Environmentally Relevant Sulfamethoxazole Induces Developmental Toxicity in Embryo-Larva of Marine Medaka (Oryzias melastigma)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Embryo Collection and SMX Exposure
2.3. Heart Rate Measurements and Morphological Observation
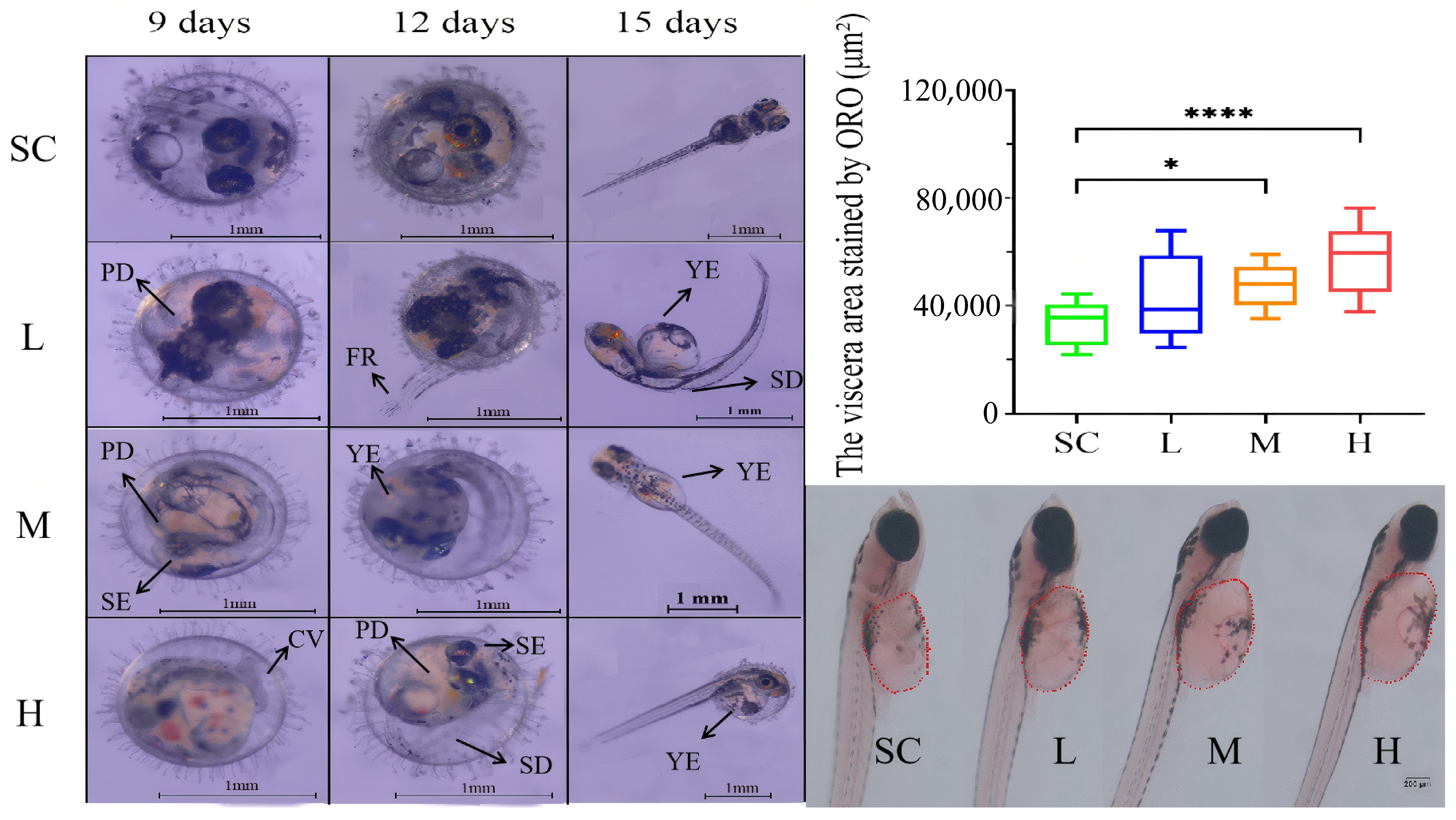
2.4. Oil Red Staining of Newly Hatched Larvae
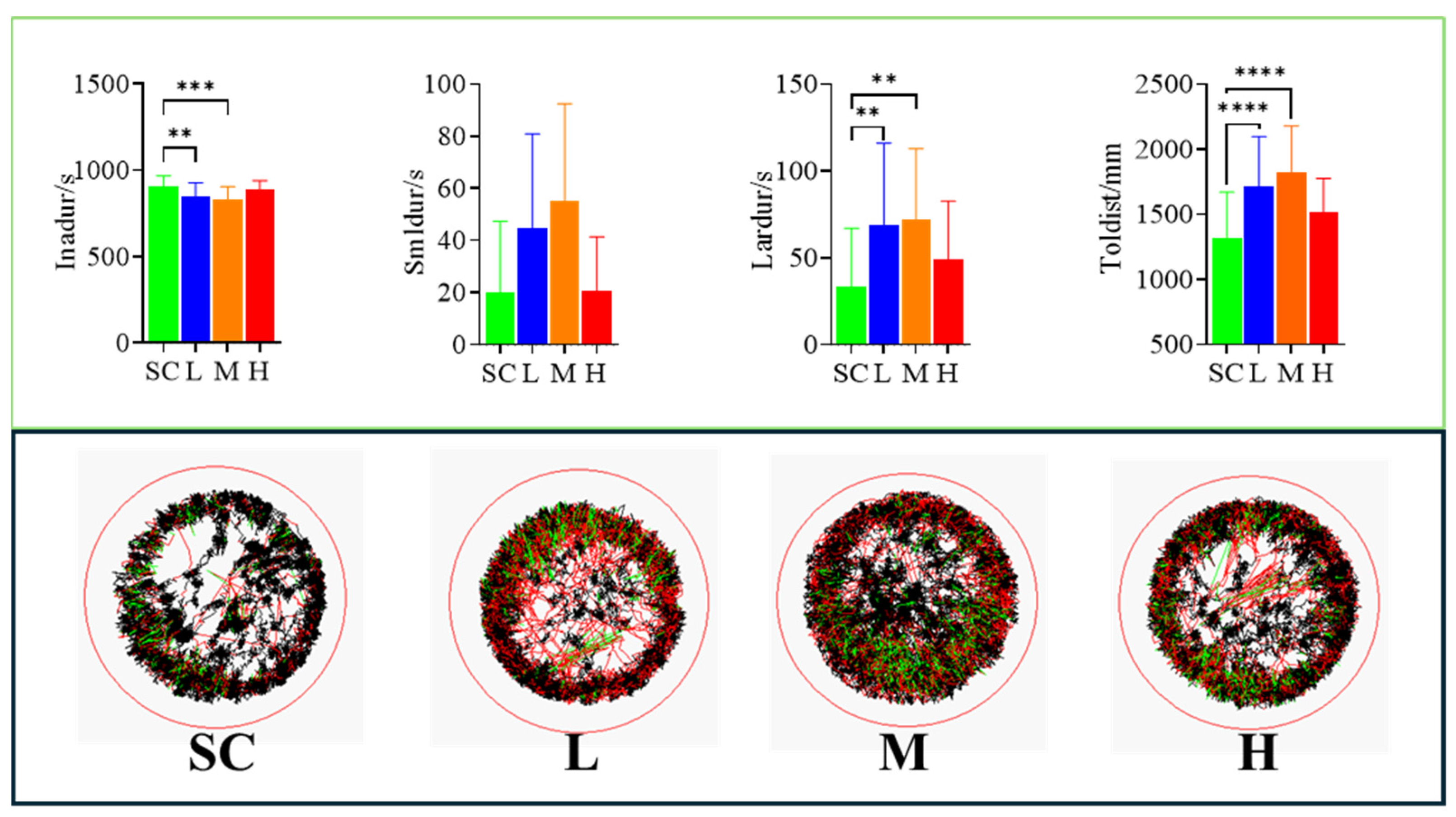
2.5. Analysis of Swimming Behavior
2.6. Total RNA Isolation, cDNA Library Construction, and Real-Time Quantitative PCR (qPCR) Analysis
2.7. 16S rRNA Amplicon Sequencing Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of SMX on the Survival Rate, Total Length, Hatching Time, and Heart Rate of Marine Medaka Embryos
3.2. Effects of SMX on the Morphology of Marine Medaka Embryos and Larvae
3.3. Effects of SMX on the Swimming Behavior of Marine Medaka
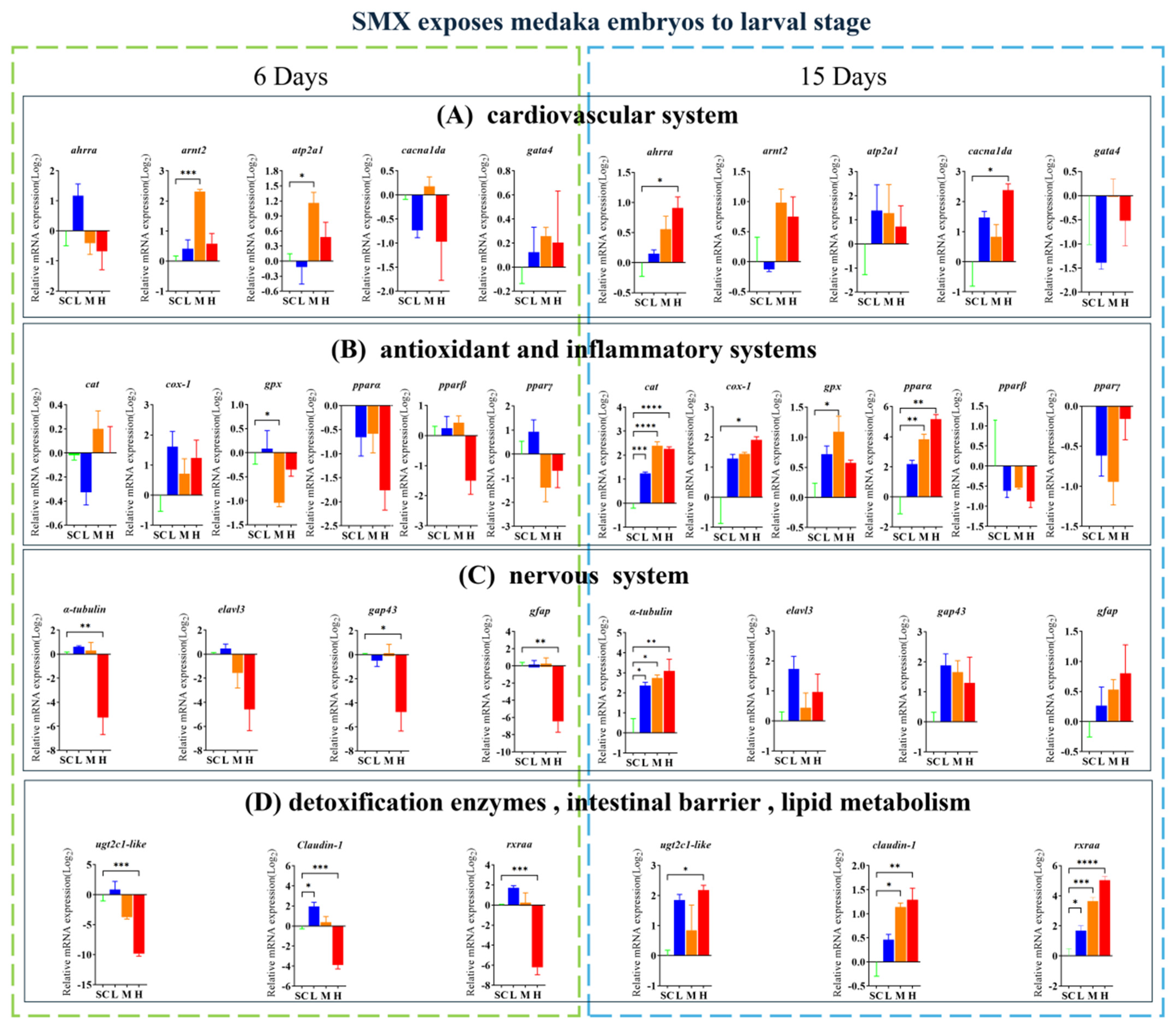
3.4. Effects of SMX on Gene Transcription in Marine Medaka Embryos and Larvae
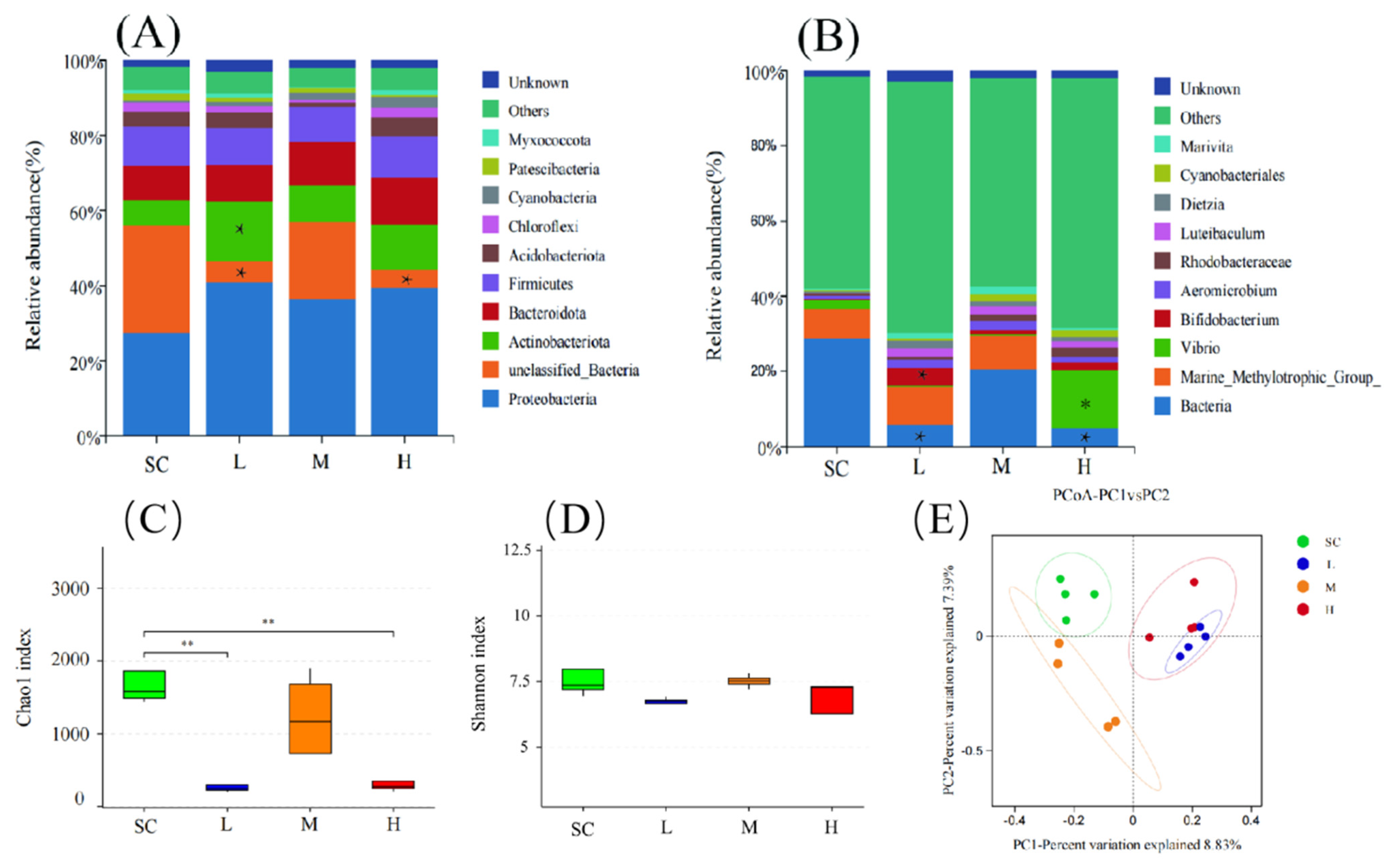
3.5. Effects of SMX on the Structure of Microbiome in Marine Medaka Larvae
4. Discussion
4.1. Effects of SMX Exposure on the Viability and Hatching of Marine Medaka Embryos
4.2. Effects of SMX Exposure on the Cardiovascular System of Marine Medaka Embryos
4.3. Effects of SMX Exposure on the Antioxidant System and Inflammatory Response in Marine Medaka Embryos
4.4. Effects of SMX Exposure on the Nervous System of Marine Medaka Embryos
4.5. Effects of SMX Exposure on the Microbial Structure of Marine Medaka Larvae
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ben, W.; Pan, X.; Qiang, Z. Occurrence and Partition of Antibiotics in the Liquid and Solid Phases of Swine Wastewater from Concentrated Animal Feeding Operations in Shandong Province, China. Environ. Sci. Process Impacts 2013, 15, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Bielen, A.; Šimatović, A.; Kosić-Vukšić, J.; Senta, I.; Ahel, M.; Babić, S.; Jurina, T.; González Plaza, J.J.; Milaković, M.; Udiković-Kolić, N. Negative Environmental Impacts of Antibiotic-Contaminated Effluents from Pharmaceutical Industries. Water Res. 2017, 126, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Lv, Z.; Zhang, Z.; Han, Y.; Liu, Z.; Zhang, H. A Review of Antibiotics, Antibiotic Resistant Bacteria, and Resistance Genes in Aquaculture: Occurrence, Contamination, and Transmission. Toxics 2023, 11, 420. [Google Scholar] [CrossRef]
