Advanced Kidney Models In Vitro Using the Established Cell Line Renal Proximal Tubular Epithelial/Telomerase Reverse Transcriptase1 for Nephrotoxicity Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Cell Culture
2.3. Generation of Tubule-like Structures
2.4. Cell Culture in the Microfluidic Device
2.5. Immunofluorescence
2.6. Quantitative Real-Time PCR
2.7. Cell Viability Assay
2.8. Statistical Analysis
3. Results
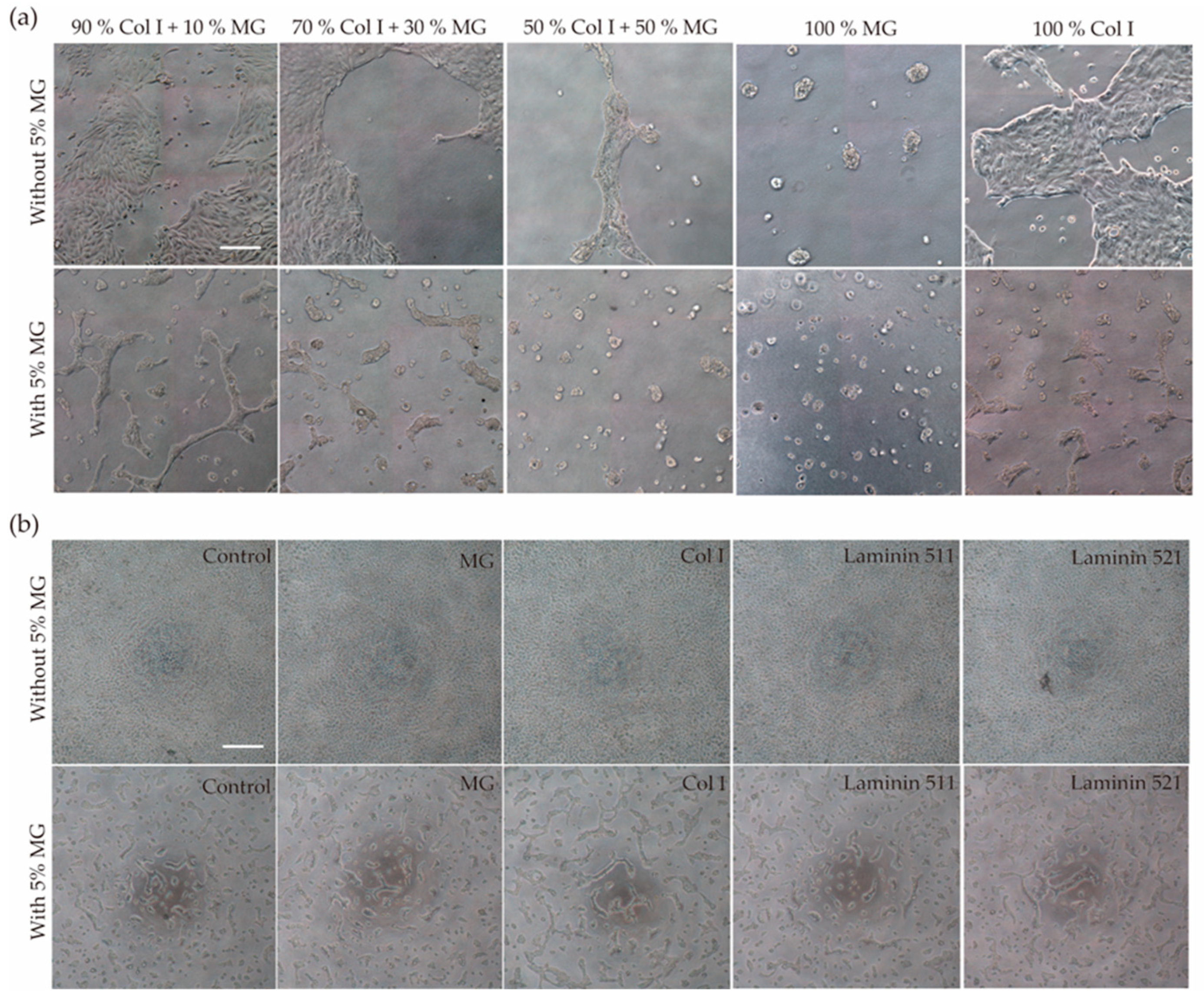
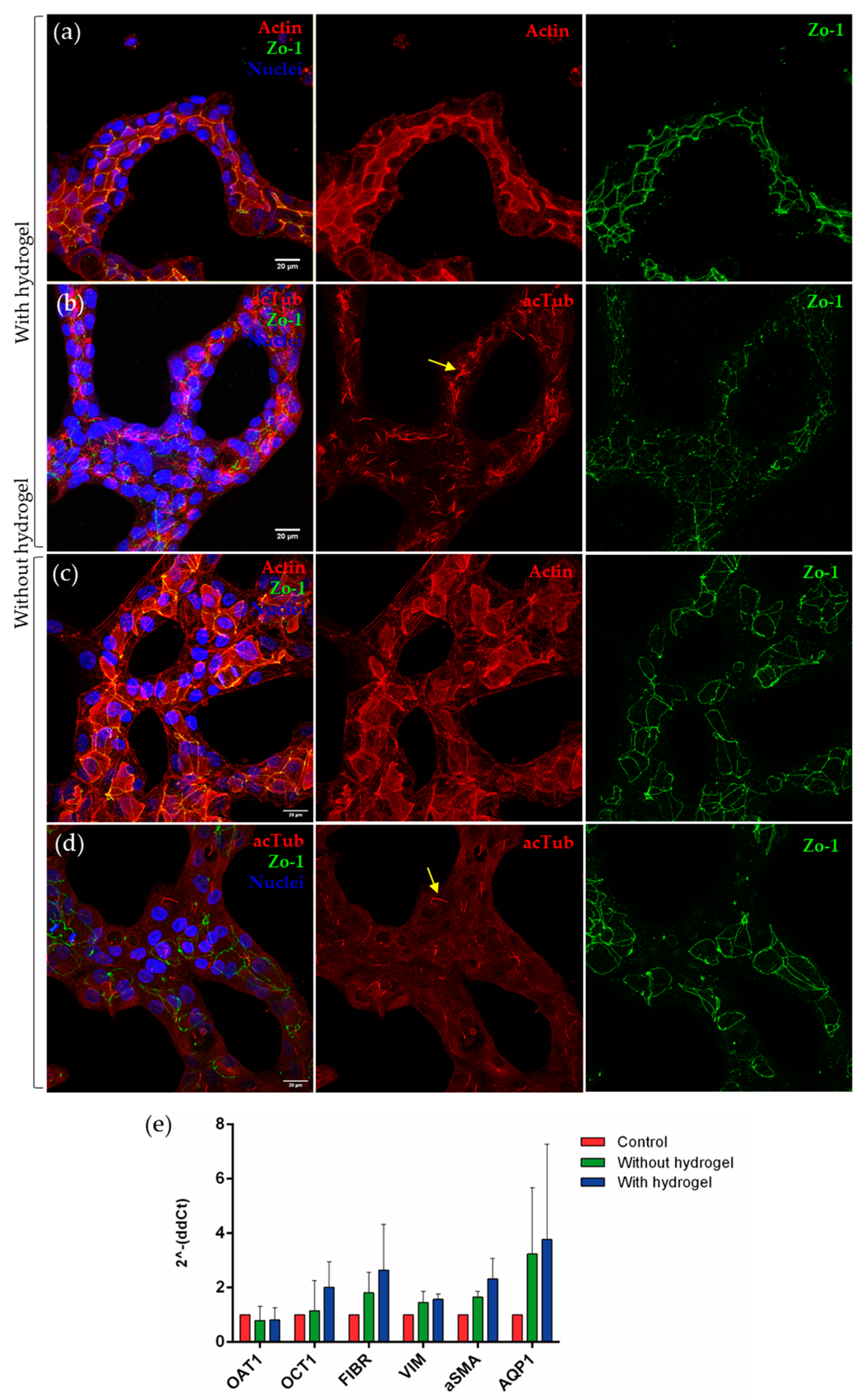
3.1. Generation of Self-Assembled Tubule-like Structures
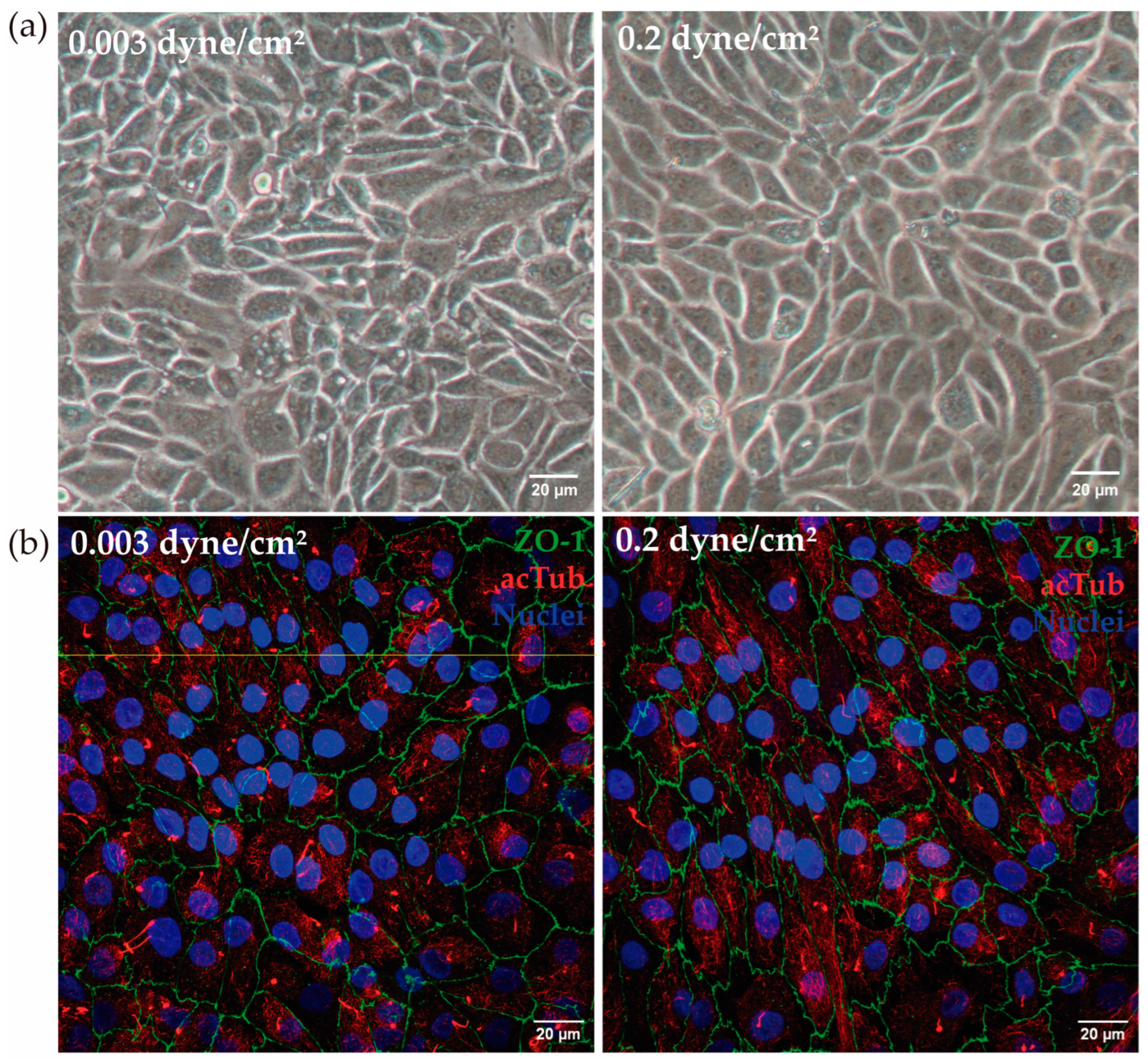
3.2. Development of a Kidney-on-a-Chip Model
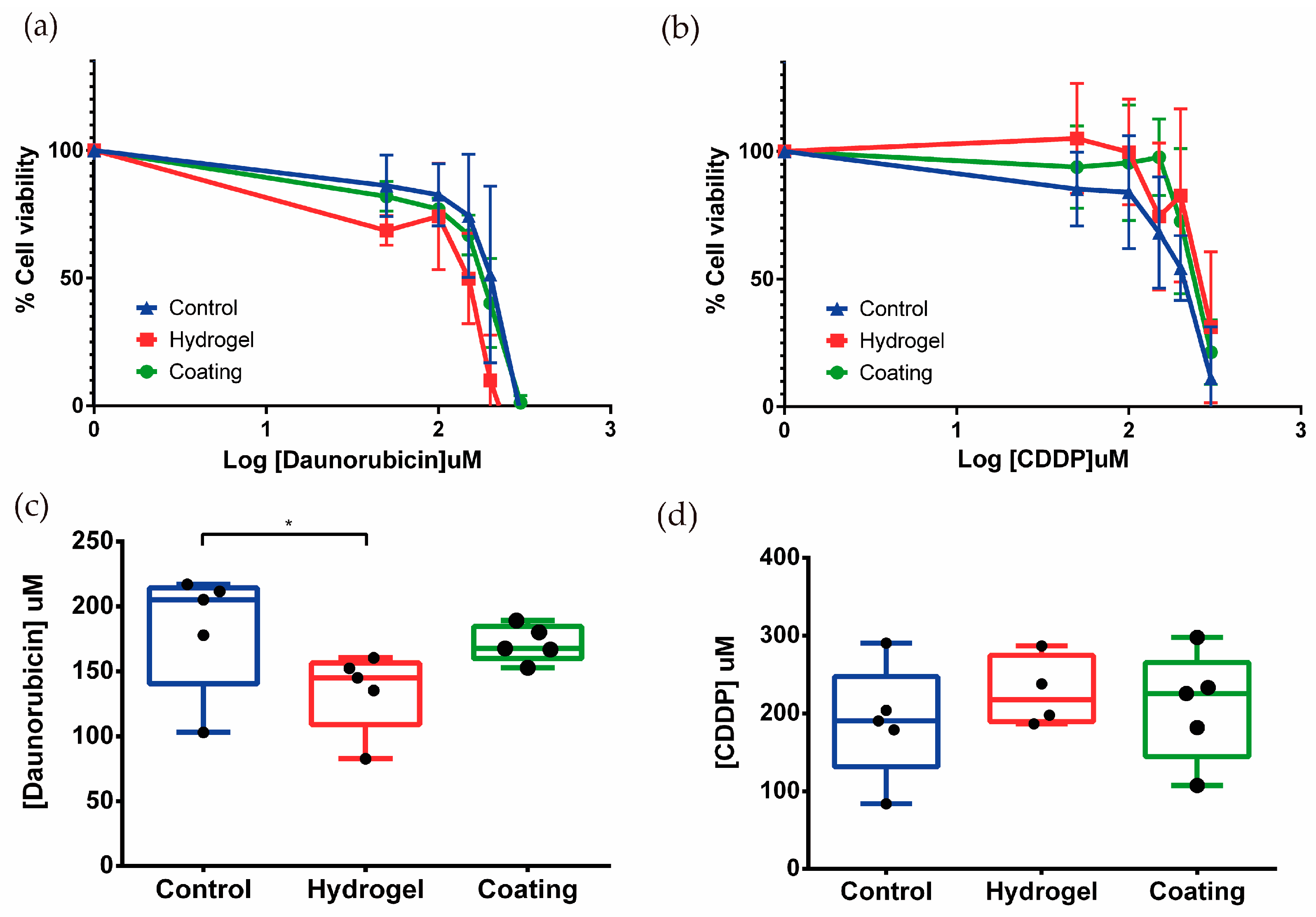
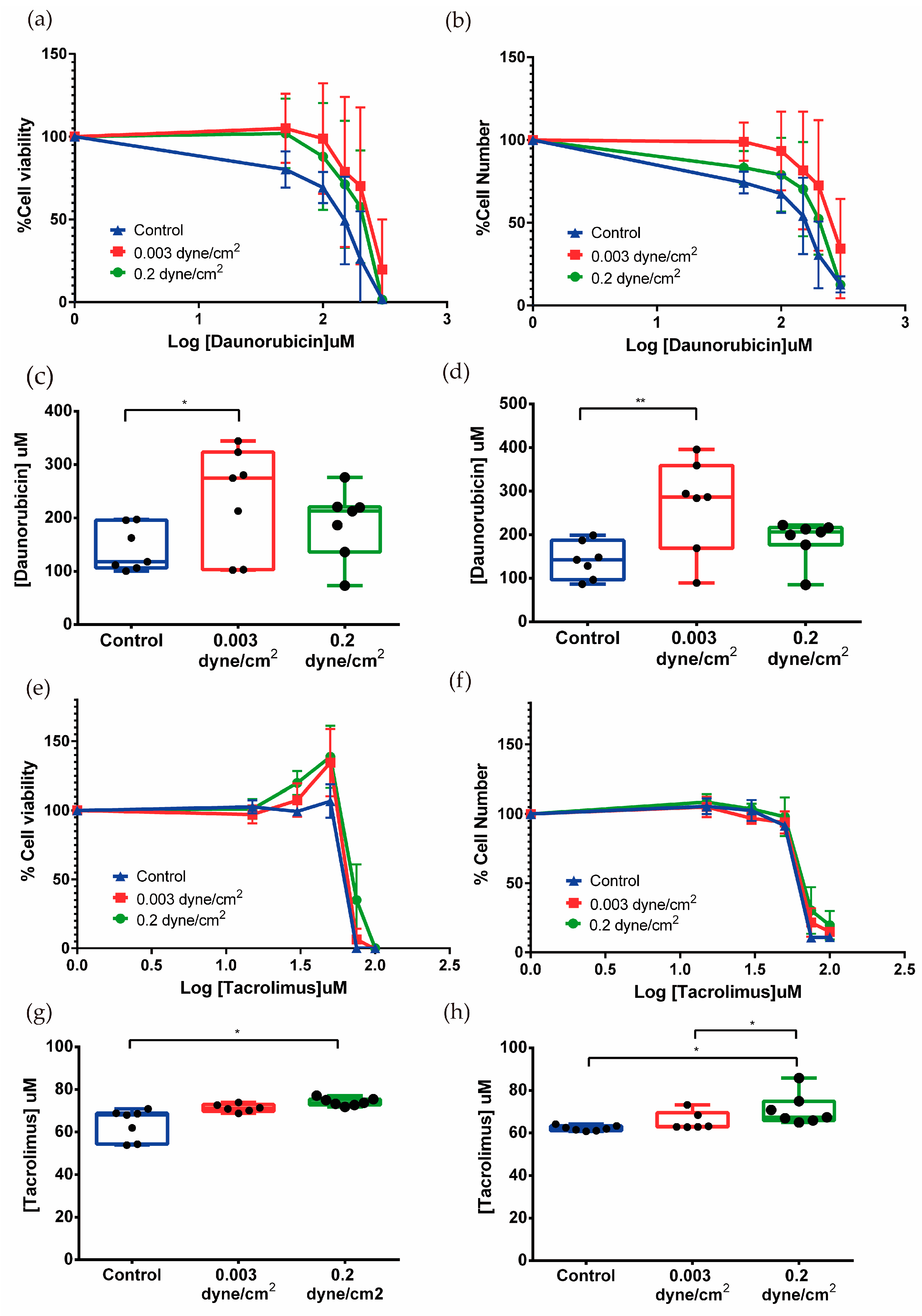
3.3. Nephrotoxicity Assays in Tubulogenesis and Kidney-on-Chip Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiong, H.Y.; Huang, P.; Xiong, S.; Li, Y.; Vathsala, A.; Zink, D. Drug-induced nephrotoxicity: Clinical impact and preclinical in vitro models. Mol. Pharm. 2014, 11, 1933–1948. [Google Scholar] [CrossRef]
- Faria, J.; Ahmed, S.; Gerritsen, K.G.F.; Mihaila, S.M.; Masereeuw, R. Kidney-based in vitro models for drug-induced toxicity testing. Arch. Toxicol. 2019, 93, 3397–3418. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Paoli, R.; Samitier, J. Mimicking the Kidney: A Key Role in Organ-on-Chip Development. Micromachines 2016, 7, 126. [Google Scholar] [CrossRef]
