Recycling of Waste Oyster Shells for Fluoride Removal from Hydrofluoric Acid Wastewater
Abstract
1. Introduction
2. Results
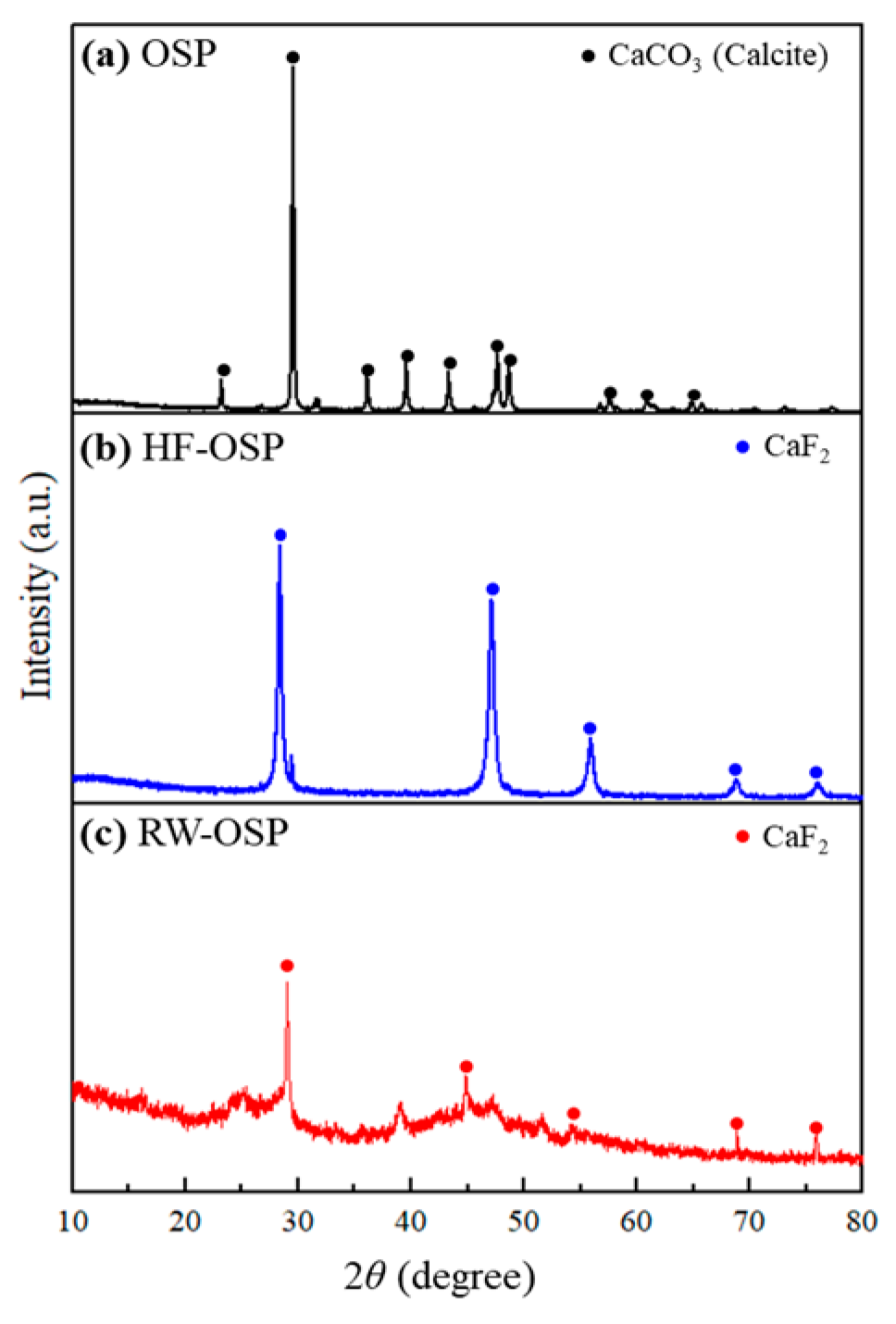
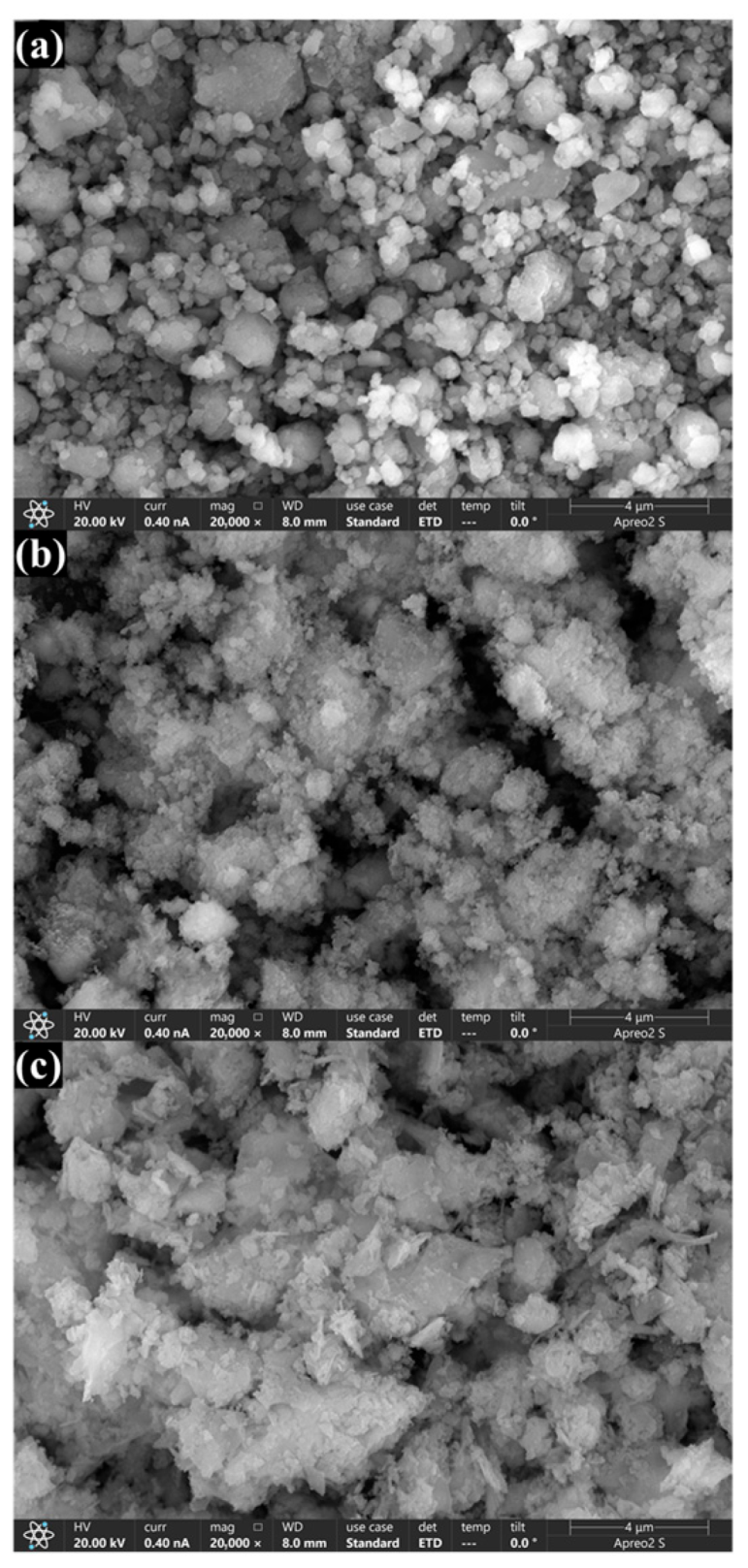
2.1. Characterization of OSP, HF-OSP, and RW-OSP
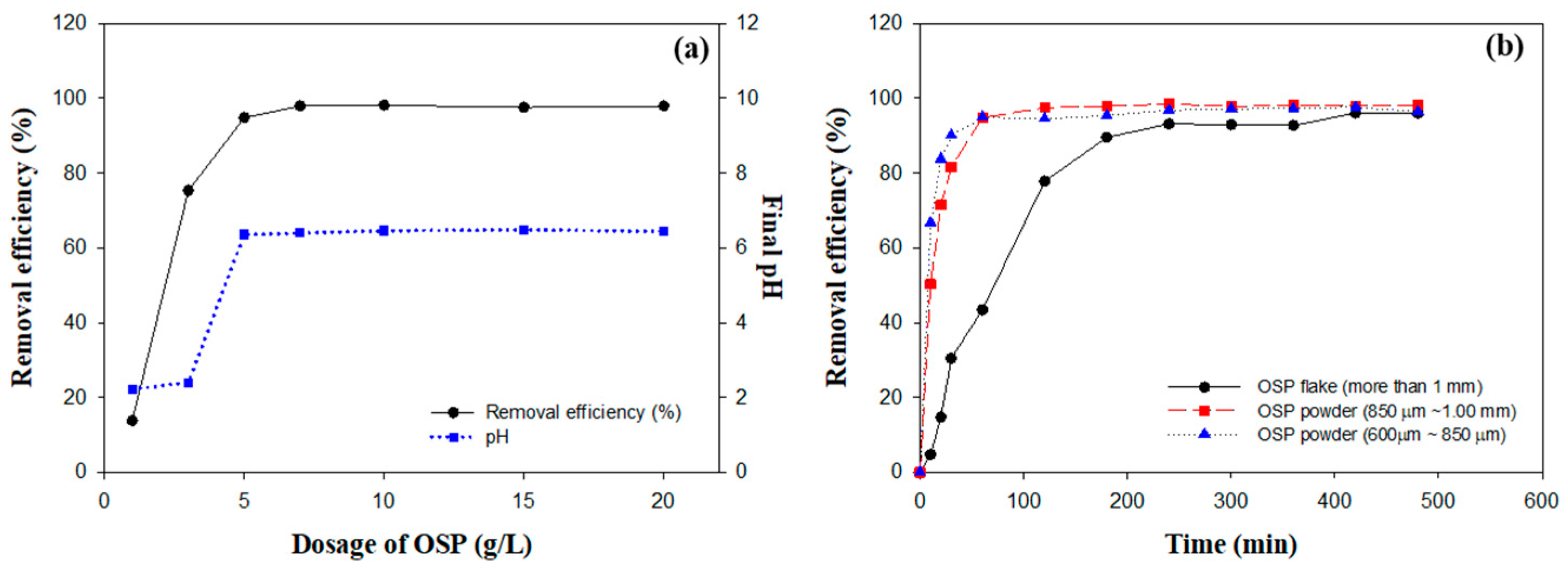
2.2. Effect of OSP Dosage on Fluoride Removal
2.3. Influence of OSP Particle Size on Fluoride Removal Efficiency
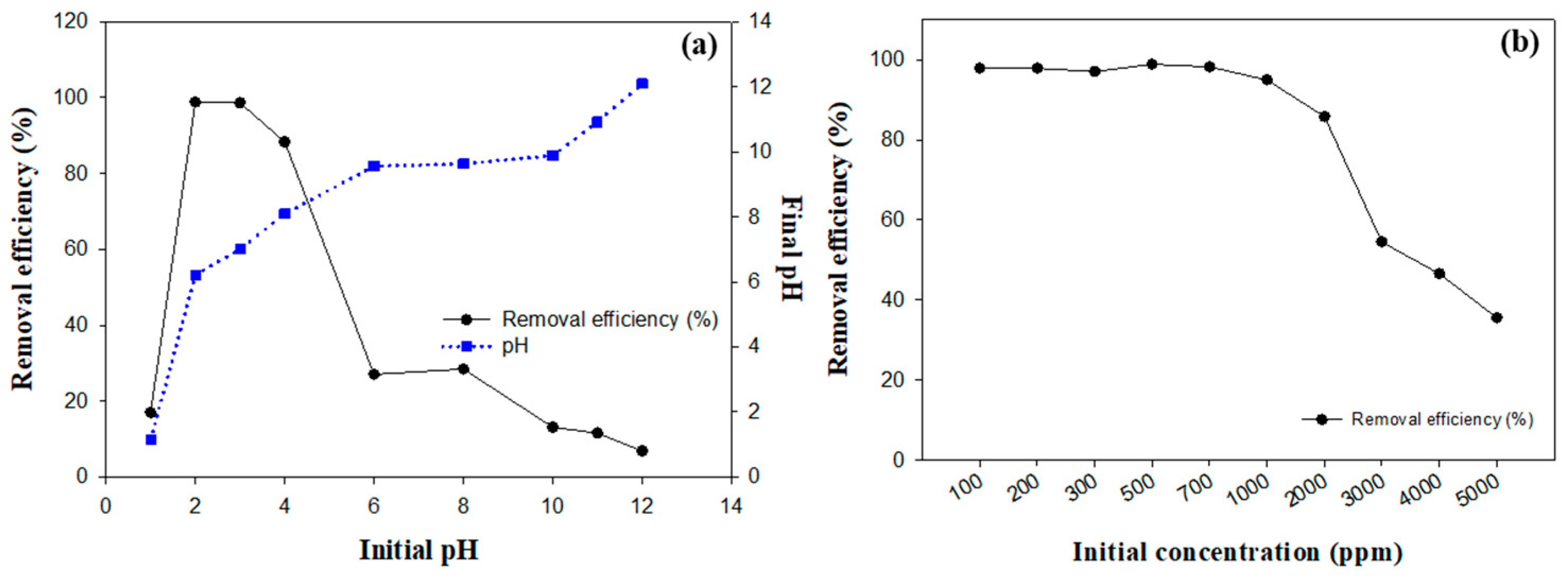
2.4. Impact of Initial pH on Fluoride Removal Efficiency