- Wang, D.; Sui, Q.; Zhao, W. Pharmaceutical and personal care products in the surface water of China: A review. Chin. Sci. Bull. 2014, 59, 743–751. (In Chinese) [Google Scholar]
- Biošić, M.; Mitrevski, M.; Babić, S. Environmental Behavior of Sulfadiazine, Sulfamethazine, and Their Metabolites. Environ. Sci. Pollut. Res. Int. 2017, 24, 9802–9812. [Google Scholar] [CrossRef]
- Hernández-Pérez, A.; Noonin, C.; Söderhäll, K.; Söderhäll, I. Environmental Concentrations of Sulfamethoxazole Increase Crayfish Pacifastacus Leniusculus Susceptibility to White Spot Syndrome Virus. Fish. Shellfish. Immunol. 2020, 102, 177–184. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Z.; Chen, Y.; Zhang, Y.; Sun, R.; Xue, Y.; Zhnag, M. Ecological risk assessment for sulfonamides in surface waters. Ecol. Environ. Sci. 2016, 25, 1508–1514. [Google Scholar]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and Personal Care Products in the Aquatic Environment in China: A Review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Shelver, W.L.; Hakk, H.; Larsen, G.L.; DeSutter, T.M.; Casey, F.X.M. Development of an Ultra-High-Pressure Liquid Chromatography-Tandem Mass Spectrometry Multi-Residue Sulfonamide Method and Its Application to Water, Manure Slurry, and Soils from Swine Rearing Facilities. J. Chromatogr. A 2010, 1217, 1273–1282. [Google Scholar] [CrossRef]
- Faleye, A.C.; Adegoke, A.A.; Ramluckan, K.; Bux, F.; Stenström, T.A. Antibiotic Residue in the Aquatic Environment: Status in Africa. Open Chem. 2018, 16, 890–903. [Google Scholar] [CrossRef]
- European Commission, Joint Research Centre. State of the Art on the Contribution of Water to Antimicrobial Resistance; Publications Office: Luxembourg, 2018. [Google Scholar]
- Bojarski, B.; Kot, B.; Witeska, M. Antibacterials in Aquatic Environment and Their Toxicity to Fish. Pharmaceuticals 2020, 13, 189. [Google Scholar] [CrossRef]
- Wahome, C.N. Contamination Levels of Groundwater, Antimicrobial Resistance Patterns, Plasmid Profiles and Chlorination Efficacy in Ongata Rongai, Kajiado North County, Kenya. Master’s Thesis, Kenyatta University, Nairobi, Kenya, 2014. [Google Scholar]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological Risk Assessment of Fifty Pharmaceuticals and Personal Care Products (PPCPs) in Chinese Surface Waters: A Proposed Multiple-Level System. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ge, F.; Huang, S.; Chen, M.; Wang, R. Occurrence of Veterinary Antibiotics in Animal Wastewater and Surface Water around Farms in Jiangsu Province, China. Chemosphere 2011, 82, 1408–1414. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wu, T.; Ma, K.; Cheng, Z.; Yi, Q.; Dai, Y.; Wang, B.; Chen, Y.; Wang, B.; et al. Characteristics of Antibiotic Resistance Genes and Microbial Community Distribution in Wanfeng Lake, Upper Pearl River, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 83214–83230. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, Z.; Qiao, Y.; Liu, D.; Feng, C.; Bai, Y. Distribution and Characterization of Typical Antibiotics in Water Bodies of the Yellow River Estuary and Their Ecological Risks. Toxics 2023, 11, 400. [Google Scholar] [CrossRef]
- Stavroulaki, A.; Tzatzarakis, M.N.; Karzi, V.; Katsikantami, I.; Renieri, E.; Vakonaki, E.; Avgenaki, M.; Alegakis, A.; Stan, M.; Kavvalakis, M.; et al. Antibiotics in Raw Meat Samples: Estimation of Dietary Exposure and Risk Assessment. Toxics 2022, 10, 456. [Google Scholar] [CrossRef]
- Qiu, W.; Fang, M.; Magnuson, J.T.; Greer, J.B.; Chen, Q.; Zheng, Y.; Xiong, Y.; Luo, S.; Zheng, C.; Schlenk, D. Maternal Exposure to Environmental Antibiotic Mixture during Gravid Period Predicts Gastrointestinal Effects in Zebrafish Offspring. J. Hazard. Mater. 2020, 399, 123009. [Google Scholar] [CrossRef]
- Fang, L.; Chen, X.; Shan, X.; Qiu, L.; Fan, L.; Meng, S.; Song, C. Antibiotic Accumulation, Growth Performance, Intestinal Diversification, and Function of Nile Tilapia (Oreochromis niloticus) Feed by Diets Supplemented with Different Doses of Sulfamethoxazole. Environ. Sci. Pollut. Res. Int. 2021, 28, 65255–65264. [Google Scholar] [CrossRef]
- Hu, F.; Dong, F.; Yin, L.; Wang, H.; Zheng, M.; Fu, S.; Zhang, W. Effects of Sulfamethoxazole on the Growth, Oxidative Stress and Inflammatory Response in the Liver of Juvenile Nile Tilapia (Oreochromis niloticus). Aquaculture 2021, 543, 736935. [Google Scholar] [CrossRef]
- Limbu, S.M.; Zhou, L.; Sun, S.-X.; Zhang, M.-L.; Du, Z.-Y. Chronic Exposure to Low Environmental Concentrations and Legal Aquaculture Doses of Antibiotics Cause Systemic Adverse Effects in Nile Tilapia and Provoke Differential Human Health Risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, N.; Hashmi, I. Assessment of Immunohematological, Hematological and Biochemical Responses in Cultivable Fish Cyprinus Carpio Exposed to an Antibiotic Sulfamethoxazole (SMX). J. Water Health 2020, 19, 108–119. [Google Scholar] [CrossRef]
