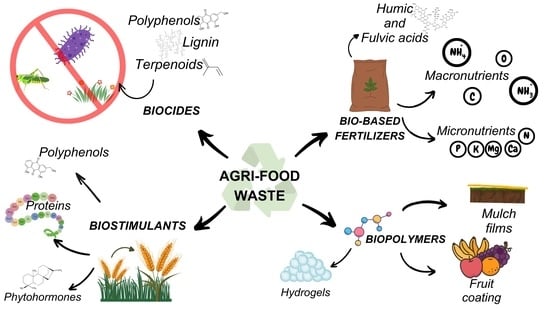
Unlocking the Potential of Agrifood Waste for Sustainable Innovation in Agriculture
Abstract
:1. Introduction
2. Biocides: From Plants, for Plants
2.1. A Sustainable and Less Toxic Alternative to Traditional Pesticides
2.2. Secondary Metabolites, Proteins, and Biopolymers as Antimicrobials
2.3. Allelopathy of By-Products: Plant Waste-Derived Herbicides for a Sustainable Agriculture
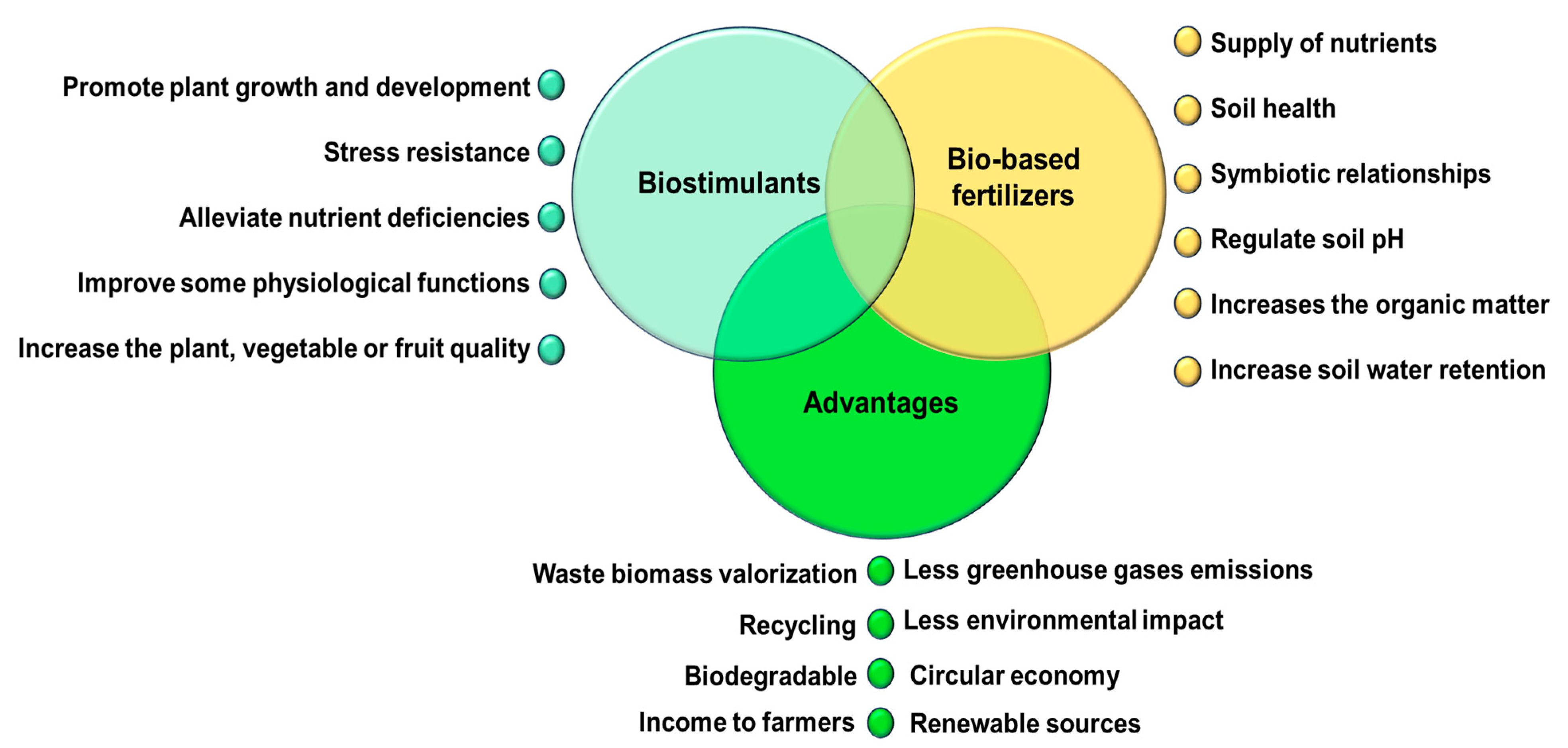
3. Importance to Convert Agri-Food Waste into Bio-Based Fertilizers
3.1. Bio-Based Fertilizers: General Aspects
3.2. Methods for Converting Agri-Food Waste into Bio-Based Fertilizers
3.3. Bio-Based Fertilizers Applied in Crops and Soil
4. Biostimulants: General Aspects
Influence on Crop Productivity with the Use of Biostimulants from Agri-Food Waste
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Ascorbic Acid |
| AD | Anaerobic Digestion |
| AFW | Agri-Food Waste |
| BBFs | Bio-Based Fertilizers |
| CFU | Colony Forming Units |
| Ch | Chitosan |
| FW | Food Waste |
| GA | Gibberellic Acid |
| GGH | Greenhouse Gas Emissions |
| Htyr | Hydroxytyrosol |
| LNP | Lignin Nanoparticles |
| OMWW | Olive Mill Wastewater |
| PBs | Plant Biostimulants |
| PLA | Polylactic Acid |
| PVA | Polyvinyl Alcohol |
| SDG | Sustainable Development Goal |
| UAE | Ultrasound-Assisted Extraction |
| UNEP | United Nations Environment Programme |
References
- Programme, United Nations Environment. Why the Global Fight to Tackle Food Waste Has Only Just Begun? Available online: https://www.unep.org/news-and-stories/story/why-global-fight-tackle-food-waste-has-only-just-begun (accessed on 25 January 2023).
