Method for Assessing the Influence of Phobic Stimuli in Virtual Simulators
Abstract
:1. Introduction
- The formalization and implementation of a method for assessing the impact of phobic influences on a person during professional training in virtual reality, which takes into account the quantitative metrics of exercise performance and characteristics of EEG signals;
- The development of practical approaches for analyzing and processing user data in virtual simulators, including collecting the quantitative metrics of professional task performance quality and extracting additional information from raw EEG signals, using clustering algorithms to separate users into statistically significant subgroups and identify abnormal subgroups predisposed to phobic influences;
- The formulation and testing of hypotheses about the degree of the influence of phobic stimuli on the effectiveness of performing professional tasks on virtual simulators.
1.1. Application of Virtual Reality Technologies and Medical Data Analysis in Professional Training
- Pulse meter: Data from these devices in the form of fitness trackers allows for collecting data on the pulse, number of steps, and calorie expenditure. Devices in this format are unobtrusive for the user and provide continuous monitoring for a sufficient amount of time. On the other hand, such devices have low accuracy, inertia, and sensitivity to skin tone and hair coverage. Additionally, most pulse meters have a private interface, which makes data collection and the subsequent transfer to third-party software difficult [29];
- Electrocardiograph: ECG graphs provide access to more detailed information about QRS complexes, which is a more informative source of data on a patient’s heart rate than the aforementioned pulse meters. However, the use of an electrocardiograph by untrained users is a problem due to the difficulties with setting up channels. An incorrect electrode placement can result in incorrect ECG data, which may affect clinical decisions [30];
- Electromyography (EMG): These devices are presented in the form of a bracelet with sensors that record data on a person’s muscle activity. The obtained data are sources of important information about the person’s condition in many areas, such as musculoskeletal rehabilitation, prosthetics, sports, etc. The main difficulty in their use is in the analysis of EMG data, as they contain a large amount of noise and have low reproducibility [31];
- Electroencephalography (EEG): This refers to devices that allow for data collection on a person’s brain activity in conjunction with VR; this technology allows the tracking of brain processes in various scenarios that can be simulated through visual and auditory stimuli. It should be noted that, when wearing a VR helmet over EEG sensors, signal distortion occurs. A partial solution to this problem is the use of special devices to fix the EEG sensors on the head [32]. However, the most common method of working with such data is pre-processing [33].
1.2. Analysis of Medical Data for the Presence of Phobic Influences
1.3. Purposes of the Study
2. Materials and Methods
2.1. Method of Assessing the Influence of Phobic Stimuli in Virtual Reality
- Mean matrix of coherence by group (the arithmetic mean of all matrix entries in the group by size ):
- The deviation of the coherence matrix from the mean matrix:
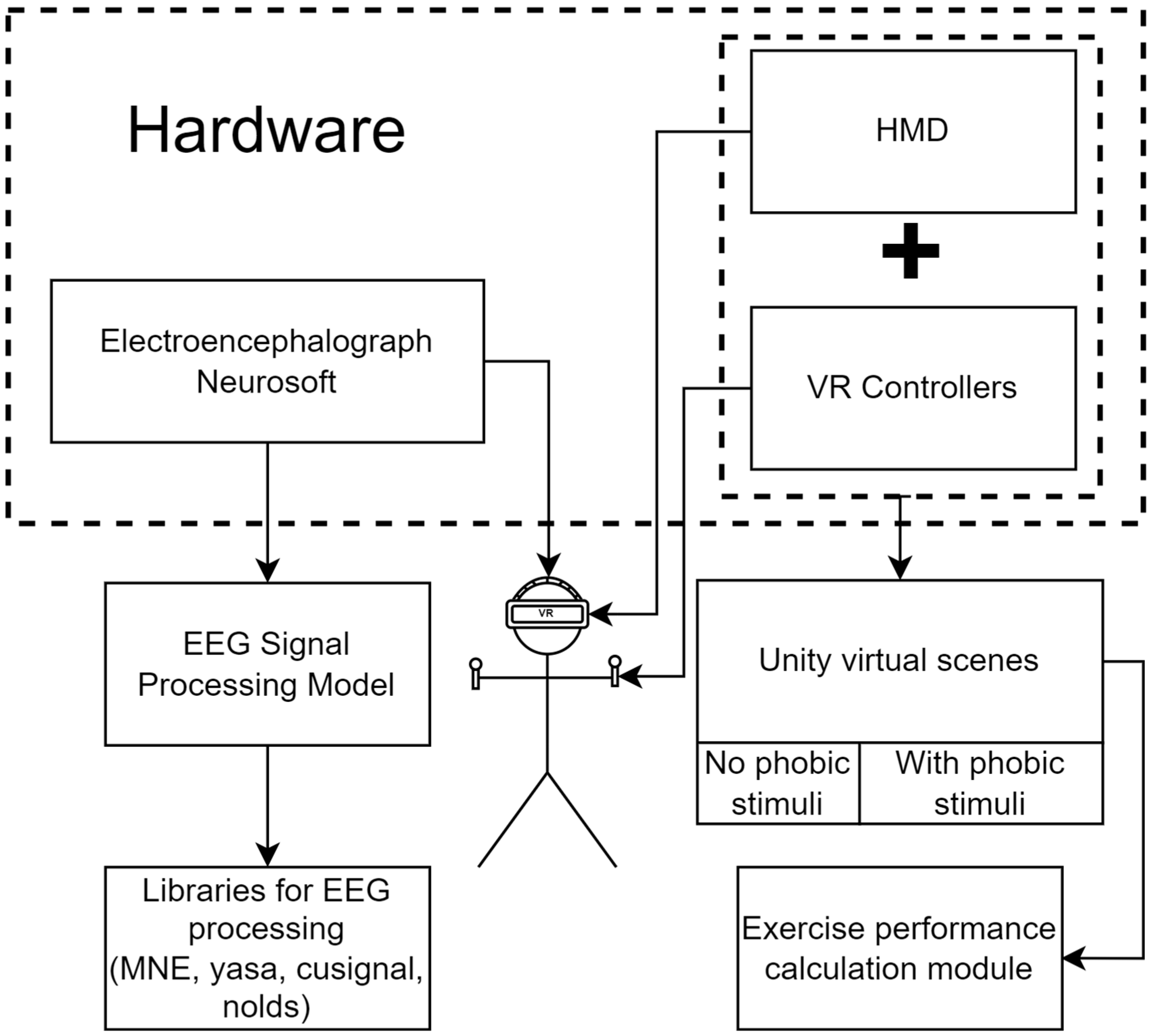
2.2. Software and Hardware for Conducting Research
- The research hardware includes medical equipment for obtaining EEG data and a virtual reality system.
- The electroencephalogram “NEURON-SPECTRUM-4/P” has the following characteristics:
- ◦
- 21 EEG channels;
- ◦
- Sampling rate: 500 Hz;
- ◦
- High-pass filter: 0.5 Hz;
- ◦
- Low-pass filter: 70 Hz.
- Numpy: For mathematical calculations and matrix processing;
- stats: Provides shapiro method for assessing the normality of data; kruskal for performing the Kruskal–Wallis test; mannwhitneyu for performing the Mann–Whitney U-test;
- nolds: hurst_rs method for calculating the Hurst index
- MNE: Provides classes and methods for the discovery, filtration, and processing of EEG and the calculation of PSD [63];
- Yasa: Method bandpower_from_psd for calculating power by frequency based on PSD;
- cluster: Cluster algorithms KMeans, SpectralClustering, and Birch;
- cusignal: The coherence method for the coherent matrix framework.
2.3. Experimental Research Design
- On the head of each participant were fixed electrodes of the electroencephalograph. Over the electrodes, a virtual reality headset was installed. The general scheme of fixation is presented in Figure 3.
- Next, software was launched. When analyzing EEG signals, the 18 channels identified earlier were used: FP1, FP2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, PZ, and OZ. The exclusion of certain channels is due to the simplification of the response assessment of the right and left hemispheres. If channel CZ is used as the middle electrode, which measures the potential difference of the others, then the potential difference CZ-CZ is equal to zero. The electrodes FPZ and FZ can be neglected, as they were located along the central line of the head, and these channels did not give information from the areas of interest in the study cortex. By analyzing EEG signals from selected channels, it was possible to identify emotions and the processes of a person’s thinking (frontal lobe), reaction to sound stimuli (temporal lobe), visual response and process of recognizing objects (occipital lobe), and processes in the sensory zone of the brain (parietal lobe) [64]. In addition, the use of the listed channels allowed for obtaining a sufficiently complete picture of brain activity to calculate intermembral asymmetry.
- Human adaptation to virtual reality takes 3–5 min.
- The respondents performed six exercises with short breaks. The performance of the scenes was recorded with a camera for subsequent verification of human actions. At the same time, video of the virtual scene and a window with the current EEG indications were recorded.
- Each respondent performed an exercise with the number of actions specified in Table 1 for the subsequent determination of differences in the quantitative metrics of the exercises.
- The collected data were processed by removing low-quality and noisy areas.
- Anonymous identifiers were assigned to respondents in the database.
- Quantitative metrics were calculated by Formulae (1)–(4).
- The reading of the EEG fragment corresponding to the exercise was carried out using the MNE software library.
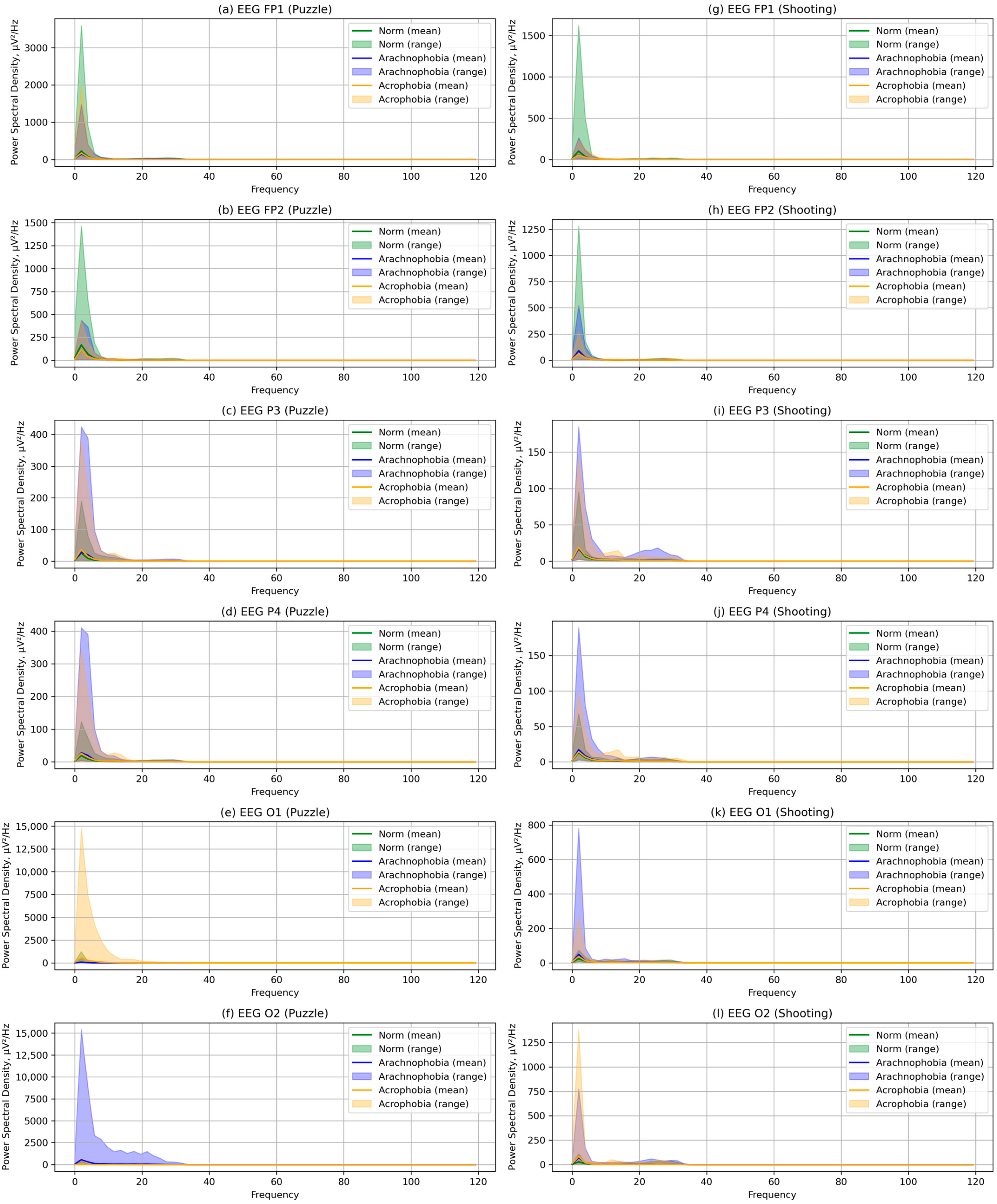
- For each EEG fragment, characteristics were calculated according to the Formulae (5)–(15). The calculation methods and libraries listed in Section 2.2 were used. PSD was calculated separately for alpha, beta, and theta rhythms. The coherence matrices were calculated in accordance with the Expressions (16)–(19).
