Sustainable Approach for the Development of TiO2-Based 3D Electrodes for Microsupercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 3D Substrate Materials
2.2. Synthesis and Deposition of TiO2 Layers on 2D and 3D Substrates
2.3. Physico-Chemical and Electrochemical Characterization
3. Results and Discussion
3.1. 2D Electrodes
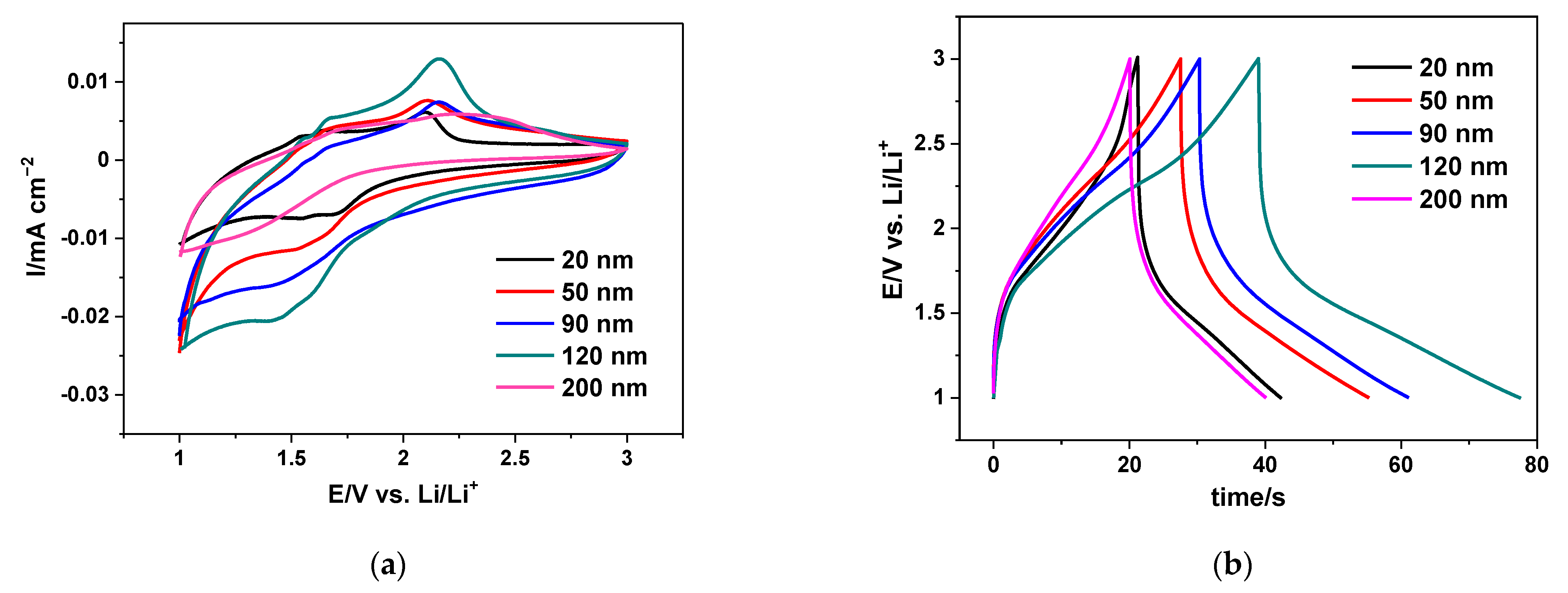
3.2. Electrochemical Characterization of 2D Electrodes
3.3. 3D Electrodes
3.3.1. 3D Substrates
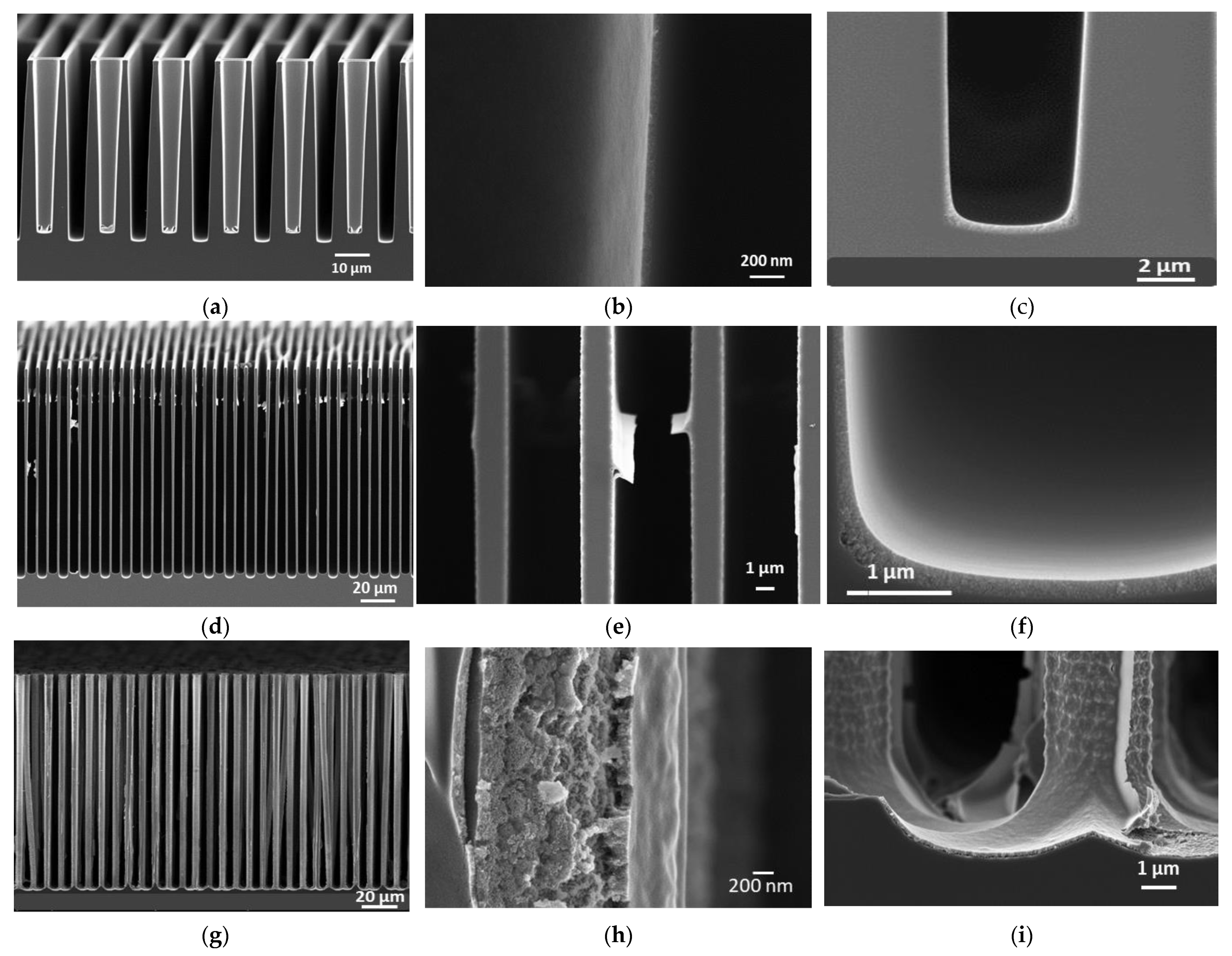
3.3.2. TiO2 Coating on 3D Substrates
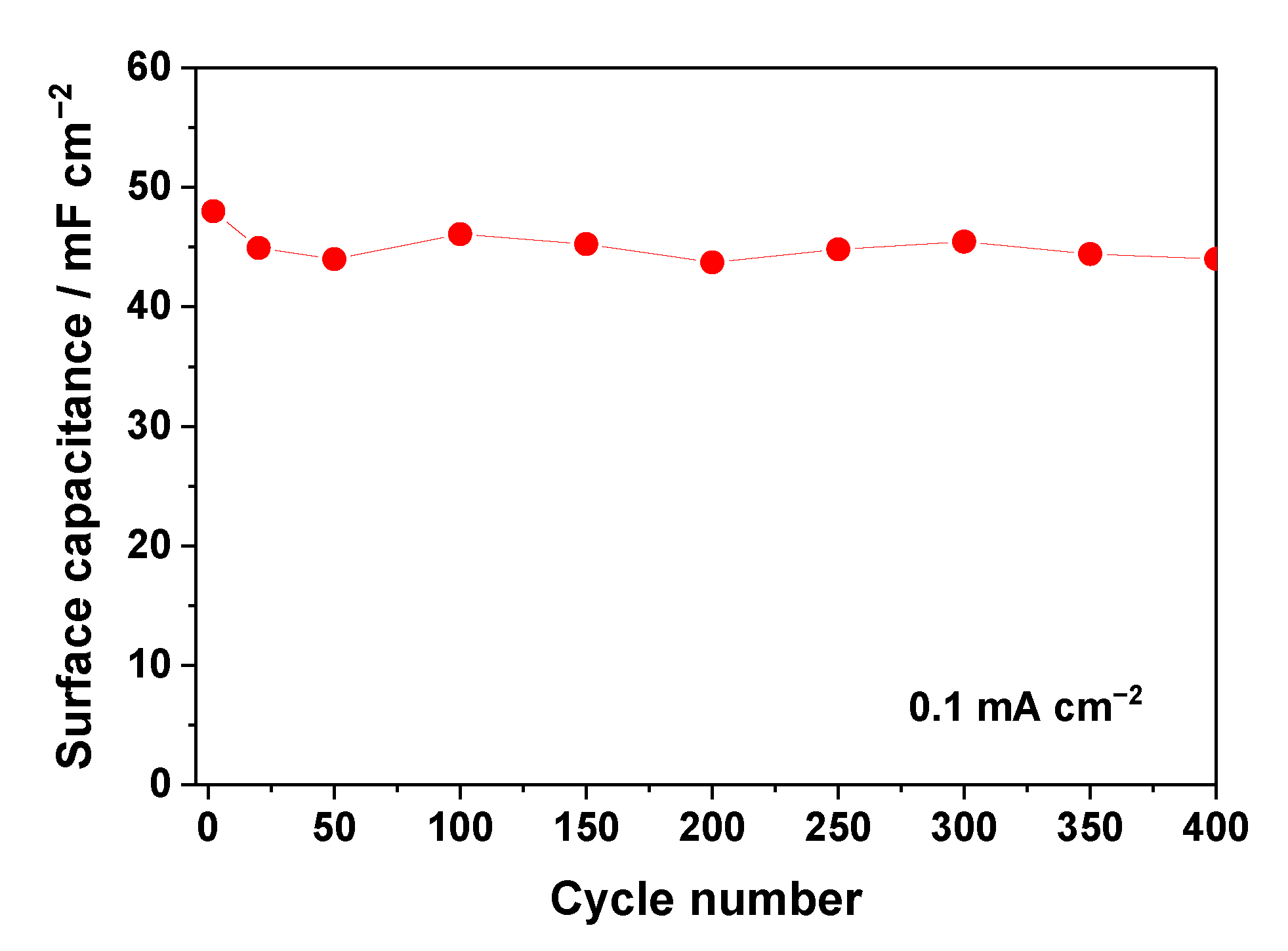
3.3.3. Electrochemical Performance of 3D Electrodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellis, B.L.; Knauth, P.; Djenizian, T. Three-Dimensional self-supported metal oxides for advanced energy storage. Adv. Mater. 2014, 26, 3368–3397. [Google Scholar] [CrossRef]
- Lethien, C.; Bideau, J.L.; Brousse, T. Challenges and prospects of 3D micro-supercapacitors for powering the internet of things. Energy Environ. Sci. 2019, 12, 96–115. [Google Scholar] [CrossRef]
- Thissandier, F.; Gentile, P.; Brousse, T.; Bidan, G.; Sadki, S. Are tomorrow’s micro-supercapacitors hidden in a forest of silicon nanotrees? J. Power Sources 2014, 269, 740–746. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, H.; Wang, F.; Shi, J.; Ci, P.; Wang, L.; Ge, L.; Wang, S.Q.; Chu, P.K. Three dimensional supercapacitors composed of Ba0.65Sr0.35TiO3 (BST)/NiSi2/silicon microchannel plates. Mat. Sci. Eng. B 2011, 176, 387–392. [Google Scholar] [CrossRef]
- Asbani, B.; Buvat, G.; Freixas, J.; Huvé, M.; Troadec, D.; Roussel, P.; Brousse, T.; Lethien, C. Ultra-high areal capacitance and high rate capability RuO2 thin film electrodes for 3D micro-supercapacitors. Energy Storage Mater. 2021, 42, 259–267. [Google Scholar] [CrossRef]
- Kyeremateng, N.A.; Brousse, T.; Pech, D. Microsupercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Technol. 2017, 12, 7–15. [Google Scholar] [CrossRef]
