Abstract
Sulfamethoxazole (SMX), a commonly used sulfonamide antibiotic, poses a threat to aquatic life due to its widespread presence in the environment. This study aims to investigate the specific effects of SMX on the development of marine medaka (Oryzias melastigma) embryos and larvae. Marine medaka embryos were exposed to SMX at concentrations of 0 (solvent control group, SC group), 1 μg/L (low concentration group, L group), 60 μg/L (middle concentration group, M group), and 1000 μg/L (high concentration group, H group). The results indicated that SMX exposure significantly accelerated the heart rate of embryos (p < 0.0001) and shortened the hatching time while also causing anomalies such as reduced pigmentation, smaller eye size, spinal curvature, and yolk sac edema. SMX also led to a decrease in the total length of the larvae. The M group and the H group exhibited a significant increase (p < 0.05) in lipid accumulation in the visceral mass of the larvae. In the L group and the M group, there was a significant increase (p < 0.0001) in the swimming distance of the larvae. At the molecular level, SMX exposure affected the transcript levels of the genes involved in the cardiovascular system (ahrra, arnt2, atp2a1, and cacan1da), antioxidant and inflammatory systems (cat, cox-1, gpx, pparα, pparβ, and pparγ), nervous system (gap43, gfap, α-tubulin), intestinal barrier function (claudin-1), detoxification enzymes (ugt2c1-like), and lipid metabolism (rxraa) in the embryos to larval stage. The microbiome analysis showed that at the phylum level, exposure to SMX resulted in an increase in the abundance of Proteobacteria. Additionally, the abundance of Actinobacteriota significantly increased in the L group (p < 0.05). At the genus level, the abundance of Bifidobacterium significantly increased in the L group (p < 0.05), while the abundance of Vibrio significantly increased in the H group (p < 0.05). The alpha diversity analysis revealed a significant decrease in the Chao1 index in the L and H groups, indicating a reduction in microbial richness. The beta diversity analysis showed differences in the microbial communities of marine medaka larvae among different SMX exposure groups. This study elucidates the negative impacts of SMX on the development of marine medaka embryos and larvae and their microbial composition, providing a scientific basis for assessing the risks of SMX in marine ecosystems.
Key Contribution:
This study reveals the effects of environmentally relevant concentrations of sulfamethoxazole on the morphology, physiology, gene expression, and microbial community structure of marine medaka embryos and larvae, providing a scientific basis for the formulation of stricter antibiotic usage regulations and environmental protection policies.
1. Introduction
The misuse of antibiotics and their release pose a potential threat to ecosystem health and biodiversity. Antibiotics enter various ecosystems through multiple pathways, including pharmaceutical wastewater, hospital discharges, agricultural and aquacultural effluents, and leachate from landfill sites [1,2]. These pathways not only lead to the accumulation of antibiotics in the environment but can also have direct or indirect impacts on organisms within the ecosystem through water bodies and food chains, thereby severely threatening the stability and biodiversity of ecosystems. Currently, concentrations of antibiotics detected in water bodies typically range from ng–μg/L, with sulfonamides, macrolides, fluoroquinolones, and tetracyclines being the most common pollutants in aquatic environments [3].
Sulfamethoxazole (SMX) is one of the most frequently detected sulfonamide antibiotics in the aquatic environment. Clinically, SMX is primarily used to treat urinary tract infections, respiratory infections, and intestinal infections caused by sensitive bacteria, and it is widely used in livestock and aquaculture industries [4]. Due to its discharge through urine and feces after use in humans and animals, SMX persists in natural ecosystems [5,6]. According to data from the U.S. Food and Drug Administration (FDA), SMX is one of the most commonly prescribed antibiotics and is frequently detected in aquatic environments [7,8]. For instance, the detection rate of SMX in 139 stream samples across 30 states in the United States was 12.5%, with the highest concentration reaching 1.9 μg/L [9]. Furthermore, the concentrations of SMX in manure slurry samples from U.S. pig farms reached a maximum of 1.47 mg/L [10]. In Africa, surface water SMX concentrations ranged from 0.5 ng/L to 39 μg/L, whereas in European inland surface waters, the range was from 0.5 ng/L to 17 μg/L [11,12]. In Asia, the concentration of SMX in surface water ranges from 2.3 ng/L to 63.66 μg/L [13,14]. In China, among the risk assessment studies of 33 potentially hazardous Pharmaceuticals and Personal Care Products (PPCPs), SMX was listed as one of the 15 priority compounds for detection in water bodies [15]. In studies of surface waters such as lakes, a total of 37 antibiotics (including 14 sulfonamides) were detected, with SMX concentrations being higher than other sulfonamides, reaching up to 940 ng/L [8], and in the vicinity of large livestock and poultry farms in Jiangsu Province, China, the detection rate of SMX in animal wastewater and surface waters was 51%, with the highest detected concentration being 63.66 μg/L [16].
SMX can accumulate in biological tissues, showing a moderate level of ecological risk to organisms [17,18]. In a random sampling of 54 raw meat samples from the market in Crete, Greece, SMX residues in pork, beef, and chicken were measured at 12.73 ± 5.44, 22.42 ± 29.78, and 8.23 ± 6.31 μg/kg, respectively [19]. A study exposed adult zebrafish (Danio rerio) to a mixture of 15 common antibiotics at concentrations of 0, 1, and 100 μg/L for four weeks, finding that SMX primarily accumulated in the swim bladder and was detected in residues in muscle, brain, blood, and ovary tissues. Notably, SMX residues were also found in unexposed F1 fertilized eggs, suggesting a potential transgenerational impact of antibiotics on the host [20]. The effects of SMX on fish encompass multiple aspects, including the reversal of growth dose effects [21], oxidative stress [22,23], immunotoxicity [24], gut microbiota, and its metabolism [20,25,26]. Zebrafish exposed to concentrations of SMX at 0, 5, 90, and 450 μg/L for 21 days exhibited pathological damage in the intestines and liver, dysbiosis of the gut microbiota, impaired liver function, and disturbances in fatty acid metabolism [27]. Nile tilapia (Oreochromis niloticus) subjected to dietary SMX at doses of 20, 200, and 1000 mg/kg·day showed that low and medium doses of SMX promoted growth, as well as increased the diversity of gut microbiota and elevated levels of short-chain fatty acids (SCFAs). In contrast, high doses of SMX inhibited growth, reduced SCFA levels, caused dysbiosis of the gut microbiota, and increased visceral fat accumulation [21]. Carp (Cyprinus carpio) exposed to SMX concentrations of 25, 50, 100, and 200 µg/L for 28 days exhibited significant changes in their hematological and biochemical parameters, characterized by a decrease in red blood cells, hemoglobin, and platelet counts, an increase in white blood cell levels, and a biphasic change in glucose, total protein, and triglyceride levels, along with a significant elevation in alanine aminotransferase levels [24]. Rainbow trout (Oncorhynchus mykiss) exposed to SMX concentrations of 0.3, 3, and 3000 µg/L showed a significant increase in the phenotypic resistance of their gut microbiota, along with an increase in the number of bacteria carrying sulfonamide resistance genes [28]. Research on SMX has primarily focused on freshwater model organisms, such as zebrafish and adult economic fish species. However, studies addressing marine fish and their early life stages remain relatively scarce. The early life stage is a critical period in fish development, encompassing various aspects such as embryonic development, hatching, and juvenile growth. During this phase, fish exhibit heightened sensitivity to environmental pollutants [29]. Given the ongoing changes in marine ecosystems and the increasing pollution issues, a comprehensive investigation into the effects of SMX on the early development of marine fish is essential. Such research will provide significant scientific evidence for the protection of marine ecosystems and the sustainable development of fisheries.
Marine medaka (Oryzias melastigma) is an ideal model organism for monitoring marine environments due to its small size, ease of maintenance, wide salinity tolerance, short generation time, high fecundity, and transparent embryos [30]. To evaluate the potential ecological risks posed by SMX to marine fish and to address the existing research gap concerning the embryonic developmental toxicity of SMX in marine species, this study exposed medaka embryos at the blastocyst stage to environmentally relevant concentrations of SMX (0, 1, 60, and 1000 μg/L) for a duration of 16 days. The research specifically examined the impact of SMX on the development of marine medaka embryos and larvae, including an analysis of the transcriptional changes in the genes related to embryonic development and modifications in the larval microbiome. This study not only provides critical mechanistic insights into the developmental toxicity pathways of SMX in marine organisms through integrated gene-microbiome analysis but also establishes an important scientific foundation for environmental risk assessment and regulatory decision-making regarding antibiotic contaminants in marine ecosystems.
2. Materials and Methods
2.1. Reagents
SMX (CAS No.: 723-46-6; purity 98%) and dimethyl sulfoxide (DMSO, CAS No.: 67-68-5; purity 99%) were purchased from Sigma-Aldrich (Shanghai, China). SMX was dissolved in DMSO to prepare a stock solution and was stored at room temperature away from light.
2.2. Embryo Collection and SMX Exposure
Adult marine medaka (with a body length of 2.15 ± 0.08 cm and body weight of 0.23 ± 0.02 g) were cultured in a controlled artificial seawater system with a salinity of 30 ‰ and a water temperature maintained at 26.0 ± 1.0 °C under a 14 h light and 10 h dark photoperiod. Freshly hatched brine shrimp nauplii were fed to the fish twice daily. Freshly fertilized eggs were collected, rinsed with seawater, and then examined under a stereomicroscope to select and gather normal developing embryos.
