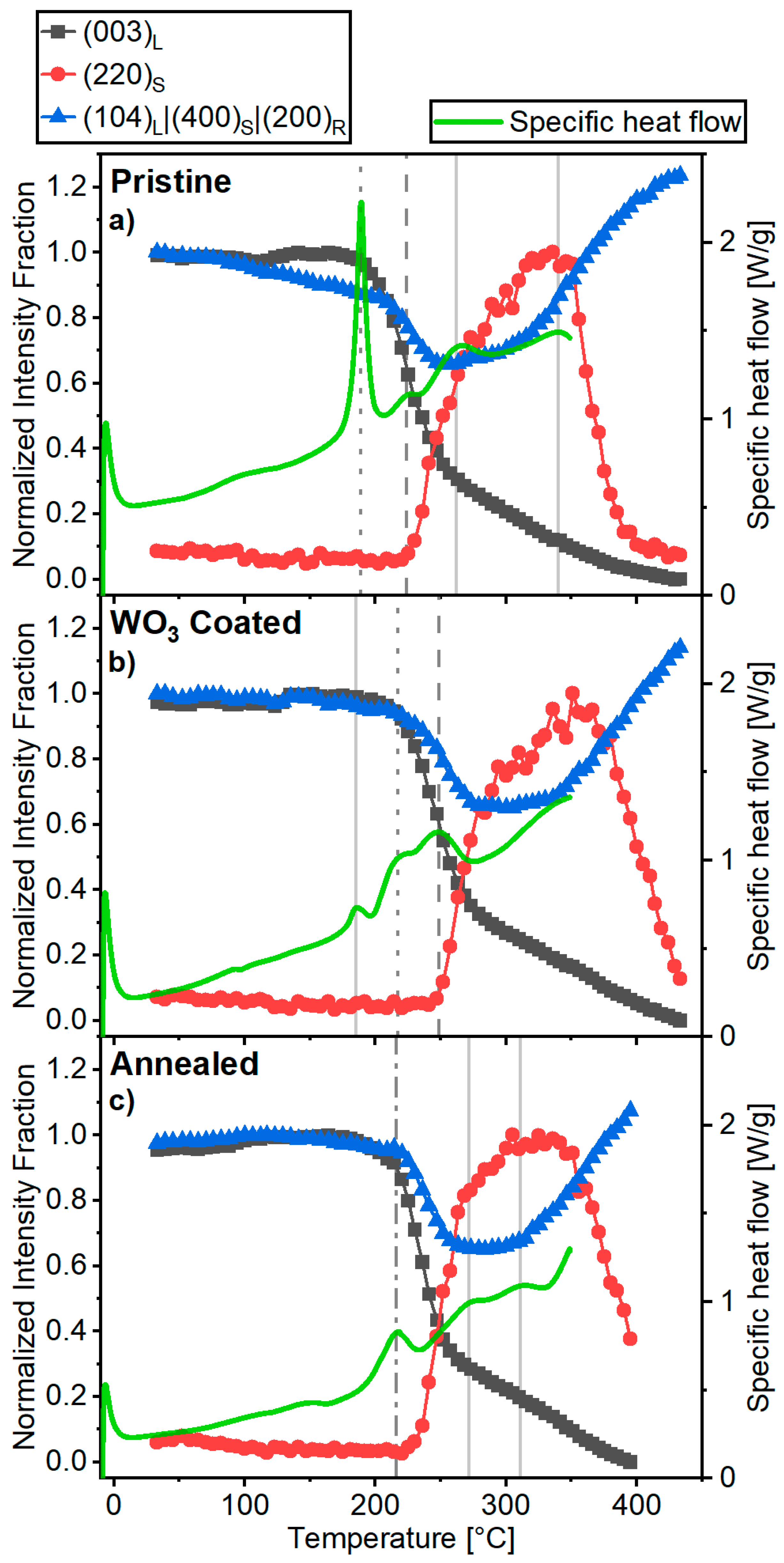
2.4. Thermal Stability of Delithiated Materials
3. Discussion
4. Conclusions
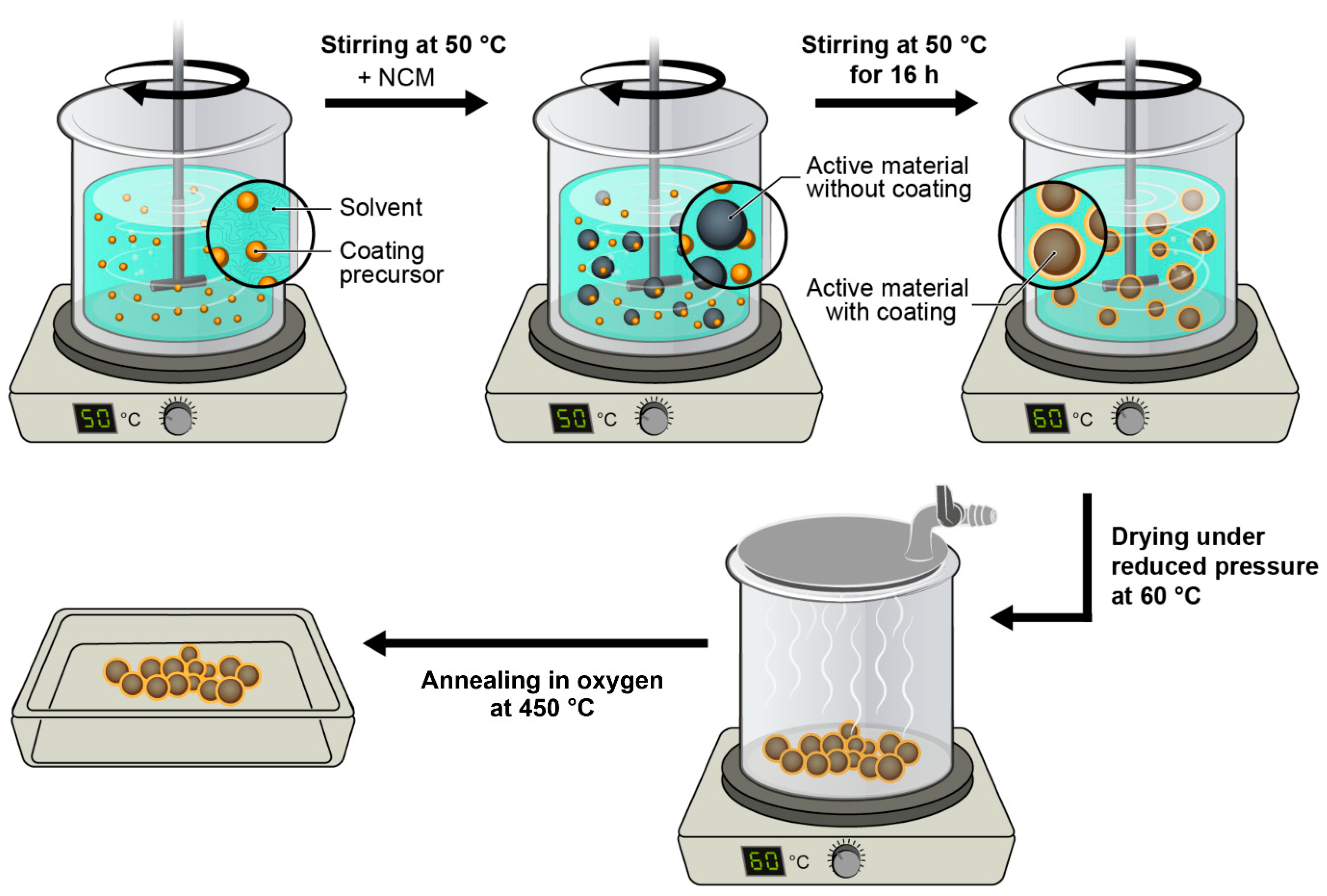
5. Experimental Section
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EUCAR. Battery Requirements for Future Automotive Applications; EUCAR: Etterbeek, Belgium, 2019. [Google Scholar]
- Andre, D.; Kim, S.-J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Betz, J.; Bieker, G.; Meister, P.; Placke, T.; Winter, M.; Schmuch, R. Theoretical versus Practical Energy: A Plea for More Transparency in the Energy Calculation of Different Rechargeable Battery Systems. Adv. Energy Mater. 2019, 9, 1900761. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Meister, P.; Jia, H.; Li, J.; Kloepsch, R.; Winter, M.; Placke, T. Best Practice: Performance and Cost Evaluation of Lithium Ion Battery Active Materials with Special Emphasis on Energy Efficiency. Chem. Mater. 2016, 28, 7203–7217. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Myung, S.-T.; Maglia, F.; Park, K.-J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-Rich Layered Cathode Materials for Automotive Lithium-Ion Batteries: Achievements and Perspectives. ACS Energy Lett. 2016, 2, 196–223. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Röser, S.; Börner, M.; Winter, M. Do Increased Ni Contents in LiNixMnyCozO2 (NMC) Electrodes Decrease Structural and Thermal Stability of Li Ion Batteries? A Thorough Look by Consideration of the Li+ Extraction Ratio. ACS Appl. Energy Mater. 2019, 2, 7733–7737. [Google Scholar] [CrossRef]
- Bak, S.-M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.-J.; Kim, K.-B.; Chung, K.Y.; Yang, X.-Q.; Nam, K.-W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation Mechanisms and Mitigation Strategies of Nickel-Rich NMC-Based Lithium-Ion Batteries. Electrochem. Energy Rev. 2020, 3, 43–80. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Chemical versus Electrochemical Electrolyte Oxidation on NMC111, NMC622, NMC811, LNMO, and Conductive Carbon. J. Phys. Chem. Lett. 2017, 8, 4820–4825. [Google Scholar] [CrossRef]