- Eustache, E.; Douard, C.; Demortière, A.; De Andrade, V.; Brachet, M.; Le Bideau, J.; Brousse, T.; Lethien, C. High Areal Energy 3D-Interdigitated Micro-Supercapacitors in Aqueous and Ionic Liquid Electrolytes. Adv. Mater. Technol. 2017, 2, 1700126. [Google Scholar] [CrossRef]
- Robert, K.; Stiévenard, D.; Deresmes, D.; Douard, C.; Iadecola, A.; Troadec, D.; Simon, P.; Nuns, N.; Marinova, M.; Huvé, M.; et al. Novel insights into the charge storage mechanism in pseudocapacitive vanadium nitride thick films for high-performance on-chip micro-supercapacitors. Energy Environ. Sci. 2020, 13, 949–957. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Wang, G.; Wang, F.; Yang, S.; Zhu, F.; Zhuang, X.; Schmidt, O.G.; Feng, X. Zn-Ion Hybrid Micro-Supercapacitors with Ultrahigh Areal Energy Density and Long-Term Durability. Adv. Mater. 2019, 31, 1806005. [Google Scholar] [CrossRef]
- Beidaghi, M.; Gogotsi, Y. Capacitive energy storage in micro-scale devices: Recent advances in design and fabrication of micro-supercapacitors. Energy Environ. Sci. 2014, 7, 867–884. [Google Scholar] [CrossRef]
- Klootwijk, J.H.; Jinesh, K.B.; Roozeboom, F. MIM in 3D: Dream or reality? Engineering 2011, 8, 1507–1513. [Google Scholar]
- Bates, J.B.; Dudney, N.J.; Lubben, D.C.; Gruzalski, G.R.; Kwak, B.S.; Yu, X.; Zuhr, R.A. Thin-film rechargeable lithium batteries. J. Power Sources 1995, 54, 58–62. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.M.; Bagetto, L.; Notten, P.H.L. All-Solid-State Lithium-Ion Microbatteries: A Review of Various Three-Dimensional Concepts. Adv. Energy Mat. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.M.; Van Dongen, T.; Niessen, R.A.H.; De Croon, M.H.J.M.; Notten, P.H.L. Low-Pressure Chemical Vapor Deposition of LiCoO2 Thin Films: A Systematic Investigation of the Deposition Parameters. J. Electrochem. Soc. 2009, 156, 69–174. [Google Scholar] [CrossRef]
- Eustache, E.; Douard, C.; Retoux, R.; Lethien, C.; Brousse, T. MnO2 Thin Films on 3D Scaffold: Microsupercapacitor Electrodes Competing with “Bulk” Carbon Electrodes. Adv. Energy Mater. 2015, 5, 18. [Google Scholar] [CrossRef]