The experiment utilized standard Petri dishes (that were 60 mm in diameter and 16 mm in height), each containing 20 mL of the exposure solution. Based on the concentrations reported in the literature [9,10,16], the study set the concentrations of SMX at 1 (low concentration group, L group), 60 (middle concentration group, M group), and 1000 (high concentration group, H group) μg/L. Based on similar studies involving SMX on adult zebrafish and embryonic development [20,31,32], the solvent control group (SC group) utilized only 0.01% DMSO, a concentration that does not impact fish reproduction or embryonic development [33]. Forty embryos at the blastocyst stage (6 h post-fertilization, hpf) were selected and placed in the Petri dishes. Each group, consisting of five replicates, was incubated in a light incubator maintained at 28 °C with a 14 h light and 10 h dark cycle. The exposure solution was replaced every 24 h, and dead embryos were removed. All newly hatched larvae were fed crushed egg yolk.
2.3. Heart Rate Measurements and Morphological Observation
Following SMX exposure, the embryos were video recorded for 30 s using a stereomicroscope on days 6 to 9, and the heart rates of three replicate groups (n = 30) were calculated based on these video data. On days 9, 12, and 15 of the experiment, embryonic development was observed and photographed using a stereomicroscope. Based on the literature [34] and our breeding experience, the incubation period for marine medaka embryos typically ranges from 8 to 16 days. Embryos that failed to hatch after 16 days of exposure to SMX were recorded as hatch failures, and the survival rates (n = 40) were calculated. Survival rate (%) = hatched larvae/initial released embryos (40) × 100%. Daily records of the number of hatched larvae were maintained to calculate the hatching time (n = 40). On the 15th day, a mixture of ice and water was used to anesthetize the larvae, after which their total length (n = 30) was measured under a stereomicroscope using NIS-Elements D 4.60.00 64-bit software.
2.4. Oil Red Staining of Newly Hatched Larvae
After 15 days of exposure to SMX, the surviving larvae (n = 15) were randomly selected from each group and were fixed in 4% paraformaldehyde at 4 °C for 24 h, followed by two washes with phosphate-buffered saline containing 0.1% Tween-20 (PBST, pH 7.4). The fixed larvae were then stained at room temperature for 3 h with a 0.5% Oil Red O (ORO, CAS: 1320-06-5) solution, washed with PBST, and immersed in 95% glycerol for at least 48 h to eliminate the background color. The ORO-stained areas in the viscera were visualized using a stereomicroscope and quantified utilizing ImageJ software (version 1.54a) to assess lipid accumulation in the hatchling larvae.
2.5. Analysis of Swimming Behavior
After 15 days of exposure to SMX, 24 larvae were randomly selected and placed in a 12-well plate (adding 1 mL of clean water to each well) and subjected to a 15 min behavioral monitoring session using a small fish behavior analysis system (Zebralab video tracking system, ViewPoint, Lyon, France). Allow the larvae to acclimate for 5 min prior to testing; this phase is not included in the results. During the measurement process, maintain a dark environment to avoid interference from external factors such as noise. During this test period, the larvae movement time, trajectory, and swimming distance were recorded. Specifically, swimming speeds below 1 mm/s were classified as resting; speeds between 1 mm/s and 10 mm/s were categorized as minor activity; and speeds reaching or exceeding 10 mm/s were considered major activity.
2.6. Total RNA Isolation, cDNA Library Construction, and Real-Time Quantitative PCR (qPCR) Analysis
On the 6th and 15th days, a total of 15 embryos (n = 15) and larvae (n = 15) were, respectively, collected for total RNA extraction. We utilized the AG RNAex Pro reagent (Precision Biotechnology (Hunan) Co., Ltd., Changsha, China) for total RNA extraction and confirmed the quality of the RNA through agarose gel electrophoresis stained with DSRed (GDSBio, Guangzhou, China). The concentration and purity of the RNA were determined using a NanoDrop 2000 spectrophotometer (ThermoFisher, Waltham, MA, USA), with all samples showing an absorbance ratio at 260/280 nm between 1.9 and 2.1. Following confirmation of the total RNA quality, cDNA templates were prepared using the TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). To investigate the molecular toxicity of SMX exposure on marine medaka embryos and larvae, qPCR was employed to examine the transcript levels of the genes associated with thyroid function, as well as the cardiovascular system, nervous system, antioxidant and inflammatory systems, and detoxification enzymes (Table S1). The transcript levels of the target genes were normalized using actb2 and rps4x [35]. All qPCR experiments were performed on the LightCycler® 96 PCR System (Roche, Basel, Switzerland). The qPCR reaction mixture (15 μL) consisted of 7.5 μL of 2 × TransStart Green qPCR SuperMix (TransGen Biotech), 0.3 μL each of forward and reverse primers (10 μM), 1.5 μL of the cDNA sample, and 5.4 μL of nuclease-free water. The qPCR program was set as follows: initial denaturation at 95 °C for 60 s, followed by 40 cycles, each consisting of denaturation at 95 °C for 5 s, annealing at 60 °C for 15 s, and extension at 72 °C for 10 s with fluorescence collection. A melting curve analysis was subsequently added to confirm the specificity of the primers. The transcript levels of each gene relative to the SC group were analyzed using the 2−ΔΔCt method and log2 transformation [36].
2.7. 16S rRNA Amplicon Sequencing Analysis
After 15 days of exposure, 15 larvae (n = 15) were randomly selected from each group. They were rinsed three times with sterile water and immediately collected into sterile centrifuge tubes, with four larvae per tube. Four replicates were set up for each group, and the samples were stored at −80 °C for preservation.
The larvae were transported via dry ice to Biomarker Technology Co., Ltd. (Beijing, China) for bacterial DNA extraction, library construction, and sequencing. DNA was extracted from the larvae using the ONREW DNA Extraction Kit (Foshan Aoweibiotech, Foshan, China) and amplified by PCR using full-length prokaryotic 16S rDNA primers (27F: AGRGTTTGATYNTGGCTCAG and 1492R: TACGGYTACCTTGTTACGACTT). The results indicated that all samples successfully amplified a single target band, meeting the quality standards for library construction. Subsequently, specific primers with barcodes were synthesized based on the full-length primer sequences for PCR amplification. The products were then purified, quantified, and normalized to form the sequencing library (SMRT Bell). After library construction, quality control was performed, and upon approval, sequencing was conducted using the Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA). The data obtained from this study have been stored in the National Center for Biotechnology Information (NCBI), with the project number being SUB14585284.
We exported the Illumina NovaSeq sequencing data into FASTQ format files. Initially, raw reads were filtered using the Trimmomatic v0.33 software. Subsequently, cutadapt 1.9.1 was employed to identify and remove the primer sequences, yielding clean reads devoid of the primer sequences. Non-chimeric reads were obtained through denoising, paired-end merging, and chimeric sequence removal using the dada2 method within the QIIME2 2020.6 platform [37,38]. The reads were clustered to form operational taxonomic units (OTUs) at a 97.0% similarity threshold using Usearch v1.2.1 software. Taxonomic annotations of the feature sequences were performed using a naive Bayes classifier in conjunction with alignment strategies, referencing the SILVA database to obtain species classification information for each feature sequence. The community composition for each sample was statistically analyzed at the phylum, class, order, family, genus, and species levels. Alpha and beta diversity analyses were conducted using QIIME2 (2020.6) to compare the similarity of species diversity among samples. Principal coordinates analysis (PCoA) with the Bray–Curtis algorithm was utilized to assess the differences in microbial diversity.
2.8. Statistical Analysis
Each solvent control group and treatment group consisted of 3 biological replicates. Statistical analysis and graph generation were performed using GraphPad Prism 9.0 software. Data are presented as the mean ± standard deviation (SD). The statistical significance of differences was evaluated using one-way ANOVA. Normality and lognormality were assessed with the Shapiro–Wilk test, and multiple comparisons were conducted using Tukey’s multiple comparisons test. A p-value of less than 0.05 (*), 0.01 (**), 0.001 (***), and 0.0001 (****) was considered statistically significant.
3. Results
3.1. Effects of SMX on the Survival Rate, Total Length, Hatching Time, and Heart Rate of Marine Medaka Embryos
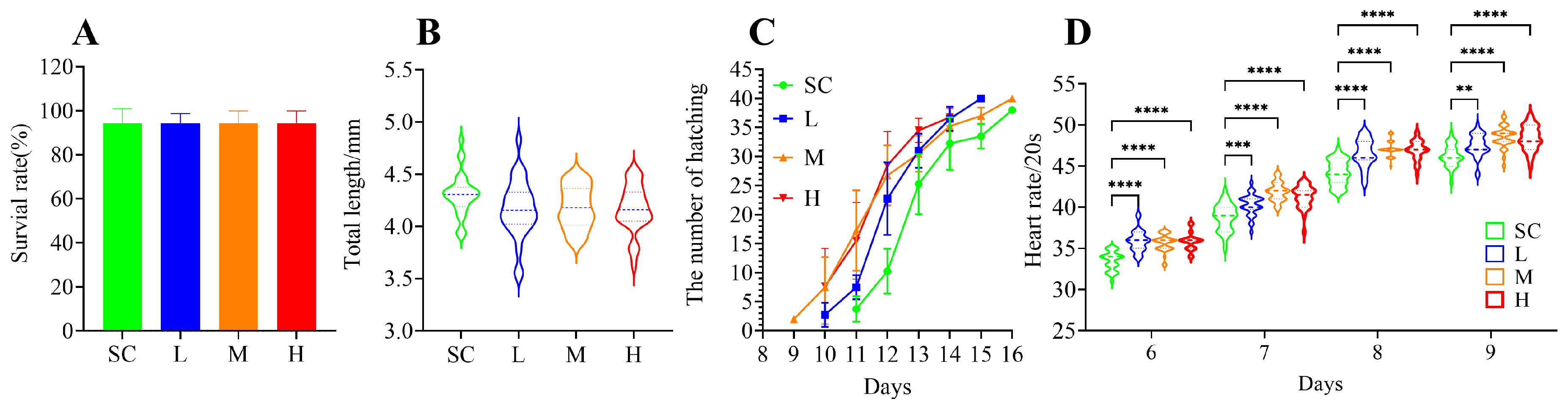
After 16 days of exposure to SMX, the survival rate of the marine medaka embryos did not exhibit significant (p > 0.05) alterations (Figure 1A). However, a slight reduction in the total length of the larvae was observed, although the difference was not statistically significant (p > 0.05) compared to the SC group (Figure 1B). Exposure to SMX did not affect the hatching rate of the marine medaka embryos, but it accelerated the hatching process. Hatching began on the 9th day in the M group, on the 10th day in the L and H groups, and on the 11th day in the SC group (Figure 1C). SMX exposure significantly accelerated the heart rate of marine medaka embryos (p < 0.0001) during the 6 to 9 days of SMX exposure; as the embryos developed, an increasing trend in heart rate was observed (Figure 1D).
