Congenital Absence of Pericardium: The Swinging Heart
Abstract
1. Introduction
2. Embryology
3. Incidence
4. Pathophysiologic Implications
5. Clinical Presentation
6. Electrocardiography
7. Non-Invasive Imaging
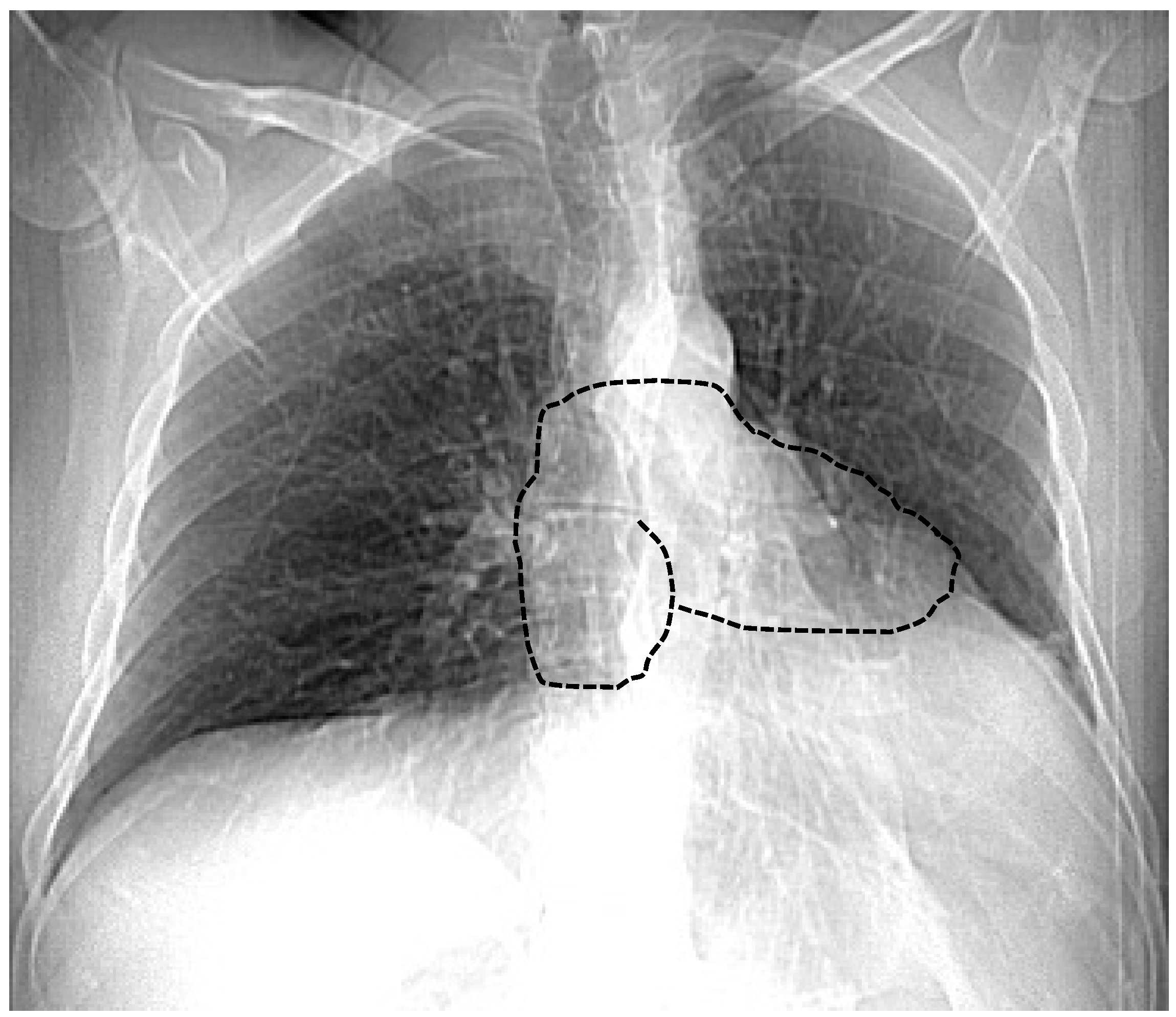
7.1. Chest X-ray
7.2. Echocardiography
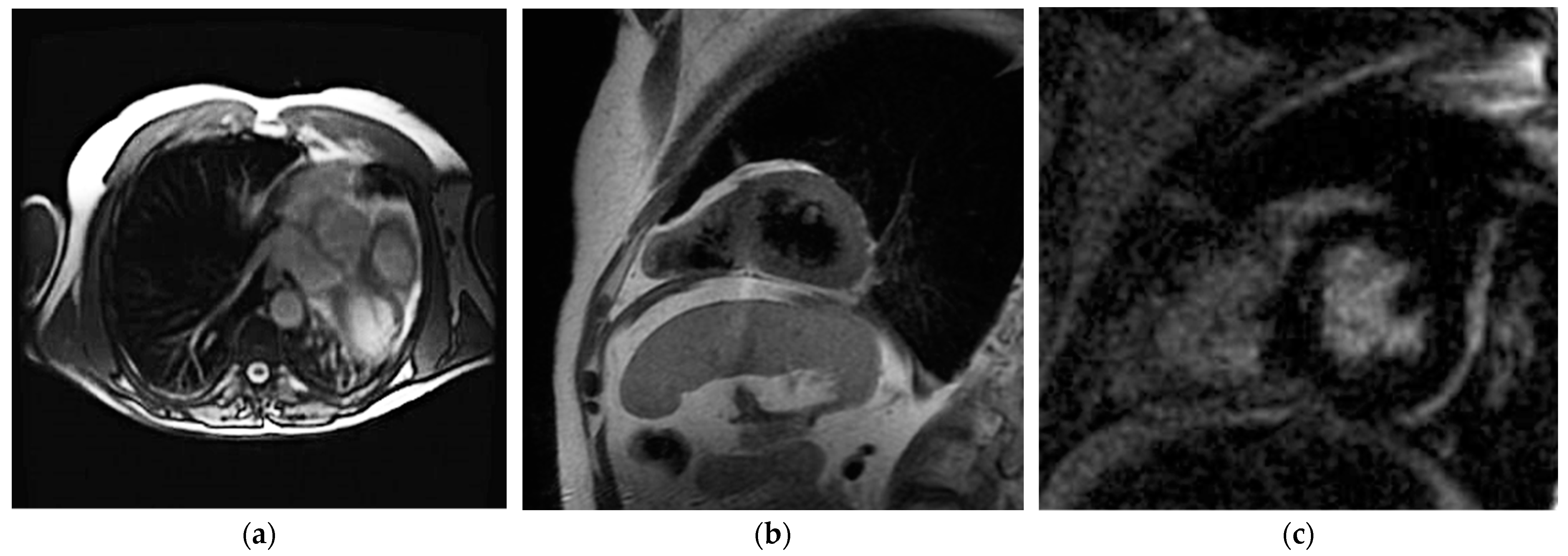
7.3. Cardiac Magnetic Resonance Imaging
7.4. Cardiac Computed Tomography
8. Diagnostic Approach and Management
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantrell, J.R.; Haller, J.A.; Ravitch, M.M. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium and heart. Surg. Gynecol. Obstet. 1958, 107, 602–614. [Google Scholar]
- Shah, A.B.; Kronzon, I. Congenital defects of the pericardium: A review. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 821–827. [Google Scholar] [CrossRef]
- Lopez, D.; Asher, C.R. Congenital Absence of the Pericardium. Prog. Cardiovasc. Dis. 2017, 59, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Schoenwolf, G.; Bleyl, S.; Brauer, P.; Francis-Wes, P. Larsen’s Human Embryology, 6th ed.; Elsevier Health; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Basu, B.N.; Pan, M. Mustafi’s Human Embryology. Calcutta 1975, 6, 109–114. [Google Scholar]
- Van Son, J.A.; Danielson, G.K.; Schaff, H.V.; Mullany, C.J.; Julsrud, P.R.; Breen, J.F. Congenital partial and complete absence of the pericardium. Mayo Clin. Proc. 1993, 68, 743–747. [Google Scholar] [CrossRef]
- Kaul, P. Left pleuropericardial agenesis and coronary artery disease. Br. J. Cardiol. 2012, 19, 124–125. [Google Scholar] [CrossRef]
- Olearchyk, A.S. Diaphragmatic defect with peritoneopericardial communication. Tex. Heart Inst. J. 2003, 30, 328–331. [Google Scholar]
- Southworth, H.; Stevenson, C.S. Congenital Defects of The Pericardium. Arch. Intern. Med. 1938, 61, 223–240. [Google Scholar] [CrossRef]
- Nasser, W.K. Congenital diseases of the pericardium. Cardiovasc. Clin. 1976, 7, 271–286. [Google Scholar]
- Klein, A.L.; Abbara, S.; Agler, D.A.; Appleton, C.P.; Asher, C.R.; Hoit, B.; Hung, J.; Garcia, M.J.; Kronzon, I.; Oh, J.K.; et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: Endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2013, 26, 965–1012. [Google Scholar] [CrossRef]
- Victor, S.; D, D.I. Congenital partial pericardial defect on the right side associated with a bronchogenic cyst. A case report. Indian Heart J. 1981, 33, 34–36. [Google Scholar] [PubMed]
