Abstract
Metallocarboxypeptidase (MCP) is a crucial protein enzyme involved in food digestion and absorption in animals, which has a potential influence on the differentiation of the trophic niche. Considering that stomatopods have raptorial appendage-specific trophic niches, the present study screened and compared the MCP M14 gene family of three stomatopods (Lysiosquillina maculata, Odontodactylus scyllarus, and Oratosquilla oratoria) with different raptorial appendage morphologies based on full-length transcriptome information. There are 13 and 17 MCP M14 gene family members identified in L. maculata and O. scyllarus, respectively, which are classified as M14A, M14B, and M14D subfamilies. However, 15 MCP M14 family members have been identified in O. oratoria, all belonging to the M14A subfamily. The physicochemical properties, phylogenetic relationships, conserved motifs, and secondary and tertiary structures of the MCP M14 amino acid sequences were also analyzed in the present study. The results revealed that each amino acid sequence had unique physicochemical properties. Ten conserved motifs were further characterized across the MCP M14 amino acid sequences, and the type and number of motifs from the same subfamily remained highly conserved. Meanwhile, we found that most of the MCP M14 gene family members have critical residues (including Zn2+ binding sites [His69, Glu72, and His196], substrate-binding residues [Arg124, Arg127, and Arg145], and disulfide bond-forming residues [Cys138 and Cys161]) involved in disulfide bond formation and enzyme activity stabilization. Furthermore, the random coil is the predominant structural feature of the MCP M14 amino acid sequence. In conclusion, these results are undoubtedly valuable for exploring the evolution and regulation mechanisms of the trophic niche in stomatopods.
Keywords:
stomatopod; Metallocarboxypeptidase M14; full-length transcriptome; trophic niche; sequence alignment Key Contribution:
Considering that stomatopods have raptorial appendage-specific trophic niches, the present study screened and compared the MCP M14 gene family of three stomatopods (Lysiosquillina maculata, Odontodactylus scyllarus, and Oratosquilla oratoria) with different raptorial appendage morphologies based on full-length transcriptome information.
1. Introduction
Stomatopods are highly specialized predatory crustaceans [], distinguished by unique morphological structures and predatory behaviors that set them apart from other taxa. They occupy a significant position within the phylogenetic system of marine crustaceans []. Although more than 450 species, classified into seven superfamilies, have been described, the phylogenetic relationships among stomatopods—particularly regarding the identity of the most primitive superfamily—remain controversial [,]. It has been hypothesized that Squilloidea may be the most primitive based on raptorial appendage morphology []. In contrast, cladistic analyses of morphological characters support Gonodactyloidea as the most basal lineage [], whereas molecular phylogenetic studies suggest that Parasquilloidea might be even more primitive [,]. These conflicting phylogenetic interpretations complicate accurate studies of stomatopod trophic niches.
The raptorial appendages of stomatopods play a crucial role in ecological adaptation []. These appendages have differentiated into two forms: “smasher” and “spearer” []. stomatopods with “smasher” raptorial appendage inhabit hard coral reef environments and feed on shellfish or crustacean [,]. To shatter the hard shells of their prey and compete for limited coral cavity resources, they have evolved thick, bulging, hammer-shaped dactylopodite. In contrast, stomatopods with “spearer” raptorial appendages live in soft sediment-bottom environments []. This environment has abundant habitat resources and less competition, but they need to dig their own caves. Additionally, to impale the fish or mollusk [,], they have evolved sharp, prickly, spear-shaped dactylopodite [].
The type of raptorial appendage correspond to distinct hunting strategies and feeding habits [,], serving as a key driver of animal evolution []. This divergence may further promote trophic niche partitioning and specialization of digestive capabilities []. To facilitate acquisition, utilization, and occupation of ecological niches, physiological [] and genetic [] characteristics related to feeding habits will evolve over the long-term evolutionary process in response to changes in the trophic niche []. Digestion and absorption are necessary processes for animals to obtain energy. Following ingestion, nutrient absorption largely depends on chemical decomposition []. As crucial biomacromolecules, digestive enzymes play a pivotal role in these processes, and their characteristics undergo significant differentiation throughout animal evolution []. The types and activities of digestive enzymes have been studied extensively []. Currently, many types of digestive enzymes have been identified in crustaceans, including proteases [], trypsin [], carboxypeptidase (CPs) [], aminopeptidase [], collagenases [], elastases [], pepsin [], chymotrypsin [], esterases [], lipases [], amylases [], cellulases [], chitinases [], maltases [], sucrases [], and laminarinases [], etc. Among these digestive enzymes, CPs are widely distributed in the tissues and organs of higher plants and animals, and fungi. Typically secreted by intestinal epithelial cells, they belong to the class of exopeptidases. CPs play an essential role in food digestion and degradation by catalyzing the hydrolysis of C-terminal amino acid residues, thereby breaking down large molecules into smaller, more readily absorbable units [,,]. Based on their catalytic mechanisms, CPs can be classified into metallocarboxypeptidases (MCPs), serine carboxypeptidases, and cysteine carboxypeptidases []. Among these, MCPs have attracted relatively greater research attention. The MCP M14 family, in particular, plays important roles in food digestion, proenzyme activation, and regulation [,], and is also the main enzyme involved in the processing of peptides and proteins during animal growth and development []. Studies have shown that the characteristics of the MCP M14 family are significantly different among different species []. Each molecule of the MCP M14 family contains a zinc ion (Zn2+), which simultaneously forms a penta-coordinated, slightly distorted tetrahedral complex with two histidine residues (His), one glutamic acid residue (Glu), and one water molecule (H2O) [,]. The MCP M14 gene family was further divided into M14A, M14B, M14C, and M14D gene subfamilies due to differences in Zn2+ binding sites [,]. Recently, the rapid development of high-throughput sequencing technology has provided opportunities for the analysis of MCP M14 family-related gene characteristics and functions in numerous animals, such as Helicoverpa armigera [], Anopheles culicifacies [], A. gambiae [], Simulium vittatum [], Trichoplusia ni [], Aedes aegypti [], and Antheraea pernyi []. However, related studies have not been carried out in stomatopods.
In order to confirm the potential characteristic differences in the MCP M14 gene family in stomatopods with different raptorial appendage morphologies, Odontodactylus scyllarus, Lysiosquillina maculata, and Oratosquilla oratoria, belonging to the Gonodactyloidea, Lysiosquilloidea, and Squilloidea, were selected as the research animals. In the context of the lack of whole genome information of stomatopod, the present study sequenced the full-length (FL) transcriptome sequences of three animals to obtain relatively complete genetic information []. Furthermore, the number of MCP M14 family members, protein physicochemical properties, phylogenetic relationships, protein sequence information and structures, as well as the differences among subfamilies of three stomatopods, were analyzed and compared. These results lay a foundation for further understanding of the digestion and absorption function of MCP M14 in stomatopod, and provide a theoretical basis for the future trophic niche differentiation study of stomatopod.