- Bagetto, L.; Niessen, R.A.H.; Roozeboom, F.; Notten, P.H.L. High energy density all-solid-state batteries: A challenging concept towards 3D integration. Avd. Funct. Mater. 2008, 18, 1057–1066. [Google Scholar] [CrossRef]
- Eustache, E.; Tilmant, P.; Morgenroth, L.; Roussel, P.; Patriarche, G.; Troadec, D.; Rolland, N.; Brousse, T.; Lethien, C. Silicon-Microtube Scaffold Decorated with Anatase TiO2 as a Negative Electrode for a 3D Litium-Ion Microbattery. Adv. Energy Mater. 2014, 4, 1301612. [Google Scholar] [CrossRef]
- Létiche, M.; Eustache, E.; Freixas, J.; Demortière, A.; De Andrade, V.; Morgenroth, L.; Tilmant, P.; Vaurette, F.; Troadec, D.; Roussel, P.; et al. Atomic Layer Deposition of Functional Layers for on Chip 3D Li-Ion All Solid State Microbattery. Adv. Energy Mater. 2017, 7, 1601402. [Google Scholar] [CrossRef]
- Mazor, H.; Golodnitsky, D.; Burstein, L.; Gladkich, A.; Peled, E. Electrophoretic deposition of lithium iron phosphate cathode for thin-film 3D-microbatteries. J. Power Sources 2012, 198, 264–272. [Google Scholar] [CrossRef]
- Golodnitsky, D.; Nathan, M.; Yufit, V.; Strauss, E.; Freedman, K.; Burstein, L.; Gladkich, A.; Peled, E. Progress in three-dimensional (3D) Li-ion microbatteries. Solid State Ion. 2006, 177, 2811–2819. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Y.; Wang, M.; Yin, M.; Tian, L.; Wang, H.; Chen, X.; Fan, Z.; Lu, L.; Li, D. Efficient and Flexible Thin Film Amorphous Silicon Solar Cells on Nanotextured Polymer Substrate Using Sol–gel Based Nanoimprinting Method. Adv. Funct. Mater. 2017, 27, 1604720. [Google Scholar] [CrossRef]
- Qiu, K.; Qiu, D.; Cai, L.; Li, S.; Wu, W.; Liang, Z.; Shen, H. Preparation of ZnS thin films and ZnS/p-Si heterojunction solar cells. Mater. Lett. 2017, 198, 23–26. [Google Scholar] [CrossRef]