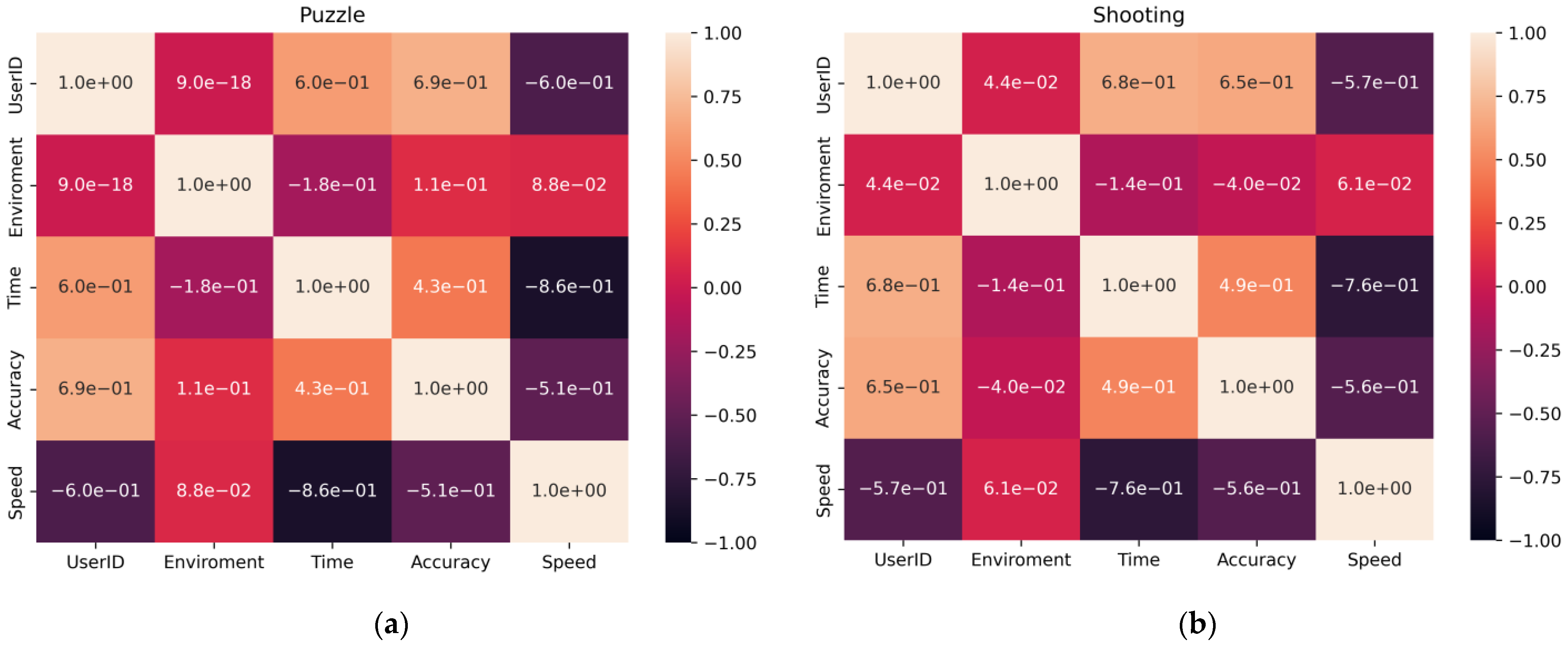
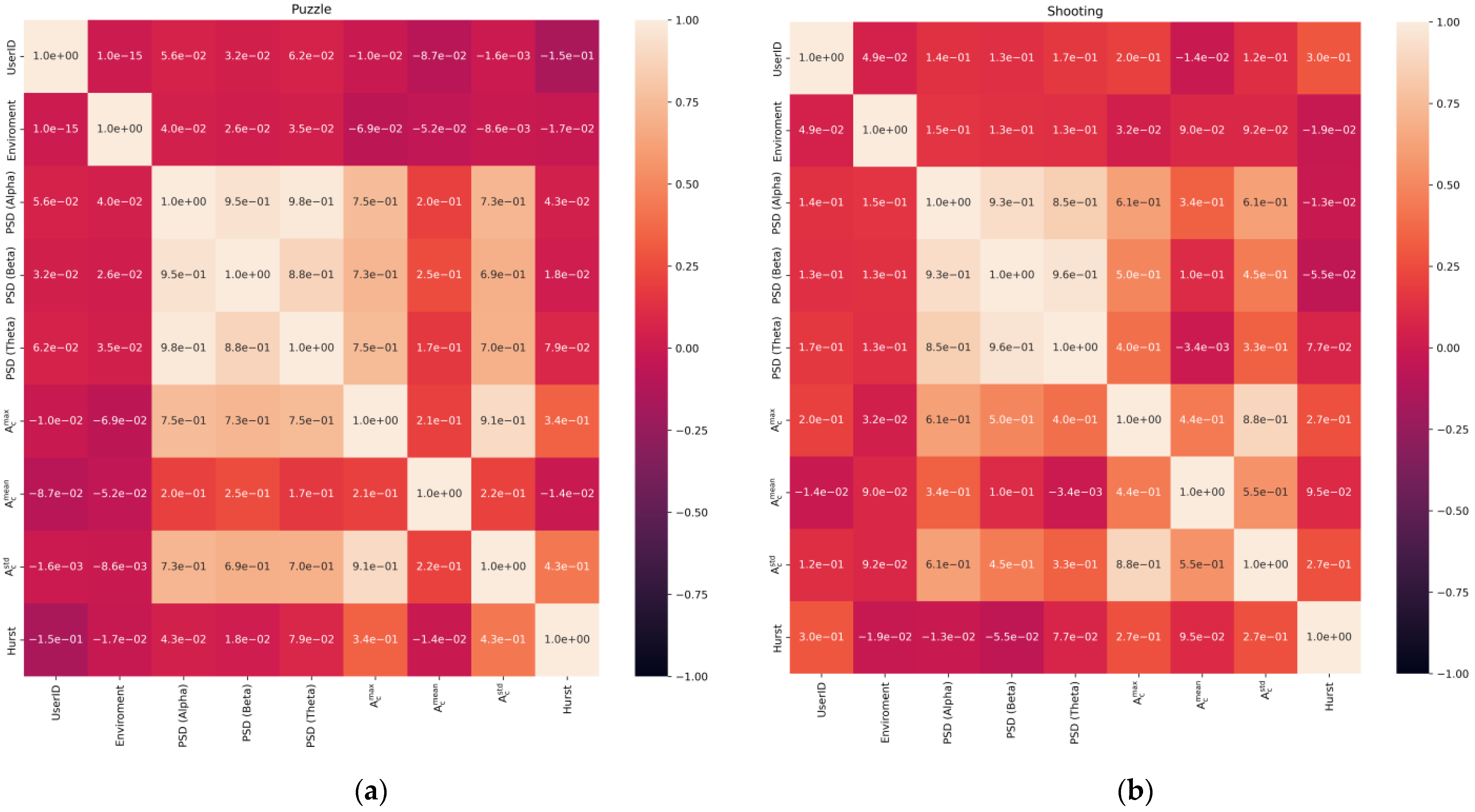
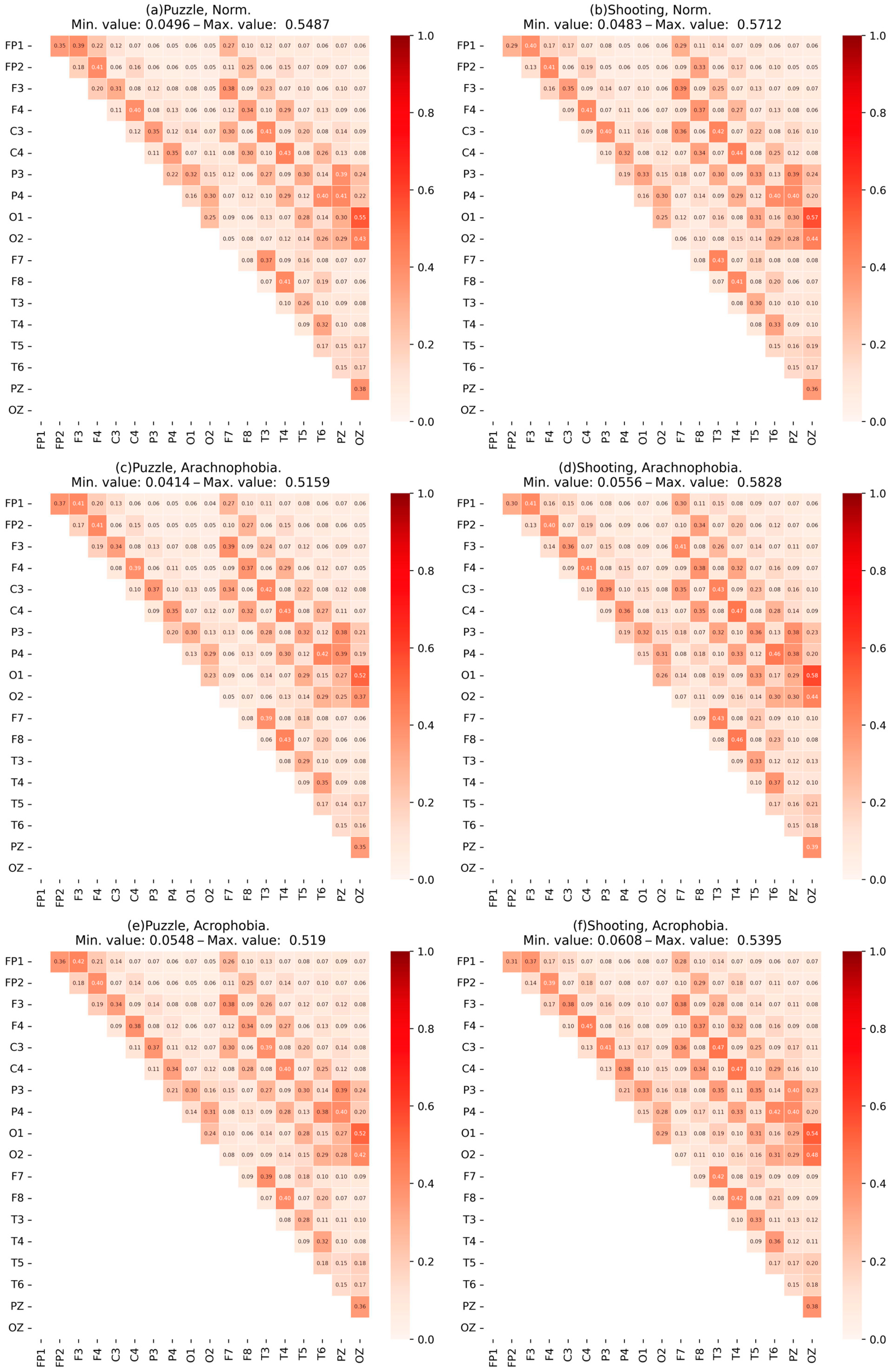
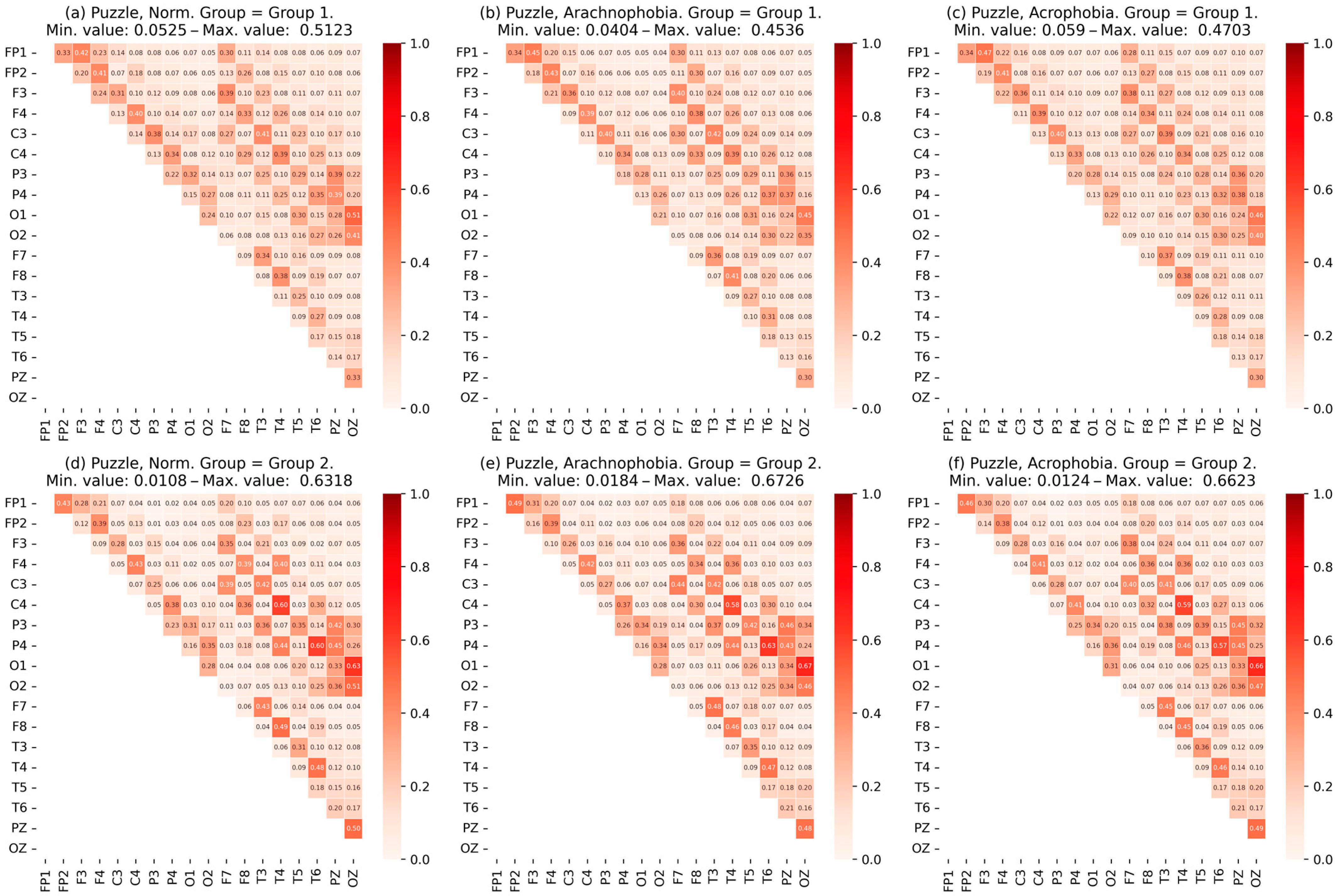
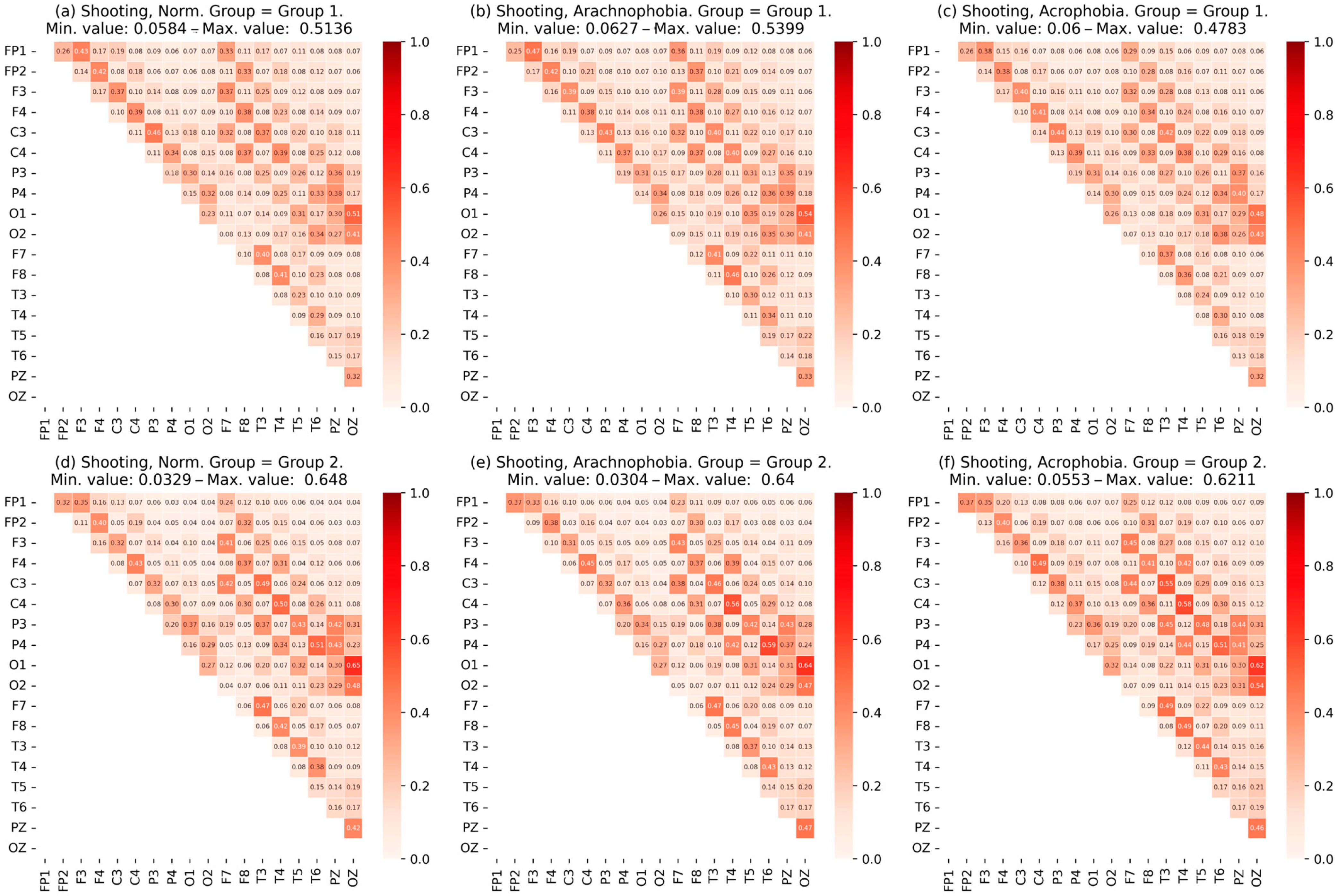
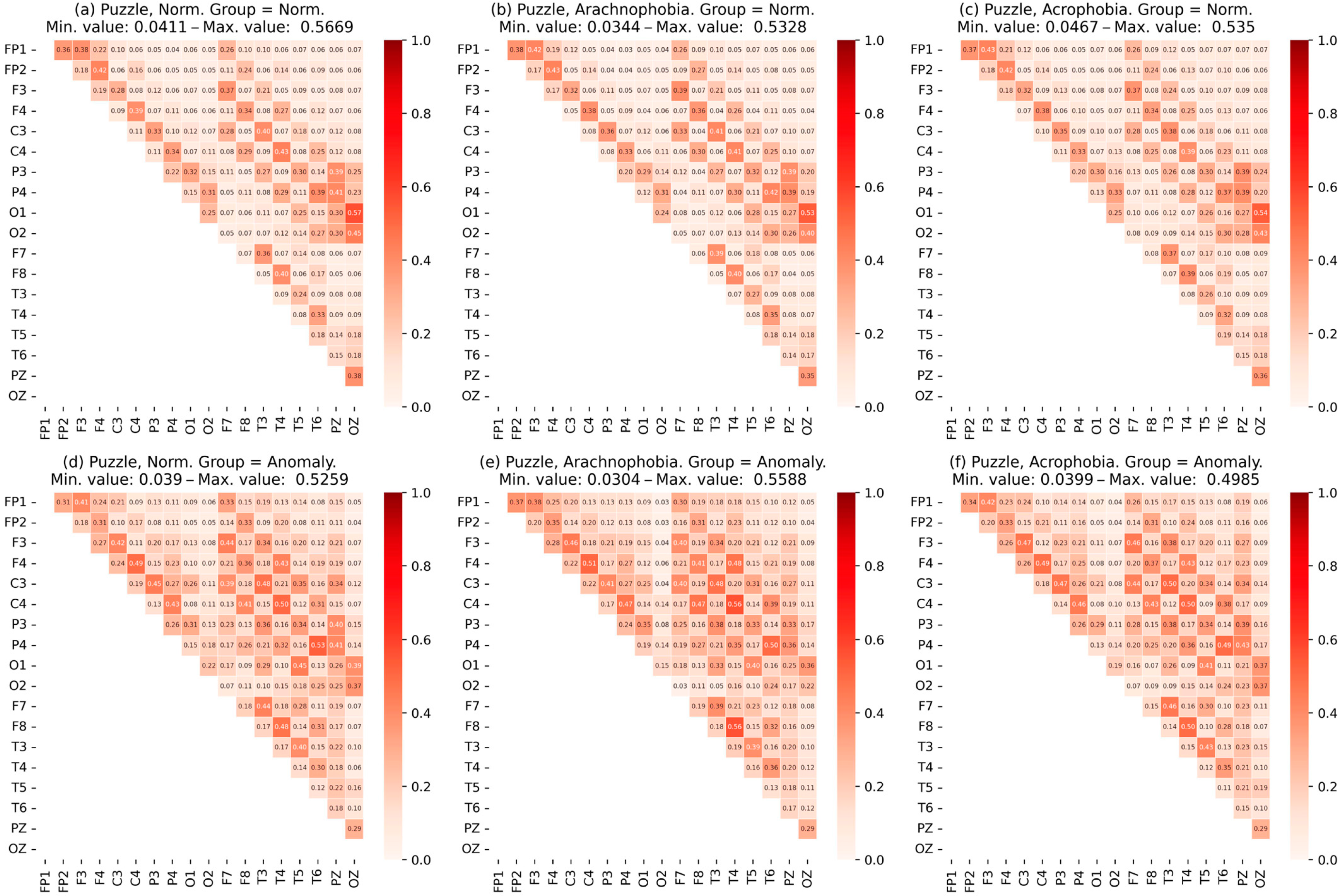
- The data were checked for accordance with the normal distribution through the use of the Shapiro–Wilk test, and thermal maps of the variables’ dependence on each other were built, which allowed for finding out the degree of dependency between them.
- The calculated metrics were combined into comparative tables for each exercise and type of environment, after which the statistical significance of the difference in samples of different scenes was determined by the Kruskal–Wallis method. If such significance was identified, then the statistical difference between individual scenes was further calculated by the Mann–Whitney U-test.
- Heat maps of the coherence matrix for each user in different scenes were arranged to assess the synchronization of EEG frequency components between different sections of the cerebral cortex in each scene. The average coherence matrices of each scene were compared to each other.
- To identify differences between users, a vector was formed from the values of time, accuracy, and speed of performance of the exercises and the mean values for the degree of IHA in alpha, beta, and theta rhythms. Vector data were processed by various clustering methods, such as Kmeans, allowing the group members to be distributed into several subgroups.
- The Kruskal–Wallis test determines the number of clusters (subgroups) for each exercise. A trained cluster algorithm that identified groups with the lowest p-value value on quantitative metrics (accuracy, time, and speed of performance of exercises) was preserved.
- Quantitative metrics and EEG characteristics were analyzed between subgroups for each scene to identify significant differences between them. During the analysis, steps 8–11 were repeated.
- The degree of IHA for each subgroup was calculated by alpha, beta, and theta rhythms for the corresponding pairs of EEG derivations from the right and left hemispheres: FP2-FP1, F4-F3, C4-C3, P4-P3, O2-O1, F8-F7, T4-T3, and T6-T5.
- Expert assessment of respondents and their division into normal and abnormal (having a reaction to phobias) subgroups with repetition of steps 8–11. The values of the degree of IHA for the normal and abnormal subgroups were assessed.
- A summary of the results obtained and an analysis of differences between subgroups were carried out.
3. Results
- Average age of the group: 20.1 ± 2.1 years;
- The group consists of 32 men and 6 women;
- All respondents do not have diagnosed phobias and have normal or corrected vision;
- The majority (80%) of the group had no experience with interaction with virtual reality.