2. Materials and Methods
2.1. Sample Collection, RNA Extraction, and FL Transcriptome Sequencing
The FL transcriptome data for O. oratoria are derived from our previously published study []. We applied an identical analytical approach—including the steps for sequencing platform, assembly, and redundancy removal—to the original O. oratoria dataset (SRR32917886) to ensure consistency with the current study. O. scyllarus and L. maculata were collected from the aquatic product market in Xiamen (China, Fujian). Healthy individuals (animals with intact bodies, normal mobility, and feeding behavior were selected) are transported back to the laboratory of the Third Institute of Oceanography in the Ministry of Natural Resources using an incubator filled with seawater. The animals were acclimatized for 7 days in the laboratory under controlled conditions designed to mimic their natural habitat in terms of water temperature, salinity, and light cycle, while being fed normally. Cryo-anesthesia was induced immediately after this acclimation period. Multiple tissues (including muscle, hepatopancreas, and gonads) were dissected from each individual and then flash frozen with liquid nitrogen and mixed in equal amounts into one sample. Furthermore, the total RNA of 3 mixed samples was extracted using Trizol Reagent Kit (Invitrogen: Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. The total RNAs were tested for integrity, purity, and quantification. RNA quality was evaluated based on concentration (Qubit, Thermo Fisher Scientific, Waltham, MA, USA), purity (NanoDrop 2000, Thermo Fisher Scientific, Waltham, MA, USA), and integrity (Qsep, BIOptic, Taipei, Taiwan, China). Only samples with a concentration >50 ng/μL, an A260/A280 ratio > 2.0, and an RIN value > 8 were processed further. mRNA was then isolated using VAHTSTM mRNA Capture Beads (Vazyme, Nanjing, China) following the manufacturer’s recommendations and subjected to FL transcriptome sequencing. Full-length cDNA was synthesized from mRNA with the Clonetech SMARTer PCR cDNA Synthesis Kit (Takara Bio, Kusatsu, Shiga, Japan), amplified, and size-selected (>1 kb) using magnetic bead purification. The cDNA was end-repaired, ligated to SMRT bell adapters, and treated with exonuclease to remove unligated products before the final purification step. The resulting SMRT-seq library was assessed for quality on an Agilent 2100 instrument (Agilent Technologies, Palo Alto, CA, USA) and sequenced on the PacBio RSII platform (Pacific Biosciences, Menlo Park, CA, USA) to generate FL transcriptome data.
2.2. FL Transcriptome Data Processing
SMRTLink software (v10.1) [] was first applied to filter out the low-quality invalid reads in the original polymerase reads produced from the PacBio RSII sequencing platform to obtain the circular consensus sequences (CCSs). In detail, adaptor sequences, subreads, and polymerase reads less than 50 bp in length, and polymerase sequences with accuracy less than 0.75 were eliminated. Furthermore, a CCS is defined as a full-length-non-chimeric read (FLNC) when it contains both 5’ and 3’ cDNA primers, and poly (A) tail, and the insertion read was in the correct position. Meanwhile, the 5’ and 3’ cDNA primers and poly (A) tails in FLNCs were removed using the Lima and Refine subroutines of the IsoSeq3 software (v3.4.0) []. The IsoSeq3 software [] was then used to filter out redundant FLNC sequences to obtain consistent FL isoforms. Meanwhile, those consistent FL isoforms with accuracy > 99% and FL sequence support number ≥ 2 are considered to be of high quality. To ensure that transcripts from the same gene can be clustered together, CD-hit software (v4.7) [] was used to cluster high-quality, consistent FL isoforms (accuracy > 99% and supported by at least two full-length sequences) again to ultimately produce non-redundant FL transcripts for subsequent analyses. Finally, the completeness of the FL transcriptome was assessed using BUSCO (v3.0.2).
2.3. The Identification MCP M14 Gene Family of L. maculata, O. scyllarus, and O. oratoria
We obtained the Hidden Markov Model (HMM) profile of the peptidase M14 domain (Pfam ID: PF00246) from the Pfam database (https://www.ebi.ac.uk/interpro/entry/pfam/; accessed on 20 December 2024). Using this profile, we performed a search for potential MCP M14 gene family sequences in L. maculata, O. scyllarus, and O. oratoria FL transcripts using HMMER software (v3.0) [], applying a stringent E-value threshold of <1.2 × 10−28. The identified MCP M14 gene family sequences were subsequently validated by comparing them against the BLAST database (database version: 2024) (https://blast.ncbi.nlm.nih.gov/; accessed on 20 December 2024) to confirm their accuracy. Finally, the domain prediction was carried out using the online SMART (https://smart.embl.de/; accessed on 20 December 2024).
2.4. Characterization of the MCP M14 Gene Family in L. maculata, O. scyllarus, and O. oratoria
The basic protein physicochemical properties of proteins belonging to the MCP M14 gene family in L. maculata, O. scyllarus, and O. oratoria were analyzed using the online ExPASy (https://web.expasy.org/protparam/; accessed on 21 December 2024). Then, clustal-W software (v2.1) [] was used to align the amino acid sequences of MCP M14 genes from L. maculata, O. scyllarus, and O. oratoria, and the phylogenetic tree was then constructed using MEGA-X software (v10.1.8) [] based on the maximum likelihood method. Meanwhile, 1000 bootstrap replicates were performed to evaluate the reliability of each ML tree clade. Subsequently, phylogenetic tree visualization was refined using the web-based Evolview (v3.0; https://www.evolgenius.info/evolview/; accessed on 22 December 2024). Conserved motifs within amino acid sequences of the MCP M14 gene family in three stomatopod species were identified using the online MEME suite (https://web.mit.edu/meme/current/share/doc/meme.html; accessed on 23 December 2024) with a maximum motif threshold of 10, followed by structural visualization in TBtools (v2.084) software. Secondary structure characterization of MCP M14 gene family proteins in three stomatopod species was performed using the SOPMA web server (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html; accessed on 25 December 2024), which quantifies structural conformations by calculating the proportional distribution of α-helices, β-strands, β-turns, and random coils. Homology modeling of the MCP M14 gene family proteins in three stomatopod species was conducted through the SWISS-MODEL workspace (https://swissmodel.expasy.org/; accessed on 28 December 2024) to predict the tertiary structure. Finally, we downloaded the amino acid sequence of Tibetan cattle carboxypeptidase BtCPA (GenBank registry number: P00730) from the Genbank database (https://www.ncbi.nlm.nih.gov/genbank/; accessed on 30 December 2024) as a reference, and compared all MPC M14 sequences of the three species based on Clustal-W [] and Genedoc (v2.7) [] software.