- Kassim, F.O.; Paul Thomas, C.L.; Afolabi, O.O.D. Integrated Conversion Technologies for Sustainable Agri-Food Waste Valorization: A Critical Review. Biomass Bioenergy 2022, 156, 106314. [Google Scholar] [CrossRef]
- Haldar, D.; Shabbirahmed, A.M.; Singhania, R.R.; Chen, C.W.; Dong, C.D.; Ponnusamy, V.K.; Patel, A.K. Understanding the Management of Household Food Waste and Its Engineering for Sustainable Valorization- a State-of-the-Art Review. Bioresour. Technol. 2022, 358, 127390. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural Colorants from Vegetable Food Waste: Recovery, Regulatory Aspects, and Stability-a Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef] [PubMed]
- Teigiserova, D.A.; Hamelin, L.; Thomsen, M. Towards Transparent Valorization of Food Surplus, Waste and Loss: Clarifying Definitions, Food Waste Hierarchy, and Role in the Circular Economy. Sci. Total Environ. 2020, 706, 136033. [Google Scholar] [CrossRef] [PubMed]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural Waste: Review of the Evolution, Approaches and Perspectives on Alternative Uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Dutta, S.; Xiong, X.; Tsang, D.C.W.; Ramadass, K.; Vinu, A.; Kirkham, M.B.; Bolan, N.S. A Review on the Valorisation of Food Waste as a Nutrient Source and Soil Amendment. Environ. Pollut. 2021, 272, 115985. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The Opportunity of Valorizing Agricultural Waste, through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Cycon, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a Strategy for the Remediation of Pesticide-Polluted Soil: A Review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Gabaston, J.; Ortiz-Somovilla, V.; Cantos-Villar, E. Wood Waste from Fruit Trees: Biomolecules and Their Applications in Agri-Food Industry. Biomolecules 2022, 12, 238. [Google Scholar] [CrossRef]
- Pawlowska, A.; Stepczynska, M. Natural Biocidal Compounds of Plant Origin as Biodegradable Materials Modifiers. J. Polym. Environ. 2022, 30, 1683–1708. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Cheng, X.; Zheng, Z.; Wu, X.; Dong, X.; Hu, Y.; Wang, X. Agricultural Jiaosu: An Eco-Friendly and Cost-Effective Control Strategy for Suppressing Fusarium Root Rot Disease in Astragalus Membranaceus. Front. Microbiol. 2022, 13, 823704. [Google Scholar] [CrossRef]
- Sparks, T.C.; Lorsbach, B.A. Insecticide Discovery-“Chance Favors the Prepared Mind”. Pestic. Biochem. Physiol. 2023, 192, 105412. [Google Scholar] [CrossRef]
- Akhter, W.; Shah, F.M.; Yang, M.; Freed, S.; Razaq, M.; Mkindi, A.G.; Akram, H.; Ali, A.; Mahmood, K.; Hanif, M. Botanical Biopesticides Have an Influence on Tomato Quality through Pest Control and Are Cost-Effective for Farmers in Developing Countries. PLoS ONE 2023, 18, e0294775. [Google Scholar] [CrossRef]
- Nicoletti, M.; Mariani, S.; Maccioni, O.; Coccioletti, T.; Murugan, K. Neem Cake: Chemical Composition and Larvicidal Activity on Asian Tiger Mosquito. Parasitol. Res. 2012, 111, 205–213. [Google Scholar] [CrossRef]
- Satyawati, S.; Verma, M.; Sharma, A. Utilization of Non Edible Oil Seed Cakes as Substrate for Growth of Paecilomyces lilacinus and as Biopesticide against Termites. Waste Biomass Valorization 2012, 4, 325–330. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Gaffney, M.; Ansari, M.; Prasad, M.; Butt, T. Neem Seed Cake Enhances the Efficacy of the Insect Pathogenic Fungus Metarhizium Anisopliae for the Control of Black Vine Weevil, Otiorhynuchs Sulcatus (Coleoptera: Curculionidae). Biol. Control 2008, 44, 111–115. [Google Scholar] [CrossRef]
- Yoon, J.; Tak, J.H. Potential Utilization of the Brewery’s Hop Wastes as an Insecticidal Synergist and Repellent against Spodoptera Frugiperda. J. Pest Sci. 2023, 96, 1441–1454. [Google Scholar] [CrossRef]
- de Carvalho, S.S.; do Prado Ribeiro, L.; Forim, M.R.; das Graças Fernandes da Silva, M.F.; Bicalho, K.U.; Fernandes, J.B.; Vendramim, J.D. Avocado Kernels, an Industrial Residue: A Source of Compounds with Insecticidal Activity against Silverleaf Whitefly. Environ. Sci. Pollut. Res. Int. 2021, 28, 2260–2268. [Google Scholar] [CrossRef]
- Fei, T.; Gwinn, K.; Leyva-Gutierrez, F.M.A.; Wang, T. Nanoemulsions of Terpene by-Products from Cannabidiol Production Have Promising Insecticidal Effect on Callosobruchus Maculatus. Heliyon 2023, 9, 15101. [Google Scholar] [CrossRef] [PubMed]
- Anastassiadou, M.; Arena, M.; Auteri, D.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; Chiusolo, A.; Marques, D.C.; Crivellente, F.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Garlic Extract. EFSA J. 2020, 18, e06116. [Google Scholar] [CrossRef] [PubMed]
- Ai Thach, N. Investigation of the Effects of Extraction Temperature and Time on Bioactive Compounds Content from Garlic (Allium sativum L.) Husk. Front. Sustain. Food Syst. 2022, 6, 1004281. [Google Scholar] [CrossRef]
- Gupta, R.; Sharmaj, N.K. A Study of the Nematicidal Activity of Allicin—An Active Principle in Garlic, Allium Sativuml, against Root-Knot Nematode, Meloidogyne Incognita (Kofoid and White, 1919) Chitwood, 1949. Int. J. Pest Manag. 1993, 39, 390–392. [Google Scholar] [CrossRef]
- Gong, B.; Bloszies, S.; Li, X.; Wei, M.; Yang, F.; Shi, Q.; Wang, X. Efficacy of Garlic Straw Application against Root-Knot Nematodes on Tomato. Sci. Hortic. 2013, 161, 49–57. [Google Scholar] [CrossRef]
- Salifu, B.; Atongi, A.A.; Yeboah, S. Efficacy of Spring Onion (Allium fistulosum) Leaf Extract for Controlling Major Field Insect Pests of Cowpea (Vigna unguiculata L.) in the Guinea Savannah Agroecological Zone of Ghana. J. Entomol. Zool. 2019, 7, 730–733. [Google Scholar]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from Micro- to Nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef]
- Haider, M.K.; Kharaghani, D.; Sun, L.; Ullah, S.; Sarwar, M.N.; Ullah, A.; Khatri, M.; Yoshiko, Y.; Gopiraman, M.; Kim, S.I. Synthesized Bioactive Lignin Nanoparticles/Polycaprolactone Nanofibers: A Novel Nanobiocomposite for Bone Tissue Engineering. Biomater. Adv. 2023, 144, 213203. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Hoyo, J.; Tzanov, T. Hybrid Tellurium-Lignin Nanoparticles with Enhanced Antibacterial Properties. ACS Appl. Mater. Interfaces 2021, 13, 14885–14893. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the Radical Scavenging Activity of Lignins—Natural Antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic Effect of Cellulose and Lignin Nanostructures in PLA Based Systems for Food Antibacterial Packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and Antibacterial Lignin Nanoparticles in Polyvinyl Alcohol/Chitosan Films for Active Packaging. Ind. Crop Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- An, L.; Heo, J.W.; Chen, J.; Kim, Y.S. Water-Soluble Lignin Quaternary Ammonium Salt for Electrospun Morphology-Controllable Antibacterial Polyvinyl Alcohol/Lignin Quaternary Ammonium Salt Nanofibers. J. Clean. Prod. 2022, 368, 133219. [Google Scholar] [CrossRef]
