Abstract
Electrical and electronic wastes (WEEEs) are a potential source of raw materials. The main challenge for scientists is to set up a reliable and eco-friendly process to recycle raw materials and precious elements from WEEEs. Today, we know that fungi could play an active role in green technologies aimed at recycling valuable elements. The bioaccumulation mechanism and bioleaching activity of filamentous fungal species have already been exploited fruitfully in extraction processes. However, not all fungal strains possess the same characteristics, and it is crucial to choose the right strains to use. In this work, we show a method to assess the precious elements’ recovery efficiency from WEEE using fungal strains. A CAS agar screening test for siderophore detection was carried out with three strains. The following plate accumulation test performed on a medium added with 120 ppm of electronic waste powder highlighted the differences in accumulation capability, growth rate, and biomass production. Among the elements in tested waste, yttrium, copper, and palladium show the highest bioconcentration factor. The results confirm the biotechnological potential of fungi to recover valuable elements at the bench scale, highlighting the importance of effective screening tests to assess the most efficient strain for each kind of waste.
1. Introduction
In 2020, according to the Global E-waste Monitor, the global production of electronic waste exceeded 50 million tons, while the annual per capita production was about 6 kg. To make this scenario worse, more than 80% of this waste was not recycled, leaving large quantities of precious materials unexploited [1].
Electronic devices are part of our everyday lives; among the raw materials essential for the production of electronic devices, there are the elements defined as “critical raw materials”. These elements, owing to rarity and difficulties in their mining, are often the limiting factor for technological development in many areas; in particular, where there are no natural deposits. According to Sustainable Development Goal 12 of Agenda 2030 established by the United Nations in 2015, we have to “Ensure sustainable consumption and production patterns”. Regarding critical raw materials, the best way to achieve this goal is a transition from traditional methods to new processes that endorse circular recycling of waste of electric and electronic equipment using environmentally friendly approaches. Indeed, traditional methods such as adsorption, crystallization, solvent extraction, coprecipitation, purification, ion exchange, and preconcentration are often expensive and detrimental to the environment [2]. A great challenge is to set up an efficient and eco-friendly method to mine precious elements from electronic waste in urban waste disposal. In this context, bio-based technologies, due to their low impact on the health of the environment and people, could be a solution; moreover, they could also be implemented in densely populated areas, decentralizing only the most dangerous parts of the process [3].
Over the past decades, many scientists worked on the isolation and selection of microorganisms to improve metallurgical processes and demonstrated their usefulness due to the production of organic acids and metal-binding molecules [4]. Today, bacteria, fungi, and algae have already been used successfully to mobilize, dissolve, and recover metals in batch reactors [4]. To date, bacteria have been the most studied organisms for this purpose. Recently, many researchers have also demonstrated the possibility of applying different species of filamentous fungi such as Aspergillus niger, Penicillium simplicissimum, and Trichoderma harzianum in extractive processes of V, Ni, Fe, and Cu [5,6,7]. In particular, bioleaching carried out by filamentous fungi showed a shorter lag phase and a higher metal-tolerance capacity than bacteria, that often are inhibited; fungi secreting organic acid allow it to function at higher pH [5,6].
In this context, our study aims to provide the basic criteria to select the most efficient fungal strains based on the specific context. Our bioaccumulation data show differences in bioconcentration yields among the strains tested on a waste powder supplied by accredited collectors. The result highlights the highest bioconcentration factor BCF for the A. tubingensis strain, confirming the importance of a reliable strain selection to design and implement efficient bioreactors for the recovery of valuable elements.
2. Materials and Methods
2.1. E-Waste Processing and Analysis
The electronic waste used in the experiments was supplied by accredited collectors. It was a semi-finished powder derived from PCs and/or mobile phones, disassembled and ground in a ball mill until the powder could be sieved at 140 mesh (particle size about 100 μm), obtaining the desired particle size. Later, to remove any polymeric residue present, the powder, added with 20 mass % wet sawdust, was processed in a microwave oven for precious metal waste carbonization—Violi S.r.L -Arezzo-Italy. More precisely, the microwave process occurred in a 30 L capacity crucible under nitrogen atmosphere, at 1 kW power, for 2 h. The syngas produced was properly treated before emission into the atmosphere. The result of the process was a fine powder enriched in precious metals and rare earths, whose elemental composition was defined using inductively coupled plasma mass spectrometry (ICP-MS) analysis. The ICP-MS measurements were performed using an axially viewed Varian (Springvale, Australia) Vista PRO. The sample introduction system consisted of a glass concentric K-style pneumatic nebulizer (Varian, Springvale, Australia) joined to a glass cyclonic spray chamber (Varian, Springvale, Australia) [8].