3. Results
3.1. FL Transcriptome Information of L. maculata and O. scyllarus
FL transcriptome data for O. oratoria have been described in our previous study (effective polymerase reads: 53.81 Gb, CCSs: 532,906, average depth: 36, mean read length: 2213, total nucleotide yield: 1,179,476,780 bp, FLNCs: 433,224, FL isoforms: 133,746, Non-redundancy FL transcripts: 102,964). In the current work, a total of 50.57 Gb and 37.36 Gb of effective polymerase reads were generated from multiple tissues of L. maculata and O. scyllarus, respectively. There were 13,567,349 effective polymerase reads in L. maculata with an average length of 3723 bp and a maximum length of 200,603 bp, although there were 10,937,122 effective polymerase reads in O. scyllarus with an average length of 3404 bp and a maximum length of 234,125 bp. From the high-quality reads of L. maculata, 403,117 CCSs were obtained, exhibiting an average depth of 26.05 passes and a mean read length of 4566 bp, with a total nucleotide yield of 1,840,833,099. In parallel, O. scyllarus produced 301,254 CCSs, with an average depth of 28.65 passes and a mean read length of 3968 bp, corresponding to 1,195,380,933 bp. Following primer and poly(A) tail removal, 390,653 (96.91%) and 288,197 (95.67%) FLNCs were retained for L. maculata and O. scyllarus, respectively. Subsequent clustering of these FLNCs generated 51,799 and 50,947 FL isoforms for L. maculata and O. scyllarus, respectively. After filtering these redundant FL isoforms, 49,467 (mean length: 3699 bp) and 49,044 (mean length: 3552 bp) non-redundancy FL transcripts were ultimately obtained from L. maculata and O. scyllarus, respectively. Given the absence of a complete reference genome for stomatopods, transcriptome completeness was assessed using BUSCO with the arthropoda_odb10 dataset. The results showed that 78.8% and 71.5% of the transcripts from O. scyllarus and L. maculata, respectively, were classified as complete.
3.2. Physicochemical Properties of Proteins Belonging to the MCP M14 Gene Family in L. maculata, O. scyllarus, and O. oratoria
The quality and depth of transcriptome sequencing are critical for the comprehensive identification of the MCP M14 gene family members. In this study, effective polymerase reads of 50.57 Gb, 37.36 Gb, and 53.81 Gb were obtained for L. maculata, O. scyllarus, and O. oratoria, respectively. The high retention rates of FLNCs further confirm the robustness of the sequencing data. These results support the reliability of cross-species comparisons of MCP M14 family member counts among the three stomatopod species. Using the HMM profile of the peptidase M14 domain, we screened FL transcripts from three stomatopod species to identify MCP M14 gene family sequences. A total of 13, 17, and 15 members were identified in L. maculata, O. scyllarus, and O. oratoria, respectively, and all contained the characteristic Zn_pept domain typical of MCP M14 proteins. Subsequently, we characterized the physicochemical properties of proteins belonging to the MCP M14 gene family across L. maculata, O. scyllarus, and O. oratoria (Table 1). For the MCP M14 gene family sequences of L. maculata, the range of amino acid length, molecular weight (MW), isoelectric point (pI), instability index, aliphatic index, and grand average of hydropathicity (GRAVY) were 271~1317 bp, 30.39~142.96 kDa, 4.99~9.39, 16.53~55.89, 65.50~89.92, and −0.608~−0.181, respectively. For the MCP M14 gene family sequences of O. scyllarus, the range of amino acid length, MW, pI, instability index, aliphatic index, and GRAVY were 206~1315bp, 23.01~142.52 kDa, 4.57~10.18, 22.87~53.37, 66.51~83.03, and −0.800~−0.148, respectively. For the MCP M14 gene family sequences of O. oratoria, the range of amino acid length, MW, pI, instability index, aliphatic index, and GRAVY were 103~349 bp, 11.04~38.98 kDa, 4.63~10.23, 16.53~80.84, 51.21~86.41, and −0.754~−0.071, respectively. This indicates that the majority of proteins belonging to the MCP M14 gene family in the three stomatopod species exhibit acidic pI (mean pI < 7), structural stability (instability index < 40 in 68% of proteins), and hydrophilic characteristics (negative GRAVY values).

Table 1.
Physicochemical properties of proteins belonging to the MCP M14 gene family in L. maculata, O. scyllarus, and O. oratoria.
3.3. Phylogenetic Analysis of the MCP M14 Gene Family in L. maculata, O. scyllarus, and O. oratoria
Phylogenetic analysis of 45 MCP M14 gene family sequences from three stomatopod species revealed three evolutionarily distinct subfamilies (Figure 1), namely M14A, M14B, and M14D. Notably, sequences from the same subfamily are clustered within conserved clades, even though they come from different species. The phylogenetic tree also showed that the MCP M14 gene family members of L. maculata and O. scyllarus were distributed in M14A (L. maculata: 7 sequences; O. scyllarus: 10 sequences), M14B (L. maculata: 3 sequences; O. scyllarus: 4 sequences), and M14D (L. maculata: 3 sequences; O. scyllarus: 3 sequences) subfamilies. In contrast, all 15 MCP M14 gene family sequences of O. oratoria were exclusively grouped within the M14A subfamily.
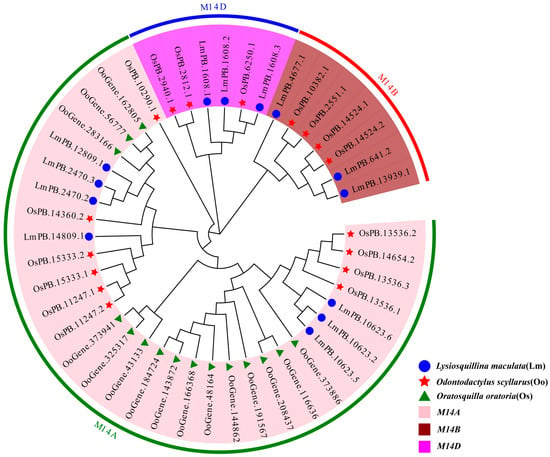
Figure 1.
Phylogenetic tree of the MCP M14 gene family in L. maculata, O. scyllarus, and O. oratoria.
3.4. Conservation Motif Analysis of the MCP M14 Gene Family in L. maculata, O. scyllarus, and O. oratoria
Our comparative analysis identified evolutionarily conserved motifs within the MCP M14 gene family across L. maculata, O. scyllaurus, and O. oratoria. Quantitative motif profiling demonstrated significant divergence in motif composition among MCP M14 subfamilies (Figure 2). The M14A subfamily exhibited the highest structural complexity with nine conserved motifs, substantially exceeding those observed in M14B (six motifs) and M14D (four motifs) subfamilies. Notably, motif 3 demonstrated universal conservation across all analyzed sequences, suggesting its fundamental role in digestive processes among the three stomatopod species. Intriguingly, motif 9 displayed subfamily specific retention, being exclusively preserved in the M14B subfamily, potentially mediating specialized functional adaptations unique to this subfamily. Additionally, sequences from the same MCP M14 gene subfamily have similar motif types.