- King, S.M.; Higgins, J.W.; Nino, C.R.; Smith, T.R.; Paffenroth, E.H.; Fairbairn, C.E.; Docuyanan, A.; Shah, V.D.; Chen, A.E.; Presnell, S.C.; et al. 3D Proximal Tubule Tissues Recapitulate Key Aspects of Renal Physiology to Enable Nephrotoxicity Testing. Front. Physiol. 2017, 8, 123. [Google Scholar] [CrossRef]
- Fedecostante, M.; Westphal, K.G.C.; Buono, M.F.; Romero, N.S.; Wilmer, M.J.; Kerkering, J.; Baptista, P.M.; Hoenderop, J.G.; Masereeuw, R. Recellularized native kidney scaffolds as a novel tool in nephrotoxicity screening. Drug Metab. Dispos. 2018, 46, 1338–1350. [Google Scholar] [CrossRef]
- Kang, H.M.; Lim, J.H.; Noh, K.H.; Park, D.; Cho, H.S.; Susztak, K.; Jung, C.R. Effective reconstruction of functional organotypic kidney spheroid for in vitro nephrotoxicity studies. Sci. Rep. 2019, 9, 17610. [Google Scholar] [CrossRef]
- Jang, K.J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119–1129. [Google Scholar] [CrossRef]
- Qu, Y.; An, F.; Luo, Y.; Lu, Y.; Liu, T.; Zhao, W.; Lin, B. A nephron model for study of drug-induced acute kidney injury and assessment of drug-induced nephrotoxicity. Biomaterials 2018, 155, 41–53. [Google Scholar] [CrossRef]