- Jung, S.-K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the Degradation Mechanisms of LiNi0.5Co0.2Mn0.3O2 Cathode Material in Lithium Ion Batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Zheng, S.; Hong, C.; Guan, X.; Xiang, Y.; Liu, X.; Xu, G.L.; Liu, R.; Zhong, G.; Zheng, F.; Li, Y.; et al. Correlation between long range and local structural changes in Ni-rich layered materials during charge and discharge process. J. Power Sources 2019, 412, 336–343. [Google Scholar] [CrossRef]
- Klein, S.; Bärmann, P.; Fromm, O.; Borzutzki, K.; Reiter, J.; Fan, Q.; Winter, M.; Placke, T.; Kasnatscheew, J. Prospects and limitations of single-crystal cathode materials to overcome cross-talk phenomena in high-voltage lithium ion cells. J. Mater. Chem. A 2021, 9, 7546–7555. [Google Scholar] [CrossRef]
- Liao, C.; Li, F.; Liu, J. Challenges and Modification Strategies of Ni-Rich Cathode Materials Operating at High-Voltage. Nanomaterials 2022, 12, 1888. [Google Scholar] [CrossRef] [PubMed]
- Langdon, J.; Manthiram, A. A perspective on single-crystal layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2021, 37, 143–160. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Chen, Z.; Noh, H.-J.; Lee, D.-J.; Jung, H.-G.; Ren, Y.; Wang, S.; Yoon, C.S.; Myung, S.-T.; Amine, K. Nanostructured high-energy cathode materials for advanced lithium batteries. Nat. Mater. 2012, 11, 942–947. [Google Scholar] [CrossRef]
- Jun, D.-W.; Yoon, C.S.; Kim, U.-H.; Sun, Y.-K. High-Energy Density Core–Shell Structured Li[Ni0.95Co0.025Mn0.025]O2 Cathode for Lithium-Ion Batteries. Chem. Mater. 2017, 29, 5048–5052. [Google Scholar] [CrossRef]
- Myung, S.-T.; Noh, H.-J.; Yoon, S.-J.; Lee, E.-J.; Sun, Y.-K. Progress in High-Capacity Core–Shell Cathode Materials for Rechargeable Lithium Batteries. J. Phys. Chem. Lett. 2014, 5, 671–679. [Google Scholar] [CrossRef]
- Wu, K.; Li, Q.; Dang, R.; Deng, X.; Chen, M.; Lee, Y.L.; Xiao, X.; Hu, Z. A novel synthesis strategy to improve cycle stability of LiNi0.8Mn0.1Co0.1O2 at high cut-off voltages through core-shell structuring. Nano Res. 2019, 12, 2460–2467. [Google Scholar] [CrossRef]