2.5. Influence of Initial Fluoride Concentration on Removal Efficiency
2.6. Verification of the Effectiveness of OSP in Actual Semiconductor Wastewater
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Pre-Treatment of Oyster Shells
4.3. Characterization
4.4. Batch Experiments
4.5. Analytical Methods and Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamakura, N. From globalising to regionalising to reshoring value chains? The case of Japan’s semiconductor industry. Camb. J. Reg. Econ. Soc. 2022, 15, 261–277. [Google Scholar] [CrossRef]
- Liu, K.T.; Chen, C.H. Formulation of research and development strategy by analysing patent portfolios of key players the semiconductor industry according to patent strength and technical function. World Pat. Inf. 2022, 70, 102125. [Google Scholar] [CrossRef]
- TSMC. TSMC 2021 Business Overview. 2021. Available online: https://investor.tsmc.com/sites/ir/annual-report/2021/2021_Business_Overview_E.pdf (accessed on 17 July 2023).
- Intel Corporation. Intel Announces Next US Site with Landmark Investment in Ohio. 2021. Available online: https://www.intel.com/content/www/us/en/newsroom/news/intel-announces-next-us-site-landmark-investment-ohio.html#gs.b12lx4 (accessed on 21 January 2022).
- Park, D.Y. Report of Investment Opportunities-Industries-Semiconductor. Invest Korea. 2023. Available online: https://www.investkorea.org/ik-en/cntnts/i-312/web.do (accessed on 7 September 2023).