- Vriend, J.; Nieskens, T.T.G.; Vormann, M.K.; van den Berge, B.T.; van den Heuvel, A.; Russel, F.G.; Suter-Dick, L.; Lanz, H.L.; Vulto, P.; Masereeuw, R.; et al. Screening of Drug-Transporter Interactions in a 3D Microfluidic Renal Proximal Tubule on a Chip. AAPS J. 2018, 20, 87. [Google Scholar] [CrossRef]
- Vormann, M.K.; Gijzen, L.; Hutter, S.; Boot, L.; Nicolas, A.; van den Heuvel, A.; Vriend, J.; Ng, C.P.; Nieskens, T.T.; van Duinen, V.; et al. Nephrotoxicity and Kidney Transport Assessment on 3D Perfused Proximal Tubules. AAPS J. 2018, 20, 90. [Google Scholar] [CrossRef]
- Bosch-fortea, M.; Rodriguez-fraticelli, A.E.; Herranz, G.; Hachimi, M.; Barea, M.D.; Young, J.; Ladoux, B.; Martin-Belmonte, F. Micropattern-based platform as a physiologically relevant model to study epithelial morphogenesis and nephrotoxicity. Biomaterials 2019, 218, 119339. [Google Scholar] [CrossRef]
- Vargas, R.; Egurbide-Sifre, A.; Medina, L. Organ-on-a-Chip systems for new drugs development. Admet. Dmpk. 2021, 9, 111–141. [Google Scholar] [CrossRef]
- Sakolish, C.; Chen, Z.; Dalaijamts, C.; Mitra, K.; Liu, Y.; Fulton, T.; Wade, T.L.; Kelly, E.J.; Rusyn, I.; Chiu, W.A. Predicting tubular reabsorption with a human kidney proximal tubule tissue-on-a-chip and physiologically-based modeling. Toxicol. Vitr. 2020, 63, 104752. [Google Scholar] [CrossRef]