Figure 2.
Conserved motifs of the MCP M14 gene family in L. maculate (Lm), O. scyllarus (Os), and O. oratoria (Oo).
3.5. Multiple Sequence Alignment of the MCP M14 Gene Family in L. maculata, O. scyllarus, and O. oratoria
Using the BtCPA sequence of Tibetan cattle as a reference sequence, we performed comparative amino acid sequence alignment of 45 MCP M14 gene family sequences to analyze the typical structure of the MCP M14 gene family (Figure 3). The grey background highlights the conserved amino acid residues of the MCP M14 gene family members. We found highly conserved Zn2+ binding sites (including His69, Glu72, and His196) in 7, 9, and 3 MCP M14 gene family sequences of L. maculata, O. scyllarus, and O. oratoria, respectively. Notably, all Zn2+ binding sites in the other MCP M14 gene family sequences in L. maculata and O. scyllarus were substituted, whereas other gene family sequences of O. oratoria had different degrees of Zn2+ binding site substitution. Meanwhile, amino acid residues (including Arg124, Arg127, and Arg145) that bind to and catalyze substrates were found in 7, 9, and 10 MCP M14 gene family sequences of L. maculata, O. scyllarus, and O. oratoria, respectively. Additionally, 7, 10, and 7 gene family sequences in L. maculata, O. scyllarus, and O. oratoria have conserved Cys138 and Cys161 residues, which form disulfide bonds and are associated with stable enzyme activity.

Figure 3.
Multiple amino acid sequences comparison of MCP M14 family members in L. maculata, O. scyllarus, and O. oratoria. Note: Partial sequence alignment reveals conserved domains characteristic of the MCP M14 gene family that mediate the substrate specificity and catalysis. BtCPA corresponds to the amino acid sequence of the MCP M14 gene family from Tibetan cattle (UniProt accession: P00730). The positions in the red, yellow, and green boxes indicate the Zn2+ binding motifs (His69, Glu72, and His196), residues involved in substrate binding and catalysis (Arg124, Arg127, and Arg145), and residues important for disulfide bond formation (Cys138 and Cys165), respectively. The grey background highlights the conserved amino acid residues of the MCP M14 gene family members.
3.6. Prediction of Secondary and Tertiary Protein Structures of the MCP M14 Gene Family in L. maculata, O. scyllarus, and O. oratoria
We predicted the secondary protein structure of the MCP M14 gene family in L. maculata, O. scyllarus, and O. oratoria (Table 2). The results showed that the largest proportion of the secondary protein structure in three stomatopods was irregular curl, which accounted for 38.22~54.35%, 36.09~53.84% and 31.62~69.23% in L. maculata, O. scyllarus, and O. oratoria, respectively. Followed by α-helices, which accounted for 16.92~37.53%, 18.45~39.54%, and 12.02~37.5% in L. maculata, O. scyllarus, and O. oratoria, respectively. However, the proportion of β-folding is the lowest in L. maculata, O. scyllarus, and O. oratoria, accounting for 5.26~9.04%, 3.38~8.5%, and 1.42~10.29%, respectively. This implies that the irregular curl is the main skeleton that constitutes the secondary protein structure of the MCP M14 gene family in three stomatopods, although the proportion of different protein secondary structures is different in different species.

Table 2.
Secondary protein structures of the MCP M14 gene family in L. maculata, O. scyllarus, and O. oratoria.
The tertiary protein structures of the MCP M14 gene family in L. maculata, O. scyllarus, and O. oratoria were constructed using SWISS-MODEL homology modeling (Figure 4, Figure 5 and Figure 6). The results show that the predicted protein and the template protein have high consistency, which means that the prediction model is reliable. Seven (including 7eqx.1.B, 1aye.1.A, 2boa.1.A, 3mn8.1.A, 2nsm.1A, 4a37.1.A, and 312n.1.A), nine (including 5om9.1.A, 7eqx.1.B, 1aye.1.A, 2boa.1.A, 1dtd.1A, 2nsm.1.A, 1uwy.1.A, 1qmu.1.A, and 312n.1.A) and five (including 7eqx.1.B, 1aye.1.A, 1kwm.2.A, 3osl.1.A, and 5mrv.1.A) models were used to predict the tertiary protein structure of the MCP M14 gene family in L. maculata, O. scyllarus and O. oratoria, respectively. The GMQE of the tertiary protein structures of the MCP M14 gene family in the three stomatopods were 0.1–0.83 (in L. maculata), 0.12–0.85 (in O. scyllarus), and 0.6–0.84 (in O. oratoria), respectively, and the QMEAN fractions were 0.42 ± 0.05 to 0.78 ± 0.05 (in L. maculata), 0.47 ± 0.05 to 0.81 ± 0.05 (in O. scyllarus), and 0.56 ± 0.06 to 0.79 ± 0.05 (in O. oratoria), respectively. This means that the model has a high degree of variability (Table 3). Additionally, our results indicated that the structural distribution presented in the tertiary protein structure of the MCP M14 gene family is consistent with the predicted secondary protein structure composition

Figure 4.
Three-dimensional protein structure prediction models for the MCP M14 gene family members of L. maculate.
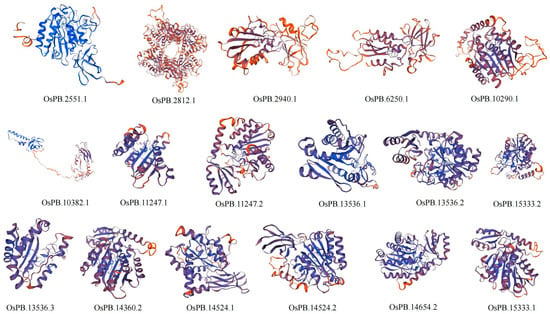
Figure 5.
Three-dimensional protein structure prediction models for the MCP M14 gene family members of O. scyllarus.
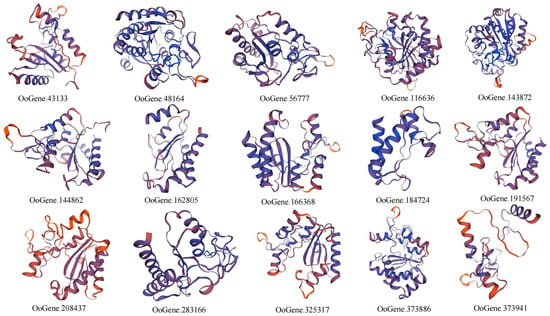
Figure 6.
