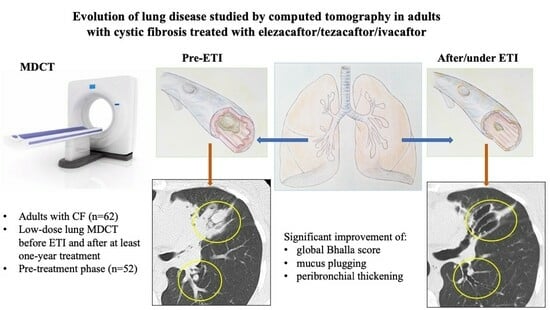
Evolution of Lung Disease Studied by Computed Tomography in Adults with Cystic Fibrosis Treated with Elexacaftor/Tezacaftor/Ivacaftor
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Functional Data Collection
2.3. CT Examinations and Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Changes in Radiology and Spirometry Parameters with ETI Treatment
3.3. Changes in CT Scores and Spirometry Parameters in the Pre-Treatment Phase
3.4. Impact of Previous Modulator Therapy on CT Scores in the Pre-Treatment Phase
3.5. Factors Influencing the Evolution of the Bhalla Score
3.6. Consistency and Reliability of Radiological Assessments
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Category | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Severity of bronchiectasis | Absent | Mild (luminal diameter slightly greater than diameter of adjacent vessel) | Moderate (lumen 2 to 3 times the diameter of adjacent vessel) | Severe (lumen > 3 times the diameter of adjacent vessel) |
| Peribronchial thickening | Absent | Mild (wall thickness equal to the diameter of adjacent vessel) | Moderate (wall thickness greater than and up to twice the diameter of adjacent vessel) | Severe (wall thickness > 2 times the diameter of the adjacent vessel) |
| Extent of bronchiectasis (number of lung segments) | Absent | 1–5 | 6–9 | >9 |
| Extent of mucus plugs (number of lung segments) | Absent | 1–5 | 6–9 | >9 |
| Abscesses or sacculations (number of lung segments) | Absent | 1–5 | 6–9 | >9 |
| Generations of bronchial division involved (bronchiectasis/plugging) | Absent | Up to 4th generation | Up to 5th generation | Up to 6th generation and distal |
| Number of bullae | Absent | Unilateral (≤4) | Bilateral (≤4) | >4 |
| Collapse/consolidation | Absent | Subsegmental | Segmental/lobar | |
| Air trapping (number of lung segments) | Absent | 1–5 | >5 |
References
- Turcios, N.L. Cystic fibrosis lung disease: An overview. Respir. Care 2020, 65, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Regard, L.; Martinand, C.; Burgel, P.R. Cystic fibrosis in 2021: “The times they are a-changin”. Arch. Bronconeumol. 2022, 58, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Hota, P.; Madan, R. Cystic fibrosis from childhood to adulthood. What is new in imaging assesment? Radiol. Clin. N. Am. 2020, 58, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Zorzo, C.; Caballero, P.; Diab, L.; Pastor, M.T.; Gómez-Punter, R.M.; Girón, R.M. Predictive value of computed tomography scoring systems evolution in adults with cystic fibrosis. Eur. Radiol. 2020, 30, 3634–3640. [Google Scholar] [CrossRef]
- Bhalla, M.; Turcios, N.; Aponte, V.; Jenkins, M.; Leitman, B.S.; McCauley, D.I.; Naidich, D.P. Cystic fibrosis: Scoring system with thin-section CT. Radiology 1991, 179, 783–788. [Google Scholar] [CrossRef]