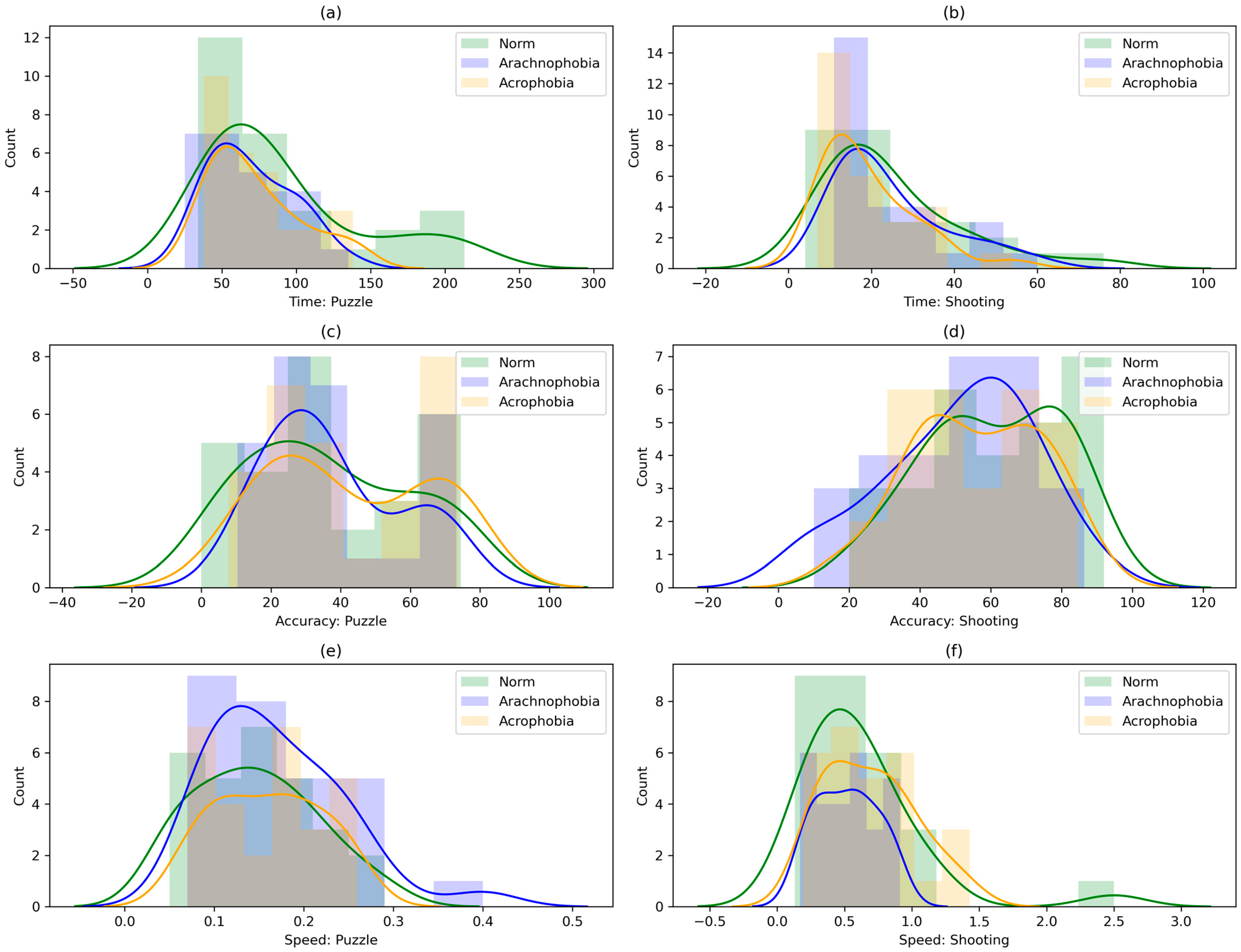
3.1. Statistical Analysis of Experimental Data
3.2. Results of Assessment of Phobic Exposure Impact on the Exercises’ Performance
3.3. Results of Subgroup Analysis Using Cluster Algorithms
3.4. Results of Subgroup Analysis by Expert Assessment Method
4. Discussion
- EEG data are very sensitive to movements; in moments of acute human reaction associated with movement, head rotation, shaking, and emotional reflection of what is happening, significant leaps were observed on all EEG channels; similar interference also occurred with sharp movements when performing exercises;
- In the first exercise, many subgroup participants experienced excitement caused by both the non-specific virtual reality environment for them and the need to master ways to interact with it; when spiders appeared, some participants switched to interaction with them, trying to remove them from the working area; some participants were distracted from performing the exercises on the transparent floor with a view from a great height;
- In the second exercise, after adding spiders to the scene, some participants switched their attention to them and began to flinch when interacting with them; at a great height, there was chaotic human interaction with objects and distraction from the surrounding environment.
4.1. Analysis of Automatically Marked Subgroups in the First Exercise
- The first subgroup showed less accuracy in the performance of the exercises; such low results may be due to the high speed of action. PSD values for all rhythms were higher than in the second subgroup;
- The second subgroup showed greater accuracy but also a greater performance time. The EEG characteristics of the second group were different: the PSD of all rhythms was lower;
- For the metrics of accuracy, time, speed, and EEG characteristics, a statistically reliable difference was obtained.
- The first subgroup slightly reduces accuracy (if the upper limit of values was estimated); PSD values increased significantly at all rhythms, although changes in the amplitude and mean matrix of coherence are not observed;
- The second subgroup improved all metrics: in the PSD, no significant changes were identified, showing the absence of a reaction to arachnophobia; the maximum amplitude of EEG was reduced;
- There was a statistically reliable difference between the performance quality metrics of the exercise, as well as some EEG characteristics.
- The first subgroup showed approximately comparable results with the second scene. The PSD was reduced but remains large enough relative to the first scene;
- The second subgroup further improved the accuracy of execution, but the time increases slightly. Reactions to phobias in the PSD values were not detected;
- Similarly to the second scene, the main metrics were statistically confirmed.
4.2. Analysis of Automatically Labeled Subgroups in the Second Exercise
- The first group showed less accuracy, but also greater speed, demonstrating a situation similar to the first exercise, as well as higher PSD values;
- The second group again showed greater accuracy through more careful performance of the exercises; PSD values correspond to the previous exercise and were significantly lower;
- The difference between the subgroups in all metrics was statistically reliable.
- The first subgroup reduced the accuracy and speed of the exercise. PSD values on alpha and theta rhythms increased slightly;
- The second subgroup deteriorated the accuracy metric; PSD increases, significantly stronger than in the first group;
- There was also a statistically reliable difference between the performance quality metrics, as well as some EEG characteristics.
- The first subgroup improved relative to the level of the first scene; PSD decreased;
- The second subgroup showed intermediate results, better than the second scene but worse than the first; PSD values remained high;
- The main metrics of the exercise performance were statistically confirmed.
4.3. Analysis of Subgroups Labeled by an Expert
4.4. Argumentation Supporting the Proposed Hypotheses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnová, S.; Duda, M.; Matulčíková, M.; Gabrhelová, G.; Hrivíková, T. Further professional on-the-job training of employees in the digital era. Int. J. Eng. Pedagog. 2022, 12, 54–67. [Google Scholar] [CrossRef]
- Chan, D.W.M.; Lam, E.W.M.; Adabre, M.A. Assessing the Effect of Pedagogical Transition on Classroom Design for Tertiary Education: Perspectives of Teachers and Students. Sustainability 2023, 15, 9177. [Google Scholar] [CrossRef]
- Paszkiewicz, A.; Salach, M.; Dymora, P.; Bolanowski, M.; Budzik, G.; Kubiak, P. Methodology of Implementing Virtual Reality in Education for Industry 4.0. Sustainability 2021, 13, 5049. [Google Scholar] [CrossRef]
- Hakami, Z. Comparison between Virtual and Traditional Learning Methods for Orthodontic Knowledge and Skills in Dental Students: A Quasi-Experimental Study. Healthcare 2021, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.H.; Godina, R.; Chowdhury, M.R.K.; Noor, C.W.M.; Wan Nik, W.M.N.; Man, M. Immersive and Non-Immersive Simulators for the Education and Training in Maritime Domain—A Review. J. Mar. Sci. Eng. 2023, 11, 147. [Google Scholar] [CrossRef]
- Makarova, I.; Mustafina, J.; Boyko, A.; Fatikhova, L.; Parsin, G.; Buyvol, P.; Shepelev, V. A Virtual Reality Lab for Automotive Service Specialists: A Knowledge Transfer System in the Digital Age. Information 2023, 14, 163. [Google Scholar] [CrossRef]
- Li, X.; Yi, W.; Chi, H.L.; Wang, X.; Chan, A.P. A critical review of virtual and augmented reality (VR/AR) applications in construction safety. Autom. Constr. 2018, 86, 150–162. [Google Scholar] [CrossRef]
- Kamińska, D.; Sapiński, T.; Wiak, S.; Tikk, T.; Haamer, R.E.; Avots, E.; Helmi, A.; Ozcinar, C.; Anbarjafari, G. Virtual Reality and Its Applications in Education: Survey. Information 2019, 10, 318. [Google Scholar] [CrossRef]
- Alenezi, M. Digital learning and digital institution in higher education. Educ. Sci. 2023, 13, 88. [Google Scholar] [CrossRef]
- Renganayagalu, S.K.; Mallam, S.C.; Nazir, S. Effectiveness of VR head mounted displays in professional training: A systematic review. Technol. Knowl. Learn. 2021, 26, 999–1041. [Google Scholar] [CrossRef]
- Krasnyanskiy, M.; Obukhov, A.; Dedov, D. Formalization of the Burning Process of Virtual Reality Objects in Adaptive Training Complexes. J. Imaging 2021, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Roldán, J.J.; Crespo, E.; Martín-Barrio, A.; Peña-Tapia, E.; Barrientos, A. A training system for Industry 4.0 operators in complex assemblies based on virtual reality and process mining. Robot. Comput. Integr. Manuf. 2019, 59, 305–316. [Google Scholar] [CrossRef]
- Barkokebas, R.; Ritter, C.; Sirbu, V.; Li, X.; Al-Hussein, M. Application of virtual reality in task training in the construction manufacturing industry. In Proceedings of the International Symposium on Automation and Robotics in Construction (ISARC 2019), Banff, AB, Canada, 21–24 May 2019; pp. 796–803. [Google Scholar]
- Marín-Morales, J.; Llinares, C.; Guixeres, J.; Alcañiz, M. Emotion recognition in immersive virtual reality: From statistics to affective computing. Sensors 2020, 20, 5163. [Google Scholar] [CrossRef]
- Krasnyansky, M.; Karpushkin, S.; Popov, A.; Obukhov, A.; Dedov, D. Methodology of forming the readiness of miners for work in extreme situations using a training complex. Int. J. Emerg. Technol. Learn. 2020, 15, 86–97. [Google Scholar] [CrossRef]
- Löcken, A.; Golling, C.; Riener, A. How should automated vehicles interact with pedestrians? A comparative analysis of interaction concepts in virtual reality. In Proceedings of the 11th International Conference on Automotive User Interfaces and Interactive Vehicular Applications (AutomotiveUI 2019), New York, NY, USA, 21–25 September 2019; pp. 262–274. [Google Scholar]