- Wang, W.; Qin, C.; Li, W.; Li, Z.; Li, J. Design of Antibacterial Cellulose Nanofibril Film by the Incorporation of Guanidine-Attached Lignin Nanoparticles. Cellulose 2022, 29, 3439–3451. [Google Scholar] [CrossRef]
- Gazzurelli, C.; Carcelli, M.; Mazzeo, P.P.; Mucchino, C.; Pandolfi, A.; Migliori, A.; Pietarinen, S.; Leonardi, G.; Rogolino, D.; Pelagatti, P. Exploiting the Reducing Properties of Lignin for the Development of an Effective Lignin@Cu2O Pesticide. Adv. Sustain. Syst. 2022, 6, 2200108. [Google Scholar] [CrossRef]
- Sinisi, V.; Pelagatti, P.; Carcelli, M.; Migliori, A.; Mantovani, L.; Righi, L.; Leonardi, G.; Pietarinen, S.; Hubsch, C.; Rogolino, D. A Green Approach to Copper-Containing Pesticides: Antimicrobial and Antifungal Activity of Brochantite Supported on Lignin for the Development of Biobased Plant Protection Products. ACS Sustain. Chem. Eng. 2018, 7, 3213–3221. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tacchini, M.; Guerrini, A.; Poli, F. Phytochemical Profile and In Vitro Bioactivities of Plant-Based By-Products in View of a Potential Reuse and Valorization. Plants 2023, 12, 795. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, E.; Caracciolo, R.; Romani, A.; Cacciola, F.; Dugo, P.; Bernini, R.; Varvaro, L.; Santi, L. An Hydroxytyrosol Enriched Extract from Olive Mill Wastewaters Exerts Antioxidant Activity and Antimicrobial Activity on Pseudomonas savastanoi pv. Savastanoi and Agrobacterium Tumefaciens. Nat. Prod. Res. 2021, 35, 2677–2684. [Google Scholar] [CrossRef]
- Ditsawanon, T.; Roytrakul, S.; Phaonakrop, N.; Charoenlappanit, S.; Thaisakun, S.; Parinthawong, N. Novel Small Antimicrobial Peptides Extracted from Agricultural Wastes Act against Phytopathogens but Not Rhizobacteria. Agronomy 2022, 12, 1841. [Google Scholar] [CrossRef]
- Giorni, P.; Bulla, G.; Leni, G.; Soldano, M.; Tacchini, M.; Guerrini, A.; Sacchetti, G.; Bertuzzi, T. Enhancement of Agri-Food by-Products: Green Extractions of Bioactive Molecules with Fungicidal Action against Mycotoxigenic Fungi and Their Mycotoxins. Front. Nutr. 2023, 10, 1196812. [Google Scholar] [CrossRef] [PubMed]
- Tzintzun-Camacho, O.; Hernández-Jiménez, V.; González-Mendoza, D.; Pérez-Pérez, J.P.; Troncoso-Rojas, R.; Durán-Hernández, D.; Ceceña-Durán, C.; Moreno-Cruz, C.F. Characterization of Grape Marc Hydrolysates and Their Antifungal Effect against Phytopathogenic Fungi of Agricultural Importance. Chil. J. Agric. Res. 2021, 81, 151–160. [Google Scholar] [CrossRef]
- Teixeira, A.; Sánchez-Hernández, E.; Noversa, J.; Cunha, A.; Cortez, I.; Marques, G.; Martín-Ramos, P.; Oliveira, R. Antifungal Activity of Plant Waste Extracts against Phytopathogenic Fungi: Allium sativum Peels Extract as a Promising Product Targeting the Fungal Plasma Membrane and Cell Wall. Horticulturae 2023, 9, 136. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J.; Vlačihová, A.; Šedo, O.; Zdráhal, Z.; Konečná, H.; Stupková, A.; Švajner, J. Proteomic Characterization and Antifungal Activity of Potato Tuber Proteins Isolated from Starch Production Waste under Different Temperature Regimes. Appl. Microbiol. Biotechnol. 2018, 102, 10551–10560. [Google Scholar] [CrossRef]
- Lachguer, K.; el Merzougui, S.; Boudadi, I.; Laktib, A.; ben El Caid, M.; Ramdan, B.; Boubaker, H.; Serghini, M.A. Major Phytochemical Compounds, in Vitro Antioxidant, Antibacterial, and Antifungal Activities of Six Aqueous and Organic Extracts of Crocus sativus L. Flower Waste. Waste Biomass Valorization 2023, 14, 1571–1587. [Google Scholar] [CrossRef]
- Farouk, A.; Hathout, A.S.; Amer, M.M.; Hussain, O.A.; Fouzy, A.S.M. The Impact of Nanoencapsulation on Volatile Constituents of Citrus sinesis L. Essential Oil and Their Antifungal Activity. Egypt. J. Chem. 2021, 65, 527–538. [Google Scholar] [CrossRef]
- Yohalem, D.; Passey, T. Amendment of Soils with Fresh and Post-Extraction Lavender (Lavandula angustifolia) and Lavandin (Lavandula × Intermedia) Reduce Inoculum of Verticillium Dahliae and Inhibit Wilt in Strawberry. Appl. Soil. Ecol. 2011, 49, 187–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Zheng, Z.; Meng, X.; Cai, Y.; Liu, J.; Hu, Y.; Yan, S.; Wang, X. A Microbial Ecosystem: Agricultural Jiaosu Achieves Effective and Lasting Antifungal Activity against Botrytis Cinerea. AMB Expr. 2020, 10, 216. [Google Scholar] [CrossRef]
- Mapuranga, J.; Zhang, N.; Zhang, L.; Chang, J.; Yang, W. Infection Strategies and Pathogenicity of Biotrophic Plant Fungal Pathogens. Front. Microbiol. 2022, 13, 799396. [Google Scholar] [CrossRef]
- Palazzolo, M.A.; Aballay, M.M.; Martinez, A.A.; Kurina-Sanz, M. Grape Stalk-Based Extracts Controlling Fruit Pathogenic Fungi as a Waste Biomass Valorization Alternative in Winemaking. Waste Biomass Valorization 2021, 13, 609–616. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research Progress on the Use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Lorenzo, P.; Guilherme, R.; Barbosa, S.; Ferreira, A.J.D.; Galhano, C. Agri-Food Waste as a Method for Weed Control and Soil Amendment in Crops. Agronomy 2022, 12, 1184. [Google Scholar] [CrossRef]
- Mallek, S.B.; Prather, T.S.; Stapleton, J.J. Interaction Effects of Allium Spp. Residues, Concentrations and Soil Temperature on Seed Germination of Four Weedy Plant Species. Appl. Soil Ecol. 2003, 37, 233–239. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Cui, J.; Du, J.; Chen, G.; Liu, H. Optimization of Ultrasonic-Assisted Extraction for Herbicidal Activity of Chicory Root Extracts. Ind. Crop Prod. 2011, 34, 1429–1438. [Google Scholar] [CrossRef]
- de Paulo Barbosa, L.M.; Oliveira Santos, J.; Mouzinho de Sousa, R.C.; Barros Furtado, J.L.; Vidinha, P.; Suller Garcia, M.A.; Aguilar Vitorino, H.; Fossatti Dall’Oglio, D. Bioherbicide from Azadirachta indica Seed Waste: Exploitation, Efficient Extraction of Neem Oil and Allelopathic Effect on Senna occidentalis. Recycling 2023, 8, 50. [Google Scholar] [CrossRef]
- El-Metwally, I.M.; Saudy, H.S.; Elewa, T.A. Natural Plant by-Products and Mulching Materials to Suppress Weeds and Improve Sugar Beet (Beta vulgaris L.) Yield and Quality. J. Soil Sci. Plant Nutr. 2022, 22, 5217–5230. [Google Scholar] [CrossRef]
- Shehata, S.A.; El-Metwally, I.M.; Abdelgawad, K.F.; Elkhawaga, F. A Efficacy of Agro-Industrial Wastes on the Weed Control, Nutrient Uptake, Growth, and Yield of Onion Crop (Allium cepa L.). J. Soil Sci. Plant Nutr. 2022, 22, 2707–2718. [Google Scholar] [CrossRef]