- Brody, A.S.; Klein, J.S.; Molina, P.L.; Quan, J.; Bean, J.A.; Wilmott, R.W. High-resolution computed tomography in young patients with cystic fibrosis: Distribution of abnormalities and correlation with pulmonary function tests. J. Pediatr. 2004, 145, 32–38. [Google Scholar] [CrossRef]
- Carpio, C.; Albi, G.; Rayon-Aledo, J.C.; Alvarez-Sala, R.; Girón, R.; Prados, C.; Caballero, P. Changes in structural lung disease in cystic fibrosis children over 4 years as evaluated by high-resolution computed tomography. Eur. Radiol. 2015, 25, 3577–3585. [Google Scholar] [CrossRef]
- Diab-Cáceres, L.; Girón-Moreno, R.M.; García-Castillo, E.; Pastor-Sanz, M.T.; Olveira, C.; García-Clemente, M.M.; Nieto-Royo, R.; Prados-Sánchez, C.; Caballero-Sánchez, P.; Olivera-Serrano, M.J.; et al. Predictive value of the modified Bhalla score for assessment of pulmonary exacerbations in adults with cystic fibrosis. Eur. Radiol. 2021, 31, 112–120. [Google Scholar] [CrossRef]
- Cademartiri, F.; Luccichenti, G.; Palumbo, A.A.; Maffei, E.; Pisi, G.; Zompatori, M.; Krestin, G.P. Predictive value of chest CT in patients with cystic fibrosis: A single-center 10-year experience. AJR. Am. J. Roentgenol. 2008, 190, 1475–1480. [Google Scholar] [CrossRef]
- David, M.; Benlala, I.; Bui, S.; Benkert, T.; Berger, P.; Laurent, F.; Macey, J.; Dournes, G. Longitudinal Evaluation of Bronchial Changes in Cystic Fibrosis Patients Undergoing Elexacaftor/Tezacaftor/Ivacaftor Therapy Using Lung MRI With Ultrashort Echo-Times. J. Magn. Reson. Imaging 2024, 60, 116–124. [Google Scholar] [CrossRef]
- Girón Moreno, R.; García-Clemente, M.; Diab-Cáceres, L.; Martínez-Vergara, A.; Martínez-García, M.A.; Gómez-Punter, R.M. Treatment of pulmonary disease of cystic fibrosis: A comprehensive review. Antibiotics 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Vivero, M.D.; Delgado Pecellín, I.; Solé Jover, A.; Álvarez Fernández, A.; Gómez Bonilla, A.; Vicente Santamaría, S.; Mondéjar López, P.; Gartner, S.; Girón, R.M.; Pérez Ruiz, E.; et al. Spanish Cystic Fibrosis Registry: Annual Report 2021; Annual Report; Sociedad Española de Fibrosis Quística: Valencia, Spain, 2023. [Google Scholar]
- Nichols, D.P.; Paynter, A.C.; Heltshe, S.L.; Donaldson, S.H.; Frederick, C.A.; Freedman, S.D.; Gelfond, D.; Hoffman, L.R.; Kelly, A.; Narkewicz, M.R.; et al. Clinical effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis. A clinical trial. Am. J. Respir. Crit. Care Med. 2022, 205, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Regard, L.; Martin, C.; Burnet, E.; Da Silva, J.; Burgel, P.R. CFTR modulators in people with cystic fibrosis: Real-world evidence in France. Cells 2022, 11, 1769. [Google Scholar] [CrossRef] [PubMed]