- Xie, S.; Yin, P.; Tian, L.; Liu, Y.; Tan, B.; Niu, J. Interactions between Dietary Lipid Levels and Chronic Exposure of Legal Aquaculture Dose of Sulfamethoxazole in Juvenile Largemouth Bass Micropterus Salmoides. Aquat. Toxicol. 2020, 229, 105670. [Google Scholar] [CrossRef]
- Yu, Z.; Shen, J.; Li, Z.; Yao, J.; Li, W.; Xue, L.; Vandenberg, L.N.; Yin, D. Obesogenic Effect of Sulfamethoxazole on Drosophila Melanogaster with Simultaneous Disturbances on Eclosion Rhythm, Glucolipid Metabolism, and Microbiota. Environ. Sci. Technol. 2020, 54, 5667–5675. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Zheng, M.; Wang, G.; Zhao, H. Exposed to Sulfamethoxazole Induced Hepatic Lipid Metabolism Disorder and Intestinal Microbiota Changes on Zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 253, 109245. [Google Scholar] [CrossRef] [PubMed]
- Labitt, R.N.; Ren, J.; Marquis, H. Emergence of Phenotypic and Genotypic Resistance in the Intestinal Microbiota of Rainbow Trout (Oncorhynchus mykiss) Exposed Long-Term to Sub-Inhibitory Concentrations of Sulfamethoxazole. Ecotoxicology 2021, 30, 2043–2054. [Google Scholar] [CrossRef]
- Mohammed, A. Why Are Early Life Stages of Aquatic Organisms More Sensitive to Toxicants than Adults? In New Insights into Toxicity and Drug Testing; Gowder, S., Ed.; InTech: Rijeka, Croatia, 2013; ISBN 978-953-51-0946-4. [Google Scholar]
- Kim, B.-M.; Kim, J.; Choi, I.-Y.; Raisuddin, S.; Au, D.W.T.; Leung, K.M.Y.; Wu, R.S.S.; Rhee, J.-S.; Lee, J.-S. Omics of the Marine Medaka (Oryzias melastigma) and Its Relevance to Marine Environmental Research. Mar. Environ. Res. 2016, 113, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, P.; Rey-Campos, M.; Figueras, A.; Novoa, B. An Environmentally Relevant Concentration of Antibiotics Impairs the Immune System of Zebrafish (Danio rerio) and Increases Susceptibility to Virus Infection. Front. Immunol. 2022, 13, 1100092. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, J.; Zhang, Y.; Shan, Z. Toxic effect sofsulfamethoxazole on zebrafish (Danio rerio) embryo/larva. Environ. Pollut. Control 2020, 42, 310–316. [Google Scholar]
- Han, X.B.; Yuen, K.W.Y.; Wu, R.S.S. Polybrominated Diphenyl Ethers Affect the Reproduction and Development, and Alter the Sex Ratio of Zebrafish (Danio rerio). Environ. Pollut. 2013, 182, 120–126. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Ran, H.; Lin, Y. Developmental stages of a marine model fish—medaka Oryzias melastigma. Oceanol. Limnol. Sin. 2016, 47, 71–82. [Google Scholar]