- Eng, C.Y.; Yan, D.; Withanage, N.; Liang, Q.; Zhou, Y. Wastewater treatment and recycle from a semiconductor industry: A demo-plant study. Water Pract. Technol. 2019, 14, 371–379. [Google Scholar] [CrossRef]
- Sim, J.; Lee, J.; Rho, H.; Park, K.D.; Choi, Y.; Kim, D.; Kim, H.; Woo, Y.C. A review of semiconductor wastewater treatment processes: Current status, challenges, and future trends. J. Clean. Prod. 2023, 429, 139570. [Google Scholar] [CrossRef]
- Chang, M.F.; Liu, J.C. Precipitation removal of fluoride from semiconductor wastewater. J. Environ. Eng. 2007, 133, 419–425. [Google Scholar] [CrossRef]
- Wald, P.H.; Jones, J.R. Semiconductor manufacturing: An introduction to processes and hazards. Am. J. Ind. Med. 1987, 11, 203–221. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Zhang, P.; Zhang, D.; Gao, F. Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chem. Eng. J. 2017, 307, 696–706. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, J.C. Coupled precipitation-ultrafiltration for treatment of high fluoride-content wastewater. J. Taiwan Inst. Chem. Eng. 2016, 58, 259–263. [Google Scholar] [CrossRef]
- Kang, W.H.; Kim, E.I.; Park, J.Y. Fluoride removal capacity of cement paste. Desalination 2007, 202, 38–44. [Google Scholar] [CrossRef]
- Lacson, C.F.Z.; Lu, M.C.; Huang, Y.H. Chemical precipitation at extreme fluoride concentration and potential recovery of CaF2 particles by fluidized-bed homogenous crystallization process. Chem. Eng. J. 2021, 415, 128917. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, J.H.; Yoo, J.H.; Park, J.H.; Bae, J.S.; Park, C.Y. Toward transformation of bivalve shell wastes into high value-added and sustainable products in South Korea: A review. J. Ind. Eng. Chem. 2024, 129, 38–52. [Google Scholar] [CrossRef]
- Korea Maritime Institute (KMI). A Study on Resources Circulation for Marine Debris of Aquaculture Farm; Office for Government Policy Coordination of Korea: Sejong-si, Republic of Korea, 2018; ISBN 979-1-18-922679-4. [Google Scholar]
- Silva, H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The potential use of oyster shell waste in new value-added by-product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- Kang, S.B.; Wang, Z.; Won, S.W. Polyethylenimine-crosslinked calcium silicate hydrate derived from oyster shell waste for removal of Reactive Yellow 2. Korean J. Chem. Eng. 2023, 40, 136–144. [Google Scholar] [CrossRef]
- Seesanong, S.; Seangarun, C.; Boonchom, B.; Laohavisuti, N.; Thompho, S.; Boonmee, W.; Mongkol, S.; Rungrojchaipon, P. Bio-green synthesis of calcium acetate from oyster shell waste at low cost and reducing the emission of greenhouse gases. Sustain. Environ. Res. 2023, 33, 26. [Google Scholar] [CrossRef]
- Asaoka, S.; Yamamoto, T.; Kondo, S.; Hayakawa, S. Removal of hydrogen sulfide using crushed oyster shell from pore water to remediate organically enriched coastal marine sediments. Bioresour. Technol. 2009, 100, 4127–4132. [Google Scholar] [CrossRef]
- Hsu, T.C. Experimental assessment of adsorption of Cu2+ and Ni2+ from aqueous solution by oyster shell powder. J. Hazard. Mater. 2009, 171, 995–1000. [Google Scholar] [CrossRef]
