Comparative Analysis of Micro-Computed Tomography and 3D Micro-Ultrasound for Measurement of the Mouse Aorta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
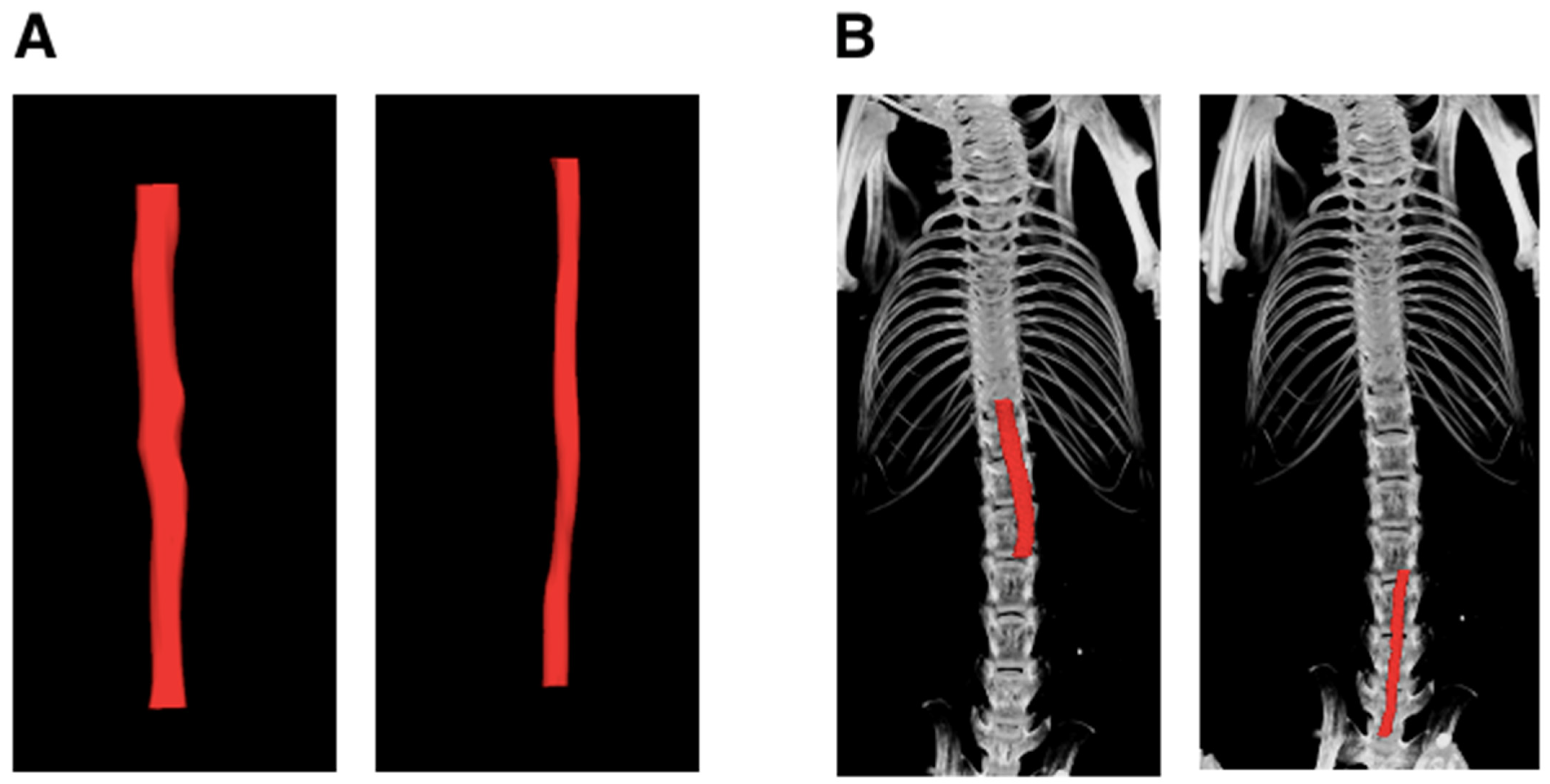
2.2. Micro-Ultrasound Scan (µUSS)
2.3. Micro-Computed Tomographic Angiography (μCTA)
2.4. Statistical Analysis
3. Results
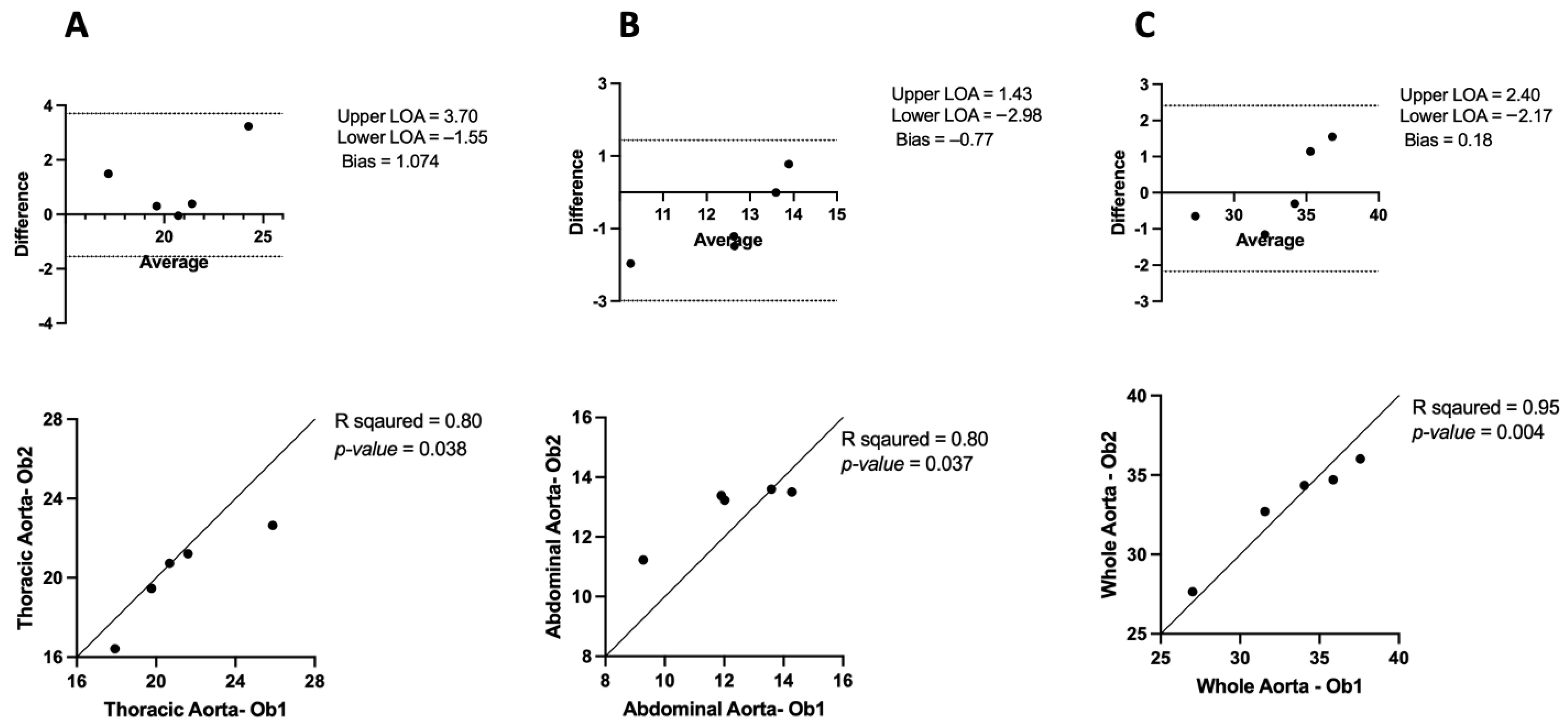
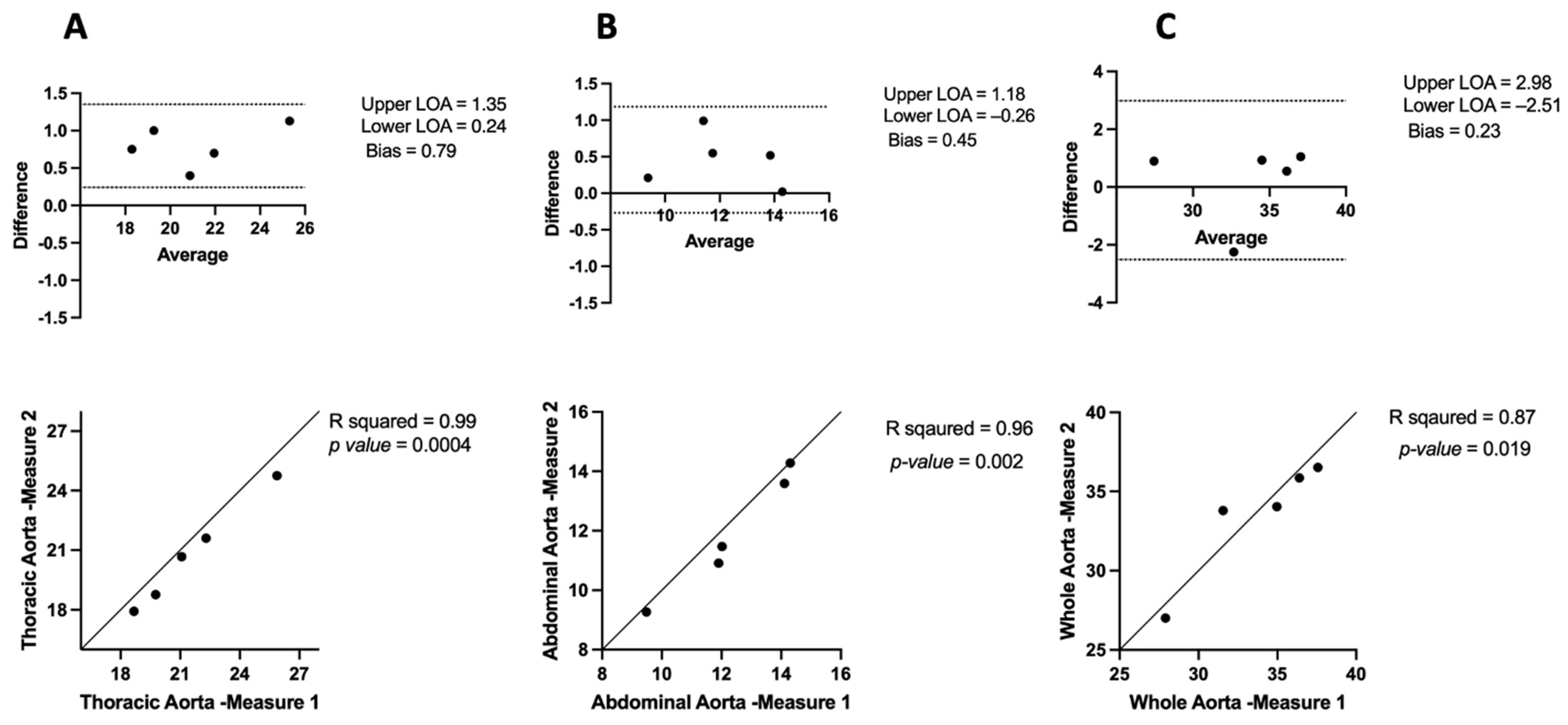
3.1. Inter-Observer and Intra-Observer Variability in μCT
3.1.1. Inter-Observer Variability
3.1.2. Intra-Observer Variability
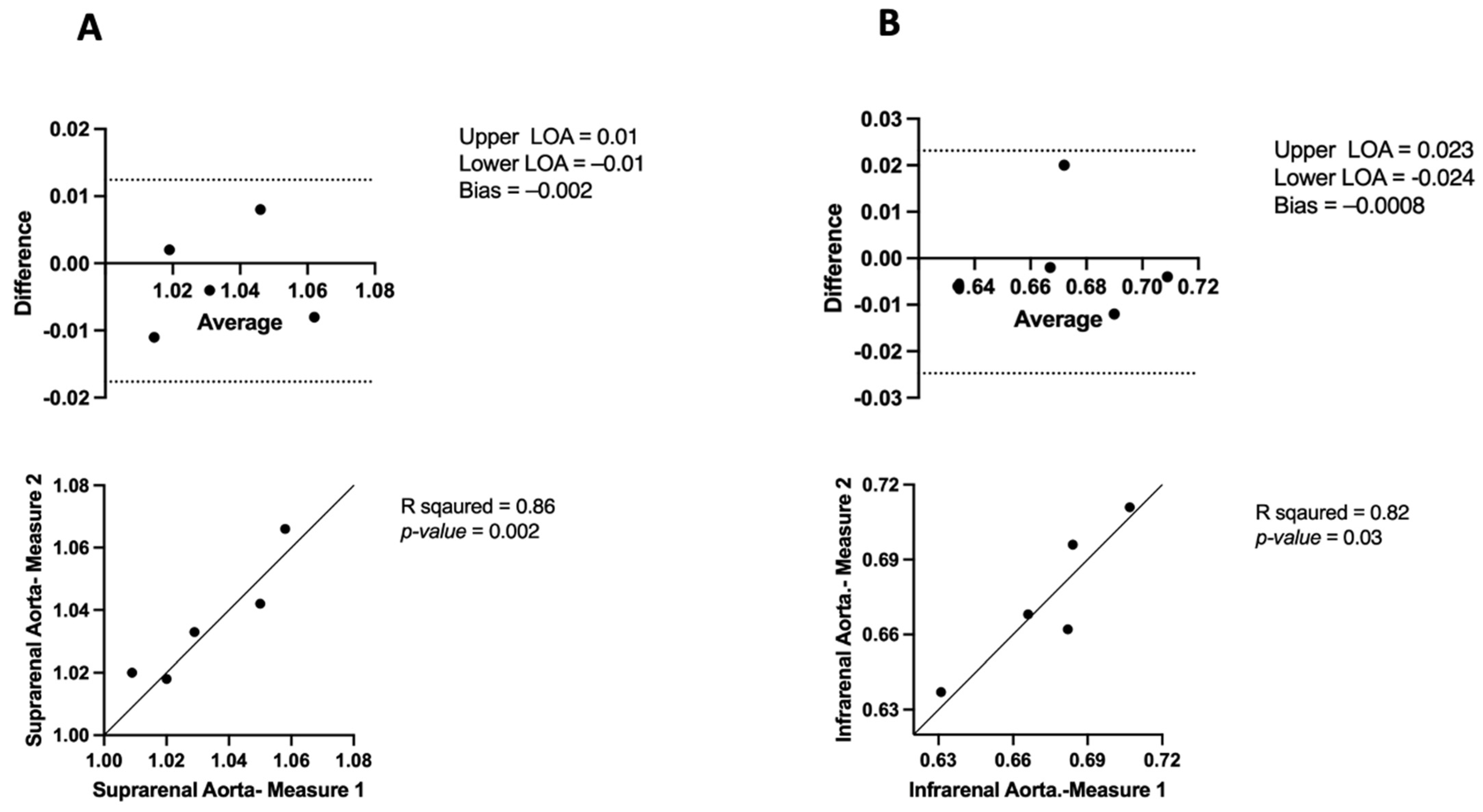
3.2. Comparing μCT and USS Aortic Measures
3.3. APmax Inter-Observer and Intra-Observer Variability in μCT
3.3.1. APmax Intra-Observer Variability in μCT
3.3.2. APmax Inter-Observer Variability in μCT
3.4. APmax in USS Intra-Observer Variability
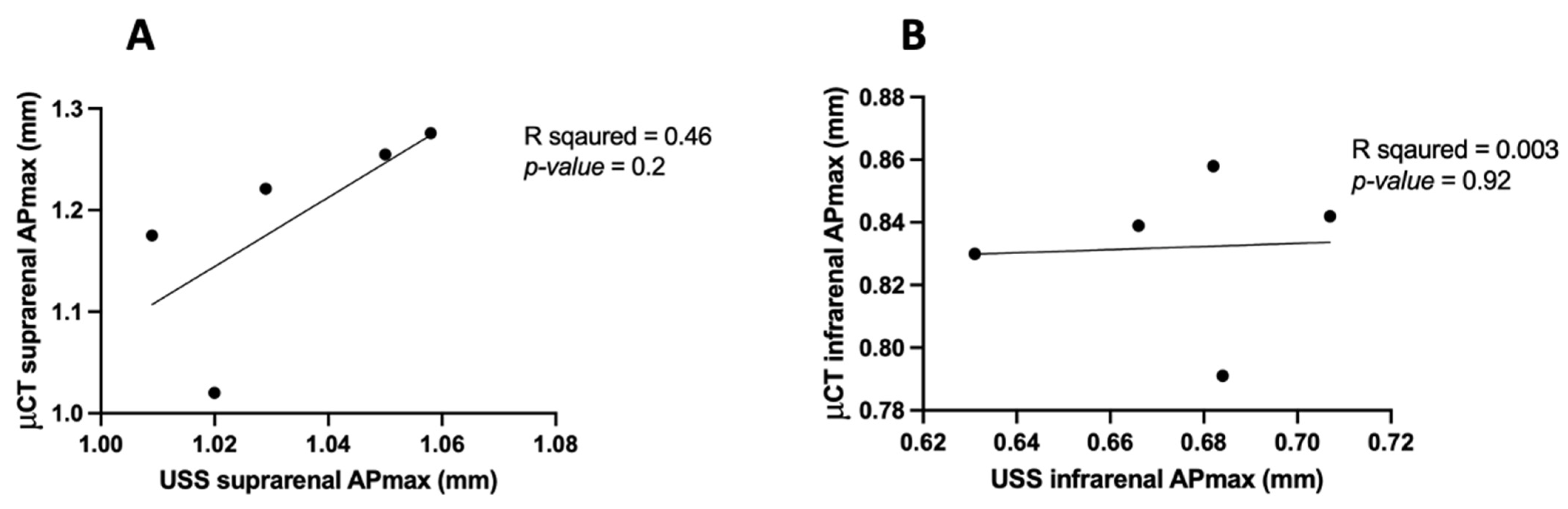
3.5. APmax in μCT vs. USS
4. Discussion
4.1. Inter- and Intra-Observer Variability
4.2. Comparison of μCT and USS
4.3. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allaire, E.; Schneider, F.; Saucy, F.; Dai, J.; Cochennec, F.; Michineau, S.; Zidi, M.; Becquemin, J.P.; Kirsch, M.; Gervais, M. New Insight in Aetiopathogenesis of Aortic Diseases. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Helmy, T.; Porembka, D.T. Aortic pathology: Aortic trauma, debris, dissection, and aneurysm. Crit. Care Med. 2007, 35, S392–S400. [Google Scholar] [CrossRef] [PubMed]
- Clift, P.F.; Cervi, E. A review of thoracic aortic aneurysm disease. Echo Res. Pract. 2020, 7, R1–R10. [Google Scholar] [CrossRef] [PubMed]
- Ramanath, V.S.; Oh, J.K.; Sundt, T.M., 3rd; Eagle, K.A. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin. Proc. 2009, 84, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Mei, X.; Chen, S.-Y. Smooth Muscle Cells in Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e247–e252. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, H.-C. Role of PET/CT in the evaluation of aortic disease. Chonnam Med. J. 2018, 54, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, J.; Lal, B.K. Abdominal aortic aneurysms. Prog. Cardiovasc. Dis. 2021, 65, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Jeanmonod, D.; Yelamanchili, V.S.; Jeanmonod, R. Abdominal Aortic Aneurysm Rupture. In StatPearls [Internet]; StatPearls Publishing: Mountain View, California, USA, 2021. [Google Scholar]
- Sakalihasan, N.; Limet, R.; Defawe, O.D. Abdominal aortic aneurysm. Lancet 2005, 365, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Pearce, W.H.; Shively, V.P. Abdominal aortic aneurysm as a complex multifactorial disease: Interactions of polymorphisms of inflammatory genes, features of autoimmunity, and current status of MMPs. Ann. N. Y. Acad. Sci. 2006, 1085, 117–132. [Google Scholar] [CrossRef]
- Zafar, M.A.; Chen, J.F.; Wu, J.; Li, Y.; Papanikolaou, D.; Abdelbaky, M.; Vinholo, T.F.; Rizzo, J.A.; Ziganshin, B.A.; Mukherjee, S.K. Natural history of descending thoracic and thoracoabdominal aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2021, 161, 498–511.e1. [Google Scholar] [CrossRef]
- Bossone, E.; Eagle, K.A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 2021, 18, 331–348. [Google Scholar] [CrossRef]
- Kessler, V.; Klopf, J.; Eilenberg, W.; Neumayer, C.; Brostjan, C. AAA Revisited: A Comprehensive Review of Risk Factors, Management, and Hallmarks of Pathogenesis. Biomedicines 2022, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.T.; Terrin, M.C.; Dalman, R.L. Medical management of small abdominal aortic aneurysms. Circulation 2008, 117, 1883–1889. [Google Scholar] [CrossRef]