Three-dimensional protein structure prediction models for the MCP M14 gene family members of O. oratoria.

Table 3.
Detailed information on the predicted three-dimensional structure of the MCP M14 gene family in L. maculata, O. scyllarus, and O. oratoria.
4. Discussion
CPs (especially the MCP) have been proven to be the critical exopeptidases in animal food digestion, decomposition, and enzyme activation [,,,,,], but related studies have not been carried out in stomatopods. Considering that the strong predatory characteristics of stomatopods are potentially differentiated with the morphological differences in the raptorial appendage [,,], it is not difficult to understand that the MCP gene characteristics of stomatopods have a morphological specificity of the raptorial appendage. In the absence of genome-wide information, FL transcriptome information can provide an opportunity for resolving the MCP gene characteristic differences in stomatopods []. In the present study, we sequenced the FL transcriptome of L. maculata and O. scyllarus for the first time. All samples achieved a sequencing depth greater than 26×, which ensured comprehensive transcriptome coverage and facilitated the capture of low-abundance transcripts. When processing the transcriptome sequencing data, we adopted exactly the same standards for transcript assembly, redundancy removal, and filtering. The number of transcripts in L. maculata and O. scyllarus was comparable; however, a significantly greater number was identified in O. oratoria. While the uniform analytical pipeline ensures comparability, this disparity may also suggest potential biological convergence between L. maculate and O. scyllarus. Conversely, the extensive transcriptome of O. oratoria could be attributed to its broad geographical distribution, which likely necessitates adaptation to diverse and variable habitats, thereby requiring a more complex genetic repertoire []. Furthermore, combined with our previously published FL transcriptome information of stomatopods, we screened the MCP M14 gene family sequences of three stomatopods and then compared their characteristic differences.
Varying numbers (13 in L. maculata, 17 in O. scyllarus, and 15 in O. oratoria) of MCP M14 gene family members have been identified in three stomatopods, and all identified members contained the characteristic Zn_pept domain of the MCP M14 family, confirming the comprehensiveness of gene identification. This seems to imply that the three stomatopods may potentially differ in their polypeptide and protein processing abilities as a result of long-term differences in hunting strategies [,,]. In fact, interspecific differences in the number of MCP M14 gene family members have been demonstrated in other animals, such as Antheraea pernyi (14 members) and A. yamamai (20 members) []. Undoubtedly, all identified MCP M14 gene family members were divided into three subfamilies (including M14A, M14B, and M14D), which is highly consistent with the findings of mammalian MCP M14 gene family []. Most proteins belonging to the MCP M14 family in three stomatopods exhibit acidic isoelectric points, structural stability, and hydrophilic properties. These characteristics suggest that the majority of MCP M14 family members likely function within the acidic environment of the digestive tract []. Furthermore, the relatively long half-life of these proteins may facilitate the maintenance of structural integrity and functional persistence in the intestinal milieu, thereby potentially enhancing digestive efficiency. It is worth noting that the MCP M14 gene family members of the three stomatopods contain the M14A subfamily, but O. oratoria lacks the M14B and M14D subfamilies, which is different from L. maculata and O. scyllarus. The M14A, M14B, and M14D subfamilies have similar spatial structure, but the amino acid sites of these subfamilies that bind Zn2+ are H69A(S)RE72...H196, H69GNE72...H196, and H69PGE72...H196, which led to large differences in zymogenic activation and catalysis, ultimately affecting protein degradation and synthesis regulation []. In the present study, a large proportion of the MCP M14 sequences are classified into the M14A subfamily, which means that M14A is more effective at the digestion of food proteins than M14B [,], although both subfamilies are critical for protein digestion [,]. Meanwhile, different types of MCP M14 subfamilies have been shown to have different cutting abilities, such as the M14A subfamily has the best cutting effect on aromatic or fatty amino acid residues, but the M14B subfamily preferentially cuts basic amino acids and also has the ability to cut some non-basic amino acid residues []. Additionally, the M14A subfamily has also been speculated to play an important role in the endodermal digestion and absorption and the formation of new cuticle during arthropod molting [,]. Therefore, we hypothesized that the species differences in the number of the M14A subfamily members may influence the growth cycle differences among the three stomatopods.
The significant variation in the number of conserved motifs among different subfamilies reflects their degree of functional specialization. The M14A subfamily, which contains the highest number (nine) of conserved motifs, may perform more complex or diverse physiological roles, such as interfering with viral maturation, processing neuropeptide hormones, and regulating cell growth [,]. In contrast, the M14B and M14D subfamilies, with fewer motifs, likely have more specialized functions. Motif 3 was universally present across all analyzed sequences, suggesting that it encodes a core domain essential for the catalytic activity of MCP M14 and is indispensable for the fundamental catalytic function of the entire gene family. On the other hand, Motif 9 exhibited subfamily specific conservation. Its exclusive and intact preservation in the M14B subfamily likely constitutes the structural basis for this subfamily’s unique functional adaptations, including substrate-specific binding and catalytic specificity []. We further analyzed the structural characteristics of the MCP M14 gene family in three stomatopods and confirmed that this gene family is highly conserved. However, we need to note that the diversity of Zn2+ binding residues still implies functional differences in MCP M14 gene families and even subfamilies of different stomatopods.
Significantly, the remaining M14C subfamily of the MCP M14 gene family has not been identified in the FL transcripts of three stomatopods. This indicates the absence of the M14C subfamily in stomatopods, consistent with the fact that M14C functions in bacterial cell-wall metabolism. Additionally, future studies also need to carry out more in vitro experiments to complete the functional verification of the MCP M14 gene family.
5. Conclusions
The present study was the first to sequence the FL transcriptomes of L. maculata and O. scyllarus, which is necessary to enrich the genetic information of stomatopods and provides a critical resource for future genome-wide analyses of gene structure and function. Combined with the published O. oratoria FL transcriptome data, we have revealed the structural characteristics of the MCP M14 gene family in three stomatopods. This study identified 13 and 17 MCP M14 gene family members in L. maculate and O. scyllarus, respectively, which were classified into the M14A, M14B, and M14D subfamilies. In contrast, 15 members identified in O. oratoria all belonged to the M14A subfamily, while the M14C subfamily was not detected in any stomatopods. All members contained the characteristic Zn_pept domain. Analysis of conserved motifs revealed both shared and distinct patterns across subfamilies. Critical functional residues were conserved in most members, including Zn2+ binding sites (His69, Glu72, and His196), substrate-binding residues (Arg124, Arg127, and Arg145), and residues involved in disulfide bond formation (Cys138 and Cys161). The tertiary structure of these proteins was predominantly composed of random coils. These results will provide valuable resources for future research on the feeding regulation mechanisms and raptorial appendage morphology-specific trophic niche evolutionary theory of stomatopods.