- Lee, Y.H.; Islam, S.M.A.; Hong, S.J.; Cho, K.M.; Math, R.K.; Heo, J.Y.; Kim, H.; Yun, H.D. Composted oyster shell as lime fertilizer is more effective than fresh oyster shell. Biosci. Biotechnol. Biochem. 2010, 74, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, C.; Sakoda, A.; Suzuki, M. Removal of phosphate by adsorption onto oyster shell powder—Kinetic studies. J. Chem. Technol. Biotechnol. 2005, 80, 356–358. [Google Scholar] [CrossRef]
- Park, W.H.; Polprasert, C. Roles of oyster shells in an integrated constructed wetland system designed for P removal. Ecol. Eng. 2008, 34, 50–56. [Google Scholar] [CrossRef]
- Baek, E.Y. Oyster shell recycling and marine ecosystems: A comparative analysis in the Republic of Korea and Japan. J. Coast. Res. 2021, 114, 350–354. [Google Scholar] [CrossRef]
- Howie, A.H.; Bishop, M.J. Contemporary oyster reef restoration: Responding to a changing world. Front. Ecol. Evol. 2021, 9, 689915. [Google Scholar] [CrossRef]
- Perricone, V.; Mutalipassi, M.; Mele, A.; Buono, M.; Vicinanza, D.; Contestabile, P. Nature-based and bioinspired solutions for coastal protection: An overview among key ecosystems and a promising pathway for new functional and sustainable designs. ICES J. Mar. Sci. 2023, 80, 1218–1239. [Google Scholar] [CrossRef]
- Kang, S.B.; Wang, Z.; Won, S.W. Recovery of palladium from acidic solution using polyethylenimine-crosslinked calcium silicate hydrate derived from oyster shell waste: Adsorption and mechanisms. Adsorpt. Sci. Technol. 2023, 2023, 6473526. [Google Scholar] [CrossRef]
- Gerward, L.; Olsen, J.S.; Steenstrup, S.; Malinowski, M.; Åsbrink, S.; Waskowska, A. X-ray diffraction investigations of CaF2 at high pressure. J. Appl. Crystallogr. 1992, 25, 578–581. [Google Scholar] [CrossRef]
- Hong, B.C.; Kawano, K. Luminescence studies of the rare earth ions-doped CaF2 and MgF2 films for wavelength conversion. J. Alloys Compd. 2006, 408–412, 838–841. [Google Scholar] [CrossRef]
- Pandurangappa, C.; Lakshminarasappa, B.N.; Nagabhushana, B.M. Synthesis and characterization of CaF2 nanocrystals. J. Alloys Compd. 2010, 489, 592–595. [Google Scholar] [CrossRef]
- Chingo Aimacaña, C.M.; Pila, K.O.; Quinchiguango Perez, D.A.; Debut, A.; Attia, M.F.; Santos-Oliveira, R.; Whitehead, D.C.; Reinoso, C.; Alexis, F.; Dahoumane, S.A. Bimodal ultrasound and X-ray bioimaging properties of particulate calcium fluoride biomaterial. Molecules 2021, 26, 5447. [Google Scholar] [CrossRef]
- Lee, S.J. A Study on the Treatment of Hydrofluoric acid Wastewater from Semiconductor Manufacturing Process and the Possibility of Reuse. Master’s Thesis, Department of Environmental Engineering Graduate School, University of Seoul, Seoul, Republic of Korea, 2019. [Google Scholar]
- Sinharoy, A.; Lee, G.Y.; Chung, C.M. Optimization of calcium fluoride crystallization process for treatment of high-concentration fluoride-containing semiconductor industry wastewater. Int. J. Mol. Sci. 2024, 25, 3960. [Google Scholar] [CrossRef]