- Ferrari, S.; Loveridge, M.; Beattie, S.D.; Jahn, M.; Dashwood, R.J.; Bhagat, R. Latest advances in the manufacturing of 3D rechargeable lithium Microbatteries. J. Power Sources 2015, 286, 25–46. [Google Scholar] [CrossRef]
- Nohren, S.; Quiroga-Gonzalez, E.; Carstensen, J.; Foll, H. Electrochemical Fabrication and Characterization of Silicon Microwire Anodes for Li Ion Batteries. J. Electroch. Soc. 2016, 163, A373–A379. [Google Scholar] [CrossRef]
- Porthault, H.; Le Cras, F.; Duffault, J.M.; Franger, S. Fast deposition of conformal LiCoO2 thin film electrodes for high capacity 3D batteries. Mat. Sci. Eng. B 2016, 213, 163–168. [Google Scholar] [CrossRef]
- Patil, M.K.; Shaikh, S.; Ganesh, I. Recent advances on TiO2 thin film based photocatalytic application (A Review). Curr. Nanosci. 2015, 11, 271–285. [Google Scholar] [CrossRef]
- Hanini, F.; Bouabellou, A.; Bouachiba, Y.; Kermiche, F.; Taabouche, A.; Hemissi, M.; Lakhdari, D. Structural, optical and electrical properties of TiO2 thin films synthesized by sol-gel technique. IOSR J. Eng. 2013, 3, 21–28. [Google Scholar]
- Liu, P.S.; Xia, F.J.; Chen, Y.M.; Cui, G. An anatase film with improved structure of titanium dioxide modified by carbon black. Mater. Lett. 2012, 72, 5–8. [Google Scholar] [CrossRef]
- Cherneva, S.; Iankov, R.; Radic, N.; Grbic, B.; Datcheva, M.; Stoychev, D. Nano-indentation investigations of the mechanical properties of thin TiO2, WO3 and their composites layers, deposited by spray pyrolysis. Mater. Technol. 2017, 51, 75–83. [Google Scholar] [CrossRef]
- Cui, Y.; Sun., J.; Hu, Z.; Yu, W.; Xu, N.; Xu, N.; Ying, Z.; Wu, J. Synthesis, phase transition and optical properties of nanocrystalline titanium dioxide films deposited by plasma assisted reactive pulsed laser deposition. Surf. Coat. Technol. 2013, 231, 180–184. [Google Scholar] [CrossRef]
- Dobromir, M.; Manole, A.V.; Ursu, L.; Ursu, C.; Neagu, M.; Luca, D. Characterization of doped TiO2 thin films obtained by pulsed laser deposition. OAM Rapid Commun. 2013, 7, 397–401. [Google Scholar]