However, this study has certain limitations. Future investigations should employ whole-genome sequencing and in vitro experiments to further elucidate the structure, function, and specific regulatory mechanisms of the MCP M14 family in stomatopods.
Author Contributions
Conceptualization, J.Z. and F.L.; methodology, F.L.; software, J.Z.; validation, X.D. (Xiuqiang Dong) and X.H.; formal analysis, J.Z.; investigation, J.Z., F.L. and X.D. (Xiaowen Duan); resources, F.L.; data curation, J.Z., F.L. and B.X.; writing—original draft preparation, J.Z.; writing—review and editing, J.Z. and F.L.; visualization, J.Z.; supervision, F.L.; project administration, F.L.; funding acquisition, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2024MD076) and the opening foundation of the Observation and Research Station of Bohai Strait Eco-Corridor, MNR (BH202401).
Institutional Review Board Statement
All procedures carried out in this study have also been approved by the Ethics Committee of Yantai University (approval code: 12443, approval date: 1 September 2025).
Data Availability Statement
The FL reads generated during the current study are available in the NCBI SRA under the accession numbers of SRR31556843, SRR31556845, and SRR32917886 under BioProject PRJNA1190238, and the reviewer can consult these reads through https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1190238.
Acknowledgments
The authors would like to extend gratitude to the Fisheries Resources and Ecological Environment Laboratory at Yantai University for the assistance in this research, and to those who took the time to review this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahyong, S.T. Raysquilla Manningi, a New Genus and Species of Stomatopod from the Australian Northwest Shelf. J. Crustac. Biol. 2000, 20, 37–41. [Google Scholar] [CrossRef]
- Sha, Z.; Wang, Y.; Cheng, J. Application of mitochondrial COI-based DNA barcoding for the identification of stomatopod species (Crustacea, Stomatopoda) in the China seas. J. Fish. Sci. China 2018, 25, 858. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Y.L.; Sha, Z.L. Progress on the systematics of Stomatopod (Crustacea: Malacostraca). Mar. Sci. 2015, 39, 173–177. [Google Scholar] [CrossRef]
- Manning, R.B. Stomatopod crustacea of Vietnam: The legacy of Raoul Serene. Crustac. Res. 1995, 4, 1–339. [Google Scholar] [CrossRef]
- Ahyong, S.T.; Harling, C. The phylogeny of the stomatopod Crustacea. Aust. J. Zool. 2000, 48, 607–642. [Google Scholar] [CrossRef]
- Ahyong, S.T. Phylogenetic Analysis of the Stomatopoda (Malacostraca). J. Crustac. Biol. 1997, 17, 695–715. [Google Scholar] [CrossRef]
- Marshall, N.J.; Land, M.F.; King, C.A.; Cronin, T.W. The compound eyes of mantis shrimps (Crustacea, Hoplocarida, Stomatopoda). II. Colour pigments in the eyes of stomatopod crustaceans: Polychromatic vision by serial and lateral filtering. Philos. Trans. R. Soc. B Biol. Sci. 1991, 334, 57–84. [Google Scholar] [CrossRef]
- Yaraghi, N.A.; Guarín-Zapata, N.; Grunenfelder, L.K.; Hintsala, E.; Bhowmick, S.; Hiller, J.M.; Betts, M.; Principe, E.L.; Jung, J.Y.; Sheppard, L.; et al. A Sinusoidally Architected Helicoidal Biocomposite. Adv. Mater. 2016, 28, 6835–6844. [Google Scholar] [CrossRef]
- Reaka, M.L. Molting in stomatopod crustaceans. I. Stages of the molt cycle, setagenesis, and morphology. J. Morphol. 1975, 146, 55–80. [Google Scholar] [CrossRef]
- Amini, S.; Masic, A.; Bertinetti, L.; Teguh, J.S.; Herrin, J.S.; Zhu, X.; Su, H.; Miserez, A. Textured fluorapatite bonded to calcium sulphate strengthen stomatopod raptorial appendages. Nat. Commun. 2014, 5, 3187. [Google Scholar] [CrossRef]
- Yaraghi, N.A.; Trikanad, A.A.; Restrepo, D.; Huang, W.; Rivera, J.; Herrera, S.; Zhernenkov, M.; Parkinson, D.Y.; Caldwell, R.L.; Zavattieri, P.D.; et al. The Stomatopod Telson: Convergent Evolution in the Development of a Biological Shield. Adv. Funct. Mater. 2019, 29, 13. [Google Scholar] [CrossRef]
- Patek, S.N.; Rosario, M.V.; Taylor, J.R.A. Comparative spring mechanics in mantis shrimp. J. Exp. Biol. 2012, 216, 1317–1329. [Google Scholar] [CrossRef]
- Devries, M.S.; Murphy, E.A.K.; Patek, S.N. Strike mechanics of an ambush predator: The spearing mantis shrimp. J. Exp. Biol. 2012, 215, 4374–4384. [Google Scholar] [CrossRef]
- Kim, S.; Cho, Y.S.; Kim, H.-M.; Chung, O.; Kim, H.; Jho, S.; Seomun, H.; Kim, J.; Bang, W.Y.; Kim, C.; et al. Comparison of carnivore, omnivore, and herbivore mammalian genomes with a new leopard assembly. Genome Biol. 2016, 17, 211. [Google Scholar] [CrossRef]
- DeVries, M.S. The role of feeding morphology and competition in governing the diet breadth of sympatric stomatopod crustaceans. Biol. Lett. 2017, 13, 20170055. [Google Scholar] [CrossRef]
- Jiao, H.W. Molecular Evolution of Taste and Trehalase Genes in Bats and Its Significance on the Dietary Adaptation; Wuhan University: Wuhan, China, 2019. [Google Scholar]
- Wu, J.W. Molecular Evolution of Lineage-Specifie Traits, Dietary Shift and Visual Function in Bats; Wuhan University: Wuhan, China, 2018. [Google Scholar]
- Chang, Y. Research on the Factors Influencing Dietary Niche Expansion and the Adaptive Evolution of Skull in Piscivorous Bats; Northeast Normal University: Changchun, China, 2020. [Google Scholar] [CrossRef]
- Youngblut, N.D.; Reischer, G.H.; Walters, W.; Schuster, N.; Farnleitner, A.H. Host diet and evolutionary history explain different aspects of gut microbiome diversity among vertebrate clades. Nat. Commun. 2019, 10, 2200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Identification of M14 Family Metal Carboxypeptidases in Antheraea pernyi and Transcriptional Response of Digestive Enzymes Triggered by Starvation; Nanyang Normal University: Nanyang, China, 2022. [Google Scholar] [CrossRef]