The metal concentration in fungal biomass after the bioaccumulation procedure was calculated as follows. The colony was dried in a non-ventilated oven at 40 °C until it reached a constant weight, which took approximately 5 days. Once dried, the samples were ground, homogenized, and an aliquot of fungal mass of 0.5 g was solubilized, according to the following protocol: 5 mL of HNO3 (63% m/m), a technical grade was added under heating on a hot plate, and, after 15 min, 2.5 mL of H2O2 (30% V/V) was added. All the reactants were of analytical grade and supplied by WWR CHEMICALS, Belgium. The solubilized samples were subsequently analyzed by ICP-MS for the determination of the absorbed metals.
2.2. Fungal Strains and Screening Test
Three fungal strains already used in bioremediation protocols were selected from the CoLD-UNIGE JRU MIRRI-IT collection of the University of Genoa. The selected strains belong to the following species: Trichoderma harzianum (code 013-B2), Penicillium glandicola (Oudem.) Seifert & Samson (code 013-B3), and Aspergillus tubingensis (code 013-B4). In particular, P. glandicola and T. harzianum were used because they have been isolated in areas polluted by heavy metals. The third strain, A. tubingensis, was chosen for its ability to produce large quantities of calcium oxalate under specific environmental conditions (data not published).
The three strains were firstly tested for their potential to produce siderophores using the CAS agar test as follows. Each fungal isolate was inoculated in duplicate on 90 mm Ø Petri dishes filled with blue CAS agar media prepared with Chrome Azurol S (CAS) and hexadecyltrimethylammonium bromide (HDTMA) according to [9]. The siderophore production was estimated measuring the ratio between the diameter of the orange halos and the diameters of the fungal colonies after 7 days of incubation at 24 °C using ImageJ software [10,11].
2.3. Fungal Growth Dish Tests
The fungal growth test was performed by inoculating 100 µL of 1 × 108 CFU × mL−1 conidial solution of each fungal strain on 90 mm Ø Petri dishes filled with malt extract agar amended with 120 ppm of e-waste powder whose composition is shown in Table 1 (MEA-120). The MEA-120 medium was prepared as follows: agar 10 g L−1, malt extract 10 g L−1, glucose 10 g L−1, peptone 1 g L−1, e-waste powder 120 mg L−1, autoclaved at 120 °C for 20 min. To prevent powder sedimentation at the plate bottom, MEA-120 was stirred during the cooling until 60 ± 5 °C, then was poured and the plates were immediately cooled on ice packs. Petri dishes filled with unamended MEA were used as a positive control. Later, to allow complete separation of the mycelial biomass free of medium residues, the medium surfaces were covered with sterile microporous (≈0.2 µm) cellophane membranes (Bio-Rad). The plates (five replicates for each strain) were incubated at 24 °C in the dark for 18 days, checked daily for contaminations, and measured and photographed every three days.

Table 1.
The composition (ppm) of the semi-finished product from WEEE.
At the end of the growth test, the specific weight of 1 mm2 of mycelium biomass was calculated as the ratio between the biomass weight and the area occupied by the colony using ImageJ [10]. More precisely, the 18-day colonies were removed using the membrane and later scratched off using sterile disposable plastic spatulas and dried in an oven at 40 °C. The dry weight was used to calculate specific weight; the specific weight was then used to calculate the biomass during the growth test. Finally, the biomass was used for chemical analysis.
2.4. Tolerance Index
In order to compare the growth in the MEA and in the MEA-120, the tolerance index was calculated based on fungal dry weight. This index corresponds to the ratio between the biomass grown on MEA and MEA-120, as reported in Equation (1), during the 18 days of growth.
TI = Biomass in MEA/Biomass in MEA-120
2.5. Bioconcentration Factor (BCF)
From the ratio between the concentration of metals in the colony growth on MEA-120 and the concentration added in MEA-120, it was possible to determine the bioconcentration factor (BCF) [12] for each metal in each strain, following the formula:
BCF = Metal concentration in biomass/Metal concentration in MEA-120.
The amount of metal bioaccumulated, related to the amount of biomass for each strain, allows the knowledge of the quantity of metal recovered from each plate.