- Chiu, K.; Ling, L.; Tripathi, V.; Ahmed, M.; Shrivastava, V. Ultrasound measurement for abdominal aortic aneurysm screening: A direct comparison of the three leading methods. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Waduud, M.A.; Kandavelu, P.; Reay, M.; Paradine, K.; Scott, D.J.; Bailey, M.A. High-Frequency Three-Dimensional Lumen Volume Ultrasound Is a Sensitive Method to Detect Early Aneurysmal Change in Elastase-Induced Murine Abdominal Aortic Aneurysm. Aorta 2021, 9, 215–220. [Google Scholar] [CrossRef]
- Clark, D.P.; Badea, C. Micro-CT of rodents: State-of-the-art and future perspectives. Phys. Medica 2014, 30, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, D.W.; Thornton, M.M. Micro-CT in small animal and specimen imaging. Trends Biotechnol. 2002, 20, S34–S39. [Google Scholar] [CrossRef]
- Kinnaman, K.A.; Kimberly, H.H.; Pivetta, E.; Platz, E.; Chudgar, A.; Adduci, A.; Stone, M.B.; Rempell, J.S. Evaluation of the aortic arch from the suprasternal notch view using focused cardiac ultrasound. J. Emerg. Med. 2016, 50, 643–650.e641. [Google Scholar] [CrossRef]