- Pradhan, S.S.; Sahoo, S.; Pradhan, S.K. Influence of annealing temperature on the structural, mechanical and wetting property of TiO2 films deposited by RF magnetron sputtering. Thin Solid Films 2010, 518, 6904–6908. [Google Scholar] [CrossRef]
- Mukherjee, S.K.; Nebatti, A.; Mohtascham, F.; Schipporeit, S.; Notthoff, C.; Mergel, D. Influence of thickness on the structural properties of radio-frequency and direct-current magnetron sputtered TiO2 anatase thin films. Thin Solid Films 2014, 558, 443–448. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Marin, G.; Karppinen, M. Titanium dioxide thin films by atomic layer deposition: A review. Semicond. Sci. Technol. 2017, 32, 093005. [Google Scholar] [CrossRef]
- Cheah, S.K.; Perre, E.; Rooth, M.; Fondell, M.; Hårsta, A.; Nyholm, L.; Boman, M.; Gustafsson, T.; Lu, J.; Simon, P.; et al. Self-Supported Three-Dimensional Nanoelectrodes for Microbattery Applications. Nano Lett. 2009, 9, 3230–3233. [Google Scholar] [CrossRef]
- Wang, W.; Tian, M.; Abdulagatov, A.; George, S.M.; Lee, Y.-C.; Yang, R. Three-Dimensional Ni/TiO2 Nanowire Network for High Areal Capacity Lithium Ion Microbattery Applications. Nano Lett. 2012, 12, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Fornasini, L.; Scaravonati, S.; Magnani, G.; Morenghi, A.; Sidoli, M.; Bersani, D.; Bertoni, G.; Aversa, L.; Verucchi, R.; Ricc, M.; et al. In situ decoration of laser-scribed graphene with TiO2 nanoparticles for scalable high-performance micro-supercapacitors. Carbon 2021, 176, 296–306. [Google Scholar] [CrossRef]
- Cai, J.; Lv, C.; Watanabe, A. High-performance all-solid-state flexible carbon/TiO2 micro-supercapacitors with photorechargeable capability. RSC Adv. 2017, 7, 415. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wu, J.; Shu, X.; Yu, C.; Cui, J.; Qin, Y.; Zhang, Y.; Ajayan, P.M.; Wu, Y. Remarkable supercapacitive performance of TiO2 nanotube arrays b introduction of oxygen vacancies. Chem. Eng. J. 2017, 313, 1071–1081. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, S.; Li, L.; Zhou, J.; Xe, F.; Wong, C.-P. Enhanced micro-supercapacitors in aqueous electrolyte based on Si nanowires coated with TiO2. J. Mater. Sci. Mater. Electron. 2019, 30, 8763–8770. [Google Scholar] [CrossRef]