- Bianchini, M.; Roca-Ayats, M.; Hartmann, P.; Brezesinski, T.; Janek, J. There and Back Again—The Journey of LiNiO2 as a Cathode Active Material. Angew. Chem. Int. Ed. 2019, 58, 10434–10458. [Google Scholar] [CrossRef]
- Li, H.; Cormier, M.; Zhang, N.; Inglis, J.; Li, J.; Dahn, J.R. Is Cobalt Needed in Ni-Rich Positive Electrode Materials for Lithium Ion Batteries? J. Electrochem. Soc. 2019, 166, A429. [Google Scholar] [CrossRef]
- Xie, Q.; Li, W.; Manthiram, A. A Mg-Doped High-Nickel Layered Oxide Cathode Enabling Safer, High-Energy-Density Li-Ion Batteries. Chem. Mater. 2019, 31, 938–946. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, H.; Lee, W.; Ahn, S.J.; Lee, E.; Yoon, W.S. Stabilizing effects of Al-doping on Ni-rich LiNi0.80Co0.15Mn0.05O2 cathode for Li rechargeable batteries. J. Power Sources 2020, 474, 228592. [Google Scholar] [CrossRef]
- Yoon, C.S.; Kim, U.H.; Park, G.T.; Kim, S.J.; Kim, K.H.; Kim, J.; Sun, Y.K. Self-Passivation of a LiNiO2 Cathode for a Lithium-Ion Battery through Zr Doping. ACS Energy Lett. 2018, 3, 1634–1639. [Google Scholar] [CrossRef]
- Bonda, M.; Holzapfel, M.; de Brion, S.; Darie, C.; Fehér, T.; Baker, P.J.; Lancaster, T.; Blundell, S.J.; Pratt, F.L. Effect of magnesium doping on the orbital and magnetic order in LiNiO2. Phys. Rev. B 2008, 78, 104409. [Google Scholar] [CrossRef]
- Sim, S.-J.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Improving the electrochemical performances using a V-doped Ni-rich NCM cathode. Sci. Rep. 2019, 9, 8952. [Google Scholar] [CrossRef] [PubMed]
- Schipper, F.; Dixit, M.; Kovacheva, D.; Talianker, M.; Haik, O.; Grinblat, J.; Erickson, E.M.; Ghanty, C.; Major, D.T.; Markovsky, B.; et al. Stabilizing nickel-rich layered cathode materials by a high-charge cation doping strategy: Zirconium-doped LiNi0.6 Co0.2Mn0.2O2. J. Mater. Chem. A 2016, 4, 16073–16084. [Google Scholar] [CrossRef]
- Park, K.; Ham, D.J.; Park, S.Y.; Jang, J.; Yeon, D.-H.; Moon, S.; Ahn, S.J. High-Ni cathode material improved with Zr for stable cycling of Li-ion rechargeable batteries. RSC Adv. 2020, 10, 26756–26764. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Martin, A.; Reissig, F.; Frankenstein, L.; Heidbüchel, M.; Winter, M.; Placke, T.; Schmuch, R. Magnesium Substitution in Ni-Rich NMC Layered Cathodes for High-Energy Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103045. [Google Scholar] [CrossRef]