- Jang, K.J.; Suh, K.Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab. Chip 2010, 10, 36–42. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef]
- DesRochers, T.M.; Suter, L.; Roth, A.; Kaplan, D.L. Bioengineered 3D Human Kidney Tissue, a Platform for the Determination of Nephrotoxicity. PLoS ONE 2013, 8, e59219. [Google Scholar] [CrossRef]
- Secker, P.F.; Luks, L.L.; Schlichenmaier, N.; Dietrich, D.R.R. RPTEC/TERT1 cells form highly differentiated tubules when cultured in a 3D matrix. Altex 2017, 35, 1–12. [Google Scholar] [CrossRef]
- Astashkina, A.I.; Mann, B.K.; Prestwich, G.D.; Grainger, D.W. A 3-D organoid kidney culture model engineered for high-throughput nephrotoxicity assays. Biomaterials 2012, 33, 4700–4711. [Google Scholar] [CrossRef]
- Kai, J.; Chuah, C.; Zink, D. Stem cell-derived kidney cells and organoids: Recent breakthroughs and emerging applications. Biotechnol. Adv. 2017, 35, 150–167. [Google Scholar] [CrossRef]
- Morizane, R.; Bonventre, J.V. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat. Protoc. 2017, 12, 195–207. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva de Sousa Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef]
- Nishinakamura, R. Human kidney organoids: Progress and remaining challenges. Nat. Rev. Nephrol. 2019, 15, 613–624. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney organoids—A new tool for kidney therapeutic development. Kidney Int. 2018, 94, 1040–1042. [Google Scholar] [CrossRef]