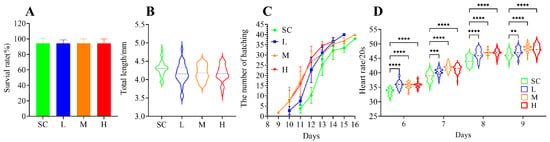
Figure 1.
Effects of SMX exposure on survival rate (A), total length of larvae (B), hatching time (C), and heart rate (D) of marine medaka. SC, 0 μg/L; L, 1 μg/L; M, 60 μg/L; H, 1000 μg/L. Statistically significant differences were observed compared to the SC group when p ≤ 0.01 (**), p ≤ 0.001 (***), and p ≤ 0.0001 (****).
3.2. Effects of SMX on the Morphology of Marine Medaka Embryos and Larvae
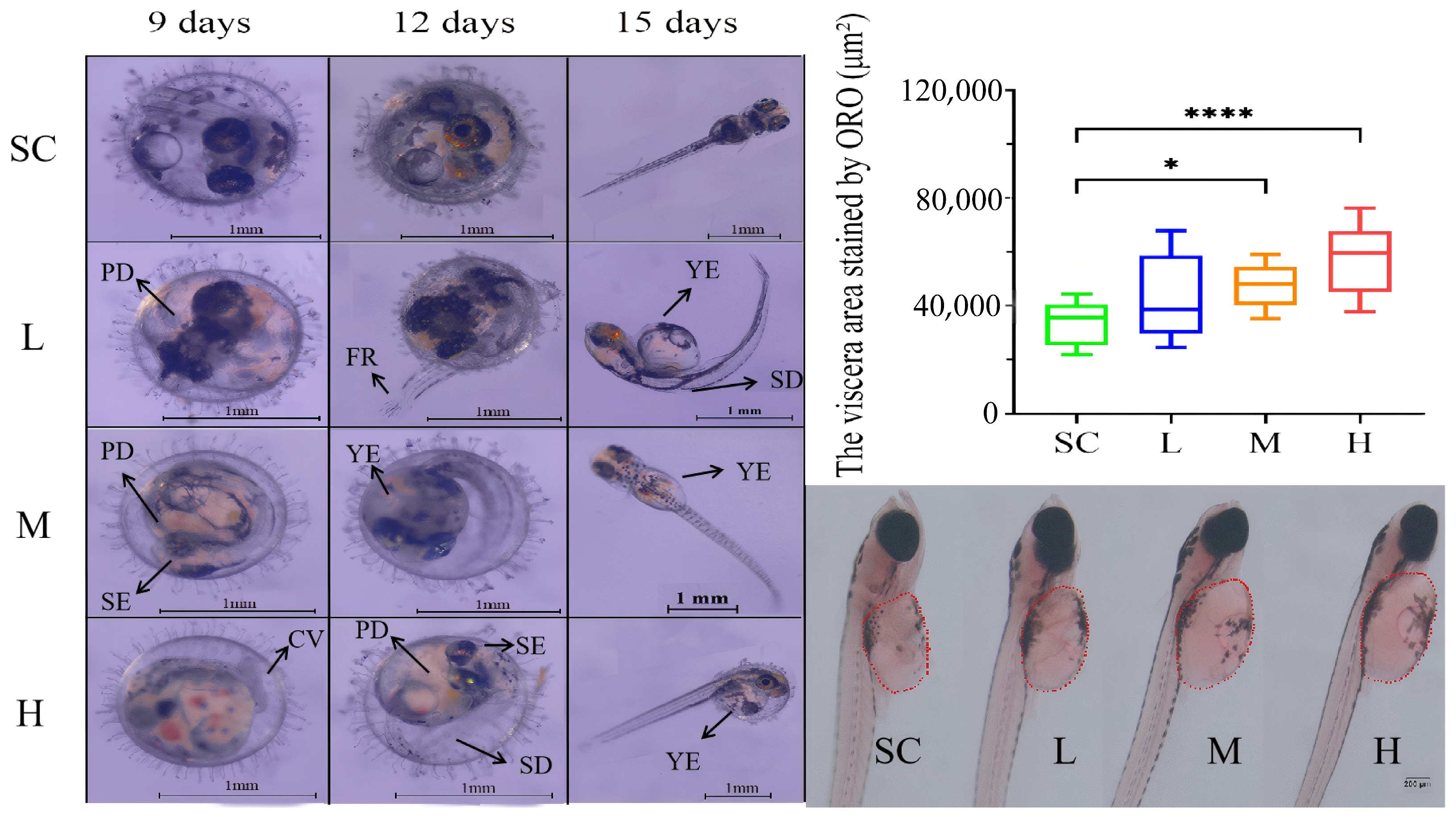
Exposure to SMX affected the development of the marine medaka embryos. After 9 days of SMX exposure, some embryos exhibited reduced pigmentation (PD), smaller eyes (SE), and curved tails (CV). By day 12, embryos were observed with spinal deformities (SD), fin rot (FR), and yolk sac edema (YE) (Figure 2). By day 15, some larvae displayed spinal deformities and yolk sac edema. Observations revealed that yolk sac edema was the predominant malformation. Consequently, Oil Red O staining was utilized to quantitatively assess lipid accumulation in the visceral region of the marine medaka larvae. The findings indicated that, in comparison to the SC group, lipid accumulation in the visceral region significantly increased in the M and H groups treated with SMX (Figure 2).
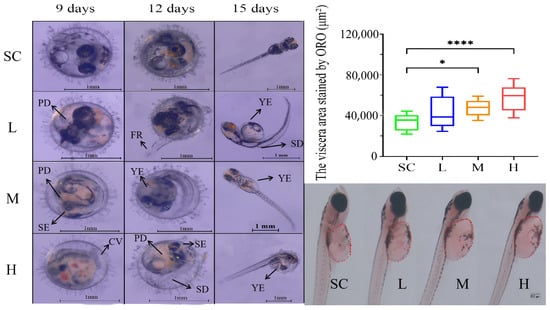
Figure 2.
Effects of SMX exposure on embryonic morphology and the visceral region of marine medaka. SC, 0 μg/L; L, 1 μg/L; M, 60 μg/L; H, 1000 μg/L. PD: pigmentation deficiency; SE: smaller eyes; CV: curved tails; SD: spinal deformities; FR: fin rot; YE: yolk sac edema. Statistically significant differences were observed compared to the SC group when p ≤ 0.05 (*) and p ≤ 0.0001 (****).
3.3. Effects of SMX on the Swimming Behavior of Marine Medaka
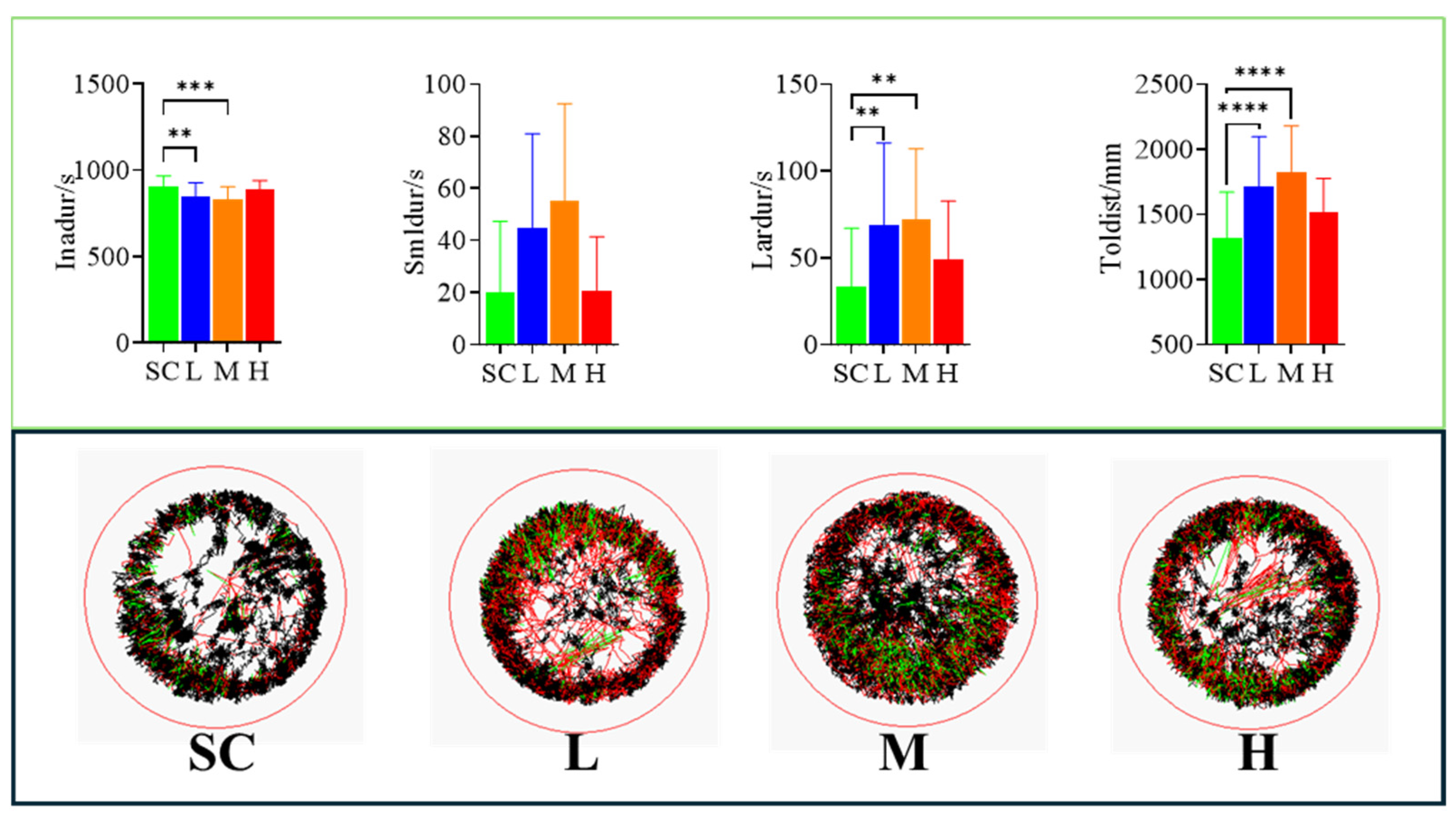
Exposure to SMX increased the swimming distance of the marine medaka larvae. Compared to the SC group, the total swimming distance was increased in all SMX-exposed groups, with particularly significant (p < 0.0001) differences observed in the L and M groups (Figure 3).
