Agar Gel as a Non-Invasive Coupling Medium for Reflectance Photoacoustic (PA) Imaging: Experimental Results on Wall-Painting Mock-Ups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
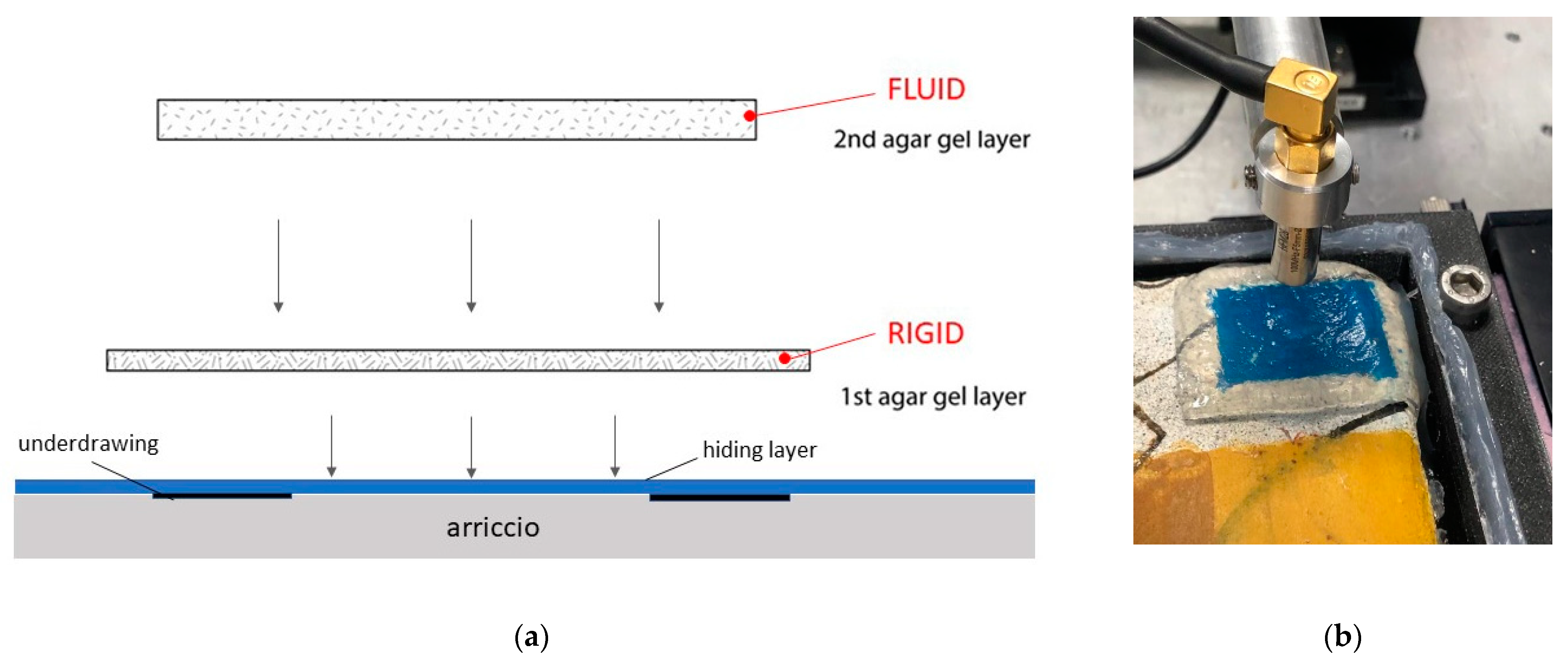
2.1.1. Wall Painting Support
2.1.2. Underdrawings
2.1.3. Hiding Layers
2.1.4. Varnish
2.2. Methods
2.2.1. Reflectance Photoacoustic Imaging
2.2.2. Coupling Media for Ultrasound Wave Propagation
3. Results
3.1. Initial Validation of Reflectance PA Imaging in Agar
3.2. Reflectance PA Imaging in Agar vs. Water
3.3. Image Contrast (CI) Ad Signal-to-Noise Ratio (SNR) Evaluation of Reflectance PA Imaging in Agar and in Water
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tserevelakis, G.J.; Chaban, A.; Klironomou, E.; Melessanaki, K.; Striova, J.; Zacharakis, G. Revealing Hidden Features in Multilayered Artworks by Means of an Epi-Illumination Photoacoustic Imaging System. J. Imaging 2021, 7, 183. [Google Scholar] [CrossRef]
- Chaban, A.; Tserevelakis, G.J.; Klironomou, E.; Fontana, R.; Zacharakis, G.; Striova, J. Revealing Underdrawings in Wall Paintings of Complex Stratigraphy with a Novel Reflectance Photoacoustic Imaging Prototype. J. Imaging 2021, 7, 250. [Google Scholar] [CrossRef]
- Tserevelakis, G.J.; Vrouvaki, I.; Siozos, P.; Melessanaki, K.; Hatzigiannakis, K.; Fotakis, C.; Zacharakis, G. Photoacoustic imaging reveals hidden underdrawings in paintings. Sci. Rep. 2017, 7, 747. [Google Scholar] [CrossRef]
- Tserevelakis, G.J.; Tsagkaraki, M.; Siozos, P.; Zacharakis, G. Uncovering the hidden content of layered documents by means of photoacoustic imaging. Strain 2019, 55, e12289. [Google Scholar] [CrossRef]
- Tserevelakis, G.J.; Pouli, P.; Zacharakis, G. Listening to laser light interactions with objects of art: A novel photoacoustic approach for diagnosis and monitoring of laser cleaning interventions. Herit. Sci. 2020, 8, 98. [Google Scholar] [CrossRef]
- Dal Fovo, A.; Tserevelakis, G.J.; Papanikolaou, A.; Zacharakis, G.; Fontana, R. Combined photoacoustic imaging to delineate the internal structure of paintings. Opt. Lett. 2019, 44, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Tserevelakis, G.J.; Dal Fovo, A.; Melessanaki, K.; Fontana, R.; Zacharakis, G. Photoacoustic signal attenuation analysis for the assessment of thin layers thickness in paintings. J. Appl. Phys. 2018, 123, 123102. [Google Scholar] [CrossRef]
- Sansonetti, A.; Bertasa, M.; Canevali, C.; Rabbolini, A.; Anzani, M.; Scalarone, D. A review in using agar gels for cleaning art surfaces. J. Cult. Herit. 2020, 44, 285–296. [Google Scholar] [CrossRef]