- Neudeck, S.; Walther, F.; Bergfeldt, T.; Suchomski, C.; Rohnke, M.; Hartmann, P.; Janek, J.; Brezesinski, T. Molecular Surface Modification of NCM622 Cathode Material Using Organophosphates for Improved Li-Ion Battery Full-Cells. ACS Appl. Mater. Interfaces 2018, 10, 20487–20498. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.P.; Fu, L.J.; Liu, H.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Cathode materials modified by surface coating for lithium ion batteries. Electrochim. Acta 2006, 51, 3872–3883. [Google Scholar] [CrossRef]
- Mohan, P.; Kalaignan, G.P. Electrochemical performance of La2O3-coated layered LiNiO2 cathode materials for rechargeable lithium-ion batteries. Ionics 2012, 19, 895–902. [Google Scholar] [CrossRef]
- Mohan, P.; Kalaignan, G.P. Electrochemical behaviour of surface modified SiO2-coated LiNiO2 cathode materials for rechargeable lithium-ion batteries. J. Nanosci. Nanotechnol. 2013, 13, 2765–2770. [Google Scholar] [CrossRef]
- Cho, J.; Kim, T.-J.; Kim, Y.J.; Park, B. High-Performance ZrO2-Coated LiNiO2 Cathode Material. Electrochem. Solid-State Lett. 2001, 4, A159. [Google Scholar] [CrossRef]
- Becker, D.; Börner, M.; Nölle, R.; Diehl, M.; Klein, S.; Rodehorst, U.; Schmuch, R.; Winter, M.; Placke, T. Surface Modification of Ni-Rich LiNi0.8Co0.1Mn0.1O2 Cathode Material by Tungsten Oxide Coating for Improved Electrochemical Performance in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 18404–18414. [Google Scholar] [CrossRef] [PubMed]
- Reissig, F.; Lange, M.A.; Haneke, L.; Placke, T.; Zeier, W.G.; Winter, M.; Schmuch, R.; Gomez-Martin, A. Synergistic Effects of Surface Coating and Bulk Doping in Ni-Rich Lithium Nickel Cobalt Manganese Oxide Cathode Materials for High-Energy Lithium Ion Batteries. ChemSusChem 2022, 15, e202102220. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, M.; Xiao, B.; Jiang, Y.; Lin, H.; Zhang, Z.; Chen, G.; Sun, Q.; He, H.; Huang, F.; et al. Highly stable Ni-rich layered oxide cathode enabled by a thick protective layer with bio-tissue structure. Energy Storage Mater. 2020, 24, 291–296. [Google Scholar] [CrossRef]