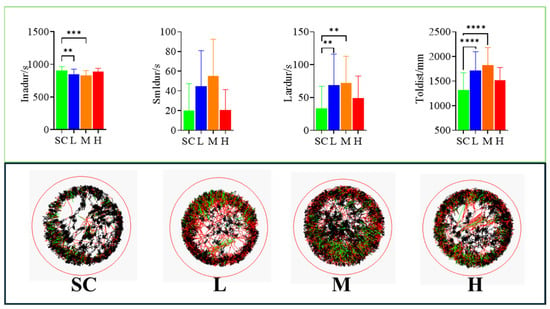
Figure 3.
Effects of SMX exposure on the swimming behavior of marine medaka larvae. SC, 0 μg/L; L, 1 μg/L; M, 60 μg/L; H, 1000 μg/L. Inadur: duration of rest in marine medaka larvae; Smldur: duration in the small-movement state; Lardur: duration in the large-movement state; Toldist: total swimming distance. Statistically significant differences compared to the SC group are indicated as p ≤ 0.01 (**), p ≤ 0.001 (***), and p ≤ 0.0001 (****).
3.4. Effects of SMX on Gene Transcription in Marine Medaka Embryos and Larvae
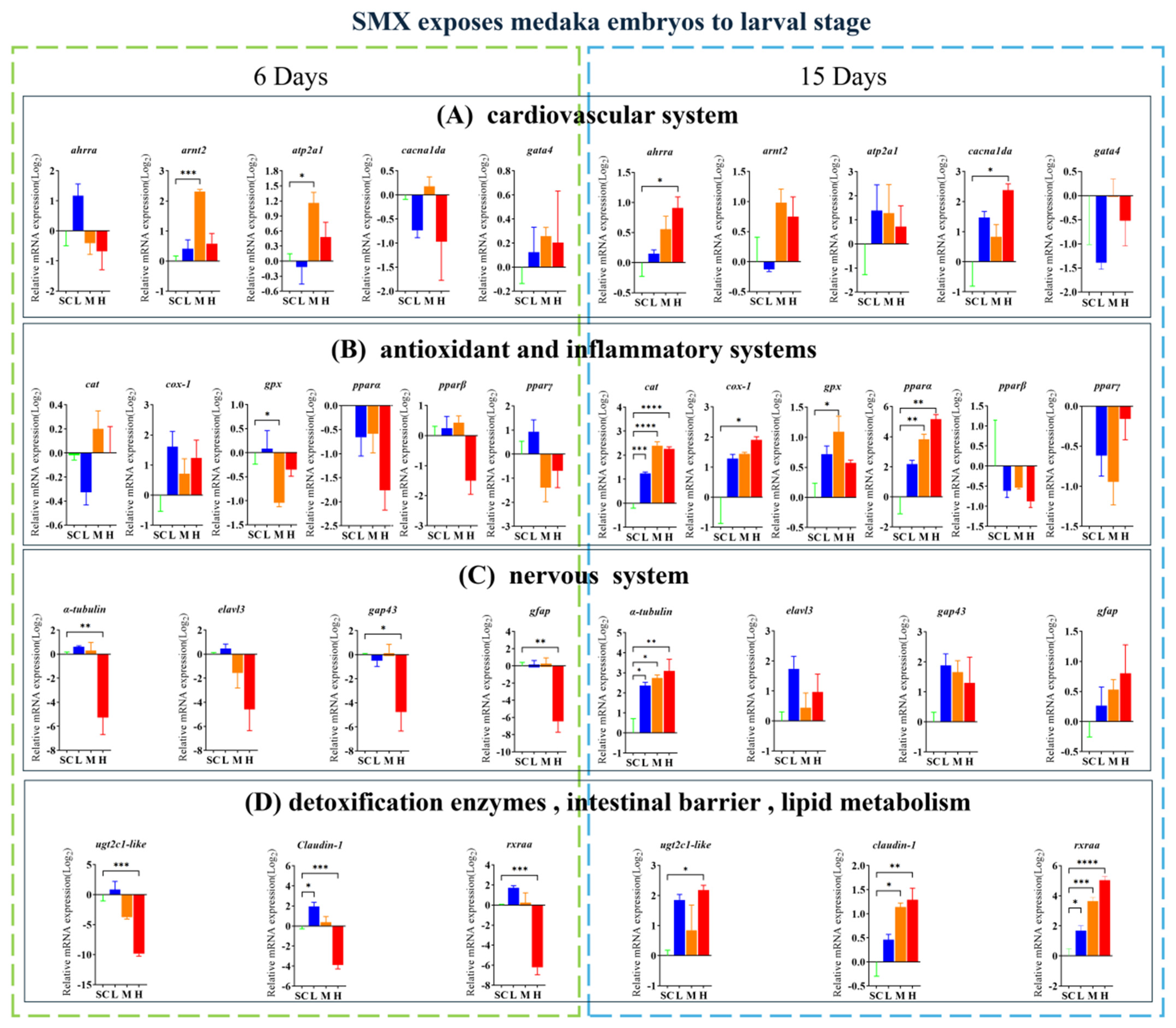
Exposure to SMX affected the transcript levels of the genes associated with the cardiovascular system (ahrra, arnt2, atp2a1, and cacnalda), antioxidant and inflammatory response systems (cat, cox-1, gpx, and pparα), nervous system (α-tubulin, gap43, and gfap), intestinal barrier function (claudin-1), detoxification enzymes (ugt2c1-like), and lipid metabolism (rxraa) in these organisms (Figure 4). On the sixth day of embryonic development, significantly upregulated genes include claudin-1 from the L group, as well as arnt2 and atp2a1 from the M group. Conversely, significantly downregulated genes comprise gpx from the M group, along with ugt2c1-like, claudin-1, and rxraa from the H group. In the hatching larvae, significantly upregulated genes include cat, α-tubulin, and rxraa from the L group; gpx, pparα, cat, α-tubulin, and rxraa from the M group; and ahrra, cacnalda, cox-1, ugt2c1-like, claudin-1, cat, α-tubulin, and rxraa from the H group. The significantly downregulated genes include α-tubulin, gap43, and gfap from the H group.
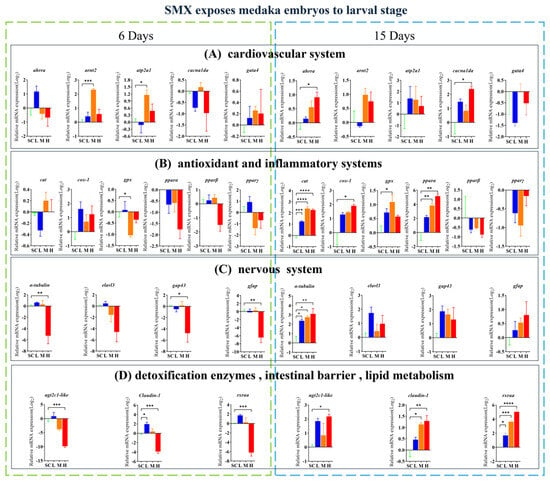
Figure 4.
Effects of SMX exposure on the transcript levels of genes involved in cardiovascular, neurological, antioxidant, inflammatory responses, detoxification enzymes, intestinal barrier, and lipid metabolism in marine medaka embryos and larvae. SC, 0 μg/L; L, 1 μg/L; M, 60 μg/L; H, 1000 μg/L. Statistically significant differences compared to the SC group are indicated when p ≤ 0.05 (*), p ≤ 0.01 (**), p ≤ 0.001 (***), and p ≤ 0.0001 (****).