- Sansonetti, A.; Casati, M.; Striova, J.; Canevali, C.; Anzani, M.; Rabbolini, A. cleaning method based on the use of agar gels: New tests and perspectives. In Proceedings of the 12th International Congress on the Deterioration and Conservation of Stone Columbia University, New York, NY, USA, 22–26 October 2012. [Google Scholar]
- Bertasa, M.; Botteon, A.; Brambilla, L.; Riedo, C.; Chiantore, O.; Poli, T.; Sansonetti, A.; Scalarone, D. Cleaning materials: A compositional multi-analytical characterization of commercial agar powders. J. Anal. Appl. Pyrol. 2017, 125, 310–317. [Google Scholar] [CrossRef]
- Prati, S.; Volpi, F.; Fontana, R.; Galletti, R.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Samorì, C.; Sciutto, G.; Tagliavini, E. Sustainability in art conservation: A novel bio-based organogel for the cleaning of water sensitive works of art. Pure Appl. Chem. 2018, 90, 239–251. [Google Scholar] [CrossRef]
- Diamond, O.; Barkovic, M.; Cross, M.; Ormsby, B. The role of agar gel in treating water stains on acrylic paintings: Case study of Composition, 1963 by Justin Knowles. J. Am. Inst. Conserv. 2019, 58, 144–157. [Google Scholar] [CrossRef]
- Bertasa, M.; Poli, T.; Riedo, C.; di Tullio, V.; Capitani, D.; Proietti, N.; Canevali, C.; Sansonetti, A.; Scalarone, D. A study of non-bounded/bounded water and water mobility in different agar gels. Microchem. J. 2018, 139, 306–314. [Google Scholar] [CrossRef]
- Bertasa, M.; Bandini, F.; Felici, A.; Lanfranchi, M.R.; Negrotti, R.; Riminesi, C.; Scalarone, D.; Sansonetti, A. A soluble salts extraction with different thickeners: Monitoring of the effects on plaster. IOP Conf. Ser. Mater. Sci. Eng. 2018, 364, 012076. [Google Scholar] [CrossRef]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar gel strength: A correlation study between chemical composition and rheological properties. Eur. Polym. J. 2020, 123, 109442. [Google Scholar] [CrossRef]
- Sullivan, M.S.; Duncan, T.T.; Berrie, B.H.; Weiss, R.G. Rigid polysaccharide gels for paper conservation: A residue study. In Gels in Conservation; Angelova, L.V., Bronwyn, O., Eds.; Archetype Publications Ltd.: London, UK, 2017; pp. 42–50. ISBN 978-1-909492-50-9. [Google Scholar]
- Medina-Esquivel, R.; Freile-Pelegrin, Y.; Quintana-Owen, P.; Yánez-Limón, J.M.; Alvarado-Gil, J.J. Measurement of the sol–gel transition temperature in agar. Int. J. Thermophys. 2008, 29, 2036. [Google Scholar] [CrossRef]