- Rossano, R.; Larocca, M.; Lamaina, A.; Viggiani, S.; Riccio, P. The hepatopancreas enzymes of the crustaceans Munida and their potential application in cheese biotechnology. LWT-Food Sci. Technol. 2011, 44, 173–180. [Google Scholar] [CrossRef]
- Fang, L.S.; Lee, B.N. Ontogenic change of digestive enzymes in Penaeus monodon. Comp. Biochem. Physiol. Part B Comp. Biochem. 1992, 103, 1033–1037. [Google Scholar] [CrossRef]
- Tsai, I.H.; Chuano, K.L.; Chuang, J.L. Chymotrypsins in digestive tracts of crustacean decapods (Shrimps). Comp. Biochem. Physiol. B Comp. Biochem. 1986, 85, 235–239. [Google Scholar] [CrossRef]
- Serrano, A.E. Ontogenetic Changes in the Activity of Chymotrypsin and Carboxypeptidases A and B in Mud Crab, Scylla serrata. Isr. J. Aquac. Bamidgeh 2013, 65, 1–6. [Google Scholar]
- Andrés, M.; Gisbert, E.; Díaz, M.; Moyano, F.J.; Rotllant, G. Ontogenetic changes in digestive enzymatic capacities of the spider crab, Maja brachydactyla (Decapoda: Majidae). J. Exp. Mar. Biol. Ecol. 2010, 389, 75–84. [Google Scholar] [CrossRef]
- Vogt, G. Synthesis of digestive enzymes, food processing, and nutrient absorption in decapod crustaceans: A comparison to the mammalian model of digestion. Zoology 2021, 147, 125945. [Google Scholar] [CrossRef]
- Le Moullac, G.; Roy, P.; Van Wormhoudt, A. Effects of trophic prophylatic factors on some digestive enzymatic activities of Penaeus vannamei larvae. In Memorias del Primer Congreso Ecuatoriano de Acuicultura, San Pedro de Manglaralto, Ecuador; CENAIM: Guayaquil, Ecuador, 1992; pp. 81–86. [Google Scholar]
- Long, Q.; Liu, J.; Sun, Y.; Yang, Z.; Tang, B.; Cheng, Y. The Effect of Food Deprivation on Foraging Behavior and Digestive and Metabolic Capacities of the Chinese Mitten Crab, Eriocheir sinensis. Fishes 2023, 8, 47. [Google Scholar] [CrossRef]
- Navarrete-Del-Toro, M.A.; García-Carreño, F.L.; Hernández-Cortés, P.; Molnár, T.; Gráf, L. Biochemical characterisation of chymotrypsin from the midgut gland of yellowleg shrimp, Penaeus californiensis. Food Chem. 2015, 173, 147–155. [Google Scholar] [CrossRef]
- Jones, D.; Kumlu, M.; Le Vay, L.; Fletcher, D. The digestive physiology of herbivorous, omnivorous and carnivorous crustacean larvae: A review. Aquaculture 1997, 155, 285–295. [Google Scholar] [CrossRef]
- Rivera-Pérez, C.; García-Carreño, F.L.; Saborowski, R. Purification and Biochemical Characterization of Digestive Lipase in Whiteleg Shrimp. Mar. Biotechnol. 2010, 13, 284–295. [Google Scholar] [CrossRef]
- Rodríguez-Viera, L.; Alpízar-Pedraza, D.; Mancera, J.M.; Perera, E. Toward a More Comprehensive View of α-Amylase across Decapods Crustaceans. Biology 2021, 10, 947. [Google Scholar] [CrossRef]
- Tsuji, A.; Sato, S.; Kondo, A.; Tominaga, K.; Yuasa, K. Purification and characterization of cellulase from North Pacific krill (Euphausia pacifica). Analysis of cleavage specificity of the enzyme. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 163, 324–333. [Google Scholar] [CrossRef]
- Proespraiwong, P.; Tassanakajon, A.; Rimphanitchayakit, V. Chitinases from the black tiger shrimp Penaeus monodon: Phylogenetics, expression and activities. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 156, 86–96. [Google Scholar] [CrossRef]
- Maugle, P.D.; Deshimaru, O.; Katayama, T.; Simpson, K.L. Digestive enzymes of the shrimp Penaeus japonicus-I. Characteristics of amylase and protease of the shrimp Penaeus japonicus. Nippon. Suisan Gakkaishi 1982, 48, 1753–1757. [Google Scholar] [CrossRef]
- Pan, L.Q.; Liu, H.Y.; Xiao, G.Q. A review on digestive enzyme of crustacean larvae. J. Fish. Sci. China 2006, 13, 492–501. [Google Scholar] [CrossRef]
- Skidgel, R.A.; Erdös, E.G. Cellular carboxypeptidases. Immunol. Rev. 1998, 161, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, A.; Sharma, R.; Gakhar, S. Identification, characterization and analysis of expression of gene encoding carboxypeptidase A in Anopheles culicifacies A (Diptera: Culicidae). Acta Trop. 2014, 139, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Fricker, L.D. Activation and membrane binding of carboxypeptidase E. J. Cell. Biochem. 1988, 38, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Isoe, J.; Zamora, J.; Miesfeld, R.L. Molecular analysis of the Aedes aegypti carboxypeptidase gene family. Insect Biochem. Mol. Biol. 2009, 39, 68–73. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Han, M.J.; Zha, X.L.; Chen, Y.; Shen, Y.H. Identification and Expression Analysis of Metallocarboxypeptidase Gene Family in Silkworm, Bombyx mori. Acta Sericologica Sin. 2016, 42, 393–403. [Google Scholar] [CrossRef]
- Ferreira, C.; Rebola, K.G.O.; Cardoso, C.; Bragatto, I.; Ribeiro, A.F.; Terra, W.R. Insect midgut carboxypeptidases with emphasis on S10 hemipteran and M14 lepidopteran carboxypeptidases. Insect Mol. Biol. 2014, 24, 222–239. [Google Scholar] [CrossRef]
- Rodriguez de la Vega, M.; Sevilla, R.G.; Hermoso, A.; Lorenzo, J.; Tanco, S.; Diez, A.; Fricker, L.D.; Bautista, J.M.; Avilés, F.X. Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 2007, 21, 851–865. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Evolutionary families of metallopeptidases. Methods Enzymol. 1995, 248, 183–228. [Google Scholar] [CrossRef]
- Bown, D.P.; Gatehouse, J.A. Characterization of a digestive carboxypeptidase from the insect pest corn earworm (Helicoverpa armigera) with novel specificity towards C-terminal glutamate residues. Eur. J. Biochem. 2004, 271, 2000–2011. [Google Scholar] [CrossRef]