- Corti, P.; Pagnoni, N.; Mangano, G.; Bordiga, G.; Lorini, G. Congenital absence of pericardium in a case of a variant of Marfan syndrome. Recenti Prog. Med. 1989, 80, 63–66. [Google Scholar]
- Nisanoglu, V.; Erdil, N.; Battaloglu, B. Complete left-sided absence of the pericardium in association with ruptured type A aortic dissection complicated by severe left hemothorax. Tex. Heart Inst. J. 2005, 32, 241–243. [Google Scholar]
- Palau, P.; Domínguez, E.; García-González, P.; Gallego, J.; Bosch, M.J.; Sieso, E. Isolated Partial Congenital Absence of the Pericardium: A Familial Presentation. Can. J. Cardiol. 2016, 32, 1039. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.K.; Jung, J.H.; Yoon, Y.E.; Kim, H.L.; Park, J.B.; Lee, S.-P.; Kim, Y.-J.; Cho, G.-Y.; Sohn, D.-W.; et al. Echocardiographic diagnosis of total or left congenital pericardial absence with positional change. Heart 2017, 103, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Beppu, S.; Matsuhisa, M.; Izumi, S.; Masuda, Y.; Nagata, S.; Park, Y.D.; Sakakibara, H.; Nimura, Y. Pericardial defect: Roles of the pericardium on kinetoanatomic changes of the heart influenced by patients’ postures. J. Cardiogr. 1986, 16, 193–205. [Google Scholar]
- Ahn, C.; Hosier, D.M.; Vasko, J.S. Congenital pericardial defect with herniation of the left atrial appendage. Ann. Thorac. Surg. 1969, 7, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Hotta, F. Congenital pericardial defect associated with cardiac incarceration: Case report. Am. Heart J. 1980, 100, 866–870. [Google Scholar] [CrossRef]
- Gatzoulis, M.A.; Munk, M.D.; Merchant, N.; Van Arsdell, G.S.; McCrindle, B.W.; Webb, G.D. Isolated congenital absence of the pericardium: Clinical presentation, diagnosis, and management. Ann. Thorac. Surg. 2000, 69, 1209–1215. [Google Scholar] [CrossRef]
- Chassaing, S.; Bensouda, C.; Bar, O.; Barbey, C.; Blanchard, D. A case of partial congenital absence of pericardium revealed by MRI. Circ. Cardiovasc. Imaging 2010, 3, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Kronzon, I.; Machnicki, S.C.; Ruiz, C.E. A constrained heart: A case of sudden onset unrelenting chest pain. Circulation 2014, 130, 1625–1631. [Google Scholar] [CrossRef]