- Silva, W.O.; Stamford, N.P.; Silva, E.V.N.; Santos, C.E.R.S.; Freitas, A.D.S.; Silva, M.V. The Impact of Biofertilizers with Diazotrophic Bacteria and Fungi Chitosan on Melon Characteristics and Nutrient Uptake as an Alternative for Conventional Fertilizers. Sci. Hortic. 2016, 209, 236–240. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Fan, J.; Yang, X.; Yi, Y.; Han, X.; Wang, D.; Zhu, P.; Peng, X. Does Animal Manure Application Improve Soil Aggregation? Insights from Nine Long-Term Fertilization Experiments. Sci. Total Environ. 2019, 660, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Chia, S.R.; Yen, H.-W.; Nomanbhay, S.; Ho, Y.-C.; Show, P.L. Transformation of Biomass Waste into Sustainable Organic Fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef]
- Havukainen, J.; Uusitalo, V.; Koistinen, L.; Liikanen, M.; Horttanainen, M. Carbon Footprint Evaluation of Biofertilizers. Int. J. Sustain. Dev. Plan. 2018, 13, 1050–1060. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhang, S.; Wang, Y. What Could Promote Farmers to Replace Chemical Fertilizers with Organic Fertilizers? J. Clean. Prod. 2018, 199, 882–890. [Google Scholar] [CrossRef]
- Su, Y.-Z.; Wang, F.; Suo, D.-R.; Zhang, Z.-H.; Du, M.-W. Long-Term Effect of Fertilizer and Manure Application on Soil-Carbon Sequestration and Soil Fertility under the Wheat–Wheat–Maize Cropping System in Northwest China. Nutr. Cycl. Agroecosystems 2006, 75, 285–295. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers Function as Key Player in Sustainable Agriculture by Improving Soil Fertility, Plant Tolerance and Crop Productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Sabier Sae, K.; Ah, S.A.; Has, I.A.; Ahme, P.H. Effect of Bio-Fertilizer and Chemical Fertilizer on Growth and Yield in Cucumber (Cucumis sativus) in Green House Condition. Pak. J. Biol. Sci. 2015, 18, 129–134. [Google Scholar] [CrossRef]
- Li, R.; Tao, R.; Ling, N.; Chu, G. Chemical, Organic and Bio-Fertilizer Management Practices Effect on Soil Physicochemical Property and Antagonistic Bacteria Abundance of a Cotton Field: Implications for Soil Biological Quality. Soil. Tillage Res. 2017, 167, 30–38. [Google Scholar] [CrossRef]
- Redding, M.R.; Lewis, R.; Kearton, T.; Smith, O. Manure and Sorbent Fertilisers Increase on-Going Nutrient Availability Relative to Conventional Fertilisers. Sci. Total Environ. 2016, 569–570, 927–936. [Google Scholar] [CrossRef]
- Diacono, M.; Persiani, A.; Testani, E.; Montemurro, F.; Ciaccia, C. Recycling Agricultural Wastes and By-products in Organic Farming: Biofertilizer Production, Yield Performance and Carbon Footprint Analysis. Sustainability 2019, 11, 3824. [Google Scholar] [CrossRef]
- Toop, T.A.; Ward, S.; Oldfield, T.; Hull, M.; Kirby, M.E.; Theodorou, M.K. Agrocycle–Developing a Circular Economy in Agriculture. Energy Procedia 2017, 123, 76–80. [Google Scholar] [CrossRef]
- Venanzi, S.; Pezzolla, D.; Cecchini, L.; Pauselli, M.; Ricci, A.; Sordi, A.; Torquati, B.; Gigliotti, G. Use of Agricultural by-Products in the Development of an Agro-Energy Chain: A Case Study from the Umbria Region. Sci. Total Environ. 2018, 627, 494–505. [Google Scholar] [CrossRef]
- Daza Serna, L.V.; Solarte Toro, J.C.; Serna Loaiza, S.; Chacón Perez, Y.; Cardona Alzate, C.A. Agricultural Waste Management through Energy Producing Biorefineries: The Colombian Case. Waste Biomass Valorization 2016, 7, 789–798. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Pilar Bernal, M. Assessment of the Fertiliser Potential of Digestates from Farm and Agroindustrial Residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Pezzolla, D.; Bol, R.; Gigliotti, G.; Sawamoto, T.; López, A.L.; Cardenas, L.; Chadwick, D. Greenhouse Gas (Ghg) Emissions from Soils Amended with Digestate Derived from Anaerobic Treatment of Food Waste. Rapid Commun. Mass. Spectrom. 2012, 26, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; Hazarika, S.; Baruah, D.C. Assessment of by-Products of Bioenergy Systems (Anaerobic digestion and Gasification) as Potential Crop Nutrient. Waste Manag. 2017, 59, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Wolka, K.; Melaku, B. Exploring Selected Plant Nutrient in Compost Prepared from Food Waste and Cattle Manure and Its Effect on Soil Properties and Maize Yield at Wondo Genet, Ethiopia. Environ. Syst. Res. 2015, 4, 31. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, S.; Zhang, X.; Chen, J.; He, X.; Zhang, Q. Effects of Pyrolysis Temperature on Soil-Plant-Microbe Responses to Solidago canadensis L.-Derived Biochar in Coastal Saline-Alkali Soil. Sci. Total Environ. 2020, 731, 138938. [Google Scholar] [CrossRef]
- Chiang, P.N.; Tong, O.Y.; Chiou, C.S.; Lin, Y.A.; Wang, M.K.; Liu, C.C. Reclamation of Zinc-Contaminated Soil Using a Dissolved Organic Carbon Solution Prepared Using Liquid Fertilizer from Food-Waste Composting. J. Hazard. Mater. 2016, 301, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami, A.I. Composting Parameters and Compost Quality: A Literature Review. Org. Agric. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Waqas, M.; Nizami, A.S.; Aburiazaiza, A.S.; Barakat, M.A.; Ismail, I.M.I.; Rashid, M.I. Optimization of Food Waste Compost with the Use of Biochar. J. Environ. Manag. 2018, 216, 70–81. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable Hydrogen Production through Biomass Gasification: A Review and Future Prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef]
- Christou, A.; Stylianou, M.; Georgiadou, E.C.; Gedeon, S.; Ioannou, A.; Michael, C.; Papanastasiou, P.; Fotopoulos, V.; Fatta-Kassinos, D. Effects of Biochar Derived from the Pyrolysis of Either Biosolids, Manure or Spent Coffee Grounds on the Growth, Physiology and Quality Attributes of Field-Grown Lettuce Plants. Environ. Technol. Innov. 2022, 26, 102263. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Nguyen, T.T.; Le, H.T.N.; Nguyen, T.T.T.; Bach, L.G.; Nguyen, T.D.; Vo, D.V.N.; Tran, T.V. The Sunflower Plant Family for Bioenergy, Environmental Remediation, Nanotechnology, Medicine, Food and Agriculture: A Review. Environ. Chem. Lett. 2021, 19, 3701–3726. [Google Scholar] [CrossRef]
- Wester-Larsen, L.; Muller-Stover, D.S.; Salo, T.; Jensen, L.S. Potential Ammonia Volatilization from 39 Different Novel Biobased Fertilizers on the European Market-a Laboratory Study Using 5 European Soils. J. Environ. Manag. 2022, 323, 116249. [Google Scholar] [CrossRef]