3. Results
3.1. E-Waste Analysis
The WEEE powder composition is shown in Table 1, the elements are listed by increasing electronegativity.
3.2. Fungal Strains and Screening Test
Figure 1 shows the blue CAS agar test of all the strains tested. In particular, A. tubingensis, as also reported in Table 2, had a stronger production of siderophores, showing a ratio of halo diameter/colony diameter more than three times greater than P. glandicola, while T. harzianum shows a partial reaction (expressed as a weak discoloration of the medium). The reactions were classified as: strong (the halo covers the entire plate and the ratio is ≥3), moderate (the halo does not cover the entire plate but exceeds the edge of the colony and the ratio is 3 ≤ 1), weak (the halo coincides with the edge of the colony or is evident as only a discoloration of the medium, and the ratio is ≥1).
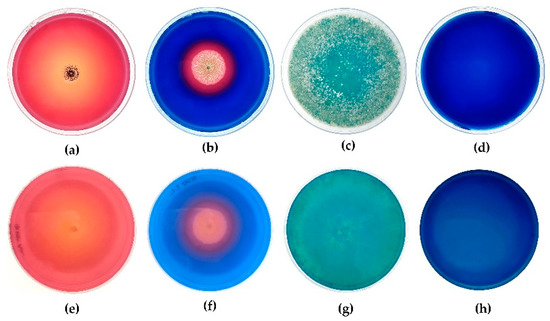
Figure 1.
CAS agar test result: A. tubingensis (a) front and (e) reverse; P. glandicola (b) front and (f) reverse; T. harzianum (c) front and (g) reverse; CAS agar control (d) front and (h) reverse.

Table 2.
CAS agar test result of the strains tested: (+++) strong, (++) moderate, (+) weak, not detectable (n.d.).
3.3. Fungal Growth Dish Tests
The growth test ended after 18 days when the growth trend of each three strains was stationary. Table 3 reports the specific weight of 1 mm2 of biomass calculated as the ratio of the dry mycelial mass scratched from the culture medium (dried in an oven at 40 °C for 48 h) to the colony surface after 18 days of growth. Figure 2 reports the growth curves of the strains monitored during 18 days of incubation at 24 °C. The curves were obtained by plotting the average biomass calculated by multiplying the average area of the five replicates grown on enriched media and controlled by the specific weight in Table 2. The graph shows the biomass difference between the three strains and the growth difference with or without WEEE in the culture medium. As shown in Figure 2, the same strain grown with or without WEEE had a similar growth rate while there is a considerable difference in biomass production among the strains.

Table 3.
Specific weight of 1 mm2 of mycelium of each species tested.
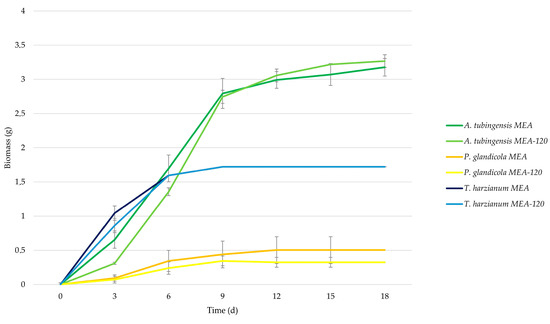
Figure 2.
Fungal biomass growth during the dish tests on medium enriched with e-waste powder MEA-120 and control MEA.
At the end of the test, the colonies were completely removed without residues of medium from the surface thanks to the use of the microporous membrane (Bio-Rad). Table 4 reports the pH value measured using a pH meter equipped with a special probe for direct soil analysis (Hanna Instrument—HI12923) at the beginning of the experiment and under the colonies after the 18-day growth period: A. tubingensis and P. glandicola acidified the medium while T. harzianum increased the pH up to 8.

Table 4.
pH at the beginning and at the end of the growth dish test.
3.4. Tolerance Index
The tolerance index (TI) was calculated as the ratio between the dry weight of the colony grown on MEA-120 against the dry weight of the colony grown on the control. Table 5 shows that A. tubingensis and T. harzianum strains are not inhibited by the presence of 120 mg L−1 of WEEE, whereas the presence of e-waste in the medium partially inhibits the growth of P. glandicola. The results are reported, and each value is reported together with its standard deviation.

Table 5.
Tolerance index.