- Dong, Z.; Li, X.; Chen, Y.; Zhang, N.; Wang, Z.; Liang, Y.-Q.; Guo, Y. Short-Term Exposure to Norethisterone Affected Swimming Behavior and Antioxidant Enzyme Activity of Medaka Larvae, and Led to Masculinization in the Adult Population. Chemosphere 2023, 310, 136844. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shan, L.; Yan, M.; Chai, X.; Hu, L.; Shao, X. Embryonic development of nibea albiflora and the effects of temperature and salinity on embryogenesis. Mar. Sci. 2017, 41, 44–50. [Google Scholar]
- Kawaguchi, M.; Yasumasu, S.; Shimizu, A.; Sano, K.; Iuchi, I.; Nishida, M. Conservation of the Egg Envelope Digestion Mechanism of Hatching Enzyme in Euteleostean Fishes. FEBS J. 2010, 277, 4973–4987. [Google Scholar] [CrossRef]
- Zhou, L.; Limbu, S.M.; Shen, M.; Zhai, W.; Qiao, F.; He, A.; Du, Z.-Y.; Zhang, M. Environmental Concentrations of Antibiotics Impair Zebrafish Gut Health. Environ. Pollut. 2018, 235, 245–254. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Gong, L.; Cheng, Y.; Yuan, Q.; He, Y. Combined Toxicity of Polystyrene Microplastics and Sulfamethoxazole on Zebrafish Embryos. Environ. Sci. Pollut. Res. Int. 2022, 29, 19273–19282. [Google Scholar] [CrossRef]
- Genge, C.; Hove-Madsen, L.; Tibbits, G.F. Functional and Structural Differences in Atria Versus Ventricles in Teleost Hearts. In New Advances and Contributions to Fish Biology; Turker, H., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0909-9. [Google Scholar]
- Tshering, G.; Plengsuriyakarn, T.; Na-Bangchang, K.; Pimtong, W. Embryotoxicity Evaluation of Atractylodin and β-Eudesmol Using the Zebrafish Model. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108869. [Google Scholar] [CrossRef]
- Iftikhar, N.; Konig, I.; English, C.; Ivantsova, E.; Souders, C.L., 2nd; Hashmi, I.; Martyniuk, C.J. Sulfamethoxazole (SMX) Alters Immune and Apoptotic Endpoints in Developing Zebrafish (Danio rerio). Toxics 2023, 11, 178. [Google Scholar] [CrossRef]
- Antkiewicz, D.S.; Peterson, R.E.; Heideman, W. Blocking Expression of AHR2 and ARNT1 in Zebrafish Larvae Protects against Cardiac Toxicity of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Toxicol. Sci. 2006, 94, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Brini, M. Calcium Pumps: Structural Basis for and Mechanism of Calcium Transmembrane Transport. Curr. Opin. Chem. Biol. 2000, 4, 152–161. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Rice, W.J.; Odermatt, A. Structure/Function Analysis of the Ca2+ Binding and Translocation Domain of SERCA1 and the Role in Brody Disease of the ATP2A1 Gene Encoding SERCA1. Ann. N. Y. Acad. Sci. 1997, 834, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Huang, S.; Wang, C.; Guo, Y.; Dong, Z.; Liu, C. Observation of Embryonic Development and Autofluorescence of Oryzias curvinotus. J. Guangdong Ocean. Univ. 2019, 39, 38–44. [Google Scholar]