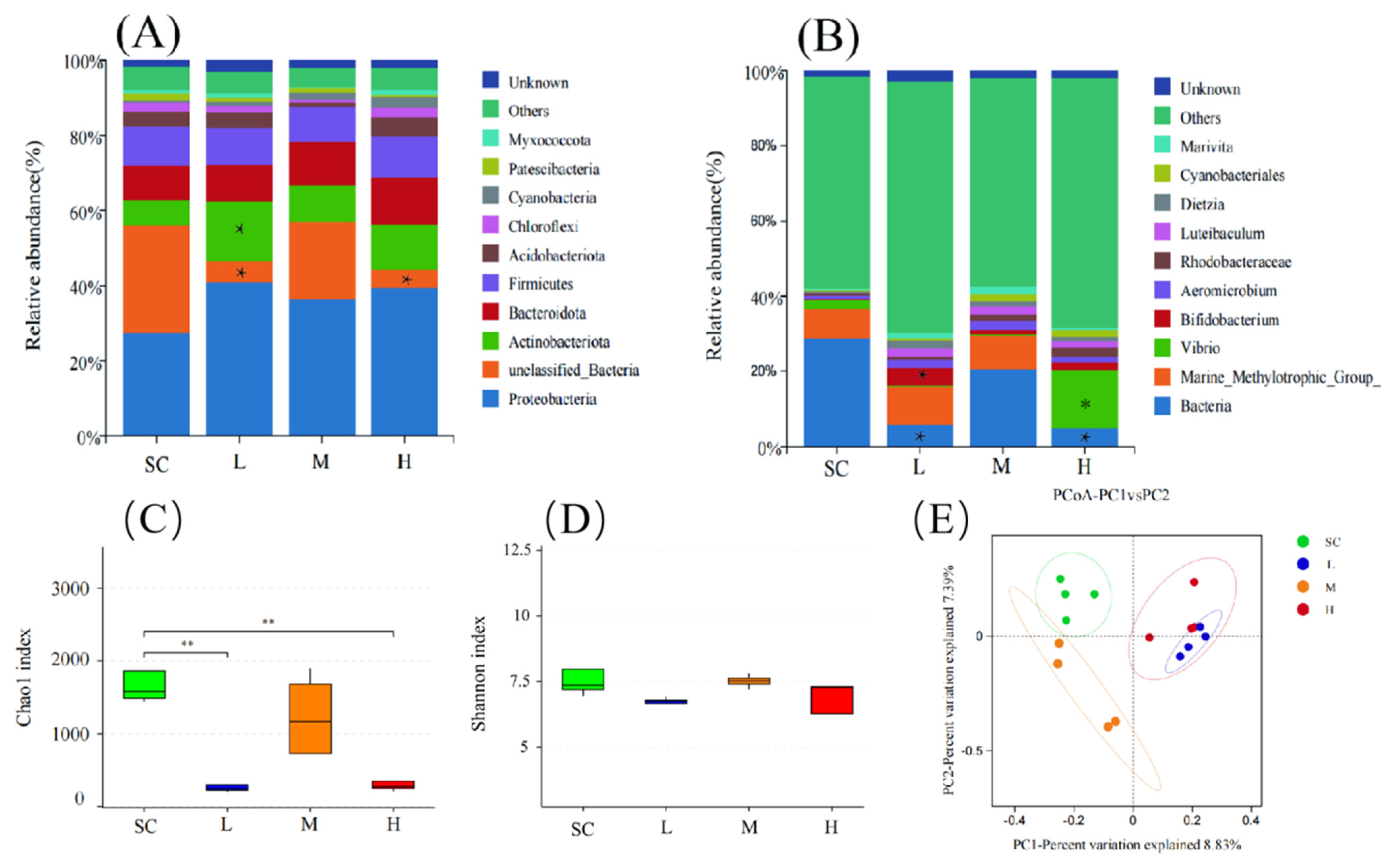
3.5. Effects of SMX on the Structure of Microbiome in Marine Medaka Larvae
At the phylum level, exposure to SMX resulted in an increase in the abundance of Proteobacteria, with Proteobacteria being considered a microbial marker of dysbiosis. Additionally, the abundance of Actinobacteriota significantly increased (p < 0.05) in the L group (Figure 5A). At the genus level, the abundance of Bifidobacterium significantly increased (p < 0.05) in the L group, while the abundance of Vibrio significantly increased (p < 0.05) in the H group (Figure 5B).
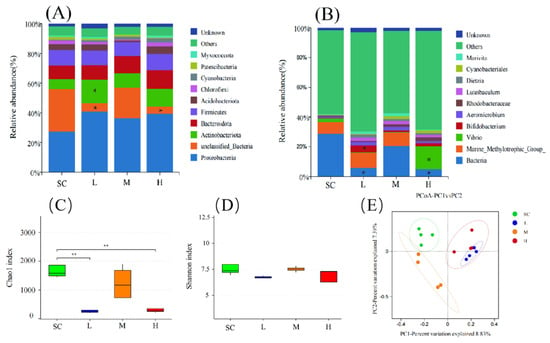
Figure 5.
Effects of SMX exposure on the species abundance and diversity of the microbial community in marine medaka larvae. Species abundance of the microbial community in marine medaka larvae at the phylum level (A) and genus level (B). The alpha diversity (C,D) and the beta diversity (E) indices of the microbial community in marine medaka larvae. SC, 0 μg/L; L, 1 μg/L; M, 60 μg/L; H, 1000 μg/L. Statistically significant differences compared to the SC group are indicated as p ≤ 0.05 (*) and p ≤ 0.01 (**).
Exposure to SMX affected the diversity of the microbiome in the marine medaka larvae. In the SMX treatment groups at L and H, the Chao1 index significantly decreased (p < 0.01), indicating a marked reduction in the richness of the microbial communities in these groups (Figure 5C). Although the Shannon index was generally lower in the SMX treatment groups compared to the SC group, this difference did not reach a significant level (Figure 5D). Principal coordinates analysis (PCoA) was employed to evaluate the effects of SMX exposure on the beta diversity of the microbiomes in marine medaka larvae. The results indicated that the SC group was predominantly distributed in the second quadrant, whereas the L and H groups were primarily located in the first quadrant, and the M group was concentrated in the third quadrant. This distribution pattern suggests that exposure to different concentrations of SMX significantly altered the microbial diversity of the marine medaka larvae (Figure 5E).
4. Discussion
4.1. Effects of SMX Exposure on the Viability and Hatching of Marine Medaka Embryos
The embryonic development in teleost fish is highly susceptible to environmental changes [39]. As a novel environmental pollutant, SMX may interfere with the normal development of fish embryos. In this study, exposure to SMX at concentrations ranging from 1 to 1000 ug/L did not significantly affect the hatching rate or total length of the marine medaka embryos; however, SMX exposure significantly accelerated the hatching process of the marine medaka embryos. The hatching process of fish consists of two stages: the digestion of the eggshell by hatching enzymes and the emergence of the embryo from the partially digested eggshell through muscular movements. In teleost fish, hatching enzymes are primarily composed of two types of metalloproteinases: High Choriolytic Enzyme (HCE) and Low Choriolytic Enzyme (LCE). For instance, the hatching enzymes in the Japanese medaka (Oryzias latipes) include HCE1, HCE2, and LCE, which work synergistically to hydrolyze the egg membrane proteins, causing the egg membrane to swell and ultimately rupture [40]. After six weeks of exposure to 260 ng/L of SMX and 420 ng/L of oxytetracycline, the activities of alkaline phosphatase (AKP) and acid phosphatase (ACP) in zebrafish were significantly reduced [41]. Our findings indicate that SMX exposure affects the expression of oxidative stress-related genes (such as cat and gpx) in marine medaka embryos, which may subsequently influence the corresponding enzyme activities. The accelerated hatching of marine medaka in response to SMX may be related to its impact on relevant hatching enzymes; however, there is currently no research investigating the specific effects of SMX on embryonic hatching enzymes, warranting further studies in the future. Additionally, SMX exposure led to various developmental abnormalities in the marine medaka embryos, including pigmentation deficiency, reduced eye size, curved tail, curved spine, fin rot, and yolk sac edema. These results are similar to those findings in zebrafish studies, where exposure to SMX also leads to abnormal development of zebrafish embryos [42].
4.2. Effects of SMX Exposure on the Cardiovascular System of Marine Medaka Embryos
The heart, as the first functional organ during embryonic development, plays a crucial role in embryonic development [43]. The heart rate is commonly used as an indicator to assess the cardiovascular toxicity of pollutants [44]. Our study revealed that exposure to SMX significantly increased the heart rate of the marine medaka embryos (p < 0.0001). Similarly, exposure to SMX at concentrations of 1 µg/L (p < 0.05) significantly increases the heart rate of zebrafish [42]. Exposure to high concentrations of SMX induces pericardial effusion in zebrafish. Furthermore, the literature indicates that SMX upregulates pro-inflammatory cytokines and apoptosis-related genes, which may impact cardiac function [45]. The RNA-Seq analysis revealed that the complement/coagulation system may be one of the most significantly affected immune mechanisms in the intestines of zebrafish exposed to SMX, with the expression of several related genes being suppressed. The impact of SMX on the immune system may indirectly influence the cardiovascular health of the fish [31].
To explore the potential mechanisms underlying SMX toxicity, this study evaluated the expression of several genes closely associated with heart development. The results indicated that by day 6 post-SMX exposure, the expression of the arnt2 was upregulated, with a significant increase in the M group. Ahr, functioning as the aryl hydrocarbon receptor, can form a complex with ARNT to regulate downstream gene expression, thereby influencing heart development [46]. Additionally, after 15 days of SMX exposure, the expression of atp2a1 and cacna1da was upregulated, with a significant increase in cacna1da expression in the H group (p < 0.05). The calcium pump encoded by atp2a1 and the L-type calcium channel encoded by cacna1da play crucial roles in the contraction of cardiomyocytes [47,48]. Prior to hatching in teleost embryos, spontaneous body movements intensify significantly, followed by the softening of the egg membrane, which is subsequently ruptured by the embryo’s tail or head. In this study, the heart rate of the mangrove killifish in the SMX group increased, which may have heightened the intensity of spontaneous body movements, leading to premature membrane rupture [49]. These findings suggest that SMX exposure may impact and potentially alter the cardiovascular system of fish embryos.