- Kunnen, S.J.; Malas, T.B.; Semeins, C.M.; Bakker, A.D.; Peters, D.J.M. Comprehensive transcriptome analysis of fluid shear stress altered gene expression in renal epithelial cells. J. Cell Physiol. 2018, 233, 3615–3628. [Google Scholar] [CrossRef]
- Ross, E.J.; Gordon, E.R.; Sothers, H.; Darji, R.; Baron, O.; Haithcock, D.; Prabhakarpandian, B.; Pant, K.; Myers, R.M.; Cooper, S.J.; et al. Three dimensional modeling of biologically relevant fluid shear stress in human renal tubule cells mimics in vivo transcriptional profiles. Sci. Rep. 2021, 11, 14053. [Google Scholar] [CrossRef]
- Chen, D.; Roberts, R.; Pohl, M.; Nigam, S.; Kreidberg, J.; Wang, Z.; Heino, J.; Ivaska, J.; Coffa, S.; Harris, R.C.; et al. Differential expression of collagen- and laminin-binding integrins mediates ureteric bud and inner medullary collecting duct cell tubulogenesis. Am. J. Physiol. Ren. Physiol. 2004, 287, 602–611. [Google Scholar] [CrossRef]
- Aschauer, L.; Carta, G.; Vogelsang, N.; Schlatter, E.; Jennings, P. Expression of xenobiotic transporters in the human renal proximal tubule cell line RPTEC/TERT1. Toxicol. Vitr. 2015, 30, 95–105. [Google Scholar] [CrossRef]