- Weber, D.; Tripković, Đ.; Kretschmer, K.; Bianchini, M.; Brezesinski, T. Surface Modification Strategies for Improving the Cycling Performance of Ni-Rich Cathode Materials. Eur. J. Inorg. Chem. 2020, 2020, 3117–3130. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Qian, D.; Meng, Y.S. Ultrathin Al2O3 Coatings for Improved Cycling Performance and Thermal Stability of LiNi0.5Co0.2Mn0.3O2 Cathode Material. Electrochim. Acta 2016, 203, 154–161. [Google Scholar] [CrossRef]
- Herzog, M.J.; Gauquelin, N.; Esken, D.; Verbeeck, J.; Janek, J. Increased Performance Improvement of Lithium-Ion Batteries by Dry Powder Coating of High-Nickel NMC with Nanostructured Fumed Ternary Lithium Metal Oxides. ACS Appl. Energy Mater. 2021, 4, 8832–8848. [Google Scholar] [CrossRef]
- Loeffler, N.; Kim, G.T.; Mueller, F.; Diemant, T.; Kim, J.K.; Behm, R.J.; Passerini, S. In Situ Coating of Li[Ni0.33Mn0.33Co0.33]O2 Particles to Enable Aqueous Electrode Processing. ChemSusChem 2016, 9, 1112–1117. [Google Scholar] [CrossRef]
- Rathore, D.; Geng, C.; Zaker, N.; Hamam, I.; Liu, Y.; Xiao, P.; Botton, G.A.; Dahn, J.; Yang, C. Tungsten Infused Grain Boundaries Enabling Universal Performance Enhancement of Co-Free Ni-Rich Cathode Materials. J. Electrochem. Soc. 2021, 168, 120514. [Google Scholar] [CrossRef]
- Geng, C.; Heino, D.; Zaker, N.; Phattharasupakun, N.; Liu, Y.; Botton, G.; Dahn, J.R. Impact of Dry Particle Fusion Coating of Tungsten Oxide on Ni-Based Positive Electrode Materials for Li-Ion Batteries. ECS Meet. Abstr. 2021, MA2021-02, 369. [Google Scholar] [CrossRef]
- Hayashi, T.; Okada, J.; Toda, E.; Kuzuo, R.; Matsuda, Y.; Kuwata, N.; Kawamura, J. Electrochemical effect of lithium tungsten oxide modification on LiCoO2 thin film electrode. J. Power Sources 2015, 285, 559–567. [Google Scholar] [CrossRef]
- Aida, T.; Tsutsui, Y.; Kanada, S.; Okada, J.; Hayashi, K.; Komukai, T. Ammonium tungstate modified Li-rich Li1+xNi0.35Co0.35Mn0.30O2 to improve rate capability and productivity of lithium-ion batteries. J. Solid State Electrochem. 2017, 21, 2047–2054. [Google Scholar] [CrossRef]