- Bencheikh, Y.; Addad, A.; Coffinier, Y.; Kumar, U.; Roussel, P.; Szunerits, S.; Hadjersi, T.; Amin, M.A.; El hak Abaidia, S.; Boukherroub, R. Silicon nanowire-hydrogenated TiO2 core-shell arrays for stable electrochemical micro-capacitors. Electrochim. Acta 2021, 396, 139198. [Google Scholar] [CrossRef]
- Salari, M.; Aboutalebi, S.H.; Konstantinov, K.; Liu, H.K. A highly ordered titania nanotube array as a supercapacitor electrode. Phys. Chem. Chem. Phys 2011, 13, 5038–5041. [Google Scholar] [CrossRef]
- Salari, M.; Aboutalebi, S.H.; Chidembo, A.T.; Nevirkovets, I.P.; Konstantinov, K.; Liu, H.K. Enhancement of the electrochemical capacitance of TiO2 nanotube arrays through controlled phase transformation of anatase to rutile. Phys. Chem. Chem. Phys. 2012, 14, 4770–4779. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Gan, J.; Tong, Y.; Li, Y. Hydrogenated TiO2 Nanotube Arrays for Supercapacitors. Nano Lett. 2012, 12, 1690–1696. [Google Scholar] [CrossRef]
- Wu, H.; Li, D.; Zhu, X.; Yang, C.; Liu, D.; Chen, X.; Song, Y.; Lu, L. High-performance and renewable supercapacitors based on TiO2 nanotube array electrodes treated by an electrochemical doping approach. Electrochim. Acta 2014, 116, 129–136. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Electrochemically Self-Doped TiO2 Nanotube Arrays for Supercapacitors. J. Phys. Chem. C 2014, 118, 5626–5636. [Google Scholar] [CrossRef]
- Adewinbi, S.A.; Buremoh, W.; Owoeye, V.A.; Ajayeoba, Y.A.; Salau, A.O.; Busari, H.K.; Tijani, M.A.; Taleatu, B.A. Preparation and characterization of TiO2 thin film electrode for optoelectronic and energy storage Potentials: Effects of Co incorporation. Chem. Phys. Lett. 2021, 779, 138854. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Poirot, N.; Rajalingam, V.; Murgu, R.N.; Omnée, R.; Raymundo-Piñero, E. Nanotexturing TiO2 over carbon nanotubes for high-energy and high-power density pseudocapacitors in organic electrolytes. Front. Mater. 2022, 9, 1011782. [Google Scholar] [CrossRef]