- Soo, J.Y.; Jansen, J.; Masereeuw, R.; Little, M.H. Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat. Rev. Nephrol. 2018, 14, 378–393. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Saorin, G.; Caligiuri, I.; Rizzolio, F. Microfluidic organoids-on-a-chip: The future of human models. Semin. Cell Dev. Biol. 2022, 144, 41–54. [Google Scholar] [CrossRef]
- Brassard, J.A.; Lutolf, M.P. Engineering Stem Cell Self-organization to Build Better Organoids. Cell Stem Cell 2019, 24, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular Matrix Scaffolds for Tissue Engineering and Regenerative Medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246. [Google Scholar] [CrossRef]
- Alhassani, R.Y.; Bagadood, R.M.; Balubaid, R.N.; Barno, H.I.; Alahmadi, M.O.; Ayoub, N.A. Drug Therapies Affecting Renal Function: An Overview. Cureus 2021, 13, e19924. [Google Scholar] [CrossRef]
- Secker, P.F.; Schlichenmaier, N.; Beilmann, M.; Deschl, U.; Dietrich, D.R. Functional transepithelial transport measurements to detect nephrotoxicity in vitro using the RPTEC/TERT1 cell line. Arch. Toxicol. 2019, 93, 1965–1978. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, C.; Chen, Y.; Tozzi, L.; Szymkowiak, S.; Li, C.; Kaplan, D.L. Developing a self-organized tubulogenesis model of human renal proximal tubular epithelial cells in vitro. J. Biomed. Mater. Res. 2019, 108, 795–804. [Google Scholar] [CrossRef]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef]