- Liu, X.; Xu, G.L.; Yin, L.; Hwang, I.; Li, Y.; Lu, L.; Xu, W.; Zhang, X.; Chen, Y.; Ren, Y.; et al. Probing the Thermal-Driven Structural and Chemical Degradation of Ni-Rich Layered Cathodes by Co/Mn Exchange. J. Am. Chem. Soc. 2020, 142, 19745–19753. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, D.; Hsu, H.; Feng, X.; Xu, G.-L.; Zhuang, M.; Gao, H.; Lu, L.; Han, X.; Chu, Z.; et al. Thermal Runaway of Lithium-Ion Batteries without Internal Short Circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H.; Song, S.H.; Lee, S.; Kim, J.; Kang, K. Unveiling the Role of Transition-Metal Ions in the Thermal Degradation of Layered Ni–Co–Mn Cathodes for Lithium Rechargeable Batteries. Adv. Funct. Mater. 2022, 32, 2108790. [Google Scholar] [CrossRef]
- Wang, L.; Maxisch, T.; Ceder, G. A First-Principles Approach to Studying the Thermal Stability of Oxide Cathode Materials. Chem. Mater. 2007, 19, 543–552. [Google Scholar] [CrossRef]
- Lee, E.; Muhammad, S.; Kim, T.; Kim, H.; Lee, W.; Yoon, W. Tracking the Influence of Thermal Expansion and Oxygen Vacancies on the Thermal Stability of Ni-Rich Layered Cathode Materials. Adv. Sci. 2020, 7, 1902413. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Lee, S.; Kim, H.; Yoon, J.; Jang, D.; Yoon, J.; Park, J.H.; Yoon, W.S. Deciphering the thermal behavior of lithium rich cathode material by in situ X-ray diffraction technique. J. Power Sources 2015, 285, 156–160. [Google Scholar] [CrossRef]
- Kaneda, H.; Koshika, Y.; Nakamura, T.; Nagata, H.; Ushio, R.; Mori, K. Improving the cycling performance and thermal stability of LiNi0.6Co0.2Mn0.2O2 cathode materials by Nb-doping and surface modification. Int. J. Electrochem. Sci. 2017, 12, 4640–4653. [Google Scholar] [CrossRef]
- Cho, W.; Kim, S.M.; Song, J.H.; Yim, T.; Woo, S.G.; Lee, K.W.; Kim, J.S.; Kim, Y.J. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Sources 2015, 282, 45–50. [Google Scholar] [CrossRef]
- Analysis of Coatings on NMC Cathode Materials|Tascon.Eu. Available online: https://www.tascon.eu/en/analysis-of-NMC-cathode-materials.html (accessed on 1 December 2022).