- Patle, D.S.; Manca, D.; Nazir, S.; Sharma, S. Operator training simulators in virtual reality environment for process operators: A review. Virtual Real. 2019, 23, 293–311. [Google Scholar] [CrossRef]
- Farra, S.L.; Gneuhs, M.; Hodgson, E.; Kawosa, B.; Miller, E.T.; Simon, A.; Timm, N.; Hausfeld, J. Comparative cost of virtual reality training and live exercises for training hospital workers for evacuation. Comput. Inform. Nurs. 2019, 37, 446. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, N. Virtual and augmented reality technologies for emergency management in the built environments: A state-of-the-art review. J. Saf. Sci. Resil. 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Feng, Z.; González, V.A.; Mutch, C.; Amor, R.; Rahouti, A.; Baghouz, A.; Cabrera-Guerrero, G. Towards a customizable immersive virtual reality serious game for earthquake emergency training. Adv. Eng. Inform. 2020, 46, 101134. [Google Scholar] [CrossRef]
- Mystakidis, S.; Besharat, J.; Papantzikos, G.; Christopoulos, A.; Stylios, C.; Agorgianitis, S.; Tselentis, D. Design, development, and evaluation of a virtual reality serious game for school fire preparedness training. Educ. Sci. 2022, 12, 281. [Google Scholar] [CrossRef]
- Gantt, P.; Gantt, R. Disaster psychology: Dispelling the myths of panic. Prof. Saf. 2012, 57, 42–49. [Google Scholar]
- Obukhov, A.; Dedov, D.; Volkov, A.; Teselkin, D. Modeling of Nonlinear Dynamic Processes of Human Movement in Virtual Reality Based on Digital Shadows. Computation 2023, 11, 85. [Google Scholar] [CrossRef]
- Krasnyanskiy, M.N.; Obukhov, A.D.; Dedov, D.L. Control System for an Adaptive Running Platform for Moving in Virtual Reality. Autom. Remote Control 2022, 83, 355–366. [Google Scholar] [CrossRef]
- Keung, C.C.W.; Kim, J.I.; Ong, Q.M. Developing a BIM-based MUVR treadmill system for architectural design review and collaboration. Appl. Sci. 2021, 11, 6881. [Google Scholar] [CrossRef]
- Hooks, K.; Ferguson, W.; Morillo, P.; Cruz-Neira, C. Evaluating the user experience of omnidirectional VR walking simulators. Entertain. Comput. 2020, 34, 100352. [Google Scholar] [CrossRef]
- Obukhov, A.; Dedov, D.; Arkhipov, A. Determination of the design parameters of the simulator breathing apparatus for training complexes. In IOP Conference Series: Materials Science and Engineering (IOP Publishing 2020); IOP Publishing: Bristol, UK; Sevastopol, Russian Federation, 3 January 2020; p. 022090. [Google Scholar]
- Delazio, A.; Nakagaki, K.; Klatzky, R.L.; Hudson, S.E.; Lehman, J.F.; Sample, A.P. Force jacket: Pneumatically-actuated jacket for embodied haptic experiences. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems (CHI 2018), Montreal, QC, Canada, 21–26 April 2018; pp. 1–12. [Google Scholar]
- Nelson, B.W.; Low, C.A.; Jacobson, N.; Areán, P.; Torous, J.; Allen, N.B. Guidelines for wrist-worn consumer wearable assessment of heart rate in biobehavioral research. NPJ Digit. Med. 2020, 3, 90. [Google Scholar] [CrossRef]
- Bifulco, P.; Narducci, F.; Vertucci, R.; Ambruosi, P.; Cesarelli, M.; Romano, M. Telemedicine supported by Augmented Reality: An interactive guide for untrained people in performing an ECG test. Biomed. Eng. Online 2014, 13, 153. [Google Scholar] [CrossRef]
- Chowdhury, R.H.; Reaz, M.B.; Ali, M.A.B.M.; Bakar, A.A.; Chellappan, K.; Chang, T.G. Surface electromyography signal processing and classification techniques. Sensors 2013, 13, 12431–12466. [Google Scholar] [CrossRef] [PubMed]
- Tauscher, J.P.; Schottky, F.W.; Grogorick, S.; Bittner, P.M.; Mustafa, M.; Magnor, M. Immersive EEG: Evaluating electroencephalography in virtual reality. In Proceedings of the 2019 IEEE Conference on Virtual Reality and 3D User Interfaces (VR) (IEEE 2019), Osaka, Japan, 27 March 2019; pp. 1794–1800. [Google Scholar]
- Gajbhiye, P.; Tripathy, R.K.; Bhattacharyya, A.; Pachori, R.B. Novel approaches for the removal of motion artifact from EEG recordings. IEEE Sens. J. 2019, 19, 10600–10608. [Google Scholar] [CrossRef]
- Jang, E.H.; Park, B.J.; Kim, S.H.; Chung, M.A.; Sohn, J.H. Correlation between Psychological and Physiological Responses during Fear. In Proceedings of the International Joint Conference on Biomedical Engineering Systems and Technologies-Volume 4 (BIOSTEC 2014), Angers, Loire Valley, France, 3–6 March 2014; pp. 104–109. [Google Scholar]
- Lin, H.P.; Lin, H.Y.; Lin, W.L.; Huang, A.C.W. Effects of stress, depression, and their interaction on heart rate, skin conductance, finger temperature, and respiratory rate: Sympathetic-parasympathetic hypothesis of stress and depression. J. Clin. Psychol. 2011, 67, 1080–1091. [Google Scholar] [CrossRef]
- Mevlevioğlu, D.; Murphy, D.; Tabirca, S. Visual respiratory feedback in virtual reality exposure therapy: A pilot study. In Proceedings of the ACM International Conference on Interactive Media Experiences (IMX 2021), New York, NY, USA, 21–23 June 2021; pp. 1–6. [Google Scholar]
- Shahimi, N.H.; Lim, R.; Mat, S.; Goh, C.H.; Tan, M.P.; Lim, E. Association between mental illness and blood pressure variability: A systematic review. BioMed. Eng. OnLine 2022, 21, 19. [Google Scholar] [CrossRef]
- Stein, D.J.; Aguilar-Gaxiola, S.; Alonso, J.; Bruffaerts, R.; de Jonge, P.; Liu, Z.; Caldas-De-Almeida, J.M.; O’neill, S.; Viana, M.C.; Al-Hamzawi, A.O.; et al. Associations between mental disorders and subsequent onset of hypertension. Gen. Hosp. Psychiatry 2014, 36, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Tychkov, A.Y.; Chernyshov, D.S.; Bofanova, N.S.; Alimuradov, A.K.; Ovchinnikov, D.L.; Sotnikov, A.M. Virtual reality implementation for assessment and treatment of phobic anxiety disorders. In Proceedings of the 2021 5th Scientific School Dynamics of Complex Networks and their Applications (DCNA), IEEE, Kaliningrad, Russia, 13−15 September 2021; pp. 202–205. [Google Scholar]
- Klem, G.; Lüders, H.; Jasper, H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. 1999, 52, 3–6. [Google Scholar]
- Trajkovic, J.; Di Gregorio, F.; Ferri, F.; Marzi, C.; Diciotti, S.; Romei, V. Resting state alpha oscillatory activity is a valid and reliable marker of schizotypy. Sci. Rep. 2021, 11, 10379. [Google Scholar] [CrossRef] [PubMed]
- Petrantonakis, P.C.; Hadjileontiadis, L.J. A novel emotion elicitation index using frontal brain asymmetry for enhanced EEG-based emotion recognition. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Winterer, G.; Egan, M.F.; Rädler, T.; Hyde, T.; Coppola, R.; Weinberger, D.R. An association between reduced interhemispheric EEG coherence in the temporal lobe and genetic risk for schizophrenia. Schizophr. Res. 2001, 49, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Haegens, S.; Cousijn, H.; Wallis, G.; Harrison, P.J.; Nobre, A.C. Inter-and intra-individual variability in alpha peak frequency. Neuroimage 2014, 92, 46–55. [Google Scholar] [CrossRef]
- Gordeev, S.A. Clinical-psychophysiological studies of patients with panic attacks with and without agoraphobic disorders. Neurosci. Behav. Physiol. 2008, 38, 633–637. [Google Scholar] [CrossRef]
- Roohi-Azizi, M.; Azimi, L.; Heysieattalab, S.; Aamidfar, M. Changes of the brain’s bioelectrical activity in cognition, consciousness, and some mental disorders. Med. J. Islam. Repub. Iran 2017, 31, 53. [Google Scholar] [CrossRef]
- Coan, J.A.; Allen, J.J.B.L.; McKnight, P.E. A capability model of individual differences in frontal EEG asymmetry. Biol. Psychol. 2006, 72, 198–207. [Google Scholar] [CrossRef]
- Obukhov, A.D.; Krasnyanskiy, M.N.; Dedov, D.L.; Nazarova, A.O. The study of virtual reality influence on the process of professional training of miners. Virtual Real. 2022, 27, 735–759. [Google Scholar] [CrossRef]
- Krysko, V.A.; Papkova, I.V.; Saltykova, O.A.; Yakovleva, T.V.; Pavlov, S.P.; Zhigalov, M.V.; Krysko, A.V. Visualization of amplitude-frequency characteristics of EEG of pathological and cognitive functions of the brain from a position of nonlinear dynamics. Proc. J. Phys. Conf. Ser. 2019, 1260, 072010. [Google Scholar] [CrossRef]