3.5. Bioconcentration Factor (BCF)
To evaluate and quantify the recovery performance, of the three strains tested, for each metal present in the WEEE the bioconcentration factor (BCF) has been used as described by Jakubiak et al. in 2014 [13]. The BCF represents the ratio between the concentration of the dried biomass and the metal concentration in the culture media.
Figure 3 reports the BCF values after 18 days of incubation for the strains grown on MEA-120. Although all the strains can concentrate some elements, A. tubingensis is the best performing. Further information about the recovery capability may result from the comparison among the bioaccumulation data, related to the total amount of biomass produced. This result is strictly related to the highest biomass production of A. tubingensis compared to the other strains (see Table 3) and confirms the BCF trend (in Figure 3); moreover, the highest BCF values make A. tubingensis the best candidate for the whole recovery process.
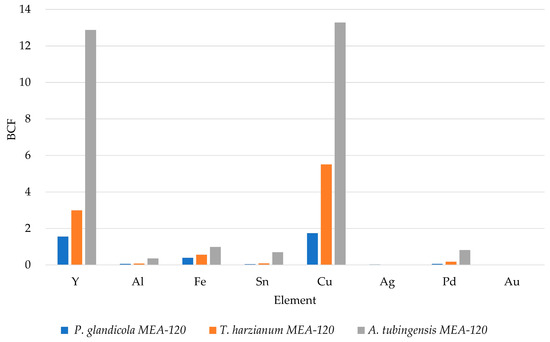
Figure 3.
Bioconcentration factor (BCF).
4. Discussions
This paper reports the fundamental steps to select efficient fungal strains exploitable in bioextraction processes from WEEE. In recent years, some studies demonstrated how fungi isolated from extreme, polluted, or mined environments could be effective in biomining applications [14]. However, most of these studies tested the accumulation of precious elements using laboratory-prepared solutions or media. These results, while confirming bioaccumulation by fungi and stimulating further research, did not consider the possible difficulties encountered if the same strain is used in applications on real waste; however, the waste composition and the possible interaction among elements can significantly affect bioaccumulation efficiency.
Our experiments were set up and carried out to screen the potential metal mobilization capability of the fungal biomass grown on real waste. These mechanisms of metal dissolution and mobilization by fungal organisms are mainly correlated to the production of organic acids and metal chelates released into the environment which favors the removal of metal from the solid or liquid matrix. The chrome azurol medium test described by Schwyn and Neilands [15] allowed us to verify the production of iron chelators and the production of siderophores [16]. We looked for the siderophores because they are well known to chelate iron that is present in high concentrations in the waste used (Fe = 7833 ppm in WEEE powder). Moreover, as highlighted by our results (Figure 2), they can also react with other elements such as Cu, Pd, Sn, and Y; this should stimulate the development and fine-tuning of new element-specific tests.
Among the fungi tested, the strain A. tubingesis showed the best BCF (Figure 3), the same strain during the growth acidified the culture media to a pH value of 1 (Table 4). This is in agreement with other authors [17,18,19,20] who found different species of the genus Aspergillus to be able to produce different acids with chelating activity. Penicillium glandicola, instead, showed a moderate production of siderophores, confirming its capability to interact with metals and survive in acidic and polluted environments [16], while Trichoderma harzianum only showed a weak discoloration of the CAS agar medium, highlighting a weak iron chelator production [21].
The growth tests conducted on waste powder-enriched media showed the great tolerance and bioconcentration capabilities of precious metals (Cu and Y) by A. tubingensis. Similar results were obtained by Becci et al. in 2020 exploiting A. niger for the extraction of Cu and Zn from printed circuit boards (PCBs) [22]. According to the assumption that the metals from the WEEE can be bioavailable after an acid attack by the fungi, the higher BCF shown by Y is due to two different reasons: (i) it is in a high concentration in waste and (ii) it has a low electronegativity. Indeed, in the WEEE powder used in our experiment there are other elements with high metallic properties (low electronegativity), and therefore well bioaccumulated, but some of these have a too low concentration in this specific sample of waste.