- Carrasco Hernández, L.; Girón Moreno, R.; Balaguer Cartagena, M.N.; Peláez, A.; Sole, A.; Alvarez Fernández, A.; Felipe Montiel, A.; Olveira, C.; Olveira, G.; Gómez Bonilla, A.; et al. Experience with elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis and advanced disease. Arch. Bronconeumol. 2023, 59, 556–565. [Google Scholar] [CrossRef]
- Bec, R.; Reynaud-Gaubert, M.; Arnaud, F.; Naud, R.; Dufeu, N.; Di Bisceglie, M.; Coiffard, B.; Gaubert, J.Y.; Bermudez, J.; Habert, P. Chest computed tomography improvement in patients with cystic fibrosis treated with elexacaftor-tezacaftor-ivacaftor: Early report. Eur. J. Radiol. 2022, 154, 110421. [Google Scholar] [CrossRef]
- Tagliati, C.; Pantano, S.; Lanni, G.; Battista, D.; Cerimele, F.; Collini, F.; Rebonato, A.; Esposito, R.; Matteo, M.; Fogante, M.; et al. Radiological and clinical evaluation of triple combination modulating therapy effectiveness in adult patients with cystic fibrosis. Meta-Radiology 2023, 1, 100025. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standarisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Fleiss, J.L. Statistical Methods for Rates and Proportions, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef]
- Carnovale, V.; Iacotucci, P.; Terlizzi, V.; Colangelo, C.; Medio, P.; Ferrillo, L.; De Gregorio, F.; Francalanci, M.; Taccetti, G.; Buonaurio, S.; et al. Effectiveness and safety of elexacaftor/ tezacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease with the Phe508del/minimal function genotype. Respir. Med. 2021, 189, 106646. [Google Scholar] [CrossRef]
- Gushue, C.; Eisner, M.; Bai, S.; Johnson, T.; Holtzlander, M.; McCoy, K.; Sheikh, S. Impact of elexacaftor–tezacaftor–ivacaftor on lung disease in cystic fibrosis. Pediatr. Pulmonol. 2023, 58, 2308–2316. [Google Scholar] [CrossRef]
- Aalbers, B.L.; Mohamed Hoesein, F.A.A.; Hofland, R.W.; Bronsveld, I.; Kruijswijk, M.A.; Schotman, S.; Slingerland, C.W.; Panhuis, H.; van der Ent, C.K.; Heijerman, H.G.M. Radiological and long-term clinical response to elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis with advanced lung disease. Pediatr. Pulmonol. 2023, 58, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Cazier, P.; Chassagnon, G.; Dhote, T.; Da Silva, J.; Kanaan, R.; Honoré, I.; Carlier, N.; Revel, M.P.; Canniff, E.; Martin, C.; et al. Reversal of cylindrical bronchial dilatations in a subset of adults with cystic fibrosis treated with elexacaftor-tezacaftor-ivacaftor. Eur. Respir. J. 2024, 63, 2301794. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.R.; Munck, A.; Durieu, I.; Chiron, R.; Mely, L.; Prevotat, A.; Murris-Espin, M.; Porzio, M.; Abely, M.; Reix, P.; et al. French Cystic Fibrosis Reference Network Study Group. Real-life safety and effectiveness of lumacaftor-ivacaftor in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.I.; Long, F.R.; McCoy, K.S.; Johnson, T.; Ryan-Wenger, N.A.; Hayes, D., Jr. Computed tomography correlates with improvement with ivacaftor in cystic fibrosis patients with G551D mutation. J. Cyst. Fibros. 2015, 14, 84–89. [Google Scholar] [CrossRef]