- Córdova, F. On the meaning of Hurst entropy applied to EEG data series. Procedia Comput. Sci. 2022, 199, 1385–1392. [Google Scholar]
- Raimundo, M.S.; Okamoto, J., Jr. Application of Hurst Exponent (H) and the R/S Analysis in the Classification of FOREX Securities. Int. J. Model. Optim. 2018, 8, 116–124. [Google Scholar] [CrossRef]
- Jokić-Begićm, N.; Begić, D. Quantitative electroencephalogram (qEEG) in combat veterans with post-traumatic stress disorder (PTSD). Nord. J. Psychiatry 2003, 57, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Li, G.; Fang, J.; Zhong, H.; Wang, J.; Sun, Y.; Shen, X. Aberrated multidimensional EEG characteristics in patients with generalized anxiety disorder: A machine-learning based analysis framework. Sensors 2022, 22, 5420. [Google Scholar] [CrossRef]
- Same, M.H.; Gandubert, G.; Gleeton, G.; Ivanov, P.; Landry, R., Jr. Simplified welch algorithm for spectrum monitoring. Appl. Sci. 2020, 11, 86. [Google Scholar] [CrossRef]
- Weiss, S.; Rappelsberger, P. Long-range EEG synchronization during word encoding correlates with successful memory performance. Cogn. Brain Res. 2000, 9, 299–312. [Google Scholar] [CrossRef]
- Gunduz, A.; Principe, J.C. Correntropy as a novel measure for nonlinearity tests. Signal Process. 2009, 89, 14–23. [Google Scholar] [CrossRef]
- Schroeter, M.L.; Bücheler, M.M.; Müller, K.; Uludağ, K.; Obrig, H.; Lohmann, G.; Tittgemeyer, M.; Villringer, A.; von Cramon, D. Towards a standard analysis for functional near-infrared imaging. NeuroImage 2004, 21, 283–290. [Google Scholar] [CrossRef]
- Chernykh, M.; Vodianyk, B.; Seleznov, I.; Harmatiuk, D.; Zyma, I.; Popov, A.; Kiyono, K. Detrending Moving Average, Power Spectral Density, and Coherence: Three EEG-Based Methods to Assess Emotion Irradiation during Facial Perception. Appl. Sci. 2022, 12, 7849. [Google Scholar] [CrossRef]
- Faes, L.; Pinna, G.D.; Porta, A.; Maestri, R.; Nollo, G. Surrogate data analysis for assessing the significance of the coherence function. IEEE Trans. Biomed. Eng. 2004, 51, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Krajčovič, M.; Gabajová, G.; Matys, M.; Grznár, P.; Dulina, Ľ.; Kohár, R. 3D Interactive Learning Environment as a Tool for Knowledge Transfer and Retention. Sustainability 2021, 13, 7916. [Google Scholar] [CrossRef]
- Polák, J.; Rádlová, S.; Janovcová, M.; Flegr, J.; Landová, E.; Frynta, D. Scary and nasty beasts: Self-reported fear and disgust of common phobic animals. Br. J. Psychol. 2020, 111, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Eaton, W.W.; Bienvenu, O.J.; Miloyan, B. Specific phobias. Lancet Psychiatry 2018, 5, 678–686. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Hämäläinen, M.S. MNE software for processing MEG and EEG data. Neuroimage 2014, 86, 446–460. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, Y.; Kong, X. Review on emotion recognition based on electroencephalography. Front. Comput. Neurosci. 2021, 15, 84. [Google Scholar] [CrossRef]



| Scene Number | Exercise Type | Environment Type |
|---|---|---|
| 1 | 1. Puzzle (assembling 10 puzzle pieces) | 1. Norm |
| 2 | 2. Arachnophobia | |
| 3 | 3. Acrophobia | |
| 4 | 2. Shooting (10 shots at moving objects) | 1. Norm |
| 5 | 2. Arachnophobia | |
| 6 | 3. Acrophobia |
| Metric | Environment | -Value (Kruskal–Wallis) | -Value (Mann–Whitney) | ||
|---|---|---|---|---|---|
| 1. Norm | 2. Arachnophobia | 3. Acrophobia | |||
| 90.321 ± 52.6 | 69.679 ± 27.885 | 72.5 ± 30.291 | 0.477 | - | |
| 36.54 ± 23.088 | 37.669 ± 18.741 | 42.641 ± 22.734 | 0.593 | - | |
| 0.145 ± 0.067 | 0.17 ± 0.074 | 0.16 ± 0.059 | 0.506 | - | |
| PSD (Alpha) | 6.678 ± 7.559 | 22.862 ± 101.149 | 10.563 ± 28.168 | 0.681 | |
| PSD (Beta) | 20.256 ± 134.652 | 21.565 ± 171.432 | 15.368 ± 51.471 | 0.927 | |
| PSD (Theta) | 9.169 ± 11.548 | 59.8 ± 535.01 | 15.483 ± 50.036 | 0.184 | |
| 130.619 ± 261.154 | 113.402 ± 342.393 | 100.746 ± 95.771 | 0.0 | 1–2 (0.0), 1–3 (0.021), 2–3 (0.001) | |
| 0.091 ± 0.415 | 0.157 ± 0.291 | 0.088 ± 0.323 | 0.0 | 1–2 (0.0), 1–3 (0.0), 2–3 (0.0) | |
| 20.069 ± 18.951 | 20.869 ± 47.739 | 18.752 ± 15.571 | - | ||
| 0.873 ± 0.045 | 0.871 ± 0.043 | 0.871 ± 0.045 | - | ||
| Metric | Environment | -Value (Kruskal–Wallis) | -Value (Mann–Whitney) | ||
|---|---|---|---|---|---|
| 1. Norm | 2. Arachnophobia | 3. Acrophobia | |||
| 24.643 ± 16.395 | 24.5 ± 13.276 | 19.143 ± 11.031 | 0.175 | - | |
| 60.617 ± 19.131 | 50.965 ± 20.79 | 55.814 ± 18.014 | 0.247 | - | |
| 0.613 ± 0.456 | 0.518 ± 0.222 | 0.687 ± 0.33 | 0.176 | - | |
| PSD (Alpha) | 4.955 ± 3.679 | 6.195 ± 8.151 | 7.688 ± 18.292 | 0.818 | |
| PSD (Beta) | 10.338 ± 49.212 | 12.02 ± 67.048 | 10.355 ± 27.168 | 0.577 | |
| PSD (Theta) | 5.847 ± 4.884 | 9.359 ± 33.705 | 18.914 ± 99.536 | 0.0 | 1–2 (0.0), 1–3 (0.009) |
| 64.476 ± 93.356 | 74.386 ± 129.27 | 61.312 ± 47.307 | 0.001 | 1–2 (0.0), 2–3 (0.011) | |
| 0.047 ± 0.716 | 0.196 ± 0.942 | 0.217 ± 1.258 | 0.0 | 1–2 (0.0), 1–3 (0.0) | |
| 14.688 ± 11.75 | 16.661 ± 18.383 | 15.52 ± 9.523 | - | ||
| 0.854 ± 0.049 | 0.855 ± 0.042 | 0.852 ± 0.05 | - | ||
| Exercise | Number of Clusters | Metric | KMeans | Birch | SpectralClustering |
|---|---|---|---|---|---|
| Puzzle | 2 | 0.0 | 0.0 | 0.036 | |
| 0.0 | 0.0 | 0.848 | |||
| 0.0 | 0.0 | 0.034 | |||
| 3 | 0.0 | 0.0 | 0.009 | ||
| 0.0 | 0.0 | 0.012 | |||
| 0.0 | 0.0 | 0.007 | |||
| Shooting | 2 | 0.0 | 0.0 | 0.0 | |
| 0.0 | 0.0 | 0.0 | |||
| 0.0 | 0.0 | 0.0 | |||
| 3 | 0.0 | 0.0 | 0.102 | ||
| 0.0 | 0.0 | 0.69 | |||
| 0.0 | 0.0 | 0.102 |
| Environment | Metric | Subgroup | * -Value | |
|---|---|---|---|---|
| First | Second | |||
| 1. Norm | 65.14 ± 23.31 | 165.86 ± 42.78 * | 0.0 | |
| 29.32 ± 21.14 | 58.19 ± 12.89 | 0.005 | ||
| 0.17 ± 0.06 | 0.07 ± 0.02 * | 0.0 | ||
| PSD (Alpha) | 13.26 ± 9.64 | 8.38 ± 3.92 | 0.0 | |
| PSD (Beta) | 4.64 ± 3.24 | 2.78 ± 1.44 | 0.0 | |
| PSD (Theta) | 14.05 ± 13.39 * | 7.77 ± 3.07 * | 0.0 | |
| 138.42 ± 96.31 * | 108.37 ± 70.9 * | 0.0 | ||
| 0.02 ± 0.17 * | −0.02 ± 0.05 * | 0.0 | ||
| 20.96 ± 10.67 * | 15.94 ± 6.36 * | 0.0 | ||
| 0.87 ± 0.04 * | 0.86 ± 0.04 * | 0.0 | ||
| 2. Arachnophobia | 59.67 ± 22.77 | 99.71 ± 18.74 * | 0.003 | |
| 30.12 ± 13.9 | 60.33 ± 11.86 | 0.001 | ||
| 0.19 ± 0.07 | 0.1 ± 0.02 * | 0.002 | ||
| PSD (Alpha) | 63.41 ± 171.31 | 8.74 ± 2.83 | 0.0 | |
| PSD (Beta) | 18.7 ± 45.73 | 2.75 ± 1.08 | 0.0 | |
| PSD (Theta) | 90.28 ± 281.38 * | 7.42 ± 1.76 * | 0.086 | |
| 131.44 ± 144.93 * | 88.33 ± 26.28 * | 0.895 | ||
| 0.08 ± 0.12 * | 0.06 ± 0.08 * | 0.234 | ||
| 21.91 ± 18.19 * | 15.24 ± 4.0 * | 0.047 | ||
| 0.87 ± 0.04 * | 0.87 ± 0.02 * | 0.895 | ||
| 3. Acrophobia | 59.95 ± 21.79 | 110.14 ± 18.87 * | 0.001 | |
| 34.07 ± 19.65 | 68.37 ± 5.17 | 0.003 | ||
| 0.18 ± 0.05 | 0.09 ± 0.02 * | 0.0 | ||
| PSD (Alpha) | 25.22 ± 46.12 | 8.68 ± 3.7 | 0.0 | |
| PSD (Beta) | 6.71 ± 10.37 | 2.75 ± 1.5 | 0.0 | |
| PSD (Theta) | 31.56 ± 83.68 * | 8.11 ± 2.87 * | 0.012 | |
| 119.6 ± 77.92 * | 97.45 ± 50.69 * | 0.005 | ||
| −0.0 ± 0.16 * | −0.02 ± 0.07 * | 0.355 | ||
| 20.52 ± 13.51 * | 16.21 ± 5.8 * | 0.047 | ||
| 0.87 ± 0.04 * | 0.87 ± 0.03 * | 0.086 | ||
| Number of participants in the subgroup | 21 | 7 | ||
| Environment | Metric | Subgroup | * -Value | |
|---|---|---|---|---|
| First | Second | |||
| 1. Norm | 18.56 ± 15.95 | 32.75 ± 13.15 | 0.002 | |
| 49.54 ± 14.86 | 75.39 ± 13.32 | 0.0 | ||
| 0.8 ± 0.51 | 0.36 ± 0.15 | 0.002 | ||
| PSD (Alpha) | 8.71 ± 3.69 | 7.11 ± 1.83 | 0.0 | |
| PSD (Beta) | 3.72 ± 2.15 * | 2.67 ± 1.05 * | 0.0 | |
| PSD (Theta) | 8.02 ± 3. 9* | 7.45 ± 3.33 * | 0.165 | |
| 70.48 ± 51.35 * | 63.42 ± 14.71 * | 0.0 | ||
| −0.05 ± 0.68 * | 0.05 ± 0.32 * | 0.0 | ||
| 14.9 ± 6.66 * | 14.63 ± 5.36 * | 0.064 | ||
| 0.83 ± 0.05 * | 0.86 ± 0.02 * | 0.0 | ||
| 2. Arachnophobia | 16.75 ± 5.12 | 34.83 ± 13.76 | 0.001 | |