- Uzün, I.; Büyük, Y.; Pakiş, I.; Doğru, A.; Calk, A.U. Sudden death due to congenital pericardial defect: An autopsy case. Am. J. Forensic Med. Pathol. 2008, 29, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Brulotte, S.; Roy, L.; Larose, E. Congenital absence of the pericardium presenting as acute myocardial necrosis. Can. J. Cardiol. 2007, 23, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeong, D.S.; Kim, I.S.; Park, B.J. Persistent Atrial Fibrillation Related to a Congenital Pericardial Defect and Left Atrial Appendage Herniation. Korean J. Thorac. Cardiovasc. Surg. 2015, 48, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Pelletier, M.J.; Perron, J.; Kumar, A.; Champagne, J. Sudden cardiac arrest due to subtotal absence of left-sided pericardium—Case report and review of the literature. Congenit. Heart Dis. 2013, 8, E92–E98. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Trevisan, R.; Lombardo, M.; Grasso, E.; Gensini, G.F.; Ambrosio, G. The influence of pectus excavatum on cardiac kinetics and function in otherwise healthy individuals: A systematic review. Int. J. Cardiol. 2023, 381, 135–144. [Google Scholar] [CrossRef]
- Castelletti, S.; Crotti, L.; Dagradi, F.; Rella, V.; Salerno, S.; Parati, G.; Cecchi, F. Partial Pericardial Agenesis Mimicking Arrhythmogenic Right Ventricular Cardiomyopathy. Clin. J. Sport Med. 2020, 30, e159–e162. [Google Scholar] [CrossRef] [PubMed]
- Bernardinello, V.; Cipriani, A.; Perazzolo Marra, M.; Motta, R.; Barchitta, A. Congenital Pericardial Agenesis in Asymptomatic Individuals: Tips for the Diagnosis. Circ. Cardiovasc. Imaging 2020, 13, e010169. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.C.; Vieira, C.; Bettencourt, N.; Pereira, V.H. Pericardial agenesis: A rare cause of chest pain. Eur. Heart J. 2021, 42, 282. [Google Scholar] [CrossRef]
- Khayata, M.; Haouzi, A.A.; Asher, C.R.; Xu, B. Multimodality Imaging Approach Evaluation of the Congenital Pericardial Defect: A Contemporary Review. Curr. Cardiol. Rep. 2023, 25, 1715–1724. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Vrettos, A.; Androulakis, E.; Kamperou, C.; Vlachopoulos, C.; Tsioufis, K.; Mohiaddin, R.; Lazaros, G. Cardiac magnetic resonance imaging of pericardial diseases: A comprehensive guide. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.J.; Rigsby, C.K.; Berhane, H.; Bollache, E.; Jarvis, K.; Barker, A.J.; Schnell, S.; Allen, B.D.; Robinson, J.D.; Markl, M. 4-D flow MRI aortic 3-D hemodynamics and wall shear stress remain stable over short-term follow-up in pediatric and young adult patients with bicuspid aortic valve. Pediatr. Radiol. 