- Chassagnon, G.; Hubert, D.; Fajac, I.; Burgel, P.R.; Revel, M.P. Long-term computed tomographic changes in cystic fibrosis patients treated with ivacaftor. Eur. Respir. J. 2016, 48, 249–252. [Google Scholar] [CrossRef]
- Arnaud, F.; Stremler-Le Bel, N.; Reynaud-Gaubert, M.; Mancini, J.; Gaubert, J.Y.; Gorincour, G. Computed tomographic changes in patients with cystic fibrosis treated by combination therapy with lumacaftor and ivacaftor. J. Clin. Med. 2021, 10, 1999. [Google Scholar] [CrossRef]
- McNally, P.; Linnane, B.; Williamson, M.; Elnazir, B.; Short, C.; Saunders, C.; Kirwan, L.; David, R.; Kemner-Van de Corput, M.P.C.; Tiddens, H.A.W.M.; et al. The clinical impact of lumacaftor-ivacaftor on structural lung disease and lung function in children aged 6–11 with cystic fibrosis in a real-world setting. Respir. Res. 2023, 24, 199. [Google Scholar] [CrossRef]
- Campredon, A.; Battistella, E.; Martin, C.; Durieu, I.; Mely, L.; Marguet, C.; Belleguic, C.; Murris-Espin, M.; Chiron, R.; Fanton, A.; et al. Using chest computed tomography and unsupervised machine learning for predicting and evaluating response to lumacaftor–ivacaftor in people with cystic fibrosis. Eur. Respir. J. 2022, 59, 2101344. [Google Scholar] [CrossRef]
- Crowley, C.; O’Connor, O.J.; Ciet, P.; Tiddens, H.A.W.M.; Maher, M.M. The evolving role of radiological imaging in cystic fibrosis. Curr. Opin. Pulm. Med. 2021, 27, 575–585. [Google Scholar] [CrossRef]
- Wucherpfennig, L.; Triphan, S.M.F.; Wege, S.; Kauczor, H.U.; Heussel, C.P.; Schmitt, N.; Wuennemann, F.; Mayer, V.L.; Sommerburg, O.; Mall, M.A.; et al. Magnetic resonance imaging detects improvements of pulmonary and paranasal sinus abnormalities in response to elexacaftor/tezacaftor/ivacaftor therapy in adults with cystic fibrosis. J. Cyst. Fibros. 2022, 21, 1053–1060. [Google Scholar] [CrossRef]
- Graeber, S.Y.; Renz, D.M.; Stahl, M.; Pallenberg, S.T.; Sommerburg, O.; Naehrlich, L.; Berges, J.; Dohna, M.; Ringshausen, F.C.; Doellinger, F.; et al. Effects of elexacaftor/tezacaftor/ivacaftor therapy on lung clearance index and magnetic resonance imaging in patients with cystic fibrosis and one or two F508del alleles. Am. J. Respir. Crit. Care Med. 2022, 206, 311–320. [Google Scholar] [CrossRef]
- Fainardi, V.; Skenderaj, K.; Ciuni, A.; Milanese, G.; Deolmi, M.; Longo, F.; Spaggiari, C.; Sverzellati, N.; Esposito, S.; Pisi, G. Structural changes in lung morphology detected by MRI after modulating therapy with elexacaftor/tezacaftor/ivacaftor in adolescent and adult patients with cystic fibrosis. Respir. Med. 2023, 216, 107328. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Dohna, M.; Graeber, S.Y.; Sommerburg, O.; Renz, D.M.; Pallenberg, S.T.; Voskrebenzev, A.; Schütz, K.; Hansen, G.; Doellinger, F.; et al. Impact of elexacaftor-tezacaftor-ivacaftor therapy on lung clearance index and magnetic resonance imaging in children with cystic fibrosis and one or two F508del alleles. Eur. Respir. J. 2024, 64, 2400004. [Google Scholar] [CrossRef] [PubMed]