- Sallaz, V.; Poulet, S.; Rouchou, J.; Boissel, J.M.; Chevalier, I.; Voiron, F.; Lamy, Y.; Oukassi, S. Hybrid All-Solid-State Thin-Film Micro-supercapacitor Based on a Pseudocapacitive Amorphous TiO2 Electrode. ACS Appl. Energy Mater. 2023, 6, 201–210. [Google Scholar] [CrossRef]
- Poirot, N.; Tillocher, T.; Raynal, P.-I. Conformal coating by liquid route on three-dimensional topology. Eur. Phys. J. Spec. Top. 2022, 231, 4245–4253. [Google Scholar] [CrossRef]
- Vincent, A.; Poirot, N. Method Depositing an Inorganic Material on a Substrate, in Particular a Micron—Or Submicron—Scale Textured. Substrate. Patent WO/2015/044582, 2014. [Google Scholar]
- Gabard, M.; Zaghrioui, M.; Chouteau, D.; Grimal, V.; Tillocher, T.; Ghamouss, F.; Poirot, N. Novel Method Based on Spin-Coating for the Preparation of 2D and 3D Si-Based Anodes for Lithium Ion Batteries. Chem. Eng. 2017, 1, 5. [Google Scholar] [CrossRef]
- Boufnichel, M.; Aachboun, S.; Grangeon, F.; Lefaucheux, P.; Ranson, P. Profile control of high aspect ratio trenches of silicon. I. Effect of process parameters on local bowing. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2002, 20, 1508–1513. [Google Scholar] [CrossRef]
- Kern, W. The Evolution of Silicon Wafer Cleaning Technology. J. Electrochem. Soc. 1990, 137, 1887–1892. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Wagemaker, M.; Kearley, G.J.; Van Well, A.A.; Mutka, H.; Mulder, F.M. Multiple Li Positions inside Oxygen Octahedra in Lithiated TiO2 Anatase. J. Am. Chem. Soc. 2003, 125, 840–848. [Google Scholar] [CrossRef]
- Baggetto, L.; Oudenhoven, J.F.M.; van Dongen, T.; Klootwijk, J.H.; Mulder, M.; Niessen, R.A.H.; de Croon, M.H.J.M.; Notten, P.H.L. On the electrochemistry of an anode stack for all-solid-state 3D-integrated batteries. J. Power Sources 2009, 189, 402–410. [Google Scholar] [CrossRef]