4.3. Effects of SMX Exposure on the Antioxidant System and Inflammatory Response in Marine Medaka Embryos
Reactive oxygen species (ROS) can lead to lipid peroxidation and DNA damage, resulting in various detrimental effects on organisms [50,51]. Catalase (CAT), glutathione (GSH), and superoxide dismutase (SOD) are crucial substances in combating ROS [52]. CAT plays a crucial role in the antioxidant defense system by facilitating the breakdown of hydrogen peroxide into water and oxygen. Additionally, the levels of glutathione peroxidase (GPX) serve as a significant marker for assessing antioxidant capacity. In this study, after 15 days of SMX exposure, the expression of cat in the marine medaka larvae significantly increased. Additionally, after 6 days of SMX exposure, the expression of gpx in the M group significantly decreased [53]. These findings suggest that SMX enhances the expression of cat and sod in marine medaka embryos, thereby increasing the activity of CAT and SOD, which triggers oxidative stress in the embryos. Previous studies have shown that exposure of Nile tilapia to water containing LECA (OTC 420 ng/L and SMX 260 ng/L) or feed with LADA (OTC 80 mg/kg/day and SMX 100 mg/kg/day) induces oxidative stress, stimulates inflammatory and detoxification responses, and leads to lipid peroxidation in the intestines and liver [23].
4.4. Effects of SMX Exposure on the Nervous System of Marine Medaka Embryos
Exposure to SMX resulted in edema of the yolk sac in the marine medaka larvae, significantly increased their swimming distance, and resulted in frequent tail-beating swimming behavior. Additionally, the L and M groups showed a particularly significant difference in SMX exposure, indicating that marine medaka larvae may exhibit anxiety-like behavior. Swimming frequency, as a measure of motor and nervous system development, has been validated [54]. Previous research has indicated that zebrafish larvae experienced a decrease in swimming frequency upon exposure to SMX, although the difference was not significant; however, under combined exposure to 1 mg/L SMX and 10 μg/L PS, swimming frequency significantly decreased [42]. The discrepancy in these results may stem from physiological differences between species.
In this study, we further analyzed the expression of several key genes (α-tubulin, gap43, and gfap) associated with neural development. The protein encoded by α-tubulin plays a critical role in microtubule assembly and is highly expressed in developing neurons [55]. Elavl3 encodes the RNA-binding protein HuC, and its absence leads to severe neurodevelopmental disorders and visual defects [56,57]. Gap43 is involved in axonal growth and synaptogenesis in neural cells and is highly expressed during neuronal development [58]. Gfap is primarily expressed in mature astrocytes. In our study, on day 6 of SMX exposure, α-tubulin expression was significantly upregulated in all exposed groups; however, on day 15 of SMX exposure, the H group showed significant downregulation of α-tubulin, gap43, and gfap expression. These results suggest that SMX may affect the motor abilities of marine medaka larvae and exhibit certain neurotoxic effects. Previous studies have shown that exposure to sulfonamide antibiotics at environmentally relevant concentrations during the early developmental stages of zebrafish can induce neurobehavioral toxicity [59]. Additionally, SMX has been reported to induce toxic effects on zebrafish brain capillaries by upregulating vascular endothelial growth factor and chemokine signaling [60].
4.5. Effects of SMX Exposure on the Microbial Structure of Marine Medaka Larvae
SMX exposure altered the microbial community structure of the marine medaka larvae. At the phylum level, the abundance of Proteobacteria increased in the SMX-treated marine medaka larvae. An increase in Proteobacteria is often considered a sign of microbial community dysbiosis. Additionally, the abundance of Actinobacteriota significantly increased in the L group. Thus far, eight types of sulfonamide and sulfonamide-like antibiotics have been isolated from Actinobacteriota [61]. Furthermore, species-specific differences in antimicrobial resistance patterns have been observed in Actinobacteriota isolates carrying methotrexate/sulfamethoxazole resistance genes [62]. Genera such as Streptomyces and Rhodococcus exhibit varying degrees of resistance to SMX [63]. The increase in Actinobacteriota may be a result of the co-adaptation of SMX with bacteria and their hosts. At the genus level, the abundance of Bifidobacterium significantly increased in the L group, while the abundance of Vibrio significantly increased in the H group. SMX competitively inhibits dihydrofolate synthase, thereby blocking bacterial folate synthesis and inhibiting DNA replication. Its antibacterial spectrum includes Gram-positive bacteria, Gram-negative bacteria, and some protozoa, but it is ineffective against most strict anaerobes, such as Bacteroides. Since Bifidobacterium are strictly anaerobic Gram-positive bacteria, they may exhibit natural resistance to SMX; however, this requires verification through specific experiments. Bifidobacterium is an important probiotic in the gut, and its sensitivity to antibiotics varies among species and strains. Broad-spectrum antimicrobial agents, such as chloramphenicol and tetracycline, exhibit significant inhibitory effects, while there is a strong resistance to aminoglycoside antibiotics (such as neomycin and kanamycin) [64]. However, current research has not directly assessed the sensitivity of Bifidobacterium to SMX. Previous studies have shown that Vibrio isolated from fish and shrimp aquaculture wastewater exhibit resistance to various antibiotics, including ciprofloxacin, penicillin, rifampicin, and vancomycin [65,66,67]. Additionally, SMX exposure impacted the microbial diversity of marine medaka larvae. The alpha diversity analysis revealed a significant decrease in the Chao 1 index in the L and H groups, indicating a significant reduction in the richness of the microbial community of marine medaka larvae. The beta diversity analysis further demonstrated differences in the microbial communities of marine medaka larvae among different SMX concentration groups, and these communities were distinct from the SC group. Studies have shown that long-term exposure to environmental concentrations of oxytetracycline and SMX significantly affects the taxonomic composition and metabolic pathways of zebrafish gut microbiota [68]. The gut microbiota is closely related to host growth and development, nutrient absorption, inflammatory responses, and metabolism [69,70,71,72,73]. Antibiotic residues may promote the emergence and spread of antibiotic-resistant bacteria (ARB) and antibiotic-resistance genes (ARGs) [74]. Research indicates that Proteobacteria are the primary carriers of ARGs, and exposure to sulfamethazine, by increasing the diversity of Gammaproteobacteria and Bacteroidetes, has promoted the distribution and enrichment of multiple ARGs involved in antibiotic inactivation in offspring, suggesting the persistent presence of antibiotic selection pressure [75]. In summary, SMX may affect the health of marine medaka by influencing changes in the microbial community.
5. Conclusions
The present study demonstrates that environmentally relevant concentrations of SMX exhibit significant toxic effects on the early developmental stages of marine medaka, manifesting as morphological malformations, shortened hatching periods, and increased heart rates. Additionally, SMX exposure inhibited the growth and development of larvae, reduced lipid consumption rates, increased their swimming distances, and influenced their microbial community structure. The findings of this study have deepened our understanding of the aquatic ecotoxicology of SMX. With the increasing concentrations of SMX in marine environments, its toxic effects on fish are becoming increasingly pronounced, potentially causing harm to marine fishery resources. This study provides a scientific basis for establishing stricter antibiotic usage regulations and environmental protection policies, which not only reduce the risk of antibiotic residues entering the food chain but also contribute to environmental conservation. Although this study uses the model organism, the marine medaka, as the experimental subject, its findings hold significant reference value for understanding the potential risks faced by other commercially relevant fish species in the context of environmental antibiotic pollution. Future research could further explore the sensitivity differences among various fish species to SMX, as well as the comprehensive impact of antibiotic contamination on fish health and ecosystems in actual aquaculture environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10030120/s1. Table S1: Primers and sequences.
Author Contributions
J.H.: Conceptualization, data curation, software, writing—original draft, writing—review and editing, and validation. L.Y.: Data curation, validation, and writing—original draft. S.H.: Data curation, validation, and writing—original draft. Z.C.: Data curation, validation, and writing—original draft. J.G.: Data curation, validation, and writing—original draft. Y.L.: Data curation, validation, and writing—original draft. Y.G.: Methodology, software, writing—original draft, and supervision. Z.W.: Software, writing—original draft, and supervision. J.L.: Software, writing—original draft, and supervision. Z.D.: Methodology, validation, writing—original draft, and supervision. N.Z.: Conceptualization, funding acquisition, writing—original draft, supervision, and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (41806195), the Postgraduate Education Innovation Project of Guangdong Ocean University (202451), the Undergraduate Innovation Team of Guangdong Ocean University (CXTD2023004), the Guangdong Provincial Program for Innovation and Entrepreneurship Training for College Students (S20241056047, S20241056070) and the Nanhai Scholar Project of Guangdong Ocean University (QNXZ201807).