- Giordano, A.; Cremonesi, P. New Methods of Applying Rigid Agar Gels: From Tiny to Large-scale Surface Areas. Stud. Conserv. 2021, 66, 437–448. [Google Scholar] [CrossRef]
- Cennini, C. The Craftman’s Handbook: II Libro dell’Arte’ Cennino a’Andrea Cennini; Thompson, D.V., Ed.; Dover: New York, NY, USA, 1960; ISBN 9780486200545. [Google Scholar]
- Wallert, A.; Hermens, E.; Peek, M. (Eds.) Historical Painting Techniques, Materials and Studio Practice. In Proceedings of the Preprints of a Symposium Held at the University of Leiden, Leiden, The Netherlands, 26–29 June 1995; Getty Conservation Institute: Malibu, CA, USA, 1995. ISBN 9780892363223. [Google Scholar]
- Basile, G. (Ed.) Giotto Nella Cappella Scrovegni: Materiali per la Tecnica Pittorica, Studi e Ricerche Dell’istituto Centrale per Il Restauro; Istituto Poligrafico e Zecca dello Stato: Rome, Italy, 2005. [Google Scholar]
- Botticelli, G. Metodologia e Restauro Delle Pitture Murali; Edizioni Centro Di: Firenze, Italy, 1992. [Google Scholar]
- Artioli, G.; Baldissin, G.; Andrianakis, M.; Angelini, I.; Asscher, Y.; Becherini, F.; Bernardi, A.; Bernhard Blümich, B.; Chaban, A.; Deiana, R.; et al. Indagini Diagnostiche Innovative e Non Invasive alla Scoperta di un Affresco di Giotto a Lungo Dimenticato, Messaggero di Sant’Antonio, «Il Santo», Padova LXI; Centro Studi Antoniani: Padua, Italy, 2021; pp. 393–406. [Google Scholar]
- Froment, F.; Tournié, A.; Colomban, P. Raman identification of natural red to yellow pigments: Ochre and iron-containing ores. J. Raman Spectrosc. 2008, 39, 560–568. [Google Scholar] [CrossRef]
- Coccato, A.; Jehlicka, J.; Moens, L.; Vandenabeele, P. Raman spectroscopy for the investigation of carbon-based black pigments. J. Raman Spectrosc. 2015, 46, 1003–1015. [Google Scholar] [CrossRef]
- Tomasini, E.P.; Halac, E.B.; Reinoso, M.; Di Liscia, E.J.; Maier, M.S. Micro-Raman Spectroscopy of Carbon-based Black Pigments. J. Raman Spectrosc. 2012, 43, 1671–1675. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Li, E.H. Optical properties of graphite. J. Appl. Phys. 1999, 85, 7404–7410. [Google Scholar] [CrossRef] [Green Version]
- Kempski, K.M.; Graham, M.T.; Gubbi, M.R.; Palmer, T.; Bell, M.A. Application of the generalized contrast-to-noise ratio to assess photoacoustic image quality. Biomed. Opt. Express 2020, 11, 3684–3698. [Google Scholar] [CrossRef]
- Rodriguez-Molares, A.; Rindal, O.M.; D’hooge, J.; Måsøy, S.E.; Austeng, A.; Bell, M.A.; Torp, H. The generalized contrast-to-noise ratio: A formal definition for lesion detectability. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 67, 745–759. [Google Scholar] [CrossRef]