- Chang, M.Y.; Huang, W.J. Production of Silicon Carbide Liquid Fertilizer by Hydrothermal Carbonization Processes from Silicon Containing Agricultural Waste Biomass. Eng. J. 2016, 20, 11–17. [Google Scholar] [CrossRef]
- Chang, M.Y.; Huang, W.J. A Practical Case Report on the Node Point of a Butterfly Model Circular Economy: Synthesis of a New Hybrid Mineral–Hydrothermal Fertilizer for Rice Cropping. Sustainability 2020, 12, 1245. [Google Scholar] [CrossRef]
- Silva, M.P.; Lobos, M.L.N.; Piloni, R.V.; Dusso, D.; Quijón, M.E.G.; Scopel, A.L.; Moyano, E.L. Pyrolytic Biochars from Sunflower Seed Shells, Peanut Shells and Spirulina Algae: Their Potential as Soil Amendment and Natural Growth Regulators. SN Appl. Sci. 2020, 2, 1926. [Google Scholar] [CrossRef]
- Altieri, R.; Esposito, A. Evaluation of the Fertilizing Effect of Olive Mill Waste Compost in Short-Term Crops. Int. Biodeterior. Biodegrad. 2010, 64, 124–128. [Google Scholar] [CrossRef]
- Sall, P.M.; Antoun, H.; Chalifour, F.P.; Beauchamp, C.J.; Moral, M.T. Potential Use of Leachate from Composted Fruit and Vegetable Waste as Fertilizer for Corn. Cogent Food Agric. 2019, 5, 1580180. [Google Scholar] [CrossRef]
- Kadir, A.A.; Rahman, N.A.; Azhari, N.W. The Utilization of Banana Peel in the Fermentation Liquid in Food Waste Composting. IOP Conf. Ser. Mater. Sci. Eng. 2016, 136, 012055. [Google Scholar] [CrossRef]
- Muniappan, V.; Hepsibha, T. Fermented Liquid Biofertilizer from Banana Waste-a Value Added Product. Innov. Agric. 2023, 6, 1–5. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Hagare, D.; Jayasena, V.; Swick, R.; Rahman, M.M.; Boyle, N.; Ghodrat, M. Recycling of Food Waste to Produce Chicken Feed and Liquid Fertiliser. Waste Manag. 2021, 131, 386–393. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Hagare, D.; Chen, Z.H.; Jayasena, V.; Shahrivar, A.A.; Panatta, O.; Liang, W.; Boyle, N. Growing Lettuce and Cucumber in A hydroponic System Using Food Waste Derived Organic Liquid Fertiliser. Environ. Sustain. 2022, 5, 325–334. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, Y.; Guo, D.; Wang, Q.; Wang, G. Chemical Properties and Microbial Responses to Biochar and Compost Amendments in the Soil under Continuous Watermelon Cropping Original Paper. Plant Soil. Environ. 2017, 63, 1–7. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shaffique, S.; Kim, L.-R.; Kwon, E.-H.; Kim, S.-H.; Lee, Y.-H.; Kalsoom, K.; Khan, M.A.; Lee, I.-J. Effects of Organic Fertilizer Mixed with Food Waste Dry Powder on the Growth of Chinese Cabbage Seedlings. Environments 2021, 8, 86. [Google Scholar] [CrossRef]
- Pensupa, N.; Jin, M.; Kokolski, M.; Archer, D.B.; Du, C. A Solid State Fungal Fermentation-Based Strategy for the Hydrolysis of Wheat Straw. Bioresour. Technol. 2013, 149, 261–267. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Mikulewicz, M. Valorisation of Agri-Food Waste to Fertilisers Is a Challenge in Implementing the Circular Economy Concept in Practice. Environ. Pollut. 2022, 312, 119906. [Google Scholar] [CrossRef]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of Commercial Bio-Inoculants to Increase Agricultural Production through Improved Phosphrous Acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Tuttobene, R.; Avola, G.; Gresta, F.; Abbate, V. Industrial Orange Waste as Organic Fertilizer in Durum Wheat. Agron. Sustain. Dev. 2009, 29, 557–563. [Google Scholar] [CrossRef]
- Bomfim, A.S.C.; Oliveira, D.M.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.J.; Rodrigue, D. Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment. Waste 2023, 1, 2–20. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Poluszyńska, J.; Miłek, D.; Szewczyk, A.; Sławińska, A. Acute Toxicity of Experimental Fertilizers Made of Spent Coffee Grounds. Waste Biomass Valorization 2018, 9, 2157–2164. [Google Scholar] [CrossRef]
- Sangamithirai, K.M.; Jayapriya, J.; Hema, J.; Manoj, R. Evaluation of in-Vessel Co-Composting of Yard Waste and Development of Kinetic Models for Co-Composting. Int. J. Recycl. Org. Waste Agric. 2015, 4, 157–165. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, J.; Huang, D.; Zhu, Q.; Liu, S.; Su, Y.; Wei, W.; Syers, J.K.; Li, Y. Improving Fertility and Productivity of a Highly-Weathered Upland Soil in Subtropical China by Incorporating Rice Straw. Plant Soil 2010, 331, 427–437. [Google Scholar] [CrossRef]
- Rady, M.M.; Semida, W.M.; Hemida, K.A.; Abdelhamid, M.T. The Effect of Compost on Growth and Yield of Phaseolus Vulgaris Plants Grown under Saline Soil. Int. J. Recycl. Org. Waste Agric. 2016, 5, 311–321. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, J.; He, M.; Zou, S.; Wang, C. Effect of Kelp Waste Extracts on the Growth and Development of Pakchoi (Brassica chinensis L.). Sci. Rep. 2016, 6, 38683. [Google Scholar] [CrossRef]
- Tartoura, K.A.H.; Youssef, S.A.; Tartoura, E.-S.A.A. Compost Alleviates the Negative Effects of Salinity Via up-Regulation of Antioxidants in Solanum lycopersicum L. Plants. Plant Growth Regul. 2014, 74, 299–310. [Google Scholar] [CrossRef]
- Cha-um, S.; Kirdmanee, C. Remediation of Salt-Affected Soil by the Addition of Organic Matter: An Investigation into Improving Glutinous Rice Productivity. Sci. Agric. 2011, 68, 406–410. [Google Scholar] [CrossRef]
- Yan, M.; Tian, H.; Song, S.; Tan, H.T.W.; Lee, J.T.E.; Zhang, J.; Sharma, P.; Tiong, Y.W.; Tong, Y.W. Effects of Digestate-Encapsulated Biochar on Plant Growth, Soil Microbiome and Nitrogen Leaching. J. Environ. Manag. 2023, 334, 117481. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Hagare, D.; Liu, M.H.; Panatta, O.; Hussain, T.; Memon, S.; Noorani, A.; Chen, Z.H. A Food Waste-Derived Organic Liquid Fertiliser for Sustainable Hydroponic Cultivation of Lettuce, Cucumber and Cherry Tomato. Foods 2023, 12, 719. [Google Scholar] [CrossRef]
- Karim, A.A.; Kumar, M.; Mohapatra, S.; Singh, S.K. Nutrient Rich Biomass and Effluent Sludge Wastes Co-Utilization for Production of Biochar Fertilizer through Different Thermal Treatments. J. Clean. Prod. 2019, 228, 570–579. [Google Scholar] [CrossRef]
- Radziemska, M.; Vaverková, M.D.; Adamcová, D.; Brtnický, M.; Mazur, Z. Valorization of Fish Waste Compost as a Fertilizer for Agricultural Use. Waste Biomass Valorization 2018, 10, 2537–2545. [Google Scholar] [CrossRef]