- Lacueva-Aparicio, A. Desarrollo y Comparativa de Modelos In Vitro de Túbulo Proximal Humano Que Mimetizan el Microambiente Físico-Químico, y su Validación en Ensayos de Nefrotoxicidad. 2023. Available online: http://zaguan.unizar.es (accessed on 13 June 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacueva-Aparicio, A.; Martínez-Gimeno, L.; Torcal, P.; Ochoa, I.; Giménez, I. Advanced Kidney Models In Vitro Using the Established Cell Line Renal Proximal Tubular Epithelial/Telomerase Reverse Transcriptase1 for Nephrotoxicity Assays. Biomimetics 2024, 9, 446. https://doi.org/10.3390/biomimetics9070446
Lacueva-Aparicio A, Martínez-Gimeno L, Torcal P, Ochoa I, Giménez I. Advanced Kidney Models In Vitro Using the Established Cell Line Renal Proximal Tubular Epithelial/Telomerase Reverse Transcriptase1 for Nephrotoxicity Assays. Biomimetics. 2024; 9(7):446. https://doi.org/10.3390/biomimetics9070446
Chicago/Turabian StyleLacueva-Aparicio, Alodia, Laura Martínez-Gimeno, Pilar Torcal, Ignacio Ochoa, and Ignacio Giménez. 2024. "Advanced Kidney Models In Vitro Using the Established Cell Line Renal Proximal Tubular Epithelial/Telomerase Reverse Transcriptase1 for Nephrotoxicity Assays" Biomimetics 9, no. 7: 446. https://doi.org/10.3390/biomimetics9070446