- Chassagnon, G.; Martin, C.; Burgel, P.R.; Hubert, D.; Fajac, I.; Paragios, N.; Zacharaki, E.I.; Legmann, P.; Coste, J.; Revel, M.P. An automated computed tomography score for the cystic fibrosis lung. Eur. Radiol. 2018, 28, 5111–5120. [Google Scholar] [CrossRef]
- Chassagnon, G.; Zacharaki, E.I.; Bommart, S.; Burgel, P.R.; Chiron, R.; Dangeard, S.; Paragios, N.; Martin, C.; Revel, M.P. Quantification of Cystic Fibrosis Lung Disease with Radiomics-based CT Scores. Radiol. Cardiothorac. Imaging 2020, 2, e200022. [Google Scholar] [CrossRef] [PubMed]
- Dournes, G.; Hall, C.S.; Willmering, M.M.; Brody, A.S.; Macey, J.; Bui, S.; Denis de Senneville, B.; Berger, P.; Laurent, F.; Benlala, I.; et al. Artificial intelligence in computed tomography for quantifying lung changes in the era of CFTR modulators. Eur. Respir. J. 2022, 59, 2100844. [Google Scholar] [CrossRef]
- Dettmer, S.; Weinheimer, O.; Sauer-Heilborn, A.; DettLammers, O.; Wielpütz, M.O.; Fuge, J.; Welte, T.; Wacker, F.; Ringshausen, F.C. Qualitative and quantitative evaluation of computed tomography changes in adults with cystic fibrosis treated with elexacaftor-tezacaftor-ivacaftor: A retrospective observational study. Front. Pharmacol. 2023, 14, 1245885. [Google Scholar] [CrossRef]
- Lv, Q.; Gallardo-Estrella, L.; Andrinopoulou, E.R.; Chen, Y.; Charbonnier, J.P.; Sandvik, R.M.; Caudri, D.; Nielsen, K.G.; de Bruijne, M.; Ciet, P.; et al. Automatic analysis of bronchus-artery dimensions to diagnose and monitor airways disease in cystic fibrosis. Thorax 2023, 79, 13–22. [Google Scholar] [CrossRef]




| Clinical Characteristics at the Time of Inclusion (n = 62) | |
|---|---|
| Sex [male] | 35 (56.5%) |
| Age | 30.4 (±7.87) |
| Type of mutation [heterozygous] | 34 (54.8%) |
| Exocrine pancreas [pancreatic insufficiency] | 51 (82.3%) |
| Endocrine pancreas [carbohydrate intolerance or diabetes] | 31 (50.0%) |
| Hepatic disease | 21 (33.9%) |
| ppFEV1 | 67.8 ±19.6 |
| ppFVC | 82.0 (±14.4) |
| BMI (kg/m2) | 22.4 (±2.6) |
| Infections | |
| Methicillin-sensitive Staphylococcus aureus | 36 (51.4%) |
| Pseudomonas aeruginosa | 20 (28.6%) |
| Methicillin-resistant Staphylococcus aureus | 3 (4.3%) |
| Haemophilus influenzae | 3 (4.3%) |
| Non-tuberculous Mycobacterium | 5 (7.1%) |
| Other | 3 (4.3%) |
| Combined infections | 8 (11.4%) |
| Prior Treatments | |
| Prior Modulator | 25 (40.3%) |
| Ivacaftor | 1 (1.6%) |
| Tezacaftor/Ivacaftor | 20 (32.3%) |
| Both | 2 (3.2%) |
| Lumacaftor/Ivacaftor | 2 (3.2%) |
| Before ETI | After ETI | p-Value | |