2019, 49, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Agalbato, C.; Melotti, E.; Marchetti, D.; Schillaci, M.; Ratti, A.; Ippolito, S.; Pancrazi, M.; Perone, F.; Cia, A.D.; et al. The Contemporary Role of Cardiac Computed Tomography and Cardiac Magnetic Resonance Imaging in the Diagnosis and Management of Pericardial Diseases. Can. J. Cardiol. 2023, 39, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Schicchi, N.; Fogante, M.; Palumbo, P.; Agliata, G.; Esposto Pirani, P.; Di Cesare, E.; Giovagnoni, A. The sub-millisievert era in CTCA: The technical basis of the new radiation dose approach. Radiol. Med. 2020, 125, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Schicchi, N.; Fogante, M.; Esposto Pirani, P.; Agliata, G.; Basile, M.C.; Oliva, M.; Agostini, A.; Giovagnoni, A. Third-generation dual-source dual-energy CT in pediatric congenital heart disease patients: State-of-the-art. Radiol. Med. 2019, 124, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Pedrotti, P.; Quattrocchi, G.; Roghi, A.; Badano, L.; Faletti, R.; Bogaert, J.; Gaita, F. Multimodality imaging of pericardial diseases. J. Cardiovasc. Med. 2016, 17, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Fadl, S.A.; Nasrullah, A.; Harris, A.; Edwards, R.; Kicska, G. Comprehensive review of pericardial diseases using different imaging modalities. Int. J. Cardiovasc. Imaging 2020, 36, 947–969. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Rajendran, K.; Baffour, F.I.; Diehn, F.E.; Ferrero, A.; Glazebrook, K.N.; Horst, K.K.; Johnson, T.F.; Leng, S.; Mileto, A.; et al. Clinical applications of photon counting detector CT. Eur. Radiol. 2023, 33, 5309–5320. [Google Scholar] [CrossRef]
- Tanaka, H.; Oishi, Y.; Mizuguchi, Y.; Miyoshi, H.; Ishimoto, T.; Nagase, N.; Yamada, H.; Oki, T. Contribution of the pericardium to left ventricular torsion and regional myocardial function in patients with total absence of the left pericardium. J. Am. Soc. Echocardiogr. 2008, 21, 268–274. [Google Scholar] [CrossRef]
- Xu, B.; Betancor, J.; Asher, C.; Rosario, A.; Klein, A. Congenital Absence of the Pericardium: A Systematic Approach to Diagnosis and Management. Cardiology 2017, 136, 270–278. [Google Scholar] [CrossRef]

| Modality | Main Characteristics | Strengths | Limitations |
|---|---|---|---|