- Téllez, H.; Aguadero, A.; Druce, J.; Burriel, M.; Fearn, S.; Ishihara, T.; McPhail, D.S.; Kilner, J.A. New perspectives in the surface analysis of energy materials by combined time-of-flight secondary ion mass spectrometry (ToF-SIMS) and high sensitivity low-energy ion scattering (HS-LEIS). J. Anal. At. Spectrom. 2014, 29, 1361–1370. [Google Scholar] [CrossRef]
- Hoskins, A.L.; McNeary, W.W.; Millican, S.L.; Gossett, T.A.; Lai, A.; Gao, Y.; Liang, X.; Musgrave, C.B.; Weimer, A.W. Nonuniform Growth of Sub-2 Nanometer Atomic Layer Deposited Alumina Films on Lithium Nickel Manganese Cobalt Oxide Cathode Battery Materials. ACS Appl. Nano Mater. 2019, 2, 6989–6997. [Google Scholar] [CrossRef]
- Moryson, Y.; Walther, F.; Sann, J.; Mogwitz, B.; Ahmed, S.; Burkhardt, S.; Chen, L.; Klar, P.J.; Volz, K.; Fearn, S.; et al. Analyzing Nanometer-Thin Cathode Particle Coatings for Lithium-Ion Batteries—The Example of TiO2 on NCM622. ACS Appl. Energy Mater. 2021, 4, 7168–7181. [Google Scholar] [CrossRef]
- Binder, J.O.; Culver, S.P.; Pinedo, R.; Weber, D.A.; Friedrich, M.S.; Gries, K.I.; Volz, K.; Zeier, W.G.; Janek, J. Investigation of Fluorine and Nitrogen as Anionic Dopants in Nickel-Rich Cathode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 44452–44462. [Google Scholar] [CrossRef] [PubMed]
- Brongersma, H.H. Low-Energy Ion Scattering. Charact. Mater. 2012. [Google Scholar] [CrossRef]
- Van Leerdam, G.C.; Ackermans, P.A.J.; Groenen, P.A.C.; Brongersma, H.H.; Schmitz, J.E.J. The surface of tungsten/silicon compounds studied by low-energy ion scattering. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1988, 35, 500–503. [Google Scholar] [CrossRef]
- Münster, P.; Diehl, M.; Frerichs, J.E.; Börner, M.; Hansen, M.R.; Winter, M.; Niehoff, P. Effect of Li plating during formation of lithium ion batteries on their cycling performance and thermal safety. J. Power Sources 2021, 484, 229306. [Google Scholar] [CrossRef]
- Zhang, M.; Gui, A.L.; Sun, W.; Becking, J.; Riedel, O.; He, X.; Berghus, D.; Siozios, V.; Zhou, D.; Placke, T.; et al. High Capacity Utilization of Li Metal Anodes by Application of Celgard Separator-Reinforced Ternary Polymer Electrolyte. J. Electrochem. Soc. 2019, 166, A2142–A2150. [Google Scholar] [CrossRef]
- Wood, K.N.; Noked, M.; Dasgupta, N.P. Lithium Metal Anodes: Toward an Improved Understanding of Coupled Morphological, Electrochemical, and Mechanical Behavior. ACS Energy Lett. 2017, 2, 664–672. [Google Scholar] [CrossRef]
- Long, B.R.; Rinaldo, S.G.; Gallagher, K.G.; Dees, D.W.; Trask, S.E.; Polzin, B.J.; Jansen, A.N.; Abraham, D.P.; Bloom, I.; Bareño, J.; et al. Enabling High-Energy, High-Voltage Lithium-Ion Cells: Standardization of Coin-Cell Assembly, Electrochemical Testing, and Evaluation of Full Cells. J. Electrochem. Soc. 2016, 163, A2999–A3009. [Google Scholar] [CrossRef]
- Faenza, N.V.; Bruce, L.; Lebens-Higgins, Z.W.; Plitz, I.; Pereira, N.; Piper, L.F.J.; Amatucci, G.G. Growth of Ambient Induced Surface Impurity Species on Layered Positive Electrode Materials and Impact on Electrochemical Performance. J. Electrochem. Soc. 2017, 164, A3727–A3741. [Google Scholar] [CrossRef]
- Sicklinger, J.; Metzger, M.; Beyer, H.; Pritzl, D.; Gasteiger, H.A. Ambient Storage Derived Surface Contamination of NCM811 and NCM111: Performance Implications and Mitigation Strategies. J. Electrochem. Soc. 2019, 166, A2322–A2335. [Google Scholar] [CrossRef]