Moreover, yttrium, included in the rare earth elements, is widely present in WEEE and it is an essential element in the electronic industry [23] but finding microorganisms able to bioaccumulate this metal is difficult. Yttrium, in fact, is an indifferent metal which plays no biological functions in fungal metabolism. This means that fungi can actively bioaccumulate it in the cells only by substitution processes with other biological metals. In fact, yttrium ions in solution compete with and displace calcium ions from the channel pore [24]. However, this is a species-specific or even intraspecific fungal property. It is known, in fact, that many fungi exclude indifferent or toxic metals without bioaccumulating them [14]. The employment of media enriched with metal salts can trick the process because of the easier dissolution of salts and the easier release of metal ions in solution, hence the importance to draw up a set of laboratory experiments to be carried out on real waste for the evaluation of fungal bioaccumulation capabilities.
The results offer good prospects in the fields of both biorecovery and bioremediation. The yttrium and copper adsorption deserves to be further investigated owing to its role in different metabolic processes.
5. Practical Implication of This Study
The tests allow an easier, rapid, and cost-effective selection of the most performant fungal strain, showing the ability of the fungal strains to extract valuable elements from real electronic waste. This result is a key step to screen fungal strains in order to select the best performing on the basis of the different wastes in other to scale up this technology from the current Technology Readiness Level, TRL 4 (technology validated in lab) to TRL 5 (technology validated in an industrially relevant environment in the case of key enabling technologies) and above. Furthermore, since the waste was obtained from a real plant, we can assume that the same technology can be applied to waste from plants treating waste with similar characteristics.
6. Conclusions
In this work, the recovery efficiency of precious elements from real electronic waste of three different fungal strains was compared thanks to a specific set of tests. The comparison of the behavior of the strains has been carried out focusing on the production of siderophores and was evaluated through the CAS agar test and the bioconcentration factor. The results allowed us to select Aspergillus tubigensis as the best candidate for the recovery of rare earth elements and precious metals from e-waste powder. Moreover, these data confirm the ability of filamentous fungal strains to accumulate precious metals from real e-waste, and they also highlight the need to correctly assess the accumulation and bioconcentration capacity by also calculating it as a function of the biomass-producing capacity of individual strains. This last aspect is crucial, especially with a view to future industrial applications.
Author Contributions
Conceptualization, S.D.P., E.R., G.C., A.M.C. and M.Z. (Mirca Zotti); methodology, S.D.P., E.R., A.M.C. and M.Z. (Mirca Zotti); validation, M.Z. (Mirca Zotti) and A.M.C.; formal analysis, investigation, S.D.P., E.R., M.M., G.C. and M.Z. (Micol Zerbini); data curation, E.R., S.D.P., M.Z. (Mirca Zotti) and G.C.; writing—original draft preparation, E.R., S.D.P. and M.Z. (Mirca Zotti); writing—review and editing, S.D.P., M.Z. (Micol Zerbini), M.M., G.C., A.M.C. and M.Z. (Mirca Zotti); supervision, A.M.C. and M.Z. (Mirca Zotti); project administration, A.M.C.; funding acquisition, A.M.C. and M.Z. (Mirca Zotti). All authors have read and agreed to the published version of the manuscript.
Funding
This research was part of the Project 4.0 environmental sector “MYRAEE-Myco Recupero di Apparecchiature Elettriche ed Elettroniche” funded by AMGA Foundation.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forti, V.; Balde, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; United Nations University/United Nations Institute for Training and Research: Bonn, Germany; International Telecommunication Union: Geneva, Switzerland; International Solid Waste Association: Rotterdam, The Netherlands, 2020; pp. 13–15.
- Yadav, A.N.; Singh, S.; Mishra, S.; Gupta, A. Recent Advancement in White Biotechnology through Fungi; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Charter, L. Leipzig Charter on Sustainable European Cities. In Proceedings of the European Conference of Ministers Responsible for Spatial/Regional Planning, Resolution, Romania, 3 November 2007; Volume 3. [Google Scholar]
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.R.; Aggarwal, S. Mechanisms of Biological Recovery of Rare-Earth Elements from Industrial and Electronic Wastes: A Review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Yu, Z.; Han, H.; Feng, P.; Zhao, S.; Zhou, T.; Kakade, A.; Kulshrestha, S.; Majeed, S.; Li, X. Recent Advances in the Recovery of Metals from Waste through Biological Processes. Bioresour. Technol. 2020, 297, 122416. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.; Di Piazza, S.; Roccotiello, E.; Lucchetti, G.; Mariotti, M.G.; Marescotti, P. Microfungi in Highly Copper-Contaminated Soils from an Abandoned Fe-Cu Sulphide Mine: Growth Responses, Tolerance and Bioaccumulation. Chemosphere 2014, 117, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Rastegar, S.O.; Mousavi, S.M.; Li, M.; Zhou, M. Advances in Bioleaching for Recovery of Metals and Bioremediation of Fuel Ash and Sewage Sludge. Bioresour. Technol. 2018, 261, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Di Piazza, S.; Cecchi, G.; Cardinale, A.M.; Carbone, C.; Mariotti, M.G.; Giovine, M.; Zotti, M. Penicillium Expansum Link Strain for a Biometallurgical Method to Recover REEs from WEEE. Waste Manag. 2017, 60, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of Blue Agar CAS Assay for Siderophore Detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ; National Institutes of Health: Bethesda, MD, USA, 1997.