- Woolley, J.F.; Stanicka, J.; Cotter, T.G. Recent Advances in Reactive Oxygen Species Measurement in Biological Systems. Trends Biochem. Sci. 2013, 38, 556–565. [Google Scholar] [CrossRef]
- Aldosari, S.; Awad, M.; Harrington, E.O.; Sellke, F.W.; Abid, M.R. Subcellular Reactive Oxygen Species (ROS) in Cardiovascular Pathophysiology. Antioxidants 2018, 7, 14. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative Stress: Molecular Perception and Transduction of Signals Triggering Antioxidant Gene Defenses. Braz J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef]
- Piner Benli, P.; Çelik, M. Glutathione and Its Dependent Enzymes’ Modulatory Responses to Neonicotinoid Insecticide Sulfoxaflor Induced Oxidative Damage in Zebrafish in Vivo. Sci. Prog. 2021, 104, 368504211028361. [Google Scholar] [CrossRef]
- Gabriel, J.P.; Ausborn, J.; Ampatzis, K.; Mahmood, R.; Eklöf-Ljunggren, E.; El Manira, A. Principles Governing Recruitment of Motoneurons during Swimming in Zebrafish. Nat. Neurosci. 2011, 14, 93–99. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Cowden, J.; Simmons, S.O.; Padilla, S.; Ramabhadran, R. Gene Expression Changes in Developing Zebrafish as Potential Markers for Rapid Developmental Neurotoxicity Screening. Neurotoxicol. Teratol. 2010, 32, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Bauer, N.M.; Schäfer, I.; White, R. Making Myelin Basic Protein -from mRNA Transport to Localized Translation. Front. Cell Neurosci. 2013, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Ueshima, E.; Muraoka, O.; Tanaka, H.; Yeo, S.Y.; Huh, T.L.; Miki, N. Zebrafish Elav/HuC Homologue as a Very Early Neuronal Marker. Neurosci. Lett. 1996, 216, 109–112. [Google Scholar] [CrossRef]
- Okada, M.; Kawagoe, Y.; Takasugi, T.; Nozumi, M.; Ito, Y.; Fukusumi, H.; Kanemura, Y.; Fujii, Y.; Igarashi, M. JNK1-Dependent Phosphorylation of GAP-43 Serine 142 Is a Novel Molecular Marker for Axonal Growth. Neurochem. Res. 2022, 47, 2668–2682. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.-B.; Jia, P.-P.; Li, W.-G.; Xie, X.-Y.; Yang, G.; Pei, D.-S. Sulfonamides (SAs) Exposure Causes Neurobehavioral Toxicity at Environmentally Relevant Concentrations (ERCs) in Early Development of Zebrafish. Aquat. Toxicol. 2023, 261, 106614. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, L.; Chen, J. Sulfamethoxazole Induces Brain Capillaries Toxicity in Zebrafish by Up-Regulation of VEGF and Chemokine Signalling. Ecotoxicol. Environ. Saf. 2022, 238, 113620. [Google Scholar] [CrossRef]