- Chojnacka, K.; Górecka, H.; Michalak, I.; Górecki, H. A Review: Valorization of Keratinous Materials. Waste Biomass Valorization 2011, 2, 317–321. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Malinowski, P.; Chojnacka, K. Valorization of Phosphorus Secondary Raw Materials by Acidithiobacillus Ferrooxidans. Molecules 2017, 22, 473. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Lehmann, J.; Solomon, D. Recycling Slaughterhouse Waste into Fertilizer: How Do Pyrolysis Temperature and Biomass Additions Affect Phosphorus Availability and Chemistry? J. Sci. Food Agric. 2015, 95, 281–288. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Sánchez-Vioque, R.; Santana-Méridas, O.; Martín-Bejerano, M.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. A Potential Use of Vine-Shoot Wastes: The Antioxidant, Antifeedant and Phytotoxic Activities of Their Aqueous Extracts. Ind. Crops Prod. 2017, 97, 120–127. [Google Scholar] [CrossRef]
- Quratul, A.; Firdaus, B.; Shafiq, M. Management of the Parthenium Hysterophorus through Biochar Formation and Its Application to Rice-Wheat Cultivation in Pakistan. Agric. Ecosyst. Environ. 2016, 235, 265–276. [Google Scholar] [CrossRef]
- Narzari, R.; Bordoloi, N.; Sarma, B.; Gogoi, L.; Gogoi, N.; Borkotoki, B.; Kataki, R. Fabrication of Biochars Obtained from Valorization of Biowaste and Evaluation of Its Physicochemical Properties. Bioresour. Technol. 2017, 242, 324–328. [Google Scholar] [CrossRef]
- Yue, Y.; Guo, W.N.; Lin, Q.M.; Li, G.T.; Zhao, X.R. Improving Salt Leaching in a Simulated Saline Soil Column by Three Biochars Derived from Rice Straw (Oryza sativa L.), Sunflower Straw (Helianthus annuus), and Cow Manure. J. Soil Water Conserv. 2016, 71, 467–475. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a Sustainable Agriculture through Plant Biostimulants: From Experimental Data to Practical Applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Van Oosten, J.M.; Pepe, O.; Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Kim, H.; Ku, K.; Choi, S.; Cardarelli, M. Vegetal-Derived Biostimulant Enhances Adventitious Rooting in Cuttings of Basil, Tomato, and Chrysanthemum Via Brassinosteroid-Mediated Processes. Agronomy 2019, 9, 74. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing Biostimulants from Agro-Food and Industrial by-Products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef]
- Rehim, A.; Bashir, M.A.; Qurat-Ul-Ain, R.; Gallagher, K.; Berlyn, G.P. Yield Enhancement of Biostimulants, Vitamin B12, and Coq10 Compared to Inorganic Fertilizer in Radish. Agronomy 2021, 11, 697. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and Biochemical Responses of Glycine max (L.) Merr. To the Use of Seaweed Extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar Applications of Biostimulants Promote Growth, Yield and Fruit Quality of Strawberry Plants Grown under Nutrient Limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Canterino, S.; Cerutti, A.K.; Bounous, G. Improving the Nutritional Value of Kiwifruit with the Application of Agroindustry Waste Extracts. J. Appl. Bot. Food Qual. 2013, 86, 11–15. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Al-Masoudi, M. Interactions between Mycorrhizal Fungi, Tea Wastes, and Algal Biomass Affecting the Microbial Community, Soil Structure, and Alleviating of Salinity Stress in Corn Yield (Zea mays L.). Plants 2018, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant Action of a Plant-Derived Protein Hydrolysate Produced through Enzymatic Hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological Activity of Vegetal Extracts Containing Phenols on Plant Metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar Applications of a Legume-Derived Protein Hydrolysate Elicit Dose-Dependent Increases of Growth, Leaf Mineral Composition, Yield and Fruit Quality in Two Greenhouse Tomato Cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Khan, A.S.; Ahmad, B.; Jaskani, M.J.; Ahmad, R.; Malik, A.U. Foliar Application of Mixture of Amino Acids and Seaweed (Ascophylum nodosum) Extract Improve Growth and Physicochemical Properties of Grapes. Int. J. Agric. Biol. 2012, 14, 383–388. [Google Scholar]
- Abou Chehade, L.; Al Chami, Z.; De Pascali, S.A.; Cavoski, I.; Fanizzi, F.P. Biostimulants from Food Processing by-Products: Agronomic, Quality and Metabolic Impacts on Organic Tomato (Solanum lycopersicum L.). J. Sci. Food Agric. 2018, 98, 1426–1436. [Google Scholar] [CrossRef]
- Ceccarini, C.; Antognoni, F.; Biondi, S.; Fraternale, A.; Verardo, G.; Gorassini, A.; Scoccianti, V. Polyphenol-Enriched Spelt Husk Extracts Improve Growth and Stress-Related Biochemical Parameters under Moderate Salt Stress in Maize Plants. Plant Physiol. Biochem. 2019, 141, 95–104. [Google Scholar] [CrossRef]
- Maqbool, N.; Sadiq, R. Allelochemicals as Growth Stimulators for Drought Stressed Maize. Am. J. Plant Sci. 2017, 8, 985–997. [Google Scholar] [CrossRef]
- Savy, D.; Canellas, L.; Vinci, G.; Cozzolino, V.; Piccolo, A. Humic-Like Water-Soluble Lignins from Giant Reed (Arundo donax L.) Display Hormone-Like Activity on Plant Growth. J. Plant Growth Regul. 2017, 36, 995–1001. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tugnoli, V.; Righi, V.; Nardi, S. Effect of Commercial Lignosulfonate-Humate on Zea mays L. Metabolism. J. Agric. Food Chem. 2011, 59, 11940–11948. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. A Potential Valorization Strategy of Wine Industry by-Products and Their Application in Cosmetics-Case Study: Grape Pomace and Grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef]
- Pardo-García, A.I.; Martínez-Gil, A.M.; Cadahía, E.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Oak Extract Application to Grapevines as a Plant Biostimulant to Increase Wine Polyphenols. Food Res. Int. 2014, 55, 150–160. [Google Scholar] [CrossRef]
- Casadesus, A.; Polo, J.; Munne-Bosch, S. Hormonal Effects of an Enzymatically Hydrolyzed Animal Protein-Based Biostimulant (Pepton) in Water-Stressed Tomato Plants. Front. Plant Sci. 2019, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Gebreluel, T.; He, M.; Zheng, S.; Zou, S.; Woldemicael, A.; Wang, C. Optimization of Enzymatic Degradation of Dealginated Kelp Waste through Response Surface Methodology. J. Appl. Phycol. 2019, 32, 529–537. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Colla, G.; Svecova, E.B.; Ruzzi, M. Foliar Application of Vegetal-Derived Bioactive Compounds Stimulates the Growth of Beneficial Bacteria and Enhances Microbiome Biodiversity in Lettuce. Front. Plant Sci. 2019, 10, 60. [Google Scholar] [CrossRef] [PubMed]
| Entry | Agri-Food Waste/ Agri-Food Waste Derived Material | Microorganism | Antibacterial Activity Testing | Ref. |
|---|---|---|---|---|
| 1 | Composite PLA film with lignin and cellulose nanostructures | Pseudomonas syringae pv. tomato | In vitro (Liquid medium test) | [33] |
| 2 | PLA film with chitosan and lignin nanoparticles | Erwinia carotovora subsp. carotovora Xanthomonas arboricola pv. pruni | In vitro (Liquid medium test) | [34] |