- Sinharoy, A.; Lee, G.Y.; Chung, C.M. Process intensification for enhanced fluoride removal and recovery as calcium fluoride using a fluidized bed reactor. Int. J. Mol. Sci. 2024, 25, 4646. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.; Zhang, N.; Li, Z.; Lin, X.; Zhu, W.; Lu, C.; Ding, W.; Zou, J. Nanostructuring of Mg-based hydrogen storage materials: Recent advances for promoting key applications. NanoMicro Lett. 2023, 15, 93. [Google Scholar] [CrossRef]
- Ran, Q.; Wang, M.; Kuang, W.; Ouyang, J.; Han, D.; Gao, Z.; Gong, J. Advances of combinative nanocrystal preparation technology for improving the insoluble drug solubility and bioavailability. Crystals 2022, 12, 1200. [Google Scholar] [CrossRef]
- Devasthali, O.S.; Shah, A.J.; Jadhav, S.V. Fluoride removal from water using filtration and chemical precipitation. In Advanced Treatment Technologies for Fluoride Removal in Water: Water Purification; Springer Nature: Cham, Switzerland, 2024; pp. 181–196. [Google Scholar] [CrossRef]
- Park, H.S.; Park, Y.S.; Jung, G.I.; Kim, J.W.; Jo, Y.M. Removal of fluoride ions from electronic industrial wastewater using lime stone slurry. Appl. Chem. Eng. 2018, 29, 258–263. [Google Scholar]
- Hu, G.; Sun, Y.; Wu, S.; Li, W.; Hu, C.; Zhuang, J.; Zhang, X.; Lei, B.; Liu, Y. Assembly of shell/core CDs@ CaF2 nanocomposites to endow polymers with multifunctional properties. Nanotechnology 2019, 30, 155601. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, Y.; Zhou, J.; Huang, T.; Sun, X. Impact of acid-base conditions on defluoridation by induced crystallization. J. Ind. Eng. Chem. 2020, 83, 35–45. [Google Scholar] [CrossRef]
- Lin, M.F.; Wu, J.L.; Chang, K.L.; Lee, W.J.; Chang, C.P.; Lin, Y.C.; Chen, P.H. Recycle of synthetic calcium fluoride and waste sulfuric acid to produce electronic grade hydrofluoric acid. Environ. Sci. Pollut. Res. 2021, 28, 40633–40639. [Google Scholar] [CrossRef]
- APHA (American Public Health Association); AWWA (American Water Works Association); WEF (Water Environment Federation). Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA/AWWA/WEF: Washington, DC, USA, 2005. [Google Scholar]

| Component | Real Wastewater | Treated Real Wastewater |
|---|---|---|
| pH | 2.15 | 6.87 |
| F− (mg/L) | 344.11 | 3.02 |
| Cl− (mg/L) | 105.54 | 103.32 |
| SO42− (mg/L) | 54.64 | 52.24 |
| PO43− (mg/L) | N.D. | N.D. |
| NH4+ (mg/L) | 25.65 | 23.31 |
| TN (mg/L) | 57.92 | 78.79 |
| TP (mg/L) | 2.9 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.B.; Ko, G.-I.; Min, B.-C.; Wang, Z.; Kim, S.M.; Won, S.W. Recycling of Waste Oyster Shells for Fluoride Removal from Hydrofluoric Acid Wastewater. Recycling 2024, 9, 86. https://doi.org/10.3390/recycling9050086
Kang SB, Ko G-I, Min B-C, Wang Z, Kim SM, Won SW. Recycling of Waste Oyster Shells for Fluoride Removal from Hydrofluoric Acid Wastewater. Recycling. 2024; 9(5):86. https://doi.org/10.3390/recycling9050086
Chicago/Turabian StyleKang, Su Bin, Gwang-Il Ko, Byeong-Chan Min, Zhuo Wang, Su Min Kim, and Sung Wook Won. 2024. "Recycling of Waste Oyster Shells for Fluoride Removal from Hydrofluoric Acid Wastewater" Recycling 9, no. 5: 86. https://doi.org/10.3390/recycling9050086
APA StyleKang, S. B., Ko, G.-I., Min, B.-C., Wang, Z., Kim, S. M., & Won, S. W. (2024). Recycling of Waste Oyster Shells for Fluoride Removal from Hydrofluoric Acid Wastewater. Recycling, 9(5), 86. https://doi.org/10.3390/recycling9050086