- Ocak, I.; Lacomis, J.M.; Deible, C.R.; Pealer, K.; Parag, Y.; Knollmann, F. The aortic root: Comparison of measurements from ECG-gated CT angiography with transthoracic echocardiography. J. Thorac. Imaging 2009, 24, 223–226. [Google Scholar] [CrossRef]
- Singh, K.; Jacobsen, B.K.; Solberg, S.; Kumar, S.; Arnesen, E. The Difference Between Ultrasound and Computed Tomography (CT) Measurements of Aortic Diameter Increases with Aortic Diameter: Analysis of Axial Images of Abdominal Aortic and Common Iliac Artery Diameter in Normal and Aneurysmal Aortas. The Tromsø Study, 1994–1995. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 158–167. [Google Scholar] [CrossRef]
- Sprouse, L.R., 2nd; Meier, G.H., 3rd; Lesar, C.J.; Demasi, R.J.; Sood, J.; Parent, F.N.; Marcinzyck, M.J.; Gayle, R.G. Comparison of abdominal aortic aneurysm diameter measurements obtained with ultrasound and computed tomography: Is there a difference? J. Vasc. Surg. 2003, 38, 466–471; discussion 471–472. [Google Scholar] [CrossRef] [PubMed]
- Pages, S.; Favre, J.-P.; Cerisier, A.; Pyneeandee, S.; Boissier, C.; Veyret, C. Comparison of color duplex ultrasound and computed tomography scan for surveillance after aortic endografting. Ann. Vasc. Surg. 2001, 15, 155–162. [Google Scholar] [CrossRef] [PubMed]