| 3 | PVA/lignin quaternary ammonium salts nanofibers | Listeria monocytogenes | In vitro (Turbidity and disc diffusion methods) | [35] |
| 4 | Lignin (from bagasse pulp black liquor) nanoparticles casted on a cellulose nanofibril | Listeria monocytogenes | In vitro (Inhibition zone method and shaking flask method) | [36] |
| 5 | Lignin@Cu nanoparticles | Listeria monocytogenes Xanthomonas campestris Pseudomonas syringae pv. actinidiae | In vitro (Agar dilution method) | [37] |
| 6 | Lignin@Cu nanoparticles | Erwinia amylovora Pseudomonas syringae Xantomonas campestris Xantomonas arboricola | In vitro (Agar dilution method) | [38] |
| 7 | Salvia sclarea L., Salvia rosmarinus Schleid, Salvia officinalis L., Helichrysum italicum and leaves of Cupressus sempervirens L. | Clavibacter michiganensis subsp. nebraskense ATCC 27822 | In vitro (Microdilution method using 96-well microtiter plates) | [39] |
| 8 | HTyr-enriched olive mill wastewater | Pseudomonas savastanoi pv. savastanoi Agrobacterium tumefaciens | In vitro (Halo inhibition assay) | [40] |
| 9 | Protein hydrolysates (<3 kDa) derived from rice (Oryza sativa) straw, bagasse (Saccharum sp.), peanut (Arachis hypogaea) seed coat, and coconut (Cocos nucifera L.) residue | Xanthomonas oryzae pv. Oryzae Xanthomonas citri Pectobacterium carotovorum Agrobacterium rhizogenes | In vitro (Broth dilution method—microplate reader) | [41] |
| Entry | Agri-Food Waste/ Agri-Food Waste Derived Material | Microorganism | Antifungal Activity Testing | Ref. |
|---|---|---|---|---|
| 1 | Extracts of red and white grape (Vitis vinifera L.) marc, grape seeds and stalks, red grapevine leaves, apple (Malus sp.), pear (Pyrus communis), tomato (Solanum lycopersicum), spent hops (Humulus lupulus) and green beans (Phaseolus vulgaris) | Aspergillus flavus Aspergillus carbonarius Fusarium graminearum Fusarium verticillioides Alternaria alternata | In vitro (Fungal growth and mycotoxin production in Petri dishes) | [42] |
| 2 | Red, pink, and white wine grape marcs hydrolysates | Fusarium oxysporum Alternaria spp. | In vitro (Growth inhibition on Petri dishes) | [43] |
| 3 | Crude extracts of peels from banana (Musa spp.), garlic (Allium sativum), brown onion (Allium cepa L.), orange (Citrus × sinensis L.), lemon (Citrus × limon L.), white potatoes (Solanum tuberosum) and pomegranate (Punica granatum), barks from Eucalyptus sp. and pine (Pinus sp.), olive (Olea europaea) leaves and pine (Pinus sp.) needles | Diplodia corticola Botrytis cinerea Colletotrichum nymphaeae Phytophthora cinnamomi | In vitro (Growth inhibition on Petri dishes) | [44] |
| 4 | Garlic (Allium sativum) peels crude extract | Colletotrichum acutatum | In vivo (Apple from “Golden”cultivar protection evaluation) | |
| 5 | Potato protease inhibitors I and II from starch manufacture effluent | Fusarium solani CCM 8079 Fusarium solani CCM 8014 Fusarium solani CCM 1036 Fusarium oxysporum CCM 17 Fusarium oxysporum CCM F65 | In vitro (Incorporation of hydrolysate in agar-media) | [45] |
| 6 | Extracts from Crocus sativus L. flower waste | Penicillium expansum Penicillium digitatum Botrytis cinerea Fusarium solani | In vitro (Disc-plate diffusion method) | [46] |
| 7 | Nanoemulsions derived from essential oil extracted from Citrus sinensis peel and Citrus sinensis essential oil alone | Fusarium spp. Aspergillus niger Penicillium spp. Aspergillus ochraceus | In vitro (Disc plate diffusion method) | [47] |
| 8 | Lignin@Cu nanoparticles | Botrytis cinerea Rhizoctonia solani | In vitro (Disc plate diffusion method) | [37] |
| Rhizoctonia solani | In vivo (In field on “Kero” variety tomato crop) | |||
| 9 | Lignin@Cu nanoparticles | Erwinia amylovora Monilinia laxa Alternaria solani Fusarium solani Botrytis cinerea Septoria tritici Rhizoctonia solani | In vitro (Agar dilution method) | [38] |
| Rhizoctonia solani | In vivo (Greenhouse italian tomato “cuore di ponente”) | |||
| 10 | Post extraction lavender (Lavandula angustifolia) and lavandin (Lavandula × intermedia) as soil amendment | Verticillium dahliae | In vivo (Field application Strawberry cv. Elsanta) | [48] |
| 11 | Agricultural Jiaosu derived from officinal plants | Fusarium oxysporum | In vitro (Agar plate diffusion method) | [13] |
| In vivo (Greenhouse Pot Experiment on Astragalus membranaceus) | ||||
| 12 | Agricultural Jiaosu derived from brown sugar and jujube (Ziziphus jujuba Mill.) wastes | Botrytis cinerea | In vitro (Colony inhibition on agar plates) | [49] |
| Method of Valorization | Source of Biomass | Type of Fertilizer | Crop Application | Principal Nutrients Found in the BBF | Ref. |
|---|---|---|---|---|---|
| Pyrolysis | Rice husks, peanut shells and sugarcane | Liquid fertilizer | * Chinese Cabbage seeds (Brassica pekinensis) | Silicon carbide | [85] |
| Sugarcane exocarp, peanut shells and rice husks | Hybrid mineral-hydrothermal fertilizer | * Rice seeds | Macro and micronutrients (carbon; oxygen; potassium; aluminum; magnesium; calcium; sodium; nickel; silicon) | [86] | |
| Biosolids (urban wastewater treatment, cattle manure coffee grounds) | Biochar | Lettuce (Lactuca sativa L.) | N.I. | [82] | |
| Sunflower seed shells, peanut shells and Spirulina algae | Biochar | Lettuce (Lactuca sativa L.) | Carbon; nitrogen; hydrogen; oxygen; fixed carbon | [87] | |
| Composting | Olive mill waste | Compost | Lettuce (Lactuca sativa L.) and tomato (Lycopersicon esculentum) | Total nitrogen; total phosphorus, potassium; total organic carbon | [88] |
| Food waste (onion, potato, cabbage) with cattle manure | Compost | * Maize | Organic carbon; available phosphorus and potassium; total nitrogen | [76] | |
| Fruit and vegetable waste | Compost (leachate part) | Cress (Lepidium sativum) and sweet corn (Zea mays cv. Luscious) | NH4; total nitrogen; total inorganic and organic nitrogen; phosphorus; phosphate; sulphate; potassium; calcium; magnesium; sodium; copper; zinc | [89] | |
| Banana peel (used as the fermentation liquid) and whilst soil and coconut husk (used as the composting medium) | Compost | N.I. (applied in soil to evaluate the nitrogen, phosphorus and potassium concentration) | Nitrogen; phosphorus; potassium | [90] | |
| Banana fruit waste with cow dung and cow urine | Compost (liquid fertilizer) | Mung bean (Vigna radiata L.) seeds | Total nitrogen; potassium, calcium; phosphorus; magnesium; iron; copper; zinc; manganese | [91] | |
| Grinding and Mincing process | Food waste | Liquid fertilizer | Not applied | Total nitrogen; nitrate; total phosphorus; calcium; magnesium; sodium; potassium | [92] |
| Grinding and mincing process | Food waste | Liquid fertilizer | Lettuce (Lactuca sativa L.) and cucumber (Cucumis sativus L.) | Total nitrogen; nitrate; total phosphorus; calcium, magnesium; sodium; potassium | [93] |