- Telenkov, S.; Mandelis, A. Signal-to-noise analysis of biomedical photoacoustic measurements in time and frequency domains. Rev. Sci. Instrum. 2010, 81, 124901. [Google Scholar] [CrossRef]
- Tserevelakis, G.J.; Mavrakis, K.G.; Kakakios, N.; Zacharakis, G. Full image reconstruction in frequency-domain photoacoustic microscopy by means of a low-cost I/Q demodulator. Opt. Lett. 2021, 46, 4718–4721. [Google Scholar] [CrossRef]




| Sample Type | Sample Number | Underdrawing | Hiding Layer | Layers | Varnish | |
|---|---|---|---|---|---|---|
| Covered | 2 | sinopia | gypsum | 1 | x | |
| charcoal | gypsum | 1 | x | |||
| 4 | sinopia | limewash | 1 | x | ||
| charcoal | limewash | 1 | x | |||
| Painted | fresco | 6 | sinopia | yellow ochre | 1 | shellac |
| charcoal | yellow ochre | 1 | shellac | |||
| 7 | sinopia | yellow ochre | 2 | shellac | ||
| charcoal | yellow ochre | 2 | shellac | |||
| 8 | sinopia | egyptian blue | 3 | x | ||
| charcoal | egyptian blue | 3 | x | |||
| tempera | 1 | graphite | egyptian blue | 3 | x | |
| charcoal | egyptian blue | 3 | x | |||
| fresco + tempera | 9 | sinopia | egyptian blue + yellow ochre | 2 + 2 | shellac | |
| charcoal | egyptian blue + yellow ochre | 2 + 2 | shellac | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaban, A.; Tserevelakis, G.J.; Klironomou, E.; Zacharakis, G.; Striova, J. Agar Gel as a Non-Invasive Coupling Medium for Reflectance Photoacoustic (PA) Imaging: Experimental Results on Wall-Painting Mock-Ups. J. Imaging 2022, 8, 235. https://doi.org/10.3390/jimaging8090235
Chaban A, Tserevelakis GJ, Klironomou E, Zacharakis G, Striova J. Agar Gel as a Non-Invasive Coupling Medium for Reflectance Photoacoustic (PA) Imaging: Experimental Results on Wall-Painting Mock-Ups. Journal of Imaging. 2022; 8(9):235. https://doi.org/10.3390/jimaging8090235
Chicago/Turabian StyleChaban, Antonina, George J. Tserevelakis, Evgenia Klironomou, Giannis Zacharakis, and Jana Striova. 2022. "Agar Gel as a Non-Invasive Coupling Medium for Reflectance Photoacoustic (PA) Imaging: Experimental Results on Wall-Painting Mock-Ups" Journal of Imaging 8, no. 9: 235. https://doi.org/10.3390/jimaging8090235
APA StyleChaban, A., Tserevelakis, G. J., Klironomou, E., Zacharakis, G., & Striova, J. (2022). Agar Gel as a Non-Invasive Coupling Medium for Reflectance Photoacoustic (PA) Imaging: Experimental Results on Wall-Painting Mock-Ups. Journal of Imaging, 8(9), 235. https://doi.org/10.3390/jimaging8090235