- d’Audiffret, A.; Desgranges, P.; Kobeiter, D.H.; Becquemin, J.-P. Follow-up evaluation of endoluminally treated abdominal aortic aneurysms with duplex ultrasonography: Validation with computed tomography. J. Vasc. Surg. 2001, 33, 42–50. [Google Scholar] [CrossRef] [PubMed]
- England, A.; Niker, A.; Redmond, C. Variability of vascular CT measurement techniques used in the assessment abdominal aortic aneurysms. Radiography 2010, 16, 173–181. [Google Scholar] [CrossRef]
- Trachet, B.; Segers, P.; Claes, F.; Berges, A. Measuring Mouse Abdominal Aorta Dimensions in Vivo: A Comparison between (3D) Ultrasound and Micro-CT. In Proceedings of the 6th World Congress of Biomechanics (WCB 2010), Singapore, 1–6 August 2010; Volume 31, pp. 418–421. [Google Scholar]
- Goldberg, A.; Pakkiri, P.; Dai, E.; Lucas, A.; Fenster, A. Measurements of aneurysm morphology determined by 3-d micro-ultrasound imaging as potential quantitative biomarkers in a mouse aneurysm model. Ultrasound Med. Biol. 2007, 33, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.H.; Reimann, C.; Brangsch, J.; Botnar, R.M.; Makowski, M.R. In vivo MR-angiography for the assessment of aortic aneurysms in an experimental mouse model on a clinical MRI scanner: Comparison with high-frequency ultrasound and histology. PLoS ONE 2017, 12, e0178682. [Google Scholar] [CrossRef]