- Seemann, R.; Brinkmann, M.; Kramer, E.; Lange, F.F.; Lipowsky, R. Wetting morphologies at microstructured surfaces. Proc. Natl. Acad. Sci. USA 2005, 102, 1848–1852. [Google Scholar] [CrossRef] [PubMed]




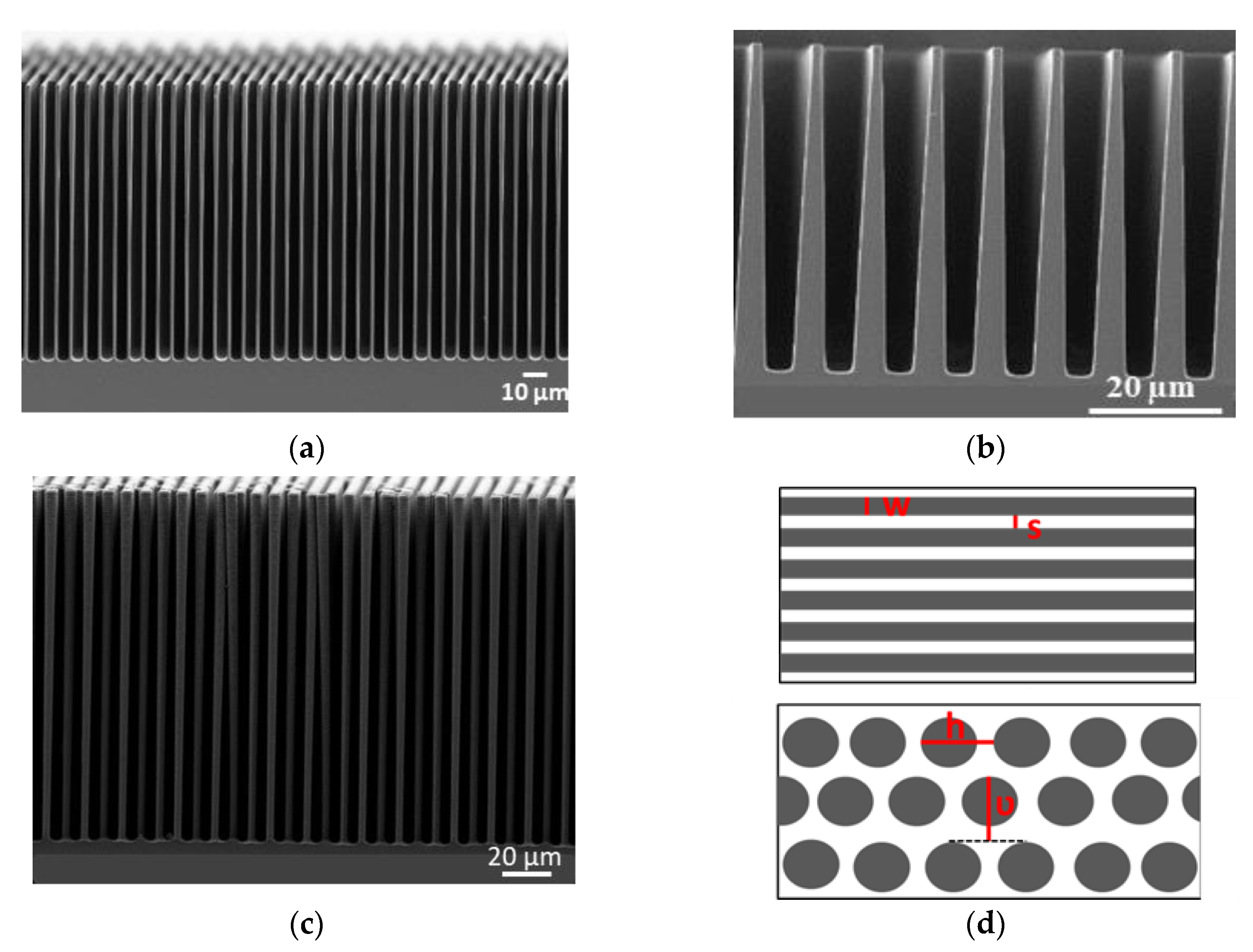


| Topology | Aspect Ratio | Etched Depth µm | Width µm | Spacing µm | Footprint Length cm | h µm | ν µm | Surface Area Enlargement |
|---|---|---|---|---|---|---|---|---|
| Trenches | 9 | 52 | 6 | 3 | 0.7 | - | - | 12.6 |
| Trenches | 29 | 117 | 4 | 3 | 0.7 | - | 34.4 | |
| Pillars | 29 | 144 | 5 | - | - | 9 | 8 | 32.4 |
| Current Density mA cm−2 | Surface Capacitance mF cm−2 | |||
|---|---|---|---|---|
| 2D | 3D AR9 Trenches | 3D AR29 Trenches | 3D AR29 Pillars | |
| 0.01 | 8.1 | 46.5 | 85.0 | 73.7 |
| 0.05 | 2.4 | 22.7 | 60.8 | 38.4 |
| 0.1 | 1.6 | 16.5 | 51.9 | 23.9 |
| 0.2 | 1.0 | 10.5 | 42.5 | 9.0 |
| 0.3 | 0.7 | 7.5 | 36.6 | 2.3 |
| 0.5 | 0.4 | 5.3 | 28.8 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poirot, N.; Gabard, M.; Boufnichel, M.; Omnée, R.; Raymundo-Piñero, E. Sustainable Approach for the Development of TiO2-Based 3D Electrodes for Microsupercapacitors. Batteries 2023, 9, 258. https://doi.org/10.3390/batteries9050258
Poirot N, Gabard M, Boufnichel M, Omnée R, Raymundo-Piñero E. Sustainable Approach for the Development of TiO2-Based 3D Electrodes for Microsupercapacitors. Batteries. 2023; 9(5):258. https://doi.org/10.3390/batteries9050258
Chicago/Turabian StylePoirot, Nathalie, Marie Gabard, Mohamed Boufnichel, Rachelle Omnée, and Encarnacion Raymundo-Piñero. 2023. "Sustainable Approach for the Development of TiO2-Based 3D Electrodes for Microsupercapacitors" Batteries 9, no. 5: 258. https://doi.org/10.3390/batteries9050258
APA StylePoirot, N., Gabard, M., Boufnichel, M., Omnée, R., & Raymundo-Piñero, E. (2023). Sustainable Approach for the Development of TiO2-Based 3D Electrodes for Microsupercapacitors. Batteries, 9(5), 258. https://doi.org/10.3390/batteries9050258