| 39.06 ± 16.95 | 66.84 ± 13.58 | 0.0 | ||
| 0.65 ± 0.17 | 0.34 ± 0.15 | 0.001 | ||
| PSD (Alpha) | 11.51 ± 10.05 | 10.24 ± 11.08 | 0.0 | |
| PSD (Beta) | 4.8 ± 3.48 * | 3.06 ± 2.46 * | 0.0 | |
| PSD (Theta) | 11.18 ± 9.98 * | 10.88 ± 9.77 * | 0.105 | |
| 75.72 ± 49.48 * | 79.81 ± 46.51 * | 0.064 | ||
| 0.33 ± 1.33 * | 0.05 ± 0.26 * | 0.064 | ||
| 16.77 ± 10.6 * | 16.07 ± 6.76 * | 0.817 | ||
| 0.82 ± 0.04 * | 0.86 ± 0.02 * | 0.0 | ||
| 3. Acrophobia | 12.75 ± 4.71 | 27.67 ± 11.28 | 0.0 | |
| 46.12 ± 14.22 | 68.74 ± 13.97 | 0.001 | ||
| 0.88 ± 0.27 | 0.43 ± 0.21 | 0.0 | ||
| PSD (Alpha) | 12.39 ± 16.53 | 17.94 ± 35.89 | 0.0 | |
| PSD (Beta) | 4.51 ± 3.46 * | 8.5 ± 20.38 * | 0.0 | |
| PSD (Theta) | 9.76 ± 7.35 * | 76.09 ± 226.87 * | 0.817 | |
| 69.93 ± 41.31 * | 75.77 ± 41.46 * | 0.0 | ||
| 0.31 ± 1.3 * | 0.05 ± 0.62 * | 0.487 | ||
| 16.9 ± 9.41 * | 17.15 ± 7.78 * | 0.064 | ||
| 0.81 ± 0.05 * | 0.87 ± 0.02 * | 0.0 | ||
| Number of participants in the subgroup | 16 | 12 | ||
| Exercise/Environment | EEG Derivations | The First Subgroup (21 Participants) | The Second Subgroup (7 Participants) | ||||
|---|---|---|---|---|---|---|---|
| Alpha | Beta | Theta | Alpha | Beta | Theta | ||
| Puzzle/ Norm | FP2-FP1 | −1.0 ± 25.3 | −1.1 ± 28.7 | 1.7 ± 31.9 | −2.4 ± 4.0 ↓ | 4.5 ± 18.9 ↑ | −8.2 ± 13.1 ↓ |
| F4-F3 | −0.4 ± 26.0 | 1.5 ± 26.9 | −6.1 ± 29.7 | 6.6 ± 13.3 ↑ | 9.8 ± 18.0 ↑ | −0.6 ± 22.8 ↑ | |
| C4-C3 | 9.5 ± 25.6 | 6.0 ± 30.0 | 5.4 ± 29.9 | 10.1 ± 20.0 ↑ | 17.8 ± 15.1 ↑ | −5.6 ± 17.7 ↓ | |
| P4-P3 | −3.2 ± 17.6 | −7.2 ± 20.4 | 1.3 ± 16.2 | −0.4 ± 9.2 ↑ | 0.3 ± 9.9 ↑ | −0.9 ± 10.4 ↓ | |
| O2-O1 | −3.3 ± 30.1 | −2.9 ± 35.9 | −2.7 ± 27.2 | 3.8 ± 25.8 ↑ | −9.4 ± 26.6 ↓ | 2.5 ± 11.2 ↑ | |
| F8-F7 | −3.2 ± 32.9 | 8.1 ± 35.0 | −2.1 ± 30.9 | 10.9 ± 15.5 ↑ | 25.4 ± 7.7 ↑ | 12.4 ± 18.0 ↑ | |
| T4-T3 | 4.4 ± 19.6 | 5.8 ± 32.1 | 4.5 ± 22.6 | −17.2 ± 18.6 ↓ | −15.7 ± 28.0 ↓ | −14.0 ± 22.4 ↓ | |
| T6-T5 | −5.2 ± 26.4 | −11.6 ± 34.0 | −5.6 ± 25.0 * | −17.4 ± 22.0 ↓ | −17.8 ± 20.2 ↓ | −27.1 ± 24.1 * ↓ | |
| Puzzle/ Arachnophobia | FP2-FP1 | −0.9 ± 20.4 | −1.2 ± 20.7 | −3.7 ± 23.6 | −1.7 ± 3.5 ↓ | 8.5 ± 22.1 ↑ | −8.9 ± 8.5 ↓ |
| F4-F3 | 8.3 ± 16.2 | 7.7 ± 20.8 | 4.1 ± 18.8 | 8.9 ± 15.1 ↑ | 9.4 ± 19.9 ↑ | 2.9 ± 20.9 ↓ | |
| C4-C3 | 16.9 ± 32.3 | 7.0 ± 35.0 | 9.4 ± 36.0 | 17.2 ± 20.0 ↑ | 15.1 ± 15.7 ↑ | 7.5 ± 19.1 ↓ | |
| P4-P3 | −0.5 ± 14.0 | −4.9 ± 20.5 | −2.2 ± 17.3 | 0.7 ± 11.6 ↑ | 3.8 ± 7.2 ↑ | 2.6 ± 7.7 ↑ | |
| O2-O1 | 2.4 ± 37.5 | 2.9 ± 39.5 | 2.6 ± 32.7 | −2.5 ± 24.1 ↓ | −12.9 ± 27.4 ↓ | 7.2 ± 17.5 ↑ | |
| F8-F7 | 3.4 ± 25.2 | 7.9 ± 26.4 | 0.9 ± 27.4 | 10.1 ± 14.5 ↑ | 27.1 ± 12.4 ↑ | 12.4 ± 18.8 ↑ | |
| T4-T3 | 0.5 ± 21.0 | 3.5 ± 31.9 | −4.9 ± 24.6 | −15.2 ± 20.1 ↓ | −16.4 ± 28.4 ↓ | −10.2 ± 18.4 ↓ | |
| T6-T5 | −8.4 ± 23.9 | −10.7 ± 32.8 | −12.2 ± 24.0 | −11.3 ± 20.0 ↓ | −15.2 ± 17.0 ↓ | −11.2 ± 11.1 ↑ | |
| Puzzle/ Acrophobia | FP2-FP1 | 0.1 ± 16.7 | 2.8 ± 21.0 | −4.5 ± 27.2 | 2.2 ± 10.7 ↑ | 12.7 ± 19.5 ↑ | −7.1 ± 18.1 ↓ |
| F4-F3 | 3.4 ± 17.6 | 5.0 ± 20.3 | 1.2 ± 20.2 | 9.7 ± 13.8 ↑ | 12.7 ± 18.8 ↑ | −1.7 ± 22.8 ↓ | |
| C4-C3 | 14.3 ± 29.3 | 5.7 ± 34.3 | 3.4 ± 39.2 | 16.9 ± 19.2 ↑ | 14.0 ± 12.3 ↑ | −1.0 ± 20.2 ↓ | |
| P4-P3 | 3.5 ± 16.0 | −2.1 ± 21.1 | −1.7 ± 15.6 | 0.9 ± 13.2 ↓ | 1.8 ± 9.0 ↑ | 1.0 ± 10.7 ↑ | |
| O2-O1 | −3.8 ± 39.3 | −1.3 ± 41.6 | −4.5 ± 32.9 | −6.6 ± 25.3 ↓ | −14.4 ± 24.9 ↓ | 2.0 ± 12.7 ↑ | |
| F8-F7 | −1.6 ± 23.7 | 13.9 ± 25.3 | −5.2 ± 19.5 | 7.0 ± 14.2 ↑ | 21.7 ± 14.9 ↑ | 2.3 ± 18.3 ↑ | |
| T4-T3 | 1.9 ± 20.2 | 3.0 ± 33.7 | −3.2 ± 24.8 | −16.7 ± 23.6 ↓ | −15.9 ± 31.8 ↓ | −12.6 ± 19.7 ↓ | |
| T6-T5 | −5.2 ± 19.8 | −10.6 ± 31.2 | −6.5 ± 22.4 | −12.5 ± 18.5 ↓ | −12.0 ± 14.2 ↓ | −13.4 ± 14.0 ↓ | |
| Exercise/Environment | EEG Derivations | The First Subgroup (21 Participants) | The Second Subgroup (7 Participants) | ||||
|---|---|---|---|---|---|---|---|
| Alpha | Beta | Theta | Alpha | Beta | Theta | ||
| Shooting/ Norm | FP2-FP1 | −1.6 ± 21.0 | 2.0 ± 27.4 | −4.2 ± 29.5 | 5.0 ± 8.5 ↑ | 7.6 ± 15.5 ↑ | 1.6 ± 12.5 ↑ |
| F4-F3 | −2.7 ± 26.4 | 2.0 ± 27.3 | −4.8 ± 29.7 | 11.6 ± 11.6 ↑ | 13.6 ± 14.3 ↑ | 5.3 ± 16.9 ↑ | |
| C4-C3 | 0.3 ± 24.9 | −2.9 ± 30.9 | −0.4 ± 24.0 | 12.1 ± 24.4 ↑ | 15.3 ± 22.6 ↑ | 4.1 ± 32.4 ↑ | |
| P4-P3 | 0.4 ± 16.5 | −8.7 ± 24.5 | −5.2 ± 11.0 | 0.5 ± 15.5 ↑ | 1.1 ± 24.0 ↑ | −0.9 ± 20.0 ↑ | |
| O2-O1 | 4.7 ± 31.3 | 3.6 ± 35.9 | −1.5 ± 16.1 | −9.6 ± 34.0 ↓ | −13.8 ± 35.9 ↓ | −6.8 ± 29.2 ↓ | |
| F8-F7 | −5.8 ± 29.3 | 0.2 ± 23.1 * | −6.2 ± 23.1 | 11.0 ± 20.7 ↑ | 23.7 ± 24.4 * ↑ | 11.4 ± 19.0 ↑ | |
| T4-T3 | 0.3 ± 27.8 | −2.2 ± 38.9 | −2.2 ± 22.2 | −6.9 ± 15.1 ↓ | −1.2 ± 31.2 ↑ | −5.6 ± 16.8 ↓ | |
| T6-T5 | −9.7 ± 23.2 | −18.3 ± 28.0 | −12.5 ± 26.2 | −16.8 ± 21.6 ↓ | −15.2 ± 26.6 ↑ | −6.7 ± 22.3 ↑ | |
| Shooting/ Arachnophobia | FP2-FP1 | 0.2 ± 18.7 | 3.3 ± 31.0 | 1.1 ± 26.8 | 7.1 ± 7.2 ↑ | 10.7 ± 17.1 ↑ | 4.5 ± 17.0 ↑ |
| F4-F3 | 6.6 ± 20.8 | 2.5 ± 28.5 | 0.6 ± 21.8 | 11.2 ± 13.2 ↑ | 13.3 ± 14.5 ↑ | 5.2 ± 16.7 ↑ | |
| C4-C3 | 6.0 ± 26.2 | −3.5 ± 25.5 | −2.7 ± 22.9 | 15.0 ± 43.0 ↑ | 15.1 ± 41.9 ↑ | 3.5 ± 46.5 ↑ | |
| P4-P3 | 2.8 ± 20.3 | −6.6 ± 24.3 | −1.1 ± 13.2 | 5.2 ± 17.0 ↑ | 4.7 ± 20.6 ↑ | −3.0 ± 17.2 ↓ | |
| O2-O1 | 4.4 ± 28.0 | −1.5 ± 35.4 | 3.1 ± 19.3 | 3.1 ± 34.7 ↓ | −5.4 ± 34.9 ↓ | −0.5 ± 31.0 ↓ | |
| F8-F7 | 4.4 ± 14.9 | 7.8 ± 15.0 * | −1.4 ± 17.9 * | 15.5 ± 21.1 ↑ | 27.8 ± 19.9 * ↑ | 15.6± 23.1 * ↑ | |
| T4-T3 | −2.2 ± 27.0 | −6.8 ± 39.4 | −1.2 ± 24.5 | −8.5 ± 24.5 ↓ | −5.3 ± 35.6 ↑ | −7.7 ± 22.1 ↓ | |
| T6-T5 | −8.8 ± 26.2 | −11.6± 32.4 | −11.7 ± 27.2 | −6.8 ± 21.0 ↑ | −4.4 ± 20.7 ↑ | −8.1 ± 12.4 ↑ | |
| Shooting/ Acrophobia | FP2-FP1 | 3.3 ± 17.8 | 7.9 ± 23.7 | −1.8 ± 24.3 | 4.8 ± 11.4 ↑ | 5.2 ± 14.4 ↓ | 0.2± 16.2 ↑ |
| F4-F3 | 1.0 ± 15.6 | 3.1 ± 22.3 | −5.8 ± 16.2 | 12.8 ± 14.1 ↑ | 11.1 ± 12.1 ↑ | 4.4 ± 12.8 ↑ | |
| C4-C3 | −0.6 ± 25.8 * | −4.8 ± 31.7* | −4.0 ± 28.5 | 25.0 ± 26.7 * ↑ | 22.8 ± 24.6* ↑ | 6.9 ± 28.6 ↑ | |
| P4-P3 | 3.8 ± 15.5 | 0.9 ± 21.2 | −0.8 ± 16.4 | 3.3 ± 16.8 ↓ | 4.4 ± 24.8 ↑ | −3.0 ± 8.8 ↓ | |
| O2-O1 | 2.1 ± 28.4 | 5.6 ± 31.4 | 3.3 ± 18.9 | −10.3 ± 31.0 ↓ | −11.8 ± 34.8 ↓ | −8.2 ± 28.9 ↓ | |
| F8-F7 | 2.5 ± 19.7 * | 7.9 ± 22.9 | −8.9 ± 26.4 * | 21.1 ± 19.2 * ↑ | 25.5 ± 25.0 ↑ | 17.4 ± 18.4 * ↑ | |
| T4-T3 | −0.0 ± 26.0 | −7.4 ± 38.7 | −7.4 ± 29.2 | −0.0 ± 17.6 ↑ | 2.4 ± 28.5 ↑ | −5.7 ± 18.6 ↑ | |
| T6-T5 | −2.7 ± 26.2 | −10.6 ± 40.3 | −13.7 ± 21.8 | −1.8 ± 36.9 ↑ | −2.0 ± 34.2 ↑ | 2.0 ± 33.5 ↑ | |
| Exercise/Environment | Metric | Subgroup | * -Value | |
|---|---|---|---|---|
| Normal | Abnormal | |||
| Puzzle/ Norm | 96.42 ± 54.1 | 53.75 ± 15.77 | 0.088 | |
| 39.34 ± 23.57 | 19.76 ± 8.41 | 0.115 | ||
| 0.14 ± 0.06 | 0.2 ± 0.05 | 0.094 | ||
| PSD (Alpha) | 10.79 ± 5.92 * | 19.55 ± 16.42 * | 0.0 | |
| PSD (Beta) | 3.79 ± 2.6 * | 6.47 ± 4.1 * | 0.0 | |
| PSD (Theta) | 10.8 ± 10.62 * | 22.57 ± 14.62 * | 0.0 | |
| 130.39 ± 95.04 * | 134.01 ± 66.85 * | 0.743 | ||
| −0.02 ± 0.11 * | 0.18 ± 0.2 * | 0.0 | ||
| 18.95 ± 10.05 * | 24.22 ± 8.45 * | 0.0 | ||
| 0.87 ± 0.04 | 0.89 ± 0.03 | 0.0 | ||
| Puzzle/ Arachnophobia | 72.79 ± 28.67 | 51.0 ± 10.17 | 0.168 | |
| 39.54 ± 19.42 | 26.42 ± 6.95 | 0.168 | ||
| 0.16 ± 0.08 | 0.2 ± 0.05 | 0.167 | ||
| PSD (Alpha) | 38.79 ± 143.33 * | 115.42 ± 172.38 * | 0.0 | |
| PSD (Beta) | 9.21 ± 30.18 * | 47.77 ± 67.65 * | 0.0 | |
| PSD (Theta) | 60.19 ± 254.07 * | 125.81 ± 183.55 * | 0.0 | |
| 104.78 ± 120.26 * | 215.96 ± 128.65 * | 0.0 | ||
| 0.06 ± 0.1 * | 0.17 ± 0.12 * | 0.0 | ||
| 17.55 ± 14.88 * | 36.4 ± 13.84 * | 0.0 | ||
| 0.86 ± 0.03 | 0.9 ± 0.04 | 0.0 | ||
| Puzzle/ Acrophobia | 77.67 ± 29.71 | 41.5 ± 2.5 | 0.005 | |
| 44.68 ± 23.6 | 30.38 ± 10.05 | 0.264 | ||
| 0.15 ± 0.05 | 0.24 ± 0.01 | 0.005 | ||
| PSD (Alpha) | 18.33 ± 38.06 * | 37.61 ± 50.4 * | 0.05 | |
| PSD (Beta) | 5.16 ± 9.08 * | 9.12 ± 8.98 * | 0.0 | |
| PSD (Theta) | 23.86 ± 77.05 * | 36.74 ± 41.66 * | 0.0 | |
| 110.48 ± 70.5 * | 135.58 ± 81.53 * | 0.05 | ||
| 0.01 ± 0.14 * | −0.09 ± 0.14 * | 0.0 | ||