- Edwards, M.J.; Lemos, F.J.A.; Donnelly-Doman, M.; Jacobs-Lorena, M. Rapid Induction by a Blood Meal of a Carboxypeptidase Gene in the Gut of the Mosquito Anopheles gambiae. Insect Biochem. Mol. Biol. 1997, 27, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Mahowald, A.; Jacobs-Lorena, M. Gut-specific genes from the black fly Simulium vittatum encoding trypsin-like and carboxypeptidase-like proteins. Insect Mol. Biol. 1993, 1, 149–163. [Google Scholar] [CrossRef]
- Wang, P.; Li, G.; Kain, W. Characterization and cDNA cloning of midgut carboxypeptidases from Trichoplusia ni. Insect Biochem. Mol. Biol. 2004, 34, 831–843. [Google Scholar] [CrossRef]
- Edwards, M.J.; Moskalyk, L.A.; Donelly-Doman, M.; Vlaskova, M.; Noriega, F.G.; Walker, V.K.; Jacobs-Lorena, M. Characterization of a carboxypeptidase A gene from the mosquito, Aedes aegypti. Insect Mol. Biol. 2000, 9, 33–38. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, P.; Li, S.; Ma, S.; Du, J.; Liang, S.; Yang, X.; Yao, L.; Duan, J. Genome-Wide Identification of M14 Family Metal Carboxypeptidases in Antheraea pernyi (Lepidoptera: Saturniidae). J. Econ. Èntomol. 2022, 115, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C.; Ahn, D.H.; Kim, S.J.; Lee, H.; Oh, T.J.; Lee, J.E.; Park, H. Advantages of Single-Molecule Real-Time Sequencing in High-GC Content Genomes. PLoS ONE 2013, 8, e68824. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.R.; Zhou, J.L.; Schunter, C.; Wang, L.; Tang, Y.Z.; Han, Z.Q.; Kang, B. How Oratosquilla oratoria compound eye response to the polarization of light: In the perspective of vision genes and related proteins. Int. J. Biol. Macromol. 2024, 259, 129053. [Google Scholar] [CrossRef]
- Gordon, S.P.; Tseng, E.; Salamov, A.; Zhang, J.; Meng, X.; Zhao, Z.; Kang, D.; Underwood, J.; Grigoriev, I.V.; Figueroa, M.; et al. Widespread Polycistronic Transcripts in Fungi Revealed by Single-Molecule mRNA Sequencing. PLoS ONE 2015, 10, e0132628. [Google Scholar] [CrossRef]
- Guizard, S.; Miedzinska, K.; Smith, J.; Smith, J.; I Kuo, R.; Davey, M.; Archibald, A.; Watson, M. nf-core/isoseq: Simple gene and isoform annotation with PacBio Iso-Seq long-read sequencing. Bioinformatics 2023, 39, btad150. [Google Scholar] [CrossRef]
- Li, W.Z.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.J.; Eddy, S.R. nhmmer: DNA homology search with profile HMMs. Bioinformatics 2013, 29, 2487–2489. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, D.P.; Lenin, E.A.; Kiruba, D.A. Intrageneric phylogenetics based on mitochondrial DNA variation among fifteen harpactorine assassin bugs with four ecotypes and three morphs (Hemiptera: Reduviidae: Harpactorinae). Zootaxa 2014, 3779, 540–550. [Google Scholar] [CrossRef]
- Goud, T.S.; Upadhyay, R.C.; Onteru, S.K.; Pichili, V.B.R.; Chadipiralla, K. Identification and sequence characterization of melanocortin 1 receptor gene (MC1R) in Bos indicus versus (Bos taurus × Bos indicus). Anim. Biotechnol. 2019, 31, 283–294. [Google Scholar] [CrossRef]
- Patnaik, B.B.; Hwang, H.J.; Baliarsingh, S.; Chung, J.M.; Sang, M.K.; Min, H.R.; Park, J.E.; Cho, H.C.; Kang, S.W.; Park, S.Y.; et al. In silico characterization of single and tandem-repeat galectin from terrestrial slug, Incilaria fruhstorferi. J. Environ. Biol. 2019, 40, 940–947. [Google Scholar] [CrossRef]
- Cool, D.R.; Loh, Y.P. Carboxypeptidase E is a sorting receptor for prohormones: Binding and kinetic studies. Mol. Cell. Endocrinol. 1998, 139, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Hourdou, M.L.; Guinand, M.; Vacheron, M.J.; Michel, G.; Denoroy, L.; Duez, C.; Englebert, S.; Joris, B.; Weber, G.; Ghuysen, J.M. Characterization of the sporulation-related gamma-D-glutamyl-(L) meso-diaminopimelic-acid-hydrolysing peptidase I of Bacillus sphaericus NCTC 9602 as a member of the metallo(zinc) carboxypeptidase A family. Modular design of the protein. Biochem. J. 1993, 292, 563–570. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Z.X.; Li, Y.; Zhang, L.W.; Hui, M.; Sha, Z.L. Rolling with the punches: Organism-environment interactions shape spatial pattern of adaptive differentiation in the widespread mantis shrimp Oratosquilla oratoria. Sci. Total Environ. 2024, 917, 170244. [Google Scholar] [CrossRef]
- Segundo, B.S.; Martínez, M.C.; Vilanova, M.; Cuchillo, C.M.; Avilés, F.X. The severed activation segment of porcine pancreatic procarboxypeptidase A is a powerful inhibitor of the active enzyme isolation and characterisation of the activation peptide. Biochim. Et Biophys. Acta 1982, 707, 74–80. [Google Scholar] [CrossRef]
- Sui, Y.P.; Liu, X.B.; Chai, L.Q.; Wang, J.X.; Zhao, X.F. Characterization and influences of classical insect hormones on the expression profiles of a molting carboxypeptidase A from the cotton bollworm (Helicoverpa armigera). Insect Mol. Biol. 2009, 18, 353–363. [Google Scholar] [CrossRef]
- Ote, M.; Mita, K.; Kawasaki, H.; Daimon, T.; Kobayashi, M.; Shimada, T. Identification of molting fluid carboxypeptidase A (MF-CPA) in Bombyx mori. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 141, 314–322. [Google Scholar] [CrossRef]
- Tham, H.W.; Balasubramaniam, V.R.; Tejo, B.A.; Ahmad, H.; Hassan, S.S. CPB1 of Aedes aegypti interacts with DENV2 E protein and regulates intracellular viral accumulation and release from midgut cells. Viruses 2014, 6, 5028–5046. [Google Scholar] [CrossRef] [PubMed]
- Settle, S.J.; Green, M.M.; Burtis, K.C. The silver gene of Drosophila melanogaster encodes multiple carboxypeptidases similar to mammalian prohormone-processing enzymes. Proc. Natl. Acad. Sci. USA 1995, 92, 9470–9474. [Google Scholar] [CrossRef] [PubMed]
- Fernández, D.; Pallarès, I.; Vendrell, J.; Avilés, F.X. Progress in metallocarboxypeptidases and their small molecular weight inhibitors. Biochimie 2010, 92, 1484–1500. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).