Institutional Review Board Statement
The research in this manuscript has been conducted under the guidance of the international ethical standards of Guangdong Ocean University.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ben, W.; Pan, X.; Qiang, Z. Occurrence and Partition of Antibiotics in the Liquid and Solid Phases of Swine Wastewater from Concentrated Animal Feeding Operations in Shandong Province, China. Environ. Sci. Process Impacts 2013, 15, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Bielen, A.; Šimatović, A.; Kosić-Vukšić, J.; Senta, I.; Ahel, M.; Babić, S.; Jurina, T.; González Plaza, J.J.; Milaković, M.; Udiković-Kolić, N. Negative Environmental Impacts of Antibiotic-Contaminated Effluents from Pharmaceutical Industries. Water Res. 2017, 126, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Lv, Z.; Zhang, Z.; Han, Y.; Liu, Z.; Zhang, H. A Review of Antibiotics, Antibiotic Resistant Bacteria, and Resistance Genes in Aquaculture: Occurrence, Contamination, and Transmission. Toxics 2023, 11, 420. [Google Scholar] [CrossRef]
- Wang, D.; Sui, Q.; Zhao, W. Pharmaceutical and personal care products in the surface water of China: A review. Chin. Sci. Bull. 2014, 59, 743–751. (In Chinese) [Google Scholar]
- Biošić, M.; Mitrevski, M.; Babić, S. Environmental Behavior of Sulfadiazine, Sulfamethazine, and Their Metabolites. Environ. Sci. Pollut. Res. Int. 2017, 24, 9802–9812. [Google Scholar] [CrossRef]
- Hernández-Pérez, A.; Noonin, C.; Söderhäll, K.; Söderhäll, I. Environmental Concentrations of Sulfamethoxazole Increase Crayfish Pacifastacus Leniusculus Susceptibility to White Spot Syndrome Virus. Fish. Shellfish. Immunol. 2020, 102, 177–184. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Z.; Chen, Y.; Zhang, Y.; Sun, R.; Xue, Y.; Zhnag, M. Ecological risk assessment for sulfonamides in surface waters. Ecol. Environ. Sci. 2016, 25, 1508–1514. [Google Scholar]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and Personal Care Products in the Aquatic Environment in China: A Review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Shelver, W.L.; Hakk, H.; Larsen, G.L.; DeSutter, T.M.; Casey, F.X.M. Development of an Ultra-High-Pressure Liquid Chromatography-Tandem Mass Spectrometry Multi-Residue Sulfonamide Method and Its Application to Water, Manure Slurry, and Soils from Swine Rearing Facilities. J. Chromatogr. A 2010, 1217, 1273–1282. [Google Scholar] [CrossRef]
- Faleye, A.C.; Adegoke, A.A.; Ramluckan, K.; Bux, F.; Stenström, T.A. Antibiotic Residue in the Aquatic Environment: Status in Africa. Open Chem. 2018, 16, 890–903. [Google Scholar] [CrossRef]
- European Commission, Joint Research Centre. State of the Art on the Contribution of Water to Antimicrobial Resistance; Publications Office: Luxembourg, 2018. [Google Scholar]
- Bojarski, B.; Kot, B.; Witeska, M. Antibacterials in Aquatic Environment and Their Toxicity to Fish. Pharmaceuticals 2020, 13, 189. [Google Scholar] [CrossRef]
- Wahome, C.N. Contamination Levels of Groundwater, Antimicrobial Resistance Patterns, Plasmid Profiles and Chlorination Efficacy in Ongata Rongai, Kajiado North County, Kenya. Master’s Thesis, Kenyatta University, Nairobi, Kenya, 2014. [Google Scholar]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological Risk Assessment of Fifty Pharmaceuticals and Personal Care Products (PPCPs) in Chinese Surface Waters: A Proposed Multiple-Level System. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ge, F.; Huang, S.; Chen, M.; Wang, R. Occurrence of Veterinary Antibiotics in Animal Wastewater and Surface Water around Farms in Jiangsu Province, China. Chemosphere 2011, 82, 1408–1414. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wu, T.; Ma, K.; Cheng, Z.; Yi, Q.; Dai, Y.; Wang, B.; Chen, Y.; Wang, B.; et al. Characteristics of Antibiotic Resistance Genes and Microbial Community Distribution in Wanfeng Lake, Upper Pearl River, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 83214–83230. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, Z.; Qiao, Y.; Liu, D.; Feng, C.; Bai, Y. Distribution and Characterization of Typical Antibiotics in Water Bodies of the Yellow River Estuary and Their Ecological Risks. Toxics 2023, 11, 400. [Google Scholar] [CrossRef]
- Stavroulaki, A.; Tzatzarakis, M.N.; Karzi, V.; Katsikantami, I.; Renieri, E.; Vakonaki, E.; Avgenaki, M.; Alegakis, A.; Stan, M.; Kavvalakis, M.; et al. Antibiotics in Raw Meat Samples: Estimation of Dietary Exposure and Risk Assessment. Toxics 2022, 10, 456. [Google Scholar] [CrossRef]
- Qiu, W.; Fang, M.; Magnuson, J.T.; Greer, J.B.; Chen, Q.; Zheng, Y.; Xiong, Y.; Luo, S.; Zheng, C.; Schlenk, D. Maternal Exposure to Environmental Antibiotic Mixture during Gravid Period Predicts Gastrointestinal Effects in Zebrafish Offspring. J. Hazard. Mater. 2020, 399, 123009. [Google Scholar] [CrossRef]
- Fang, L.; Chen, X.; Shan, X.; Qiu, L.; Fan, L.; Meng, S.; Song, C. Antibiotic Accumulation, Growth Performance, Intestinal Diversification, and Function of Nile Tilapia (Oreochromis niloticus) Feed by Diets Supplemented with Different Doses of Sulfamethoxazole. Environ. Sci. Pollut. Res. Int. 2021, 28, 65255–65264. [Google Scholar] [CrossRef]
- Hu, F.; Dong, F.; Yin, L.; Wang, H.; Zheng, M.; Fu, S.; Zhang, W. Effects of Sulfamethoxazole on the Growth, Oxidative Stress and Inflammatory Response in the Liver of Juvenile Nile Tilapia (Oreochromis niloticus). Aquaculture 2021, 543, 736935. [Google Scholar] [CrossRef]
- Limbu, S.M.; Zhou, L.; Sun, S.-X.; Zhang, M.-L.; Du, Z.-Y. Chronic Exposure to Low Environmental Concentrations and Legal Aquaculture Doses of Antibiotics Cause Systemic Adverse Effects in Nile Tilapia and Provoke Differential Human Health Risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, N.; Hashmi, I. Assessment of Immunohematological, Hematological and Biochemical Responses in Cultivable Fish Cyprinus Carpio Exposed to an Antibiotic Sulfamethoxazole (SMX). J. Water Health 2020, 19, 108–119. [Google Scholar] [CrossRef]
- Xie, S.; Yin, P.; Tian, L.; Liu, Y.; Tan, B.; Niu, J. Interactions between Dietary Lipid Levels and Chronic Exposure of Legal Aquaculture Dose of Sulfamethoxazole in Juvenile Largemouth Bass Micropterus Salmoides. Aquat. Toxicol. 2020, 229, 105670. [Google Scholar] [CrossRef]
- Yu, Z.; Shen, J.; Li, Z.; Yao, J.; Li, W.; Xue, L.; Vandenberg, L.N.; Yin, D. Obesogenic Effect of Sulfamethoxazole on Drosophila Melanogaster with Simultaneous Disturbances on Eclosion Rhythm, Glucolipid Metabolism, and Microbiota. Environ. Sci. Technol. 2020, 54, 5667–5675. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Zheng, M.; Wang, G.; Zhao, H. Exposed to Sulfamethoxazole Induced Hepatic Lipid Metabolism Disorder and Intestinal Microbiota Changes on Zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 253, 109245. [Google Scholar] [CrossRef] [PubMed]
- Labitt, R.N.; Ren, J.; Marquis, H. Emergence of Phenotypic and Genotypic Resistance in the Intestinal Microbiota of Rainbow Trout (Oncorhynchus mykiss) Exposed Long-Term to Sub-Inhibitory Concentrations of Sulfamethoxazole. Ecotoxicology 2021, 30, 2043–2054. [Google Scholar] [CrossRef]
- Mohammed, A. Why Are Early Life Stages of Aquatic Organisms More Sensitive to Toxicants than Adults? In New Insights into Toxicity and Drug Testing; Gowder, S., Ed.; InTech: Rijeka, Croatia, 2013; ISBN 978-953-51-0946-4. [Google Scholar]
- Kim, B.-M.; Kim, J.; Choi, I.-Y.; Raisuddin, S.; Au, D.W.T.; Leung, K.M.Y.; Wu, R.S.S.; Rhee, J.-S.; Lee, J.-S. Omics of the Marine Medaka (Oryzias melastigma) and Its Relevance to Marine Environmental Research. Mar. Environ. Res. 2016, 113, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, P.; Rey-Campos, M.; Figueras, A.; Novoa, B. An Environmentally Relevant Concentration of Antibiotics Impairs the Immune System of Zebrafish (Danio rerio) and Increases Susceptibility to Virus Infection. Front. Immunol. 2022, 13, 1100092. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, J.; Zhang, Y.; Shan, Z. Toxic effect sofsulfamethoxazole on zebrafish (Danio rerio) embryo/larva. Environ. Pollut. Control 2020, 42, 310–316. [Google Scholar]
- Han, X.B.; Yuen, K.W.Y.; Wu, R.S.S. Polybrominated Diphenyl Ethers Affect the Reproduction and Development, and Alter the Sex Ratio of Zebrafish (Danio rerio). Environ. Pollut. 2013, 182, 120–126. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Ran, H.; Lin, Y. Developmental stages of a marine model fish—medaka Oryzias melastigma. Oceanol. Limnol. Sin. 2016, 47, 71–82. [Google Scholar]