- Kagadis, G.C.; Loudos, G.; Katsanos, K.; Langer, S.G.; Nikiforidis, G.C. In vivo small animal imaging: Current status and future prospects. Med. Phys. 2010, 37, 6421–6442. [Google Scholar] [CrossRef]


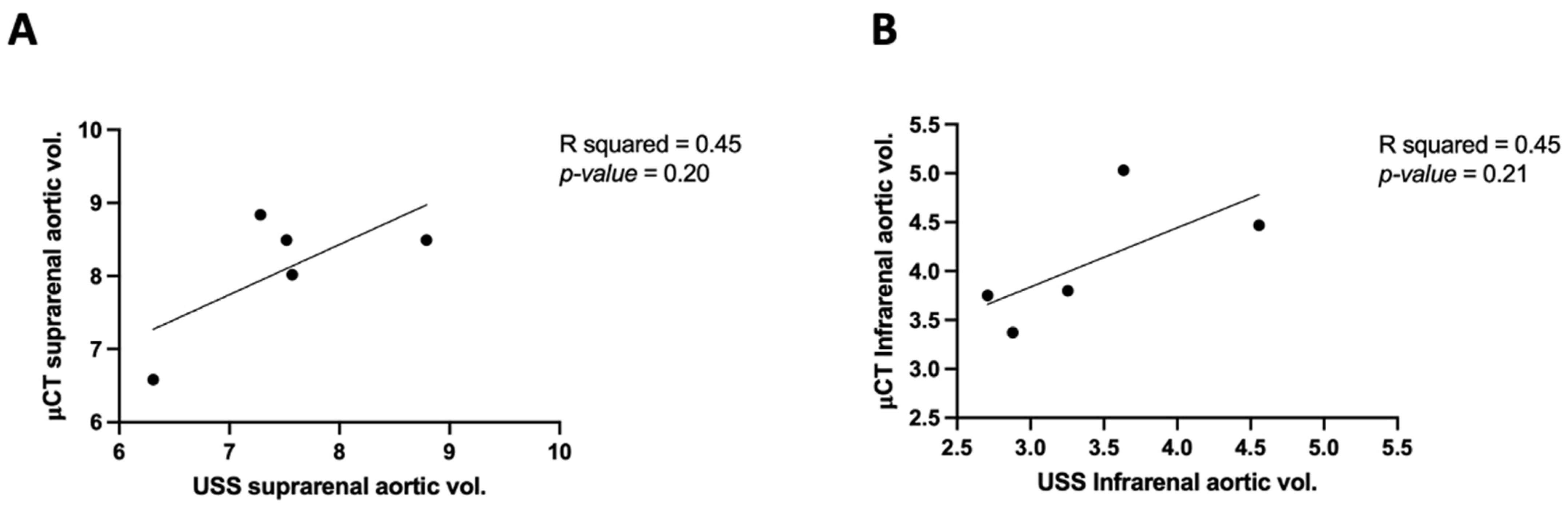


| USS | μCT | |||
|---|---|---|---|---|
| Suprarenal Aorta | Infrarenal Aorta | Suprarenal Aorta | Infrarenal Aorta | |
| Aortic volume | 7.57 | 3.254 | 8.02 | 3.8 |
| 7.52 | 4.558 | 8.49 | 4.47 | |
| 7.28 | 2.707 | 8.84 | 3.75 | |
| 6.308 | 2.879 | 6.58 | 3.37 | |
| 8.79 | 3.634 | 8.49 | 5.03 | |
| Mean ± SEM | 7.5 ± 0.39 | 3.4 ± 0.32 | 8.1 ± 0.39 | 4.1 ± 0.29 |
| Aortic Region | Measurement Modality | Mean APmax (mm) | Standard Deviation (mm) | Correlation (R2) | p-Value |
|---|---|---|---|---|---|
| Suprarenal Aorta | μCT—Inter Observer | 1.18 | 0.10 | 0.84 | 0.02 |
| μCT—Intra Observer | 1.18 | 0.10 | 0.97 | 0.001 | |
| USS—Intra Observer | 1.03 | 0.02 | 0.86 | 0.02 | |
| Infrarenal Aorta | μCT—Inter Observer | 0.83 | 0.02 | 0.90 | 0.01 |
| μCT—Intra Observer | 0.83 | 0.02 | 0.99 | 0.0002 | |