- Ghosh, S.K.; Banerjee, S.; Sengupta, C. Siderophore Production by Antagonistic Fungi (Coleoptera: Chrysomelidae: Bruchinae) © 527 Bioassay, Characterization and Estimation of Siderophores from Some Important Antagonistic Fungi. J. Biopest. 2017, 10, 105–112. [Google Scholar]
- Cecchi, G.; Roccotiello, E.; Di Piazza, S.; Riggi, A.; Mariotti, M.G.; Zotti, M. Assessment of Ni Accumulation Capability by Fungi for a Possible Approach to Remove Metals from Soils and Waters. J. Environ. Sci. Health Part B 2017, 52, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, M.; Giska, I.; Asztemborska, M.; Bystrzejewska-Piotrowska, G. Bioaccumulation and Biosorption of Inorganic Nanoparticles: Factors Affecting the Efficiency of Nanoparticle Mycoextraction by Liquid-Grown Mycelia of Pleurotus Eryngii and Trametes Versicolor. Mycol. Prog. 2014, 13, 525–532. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomycology: Biogeochemical Transformations of Rocks, Minerals, Metals and Radionuclides by Fungi, Bioweathering and Bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Cecchi, G.; Ceci, A.; Marescotti, P.; Persiani, A.M.; Di Piazza, S.; Zotti, M. Interactions among Microfungi and Pyrite-Chalcopyrite Mineralizations: Tolerance, Mineral Bioleaching, and Metal Bioaccumulation. Mycol. Prog. 2019, 18, 415–423. [Google Scholar] [CrossRef]
- Osman, Y.; Gebreil, A.; Mowafy, A.M.; Anan, T.I.; Hamed, S.M. Characterization of Aspergillus Niger Siderophore That Mediates Bioleaching of Rare Earth Elements from Phosphorites. World J. Microbiol. Biotechnol. 2019, 35, 93. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; John, P.J.; Ledwani, L. Microbial Siderophores an Envisaged Tool for Asbestos Bioremediation—A Microcosm Approach. Mater. Today Proc. 2021, 43, 3110–3116. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, W.; Liu, D.; Lu, C.; Zhang, D.; Wu, H.; Dong, D.; Meng, L. Identification and Evaluation of Aspergillus Tubingensis as a Potential Biocontrol Agent against Grey Mould on Tomato. J. Gen. Plant Pathol. 2018, 84, 148–159. [Google Scholar] [CrossRef]
- Machuca, A.; Milagres, A.M.F. Use of CAS-Agar Plate Modified to Study the Effect of Different Variables on the Siderophore Production by Aspergillus. Lett. Appl. Microbiol. 2003, 36, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elyousr, K.A.M.; Marei Almasaudi, N. Application of Trichoderma Harzianum Strain KABOFT4 for Management of Tomato Bacterial Wilt Under Greenhouse Conditions. Gesunde Pflanz. 2022, 74, 413–421. [Google Scholar] [CrossRef]
- Becci, A.; Karaj, D.; Merli, G.; Beolchini, F. Biotechnology for Metal Recovery from End-of-Life Printed Circuit Boards with Aspergillus niger. Sustainability 2020, 12, 6482. [Google Scholar] [CrossRef]
- Wang, W.; Xu, C.; Jin, Y.; Zhang, Z.; Yan, R.; Zhu, D. The Accumulation of Rare-Earth Yttrium Ions by Penicillium sp. ZD28. AMB Express 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jakubek, L.M.; Marangoudakis, S.; Raingo, J.; Liu, X.; Lipscombe, D.; Hurt, R.H. The Inhibition of Neuronal Calcium Ion Channels by Trace Levels of Yttrium Released from Carbon Nanotubes. Biomaterials 2009, 30, 6351–6357. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).