| 17.92 ± 10.37 * | 28.58 ± 17.28 * | 0.0 | ||
| 0.86 ± 0.04 | 0.89 ± 0.03 | 0.0 | ||
| Shooting/ Norm | 26.38 ± 16.91 | 14.25 ± 6.3 | 0.211 | |
| 62.8 ± 18.67 | 47.5 ± 16.39 | 0.222 | ||
| 0.54 ± 0.3 | 1.06 ± 0.83 | 0.211 | ||
| PSD (Alpha) | 7.19 ± 2.04 * | 13.01 ± 3.86 * | 0.0 | |
| PSD (Beta) | 2.81 ± 1.39 * | 6.03 ± 1.81 * | 0.0 | |
| PSD (Theta) | 6.78 ± 2.7* | 13.75 ± 3.06 * | 0.0 | |
| 63.39 ± 40.45 * | 91.85 ± 27.78 * | 0.0 | ||
| 0.01 ± 0.53 * | −0.16 ± 0.7 * | 0.513 | ||
| 13.88 ± 6.1 * | 20.19 ± 2.54 * | 0.0 | ||
| 0.84 ± 0.04 * | 0.84 ± 0.05 * | 0.022 | ||
| Shooting/ Arachnophobia | 26.17 ± 13.62 | 14.5 ± 2.06 | 0.07 | |
| 51.54 ± 21.96 | 47.5 ± 10.9 | 0.489 | ||
| 0.49 ± 0.22 | 0.7 ± 0.1 | 0.07 | ||
| PSD (Alpha) | 9.74 ± 8.6 * | 18.31 ± 16.36 * | 0.0 | |
| PSD (Beta) | 3.67 ± 2.98 * | 6.35 ± 3.5 * | 0.0 | |
| PSD (Theta) | 9.43 ± 7.51 * | 20.77 ± 15.37 * | 0.0 | |
| 72.27 ± 38.21 * | 108.7 ± 80.08 * | 0.0 | ||
| 0.01 ± 0.28 * | 1.43 ± 2.28 * | 0.0 | ||
| 14.96 ± 5.77 * | 25.54 ± 17.09 * | 0.0 | ||
| 0.84 ± 0.03 * | 0.83 ± 0.05* | 0.743 | ||
| Shooting/ Acrophobia | 19.92 ± 11.52 | 14.5 ± 5.55 | 0.411 | |
| 55.12 ± 19.15 | 60.0 ± 7.07 | 0.62 | ||
| 0.67 ± 0.34 | 0.77 ± 0.21 | 0.411 | ||
| PSD (Alpha) | 13.09 ± 25.92 * | 24.84 ± 29.34 * | 0.0 | |
| PSD (Beta) | 6.06 ± 14.67 * | 7.16 ± 5.38 * | 0.0 | |
| PSD (Theta) | 41.64 ± 164.09 * | 17.48 ± 10.53 * | 0.0 | |
| 67.84 ± 35.92 * | 100.02 ± 58.45 * | 0.0 | ||
| 0.01 ± 0.58 * | 1.35 ± 2.1 * | 0.0 | ||
| 15.87 ± 7.01 * | 23.86 ± 13.67 * | 0.0 | ||
| 0.84 ± 0.05 * | 0.84 ± 0.06 * | 0.513 | ||
| Number of participants in the subgroup | 24 | 4 | ||
| Exercise/Environment | EEG Derivation | Normal Subgroup (24 Participants) | Abnormal Subgroup (4 Participants) | ||||
|---|---|---|---|---|---|---|---|
| Alpha | Beta | Theta | Alpha | Beta | Theta | ||
| Puzzle/ Norm | FP2-FP1 | −0.2 ± 20.4 | −0.3 ± 26.2 | 1.7 ± 28.3 | −8.4 ± 28.9 ↓ | 4.0 ± 29.1 ↑ | −15.4 ± 27.0 ↓ |
| F4-F3 | 0.5 ± 25.4 | 2.6 ± 26.3 | −6.7 ± 29.7 | 6.2 ± 1.4 ↑ | 9.7 ± 15.3 ↑ | 7.6 ± 10.3 ↑ | |
| C4-C3 | 12.9 ± 23.7 | 12.1 ± 26.3 | 7.4 ± 26.5 * | −9.9 ± 18.0 ↓ | −10.4 ± 26.8 ↓ | −25.8 ± 15.2 * ↓ | |
| P4-P3 | −1.6 ± 16.7 | −5.3± 19.4 | 0.4 ± 16.1 | −7.8 ± 8.6 ↓ | −5.5 ± 13.1 ↓ | 2.8 ± 4.7 ↑ | |
| O2-O1 | −2.6 ± 30.8 | −8.1± 34.2 | −2.1 ± 25.1 | 5.1 ± 14.9 ↑ | 16.8 ± 22.6 ↑ | 2.8 ± 18.3 ↑ | |
| F8-F7 | 0.9 ± 32.3 | 13.5 ± 33.1 | 3.4 ± 30.5 | −3.2 ± 9.1 ↓ | 6.4 ± 17.3 ↓ | −9.9 ± 11.7 ↓ | |
| T4-T3 | −1.9 ± 20.0 | 1.1 ± 32.2 | −2.8 ± 21.3 | 4.5 ± 28.2 ↑ | −3.7 ± 34.2 ↓ | 16.3 ± 31.3 ↑ | |
| T6-T5 | −8.3 ± 24.0 | −14.8 ± 27.5 | −11.0 ± 27.4 | −8.1 ± 35.2 ↑ | −2.9 ± 46.4 ↑ | −11.0 ± 20.0 ↑ | |
| Puzzle/ Arachnophobia | FP2-FP1 | −0.2 ± 15.2 | 2.3 ± 20.2 | −3.9 ± 21.3 | −6.7 ± 28.2 ↓ | −5.3 ± 27.0 ↓ | −11.9 ± 17.7 ↓ |
| F4-F3 | 9.0 ± 17.0 | 8.8 ± 19.8 | 3.3 ± 20.2 | 5.2 ± 5.8 ↓ | 4.3 ± 24.4 ↓ | 6.9 ± 13.3 ↑ | |
| C4-C3 | 21.7 ± 27.2 | 12.0 ± 30.6 | 15.4 ± 29.4 * | −11.4 ± 28.3 ↓ | −8.8 ± 31.0 ↓ | −29.9 ± 22.0 * ↓ | |
| P4-P3 | 0.4 ± 14.4 | −3.4 ± 19.3 | −2.9 ± 16.0 | −3.7 ± 2.8 ↓ | 1.8 ± 11.3 ↑ | 10.4 ± 5.8 ↑ | |
| O2-O1 | −3.8 ± 30.3 | −8.7 ± 31.9 * | −0.1 ± 24.2 | 31.5 ± 43.2 ↑ | 44.6 ± 36.4 * ↑ | 26.9 ± 45.1 ↑ | |
| F8-F7 | 5.9 ± 24.6 | 14.3 ± 25.9 | 6.6 ± 26.8 | 0.1 ± 8.7 ↓ | 2.9 ± 17.1 ↓ | −13.3 ± 9.3 ↓ | |
| T4-T3 | −2.4 ± 22.4 | −0.4 ± 31.7 | −6.3 ± 24.6 | −9.6 ± 16.7 ↓ | −8.2 ± 34.5 ↓ | −6.1 ± 13.5 ↑ | |
| T6-T5 | −7.8 ± 20.9 | −13.9 ± 26.2 | −9.7 ± 18.2 | −17.1 ± 31.8 ↓ | 0.9 ± 43.1 ↑ | −25.4 ± 32.3 ↓ | |
| Puzzle/ Acrophobia | FP2-FP1 | 1.5 ± 14.6 | 5.2 ± 21.6 | −3.0 ± 25.0 | −5.1 ± 19.0 ↓ | 6.1 ± 17.7 ↑ | −17.7 ± 23.1 ↓ |
| F4-F3 | 4.7 ± 17.9 | 6.6 ± 21.6 | −1.2 ± 21.1 | 6.6 ± 8.6 ↑ | 8.6 ± 8.7 ↑ | 10.7 ± 16.6 ↑ | |
| C4-C3 | 18.5 ± 25.5 | 9.7 ± 30.5 | 7.6 ± 35.1 * | −6.3 ± 27.0 ↓ | −4.1 ± 28.3 ↓ | −29.7 ± 14.8 * ↓ | |
| P4-P3 | 2.8 ± 16.6 | −1.7 ± 20.1 | −2.0 ± 15.5 | 3.0 ± 2.7 ↑ | 2.7 ± 6.9 ↑ | 4.9 ± 3.7 ↑ | |
| O2-O1 | −6.4 ± 38.2 | −9.8 ± 38.8 | −3.8 ± 31.0 | 6.9 ± 18.9 ↑ | 26.8 ± 15.8 ↑ | 2.4 ± 15.0 ↑ | |
| F8-F7 | 0.5 ± 22.7 | 16.7 ± 23.5 | −2.4 ± 20.6 | 0.7 ± 18.0 ↑ | 10.7 ± 22.1 ↓ | −8.4 ± 8.9 ↓ | |
| T4-T3 | −2.5 ± 22.5 | 0.5 ± 34.4 | −8.4 ± 20.7 | −3.9 ± 22.8 ↓ | −14.7 ± 30.3 ↓ | 11.5 ± 33.4 ↑ | |
| T6-T5 | −6.2 ± 17.2 | −11.9 ± 25.7 | −6.8 ± 19.0 | −12.0 ± 30.2 ↓ | −5.3 ± 38.0 ↑ | −16.3 ± 28.5 ↓ | |
| Shooting/ Norm | FP2-FP1 | 0.2 ± 17.3 | 5.3 ± 22.2 | 0.3 ± 23.6 | 7.1 ± 14.8 ↑ | −0.7 ± 28.0 ↓ | −13.9 ± 22.1 ↓ |
| F4-F3 | 2.7 ± 23.4 | 6.6 ± 24.7 | −1.9 ± 25.4 | 8.1 ± 15.2 ↑ | 8.8 ± 12.7 ↑ | 8.1 ± 24.9 ↑ | |
| C4-C3 | 8.9 ± 24.0 | 9.3 ± 26.9 | 3.3 ± 29.6 | −15.6 ± 23.0 ↓ | −21.6 ± 27.4 ↓ | −9.1 ± 11.0 ↓ | |
| P4-P3 | −0.1 ± 16.7 | −4.2 ± 25.6 | −2.9 ± 16.5 | 3.3 ± 10.8 ↑ | −6.2 ± 18.8 ↓ | −5.9 ± 8.4 ↓ | |
| O2-O1 | −4.4 ± 32.5 | −8.5 ± 34.9 | −3.9 ± 23.9 | 16.6 ± 32.0 ↑ | 24.0 ± 36.7 ↑ | −2.7 ± 14.7 ↑ | |
| F8-F7 | −1.4 ± 26.8 | 10.6 ± 25.6 | 0.9 ± 23.2 | 18.5 ± 23.5 ↑ | 8.0 ± 30.3 ↓ | 4.3 ± 22.3 ↑ | |
| T4-T3 | −1.7 ± 24.9 | −0.4 ± 36.6 | −6.7 ± 16.2 | −9.5 ± 9.7 ↓ | −9.7 ± 28.8 ↓ | 14.5 ± 29.6 ↑ | |
| T6-T5 | −11.0 ± 20.2 | −16.2 ± 28.1 | −8.0 ± 21.0 | −23.5 ± 32.4 ↓ | −21.6 ± 23.1 ↓ | −22.2 ± 38.4 ↓ | |
| Shooting/ Arachnophobia | FP2-FP1 | 2.5 ± 15.3 | 7.7 ± 25.3 | 2.5 ± 23.5 | 7.2 ± 14.3 ↑ | −1.1 ± 29.8 ↓ | 3.0 ± 20.8 ↑ |
| F4-F3 | 8.9 ± 16.6 | 9.6 ± 22.0 | 1.2 ± 20.9 | 6.5 ± 24.9 ↓ | −7.9 ± 30.0 ↓ | 10.9 ± 7.8 ↑ | |
| C4-C3 | 10.9 ± 36.6 | 5.9 ± 36.5 | 2.2 ± 36.5 | 3.2 ± 18.9 ↓ | −3.9 ± 20.1 ↓ | −13.4 ± 20.4 ↓ | |
| P4-P3 | 2.4 ± 19.1 | −1.9 ± 24.3 | −2.9 ± 15.9 | 12.4 ± 16.0 ↑ | −0.8 ± 17.6 ↑ | 4.4 ± 6.2 ↑ | |
| O2-O1 | 1.3 ± 31.9 | −8.9 ± 34.1 * | 1.2 ± 26.5 | 19.0 ± 19.5 ↑ | 31.2 ± 18.0 * ↑ | 4.0 ± 13.1 ↑ | |
| F8-F7 | 10.2 ± 18.6 | 17.8 ± 19.7 | 9.3 ± 20.9 * | 2.9 ± 17.5 ↓ | 7.8 ± 18.9 ↓ | −15.1 ± 16.0 * ↓ | |
| T4-T3 | −5.6 ± 27.4 | −4.5 ± 39.5 | −7.2 ± 19.8 | −0.7 ± 16.7 ↑ | −15.6 ± 23.5 ↓ | 15.2 ± 34.0 ↑ | |
| T6-T5 | −4.9 ± 18.5 | −6.4 ± 27.5 | −7.1 ± 16.3 | −26.6 ± 40.2 ↓ | −21.3 ± 29.2 ↓ | −28.6 ± 38.2 ↓ | |
| Shooting/ Acrophobia | FP2-FP1 | 5.2 ± 13.9 | 7.1 ± 20.4 | 0.7 ± 20.6 | −3.7 ± 21.0 ↓ | 4.4 ± 19.8 ↓ | −11.2 ± 21.8 ↓ |
| F4-F3 | 6.1 ± 16.8 | 7.0 ± 19.9 | −2.3 ± 16.3 | 5.4 ± 10.6 ↓ | 3.6 ± 12.9 ↓ | 3.7 ± 9.7 ↑ | |
| C4-C3 | 14.1 ± 28.6 | 10.0 ± 32.9 | 3.7 ± 29.1 | −11.9 ± 21.2 ↓ | −10.9 ± 16.8 ↓ | −17.5 ± 20.6 ↓ | |
| P4-P3 | 2.3 ± 15.8 | 1.5 ± 23.9 | −2.4 ± 13.8 | 11.7 ± 15.5 ↑ | 8.0 ± 14.6 ↑ | 2.6 ± 12.1 ↑ | |
| O2-O1 | −6.4 ± 29.8 | −9.3 ± 30.5 * | −2.8 ± 25.9 | 16.0 ± 25.1 ↑ | 42.8 ± 13.2* ↑ | 5.1 ± 9.9 ↑ | |
| F8-F7 | 10.3 ± 22.4 | 17.3 ± 24.1 | 5.2 ± 27.4 | 11.5 ± 15.7 ↑ | 4.7 ± 29.6 ↓ | −15.0 ± 11.1 ↓ | |
| T4-T3 | −1.7 ± 22.5 | −3.1 ± 35.8 | −9.4 ± 22.6 | 10.2 ± 21.9 ↑ | −4.0 ± 29.6 ↓ | 9.5 ± 33.0 ↑ | |
| T6-T5 | −4.7 ± 30.2 | −9.7 ± 35.9 | −6.3 ± 29.9 | 11.9 ± 33.5 ↑ | 9.9 ± 45.7 ↑ | −10.9 ± 17.0 ↓ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obukhov, A.; Krasnyanskiy, M.; Volkov, A.; Nazarova, A.; Teselkin, D.; Patutin, K.; Zajceva, D. Method for Assessing the Influence of Phobic Stimuli in Virtual Simulators. J. Imaging 2023, 9, 195. https://doi.org/10.3390/jimaging9100195
Obukhov A, Krasnyanskiy M, Volkov A, Nazarova A, Teselkin D, Patutin K, Zajceva D. Method for Assessing the Influence of Phobic Stimuli in Virtual Simulators. Journal of Imaging. 2023; 9(10):195. https://doi.org/10.3390/jimaging9100195
Chicago/Turabian StyleObukhov, Artem, Mikhail Krasnyanskiy, Andrey Volkov, Alexandra Nazarova, Daniil Teselkin, Kirill Patutin, and Darya Zajceva. 2023. "Method for Assessing the Influence of Phobic Stimuli in Virtual Simulators" Journal of Imaging 9, no. 10: 195. https://doi.org/10.3390/jimaging9100195
APA StyleObukhov, A., Krasnyanskiy, M., Volkov, A., Nazarova, A., Teselkin, D., Patutin, K., & Zajceva, D. (2023). Method for Assessing the Influence of Phobic Stimuli in Virtual Simulators. Journal of Imaging, 9(10), 195. https://doi.org/10.3390/jimaging9100195