|---|---|---|---|
| CT0 | CT1 | ||
| Bhalla CT score (global) | 12.2 (±2.8) | 14.0 (±2.8) | <0.001 |
| Severity of bronchiectasis | 2.0 (±0.8) | 2.0 (±0.8) | 0.824 |
| Peribronchial thickening | 1.4 (±0.6) | 1.0 (±0.4) | <0.001 |
| Extent of the bronchiectasis | 2.9 (±0.4) | 2.8 (±0.5) | 0.234 |
| Extent of mucus plugs | 1.6 (±0.7) | 0.8 (±0.6) | <0.001 |
| Abscesses or sacculations | 0.2 (±0.5) | 0.1 (±0.3) | 0.078 |
| Generations of bronchial division involved | 2.4 (±0.6) | 2.3 (±0.6) | 0.306 |
| Number of bullae | 0.1 (±0.3) | 0.1 (±0.3) | 0.474 |
| Collapse/consolidation | 1.6 (±0.7) | 1.6 (±0.6) | 0.814 |
| Air trapping | 0.6 (±0.7) | 0.4 (±0.7) | 0.096 |
| Spirometry values | |||
| ppFEV1 | 66.5 (±19.8) | 77.0 (±20.4) | 0.005 |
| ppFVC | 80.6 (±16.4) | 91.6 (±14.1) | <0.001 |
| BMI (kg/m2) | 22.4 (±2.6) | 23.6 (±2.6) | 0.011 |
| Radiology Items | CT-1 | CT0 | p-Value |
|---|---|---|---|
| Bhalla score (global) | 12.6 (±2.8) | 12.1 (±2.9) | 0.287 |
| Severity of bronchiectasis | 1.9 (±0.8) | 2.1 (±0.8) | 0.382 |
| Peribronchial thickening | 1.3 (±0.6) | 1.4 (±0.6) | 0.406 |
| Extent of the bronchiectasis | 2.9 (±0.4) | 2.9 (±0.4) | 0.759 |
| Extent of mucus plugs | 1.3 (±0.7) | 1.7 (±0.7) | 0.027 |
| Abscesses or sacculations | 0.2 (±0.4) | 0.2 (±0.5) | 0.767 |
| Generations of bronchial division involved | 2.3 (±0.6) | 2.4 (±0.6) | 0.718 |
| Number of bullae | 0.1 (±0.4) | 0.1 (±0.3) | 0.246 |
| Collapse/consolidation | 0.6 (±0.7) | 0.6 (±0.7) | 0.793 |
| Air trapping | 1.7 (±0.5) | 1.6 (±0.6) | 0.597 |
| Spirometry values | |||
| ppFEV1 | 67.8 (±19.6) | 65.8 (±18.7) | 0.627 |
| ppFVC | 82.0 (±14.4) | 80.7 (±15.8) | 0.670 |
| With Prior Modulator (N = 22) | CT-1 | CT0 | p-Value |
|---|---|---|---|
| Bhalla score (global) | 11.5 (±1.9) | 11.4 (±2.5) | 0.886 |
| Severity of bronchiectasis | 2.2 (±0.7) | 2.2 (±0.8) | 0.954 |
| Peribronchial thickening | 1.5 (±0.6) | 1.6 (±0.6) | 0.489 |
| Extent of the bronchiectasis | 3.0 (±0.2) | 3.0 (±0.2) | 0.927 |
| Extent of mucus plugs | 1.6 (±0.5) | 1.7 (±0.8) | 0.759 |
| Abscesses or sacculations | 0.2 (±0.4) | 0.2 (±0.4) | 0.630 |
| Generations of bronchial division involved | 2.6 (±0.5) | 2.5 (±0.5) | 0.863 |
| Number of bullae | 0.1 (±0.5) | 0.1 (±0.4) | 0.895 |
| Collapse/consolidation | 0.5 (±0.6) | 0.6 (±0.6) | 0.624 |
| Air trapping | 1.9 (±0.3) | 1.7 (±0.6) | 0.270 |
| Without Prior Modulator (N = 30) | CT-1 | CT0 | p-Value |
| Bhalla score (global) | 13.4 (±3.0) | 12.8 (±3.0) | 0.361 |
| Severity of bronchiectasis | 1.7 (±0.8) | 1.9 (±0.8) | 0.424 |
| Peribronchial thickening | 1.2 (±0.5) | 1.2 (±0.5) | 0.859 |
| Extent of the bronchiectasis | 2.8 (±0.5) | 2.9 (±0.5) | 0.529 |
| Extent of mucus plugs | 1.1 (±0.8) | 1.6 (±0.7) | 0.019 |
| Abscesses or sacculations | 0.2 (±0.4) | 0.2 (±0.5) | 0.776 |
| Generations of bronchial division involved | 2.2 (±0.6) | 2.3 (±0.6) | 0.271 |