| Pyrolysis + composting | Pig manure and rice straw | Biochar (rice straw) and compost (pig manure) | * Watermelon | Organic carbon; nitrogen total; phosphorus available; potassium available | [94] |
| No treatments for conversion | Food waste | Liquid fertilizer | Chinese cabbage (Brassica pekinensis) | N.I. | [95] |
| Agri-Food Waste | Application Crop | Group | Application Method | Plant Response | Ref. |
|---|---|---|---|---|---|
| Vegetal and seaweed (commercial biostimulants) | Lettuce (Lactuca sativa L.) | Protein hydrolysates | Hydroponic | Increased yield of leafy vegetables and improved physiology and biochemical composition. | [126] |
| Seaweeds | Soybean (Glycine max (L.) Merrill.) | Phytohormones (auxins, cytokinins) Amino acids, vitamin B1, B2, C and E. Minerals (N, P, K, Mg, Fe, Mn, B, Zn, and Cu) | Foliar | Alteration of the nutraceutical and antioxidative potential and improved the growth and yield | [127] |
| Ten biostimulants from different biological sources (alfalfa and seaweeds) | Strawberry (Fragaria × ananassa Duch.) cv. Elsant | Humic acids, alfalfa hydrolysate, macro seaweed extract and microalga hydrolysate, amino acids alone or in combination with zinc, B-group vitamins, chitosan, and a commercial product containing silicon (10 different biostimulants) | Foliar | Greater pulp consistency, yield and improved fruit quality | [128] |
| Vegetal based | Radish (Raphanus sativus L.) | Vitamin B12, and CoQ10 | Soil | Increased root and shoot biomass | [125] |
| Rape seed, apple seeds, and rice husks | Kiwi fruit (Actinidia deliciosa, c.v Hayward and Green Light) | Auxins, cytokinins, gibberellins, amino acids, protein, and minerals. | Foliar | Increased the fruit weight Increase of the vitamin C content in the fruits | [129] |
| Mycorrhizal Fungi, Tea Wastes, and Algal Biomass | Corn (Zea mays L.) | Polyphenols acids, protein, nutrients, carbohydrates, amino acids and organic carbon | Soil | Improved soil microbial activity; increased resistance to saline environments; highly efficient in improving soil mean weight diameter; increased soil-organic carbon, microbiota and increased grain productivity. | [130] |
| Plants | Corn (Zea mays L.) | Nitrogen, protein hydrolysate, amino acids (alanine, arginine, aspartic acid, cysteine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine and valine) and soluble peptides | Stem was immersed for few minutes into the biostimulant solution | Increase the shoot length, total biomass, root and nitrogen content. | [131] |
| Red grape, blueberry fruits and hawthorn leaves | Corn (Zea mays L.) | Indoleacetic acid and isopentenyladenosine auxin and gibberellin Nitrogen | Soil | Increased protein and fructose content in the roots; Increased protein and glucose in leaves; increased the maize plant dry weight was found in both roots and leaves; the treatments with the extracts in separated or together increased the phenolic acids in the plants (p-coumaric, gallic acids, vanillic, caffeic). | [132] |
| Legumes | Tomato (Solanum lycopersicum L.) | Protein hydrolysate | Foliar | Improvement in yield (fruit weight); foliar nutrition (K and Mg); Greater assimilation of CO2; increase in antioxidant activity; total soluble solids and increase in lycopene and ascorbic acid. | [133] |
| Seaweed | Grapes (Vitis vinifera L.) cv. ‘Perlette) | Amino acids | Foliar | Higher leaf size, chlorophyll content, berry setting, number of bunches per cane, rachis length, berry weight, berry size, soluble solid concentrations, total sugars and reducing sugars with reduced berry drop and ascorbic acid. | [134] |
| Fennel processing residues, lemon processing residues and brewer’s spent grain | Tomato (Solanum lycopersicum L.) | Organic acids; sugars and flavonoids; organic acids (citric, gallic, malic, fumaric and tartaric acids) and their conjugates (lactates); free amino acids (proline, glutamine and asparagine). | Irrigation (soil) | Increased the shoot growth and dry matter; increased fresh fruit yield; increased the vitamin C concentration on the fruit. | [135] |
| Vine-shoot wastes | Lettuce (Lactuca sativa L.) | Phenolic compounds (phenolic acids, stilbenes, flavanols, (+)-catechin and (−)-epicatechin pyrogallol and hydroxybenzoic acids (ellagic and gallic)). | N.I. | The tested extracts did not affect the germination of lettuce seeds, but the extracts stimulated root elongation. | [115] |
| Spelt (Triticum dicoccum L.) husks | Maize (Zea mays L.) | Polyphenol (p-hydroxybenzoic, syringic acids, ferulic, p-coumaric, and caffeic). | Soil | Recovery of shoot growth to control levels and reduction in stress-induced proline accumulation; mitigating salt and oxidative stress. | [136] |
| Sorghum leaves | Maize (Zea mays L.) | Phenolic compounds | Foliar | Improved germination and plant growth and when the extract was applied (0.75 mL/L) in the tenuous absence of water increased stem diameter as well as leaf area. | [137] |
| Giant Reed | Tomato (Solanum lycopersicum L. cv. MT), watercress (Lepidium sativum L.) and chicory seeds (Cichorium intybus L.) | Humic-like lignins | Seeds hydration | Positively seed development by either directly acting as gibberellin (GA) molecules or by positively perturbing GA-related hormonal balances and, thus, influencing GA-mediated physiological mechanisms. | [138] |
| Vegetal | Tomato (Solanum lycopersicum L.), basil (Ocimum basilicum L.), and Chrysanthemum (Chrysanthemum indicum L.) | Auxin | Stem was immersed into the biostimulant solution | Enhances Adventitious Rooting | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voss, M.; Valle, C.; Calcio Gaudino, E.; Tabasso, S.; Forte, C.; Cravotto, G. Unlocking the Potential of Agrifood Waste for Sustainable Innovation in Agriculture. Recycling 2024, 9, 25. https://doi.org/10.3390/recycling9020025
Voss M, Valle C, Calcio Gaudino E, Tabasso S, Forte C, Cravotto G. Unlocking the Potential of Agrifood Waste for Sustainable Innovation in Agriculture. Recycling. 2024; 9(2):25. https://doi.org/10.3390/recycling9020025
Chicago/Turabian StyleVoss, Monica, Carlotta Valle, Emanuela Calcio Gaudino, Silvia Tabasso, Claudio Forte, and Giancarlo Cravotto. 2024. "Unlocking the Potential of Agrifood Waste for Sustainable Innovation in Agriculture" Recycling 9, no. 2: 25. https://doi.org/10.3390/recycling9020025
APA StyleVoss, M., Valle, C., Calcio Gaudino, E., Tabasso, S., Forte, C., & Cravotto, G. (2024). Unlocking the Potential of Agrifood Waste for Sustainable Innovation in Agriculture. Recycling, 9(2), 25. https://doi.org/10.3390/recycling9020025