| Electrocardiography | Incomplete right bundle branch block Right QRS axis deviation Leftward displacement of the precordial transitional zone Peaked P waves Postural changes in the QRS vector | Availability Low cost Bed-side evaluation | No imaging evaluation |
| Echocardiography | Heart displacement Clockwise rotation Apparent right ventricular enlargement Paradoxical septal motion High movement of the heart | Availability Low cost High temporal resolution Bed-side evaluation | Operator dependent Low spatial resolution Low tissue characterization |
| Chest X-ray | Leftward heart displacement (Snoopy sign) Prominent main pulmonary artery Radiolucency between the heart and diaphragm | Availability Low cost Bed-side evaluation | Low sensibility Low specificity |
| CCT | Evaluation of heart position Evaluation of associated cardiac disease | Spatial resolution Temporal resolution | Radiation dose Low tissue characterization Artifact from metallic implantable devices |
| CMR | Direct pericardium visualization Evaluation of heart position Evaluation of heart function | Temporal resolution Tissue characterization | Long acquisition time Claustrophobia Presence of devices (compatibility, artifacts) High cost |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzullo, R.; Capestro, A.; Cosimo, R.; Fogante, M.; Aprile, A.; Balardi, L.; Giordano, M.; Gaio, G.; Gauderi, G.; Russo, M.G.; et al. Congenital Absence of Pericardium: The Swinging Heart. J. Imaging 2024, 10, 199. https://doi.org/10.3390/jimaging10080199
Marzullo R, Capestro A, Cosimo R, Fogante M, Aprile A, Balardi L, Giordano M, Gaio G, Gauderi G, Russo MG, et al. Congenital Absence of Pericardium: The Swinging Heart. Journal of Imaging. 2024; 10(8):199. https://doi.org/10.3390/jimaging10080199
Chicago/Turabian StyleMarzullo, Raffaella, Alessandro Capestro, Renato Cosimo, Marco Fogante, Alessandro Aprile, Liliana Balardi, Mario Giordano, Gianpiero Gaio, Gabriella Gauderi, Maria Giovanna Russo, and et al. 2024. "Congenital Absence of Pericardium: The Swinging Heart" Journal of Imaging 10, no. 8: 199. https://doi.org/10.3390/jimaging10080199
APA StyleMarzullo, R., Capestro, A., Cosimo, R., Fogante, M., Aprile, A., Balardi, L., Giordano, M., Gaio, G., Gauderi, G., Russo, M. G., & Schicchi, N. (2024). Congenital Absence of Pericardium: The Swinging Heart. Journal of Imaging, 10(8), 199. https://doi.org/10.3390/jimaging10080199