- Pritzl, D.; Teufl, T.; Freiberg, A.T.S.; Strehle, B.; Sicklinger, J.; Sommer, H.; Hartmann, P.; Gasteiger, H.A. Washing of Nickel-Rich Cathode Materials for Lithium-Ion Batteries: Towards a Mechanistic Understanding. J. Electrochem. Soc. 2019, 166, A4056–A4066. [Google Scholar] [CrossRef]
- Chen, Z.; Dahn, J.R. Improving the Capacity Retention of LiCoO2 Cycled to 4.5 V by Heat-Treatment. Electrochem. Solid-State Lett. 2004, 7, A11. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Y.-C.; Jenkins, D.M.; Chernova, N.A.; Chung, Y.; Radhakrishnan, B.; Chu, I.-H.; Fang, J.; Wang, Q.; Omenya, F.; et al. Thermal Stability and Reactivity of Cathode Materials for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 7013–7021. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Dahn, J.R. The Reaction of Charged Cathodes with Nonaqueous Solvents and Electrolytes: I. Li0.5CoO2. J. Electrochem. Soc. 2001, 148, A1205. [Google Scholar] [CrossRef]
- Dahn, J.R.; Fuller, E.W.; Obrovac, M.; von Sacken, U. Thermal stability of LixCoO2, LixNiO2 and λ-MnO2 and consequences for the safety of Li-ion cells. Solid State Ion. 1994, 69, 265–270. [Google Scholar] [CrossRef]
- Nam, K.W.; Bak, S.M.; Hu, E.; Yu, X.; Zhou, Y.; Wang, X.; Wu, L.; Zhu, Y.; Chung, K.Y.; Yang, X.Q. Combining in situ synchrotron X-Ray diffraction and absorption techniques with transmission electron microscopy to study the origin of thermal instability in overcharged cathode materials for lithium-ion batteries. Adv. Funct. Mater. 2013, 23, 1047–1063. [Google Scholar] [CrossRef]
- Fauth, F.; Peral, I.; Popescu, C.; Knapp, M. The new Material Science Powder Diffraction beamline at ALBA Synchrotron. Powder Diffr. 2013, 28, S360–S370. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++: An. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Chupas, P.J.; Chapman, K.W.; Kurtz, C.; Hanson, J.C.; Lee, P.L.; Grey, C.P. A versatile sample-environment cell for non-ambient X-ray scattering experiments. J. Appl. Crystallogr. 2008, 41, 822–824. [Google Scholar] [CrossRef]
- Reissig, F.; Puls, S.; Placke, T.; Winter, M.; Schmuch, R.; Gomez-Martin, A. Investigation of Lithium Polyacrylate Binders for Aqueous Processing of Ni-Rich Lithium Layered Oxide Cathodes for Lithium-Ion Batteries. ChemSusChem 2022, 15, e202200401. [Google Scholar] [CrossRef]



| Onset T [°C] | |
|---|---|
| Pristine | 192 |
| WO3 coated | 199 |
| Annealed | 190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reissig, F.; Ramirez-Rico, J.; Placke, T.J.; Winter, M.; Schmuch, R.; Gomez-Martin, A. The Role of Protective Surface Coatings on the Thermal Stability of Delithiated Ni-Rich Layered Oxide Cathode Materials. Batteries 2023, 9, 245. https://doi.org/10.3390/batteries9050245
Reissig F, Ramirez-Rico J, Placke TJ, Winter M, Schmuch R, Gomez-Martin A. The Role of Protective Surface Coatings on the Thermal Stability of Delithiated Ni-Rich Layered Oxide Cathode Materials. Batteries. 2023; 9(5):245. https://doi.org/10.3390/batteries9050245
Chicago/Turabian StyleReissig, Friederike, Joaquin Ramirez-Rico, Tobias Johannes Placke, Martin Winter, Richard Schmuch, and Aurora Gomez-Martin. 2023. "The Role of Protective Surface Coatings on the Thermal Stability of Delithiated Ni-Rich Layered Oxide Cathode Materials" Batteries 9, no. 5: 245. https://doi.org/10.3390/batteries9050245
APA StyleReissig, F., Ramirez-Rico, J., Placke, T. J., Winter, M., Schmuch, R., & Gomez-Martin, A. (2023). The Role of Protective Surface Coatings on the Thermal Stability of Delithiated Ni-Rich Layered Oxide Cathode Materials. Batteries, 9(5), 245. https://doi.org/10.3390/batteries9050245