| USS—Intra Observer | 0.67 | 0.02 | 0.82 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alenezi, H.A.; Hemmings, K.E.; Kandavelu, P.; Koch-Paszkowski, J.; Bailey, M.A. Comparative Analysis of Micro-Computed Tomography and 3D Micro-Ultrasound for Measurement of the Mouse Aorta. J. Imaging 2024, 10, 145. https://doi.org/10.3390/jimaging10060145
Alenezi HA, Hemmings KE, Kandavelu P, Koch-Paszkowski J, Bailey MA. Comparative Analysis of Micro-Computed Tomography and 3D Micro-Ultrasound for Measurement of the Mouse Aorta. Journal of Imaging. 2024; 10(6):145. https://doi.org/10.3390/jimaging10060145
Chicago/Turabian StyleAlenezi, Hajar A., Karen E. Hemmings, Parkavi Kandavelu, Joanna Koch-Paszkowski, and Marc A. Bailey. 2024. "Comparative Analysis of Micro-Computed Tomography and 3D Micro-Ultrasound for Measurement of the Mouse Aorta" Journal of Imaging 10, no. 6: 145. https://doi.org/10.3390/jimaging10060145
APA StyleAlenezi, H. A., Hemmings, K. E., Kandavelu, P., Koch-Paszkowski, J., & Bailey, M. A. (2024). Comparative Analysis of Micro-Computed Tomography and 3D Micro-Ultrasound for Measurement of the Mouse Aorta. Journal of Imaging, 10(6), 145. https://doi.org/10.3390/jimaging10060145