- Dong, Z.; Li, X.; Chen, Y.; Zhang, N.; Wang, Z.; Liang, Y.-Q.; Guo, Y. Short-Term Exposure to Norethisterone Affected Swimming Behavior and Antioxidant Enzyme Activity of Medaka Larvae, and Led to Masculinization in the Adult Population. Chemosphere 2023, 310, 136844. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shan, L.; Yan, M.; Chai, X.; Hu, L.; Shao, X. Embryonic development of nibea albiflora and the effects of temperature and salinity on embryogenesis. Mar. Sci. 2017, 41, 44–50. [Google Scholar]
- Kawaguchi, M.; Yasumasu, S.; Shimizu, A.; Sano, K.; Iuchi, I.; Nishida, M. Conservation of the Egg Envelope Digestion Mechanism of Hatching Enzyme in Euteleostean Fishes. FEBS J. 2010, 277, 4973–4987. [Google Scholar] [CrossRef]
- Zhou, L.; Limbu, S.M.; Shen, M.; Zhai, W.; Qiao, F.; He, A.; Du, Z.-Y.; Zhang, M. Environmental Concentrations of Antibiotics Impair Zebrafish Gut Health. Environ. Pollut. 2018, 235, 245–254. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Gong, L.; Cheng, Y.; Yuan, Q.; He, Y. Combined Toxicity of Polystyrene Microplastics and Sulfamethoxazole on Zebrafish Embryos. Environ. Sci. Pollut. Res. Int. 2022, 29, 19273–19282. [Google Scholar] [CrossRef]
- Genge, C.; Hove-Madsen, L.; Tibbits, G.F. Functional and Structural Differences in Atria Versus Ventricles in Teleost Hearts. In New Advances and Contributions to Fish Biology; Turker, H., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0909-9. [Google Scholar]
- Tshering, G.; Plengsuriyakarn, T.; Na-Bangchang, K.; Pimtong, W. Embryotoxicity Evaluation of Atractylodin and β-Eudesmol Using the Zebrafish Model. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108869. [Google Scholar] [CrossRef]
- Iftikhar, N.; Konig, I.; English, C.; Ivantsova, E.; Souders, C.L., 2nd; Hashmi, I.; Martyniuk, C.J. Sulfamethoxazole (SMX) Alters Immune and Apoptotic Endpoints in Developing Zebrafish (Danio rerio). Toxics 2023, 11, 178. [Google Scholar] [CrossRef]
- Antkiewicz, D.S.; Peterson, R.E.; Heideman, W. Blocking Expression of AHR2 and ARNT1 in Zebrafish Larvae Protects against Cardiac Toxicity of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Toxicol. Sci. 2006, 94, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Brini, M. Calcium Pumps: Structural Basis for and Mechanism of Calcium Transmembrane Transport. Curr. Opin. Chem. Biol. 2000, 4, 152–161. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Rice, W.J.; Odermatt, A. Structure/Function Analysis of the Ca2+ Binding and Translocation Domain of SERCA1 and the Role in Brody Disease of the ATP2A1 Gene Encoding SERCA1. Ann. N. Y. Acad. Sci. 1997, 834, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Huang, S.; Wang, C.; Guo, Y.; Dong, Z.; Liu, C. Observation of Embryonic Development and Autofluorescence of Oryzias curvinotus. J. Guangdong Ocean. Univ. 2019, 39, 38–44. [Google Scholar]
- Woolley, J.F.; Stanicka, J.; Cotter, T.G. Recent Advances in Reactive Oxygen Species Measurement in Biological Systems. Trends Biochem. Sci. 2013, 38, 556–565. [Google Scholar] [CrossRef]
- Aldosari, S.; Awad, M.; Harrington, E.O.; Sellke, F.W.; Abid, M.R. Subcellular Reactive Oxygen Species (ROS) in Cardiovascular Pathophysiology. Antioxidants 2018, 7, 14. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative Stress: Molecular Perception and Transduction of Signals Triggering Antioxidant Gene Defenses. Braz J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef]
- Piner Benli, P.; Çelik, M. Glutathione and Its Dependent Enzymes’ Modulatory Responses to Neonicotinoid Insecticide Sulfoxaflor Induced Oxidative Damage in Zebrafish in Vivo. Sci. Prog. 2021, 104, 368504211028361. [Google Scholar] [CrossRef]
- Gabriel, J.P.; Ausborn, J.; Ampatzis, K.; Mahmood, R.; Eklöf-Ljunggren, E.; El Manira, A. Principles Governing Recruitment of Motoneurons during Swimming in Zebrafish. Nat. Neurosci. 2011, 14, 93–99. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Cowden, J.; Simmons, S.O.; Padilla, S.; Ramabhadran, R. Gene Expression Changes in Developing Zebrafish as Potential Markers for Rapid Developmental Neurotoxicity Screening. Neurotoxicol. Teratol. 2010, 32, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Bauer, N.M.; Schäfer, I.; White, R. Making Myelin Basic Protein -from mRNA Transport to Localized Translation. Front. Cell Neurosci. 2013, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Ueshima, E.; Muraoka, O.; Tanaka, H.; Yeo, S.Y.; Huh, T.L.; Miki, N. Zebrafish Elav/HuC Homologue as a Very Early Neuronal Marker. Neurosci. Lett. 1996, 216, 109–112. [Google Scholar] [CrossRef]
- Okada, M.; Kawagoe, Y.; Takasugi, T.; Nozumi, M.; Ito, Y.; Fukusumi, H.; Kanemura, Y.; Fujii, Y.; Igarashi, M. JNK1-Dependent Phosphorylation of GAP-43 Serine 142 Is a Novel Molecular Marker for Axonal Growth. Neurochem. Res. 2022, 47, 2668–2682. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.-B.; Jia, P.-P.; Li, W.-G.; Xie, X.-Y.; Yang, G.; Pei, D.-S. Sulfonamides (SAs) Exposure Causes Neurobehavioral Toxicity at Environmentally Relevant Concentrations (ERCs) in Early Development of Zebrafish. Aquat. Toxicol. 2023, 261, 106614. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, L.; Chen, J. Sulfamethoxazole Induces Brain Capillaries Toxicity in Zebrafish by Up-Regulation of VEGF and Chemokine Signalling. Ecotoxicol. Environ. Saf. 2022, 238, 113620. [Google Scholar] [CrossRef]
- Awakawa, T.; Barra, L.; Abe, I. Biosynthesis of Sulfonamide and Sulfamate Antibiotics in Actinomycete. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab001. [Google Scholar] [CrossRef]
- Rahdar, H.A.; Mahmoudi, S.; Bahador, A.; Ghiasvand, F.; Sadeghpour Heravi, F.; Feizabadi, M.M. Molecular Identification and Antibiotic Resistance Pattern of Actinomycetes Isolates among Immunocompromised Patients in Iran, Emerging of New Infections. Sci. Rep. 2021, 11, 10745. [Google Scholar] [CrossRef]
- Watson, A.K.; Kepplinger, B.; Bakhiet, S.M.; Mhmoud, N.A.; Chapman, J.; Allenby, N.E.; Mickiewicz, K.; Goodfellow, M.; Fahal, A.H.; Errington, J. Systematic Whole-Genome Sequencing Reveals an Unexpected Diversity among Actinomycetoma Pathogens and Provides Insights into Their Antibacterial Susceptibilities. PLoS. Negl. Trop. Dis. 2022, 16, e0010128. [Google Scholar] [CrossRef]
- Miller, L.G.; Finegold, S.M. Antibacterial Sensitivity of Bifidobacterium (Lactobacillus bifidus). J. Bacteriol. 1967, 93, 125–130. [Google Scholar] [CrossRef]
- Igbinosa, E.O. Detection and Antimicrobial Resistance of Vibrio Isolates in Aquaculture Environments: Implications for Public Health. Microb. Drug Resist. 2016, 22, 238–245. [Google Scholar] [CrossRef]
- Lin, M.; Wu, X.; Yan, Q.; Ma, Y.; Huang, L.; Qin, Y.; Xu, X. Incidence of Antimicrobial-Resistance Genes and Integrons in Antibiotic-Resistant Bacteria Isolated from Eels and Aquaculture Ponds. Dis. Aquat. Organ. 2016, 120, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Stalin, N.; Srinivasan, P. Molecular Characterization of Antibiotic Resistant Vibrio Harveyi Isolated from Shrimp Aquaculture Environment in the South East Coast of India. Microb. Pathog. 2016, 97, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Kayani, M.U.R.; Yu, K.; Qiu, Y.; Shen, Y.; Gao, C.; Feng, R.; Zeng, X.; Wang, W.; Chen, L.; Su, H.L. Environmental Concentrations of Antibiotics Alter the Zebrafish Gut Microbiome Structure and Potential Functions. Environ. Pollut. 2021, 278, 116760. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Nell, S.; Suerbaum, S.; Josenhans, C. The Impact of the Microbiota on the Pathogenesis of IBD: Lessons from Mouse Infection Models. Nat. Rev. Microbiol. 2010, 8, 564–577. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Jin, C.; Yuan, X.; Wang, C.; Fu, Z.; Jin, Y. Maternal Exposure to Imazalil Disrupts Intestinal Barrier and Bile Acids Enterohepatic Circulation Tightly Related IL-22 Expression in F0, F1 and F2 Generations of Mice. J. Hazard. Mater. 2021, 403, 123668. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, Z.; Wang, X.; Shen, M.; Zhou, J.; Fu, Z.; Jin, Y. Short-Term Propamocarb Exposure Induces Hepatic Metabolism Disorder Associated with Gut Microbiota Dysbiosis in Adult Male Zebrafish. Acta Biochim. Biophys. Sin. 2019, 51, 88–96. [Google Scholar] [CrossRef]
- Zhao, X.; Su, H.; Xu, W.; Hu, X.; Xu, Y.; Wen, G.; Cao, Y. Removal of Antibiotic Resistance Genes and Inactivation of Antibiotic-Resistant Bacteria by Oxidative Treatments. Sci. Total Environ. 2021, 778, 146348. [Google Scholar] [CrossRef]
- Yin, X.; Zheng, J.; Liu, Y.; Li, Y.; Yu, X.; Li, Y.; Wang, X. Metagenomic Evidence for Increasing Antibiotic Resistance in Progeny upon Parental Antibiotic Exposure as the Cost of Hormesis. Chemosphere 2022, 309, 136738. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).