| Number of bullae | 0.1 (±0.4) | 0.0 (±0.2) | 0.208 |
| Collapse/consolidation | 0.6 (±0.7) | 0.6 (±0.7) | 0.653 |
| Air trapping | 1.6 (±0.6) | 1.5 (±0.7) | 0.994 |
| Item | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR [CI 95%] | p-Value | OR [CI 95%] | p-Value | |
| Mutation type [heterozygous] | 1.4 [0.5–3.7] | 0.513 | 3.3 [0.9–13.7] | 0.081 |
| Endocrine pancreas [carbohydrate intolerance or diabetes] | 1.1 [0.4–2.8] | 0.864 | 0.3 [0.1–1.2] | 0.098 |
| BMI after ETI | 1.0 [0.8–1.2] | 0.927 | 1.2 [0.9–1.5] | 0.151 |
| Exocrine pancreas [pancreatic insufficiency] | 2.6 [0.7–11] | 0.171 | 5.3 [1.0–34.2] | 0.062 |
| Oral antibiotic [1 cycle] before ETI | 1.4 [0.3–0.2] | 0.647 | 1.0 [0.2–7.1] | 0.964 |
| Oral antibiotic [>2 cycles] before ETI | 0.6 [0.3–0.2] | 0.519 | 0.2 [0.0–0.9] | 0.047 |
| Intravenous antibiotic [>2 cycles] before ETI | 1.6 [0.6–4.4] | 0.349 | 6.2 [1.7–26.9] | 0.010 |
| Chronic bronchial infection with MSSA | 2.2 [0.8–5.9] | 0.122 | 3.9 [1.2–13.7] | 0.027 |
| Chronic bronchial infection with Pseudomonas | 1.0 [0.3–2.6] | 0.928 | 2.8 [0.8–9.9] | 0.108 |
| ppFEV1 prior to ETI | 1.0 [1.0–1.0] | 0.914 | 0.9 [0.8–1.0] | 0.005 |
| ppFEV1 after ETI | 1.0 [1.0–1.0] | 0.194 | 1.1 [1.1–1.3] | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Muñiz, S.; Caballero, P.; Peláez, A.; Solís-García, M.; de Benavides, C.; Collada, J.; Díaz-Lorenzo, I.; Zorzo, C.; Gómez-Punter, R.M.; Girón, R.M. Evolution of Lung Disease Studied by Computed Tomography in Adults with Cystic Fibrosis Treated with Elexacaftor/Tezacaftor/Ivacaftor. J. Imaging 2025, 11, 124. https://doi.org/10.3390/jimaging11040124
Hernández-Muñiz S, Caballero P, Peláez A, Solís-García M, de Benavides C, Collada J, Díaz-Lorenzo I, Zorzo C, Gómez-Punter RM, Girón RM. Evolution of Lung Disease Studied by Computed Tomography in Adults with Cystic Fibrosis Treated with Elexacaftor/Tezacaftor/Ivacaftor. Journal of Imaging. 2025; 11(4):124. https://doi.org/10.3390/jimaging11040124
Chicago/Turabian StyleHernández-Muñiz, Susana, Paloma Caballero, Adrián Peláez, Marta Solís-García, Carmen de Benavides, Javier Collada, Ignacio Díaz-Lorenzo, Cristina Zorzo, Rosa Mar Gómez-Punter, and Rosa María Girón. 2025. "Evolution of Lung Disease Studied by Computed Tomography in Adults with Cystic Fibrosis Treated with Elexacaftor/Tezacaftor/Ivacaftor" Journal of Imaging 11, no. 4: 124. https://doi.org/10.3390/jimaging11040124
APA StyleHernández-Muñiz, S., Caballero, P., Peláez, A., Solís-García, M., de Benavides, C., Collada, J., Díaz-Lorenzo, I., Zorzo, C., Gómez-Punter, R. M., & Girón, R. M. (2025). Evolution of Lung Disease Studied by Computed Tomography in Adults with Cystic Fibrosis Treated with Elexacaftor/Tezacaftor/Ivacaftor. Journal of Imaging, 11(4), 124. https://doi.org/10.3390/jimaging11040124