- Awakawa, T.; Barra, L.; Abe, I. Biosynthesis of Sulfonamide and Sulfamate Antibiotics in Actinomycete. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab001. [Google Scholar] [CrossRef]
- Rahdar, H.A.; Mahmoudi, S.; Bahador, A.; Ghiasvand, F.; Sadeghpour Heravi, F.; Feizabadi, M.M. Molecular Identification and Antibiotic Resistance Pattern of Actinomycetes Isolates among Immunocompromised Patients in Iran, Emerging of New Infections. Sci. Rep. 2021, 11, 10745. [Google Scholar] [CrossRef]
- Watson, A.K.; Kepplinger, B.; Bakhiet, S.M.; Mhmoud, N.A.; Chapman, J.; Allenby, N.E.; Mickiewicz, K.; Goodfellow, M.; Fahal, A.H.; Errington, J. Systematic Whole-Genome Sequencing Reveals an Unexpected Diversity among Actinomycetoma Pathogens and Provides Insights into Their Antibacterial Susceptibilities. PLoS. Negl. Trop. Dis. 2022, 16, e0010128. [Google Scholar] [CrossRef]
- Miller, L.G.; Finegold, S.M. Antibacterial Sensitivity of Bifidobacterium (Lactobacillus bifidus). J. Bacteriol. 1967, 93, 125–130. [Google Scholar] [CrossRef]
- Igbinosa, E.O. Detection and Antimicrobial Resistance of Vibrio Isolates in Aquaculture Environments: Implications for Public Health. Microb. Drug Resist. 2016, 22, 238–245. [Google Scholar] [CrossRef]
- Lin, M.; Wu, X.; Yan, Q.; Ma, Y.; Huang, L.; Qin, Y.; Xu, X. Incidence of Antimicrobial-Resistance Genes and Integrons in Antibiotic-Resistant Bacteria Isolated from Eels and Aquaculture Ponds. Dis. Aquat. Organ. 2016, 120, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Stalin, N.; Srinivasan, P. Molecular Characterization of Antibiotic Resistant Vibrio Harveyi Isolated from Shrimp Aquaculture Environment in the South East Coast of India. Microb. Pathog. 2016, 97, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Kayani, M.U.R.; Yu, K.; Qiu, Y.; Shen, Y.; Gao, C.; Feng, R.; Zeng, X.; Wang, W.; Chen, L.; Su, H.L. Environmental Concentrations of Antibiotics Alter the Zebrafish Gut Microbiome Structure and Potential Functions. Environ. Pollut. 2021, 278, 116760. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Nell, S.; Suerbaum, S.; Josenhans, C. The Impact of the Microbiota on the Pathogenesis of IBD: Lessons from Mouse Infection Models. Nat. Rev. Microbiol. 2010, 8, 564–577. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Jin, C.; Yuan, X.; Wang, C.; Fu, Z.; Jin, Y. Maternal Exposure to Imazalil Disrupts Intestinal Barrier and Bile Acids Enterohepatic Circulation Tightly Related IL-22 Expression in F0, F1 and F2 Generations of Mice. J. Hazard. Mater. 2021, 403, 123668. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, Z.; Wang, X.; Shen, M.; Zhou, J.; Fu, Z.; Jin, Y. Short-Term Propamocarb Exposure Induces Hepatic Metabolism Disorder Associated with Gut Microbiota Dysbiosis in Adult Male Zebrafish. Acta Biochim. Biophys. Sin. 2019, 51, 88–96. [Google Scholar] [CrossRef]
- Zhao, X.; Su, H.; Xu, W.; Hu, X.; Xu, Y.; Wen, G.; Cao, Y. Removal of Antibiotic Resistance Genes and Inactivation of Antibiotic-Resistant Bacteria by Oxidative Treatments. Sci. Total Environ. 2021, 778, 146348. [Google Scholar] [CrossRef]
- Yin, X.; Zheng, J.; Liu, Y.; Li, Y.; Yu, X.; Li, Y.; Wang, X. Metagenomic Evidence for Increasing Antibiotic Resistance in Progeny upon Parental Antibiotic Exposure as the Cost of Hormesis. Chemosphere 2022, 309, 136738. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Ye, L.; Huang, S.; Chen, Z.; Gao, J.; Li, Y.; Guo, Y.; Wang, Z.; Liao, J.; Dong, Z.; et al. Environmentally Relevant Sulfamethoxazole Induces Developmental Toxicity in Embryo-Larva of Marine Medaka (Oryzias melastigma). Fishes 2025, 10, 120. https://doi.org/10.3390/fishes10030120
Huang J, Ye L, Huang S, Chen Z, Gao J, Li Y, Guo Y, Wang Z, Liao J, Dong Z, et al. Environmentally Relevant Sulfamethoxazole Induces Developmental Toxicity in Embryo-Larva of Marine Medaka (Oryzias melastigma). Fishes. 2025; 10(3):120. https://doi.org/10.3390/fishes10030120
Chicago/Turabian StyleHuang, Jianxuan, Lei Ye, Siyi Huang, Zuchun Chen, Jiahao Gao, Yangmei Li, Yusong Guo, Zhongduo Wang, Jian Liao, Zhongdian Dong, and et al. 2025. "Environmentally Relevant Sulfamethoxazole Induces Developmental Toxicity in Embryo-Larva of Marine Medaka (Oryzias melastigma)" Fishes 10, no. 3: 120. https://doi.org/10.3390/fishes10030120
APA StyleHuang, J., Ye, L., Huang, S., Chen, Z., Gao, J., Li, Y., Guo, Y., Wang, Z., Liao, J., Dong, Z., & Zhang, N. (2025). Environmentally Relevant Sulfamethoxazole Induces Developmental Toxicity in Embryo-Larva of Marine Medaka (Oryzias melastigma). Fishes, 10(3), 120. https://doi.org/10.3390/fishes10030120





