You Are What You Eat: California Sea Cucumbers Become “Fishier” After Integrated Multi-Trophic Aquaculture with Chinook Salmon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Farm Parameters, and Source of Sea Cucumbers
2.2. Experimental Design and Tissue Sampling
2.2.1. Fatty Acids
2.2.2. Nitrogenous Metabolites
2.3. Statistical Analysis
3. Results
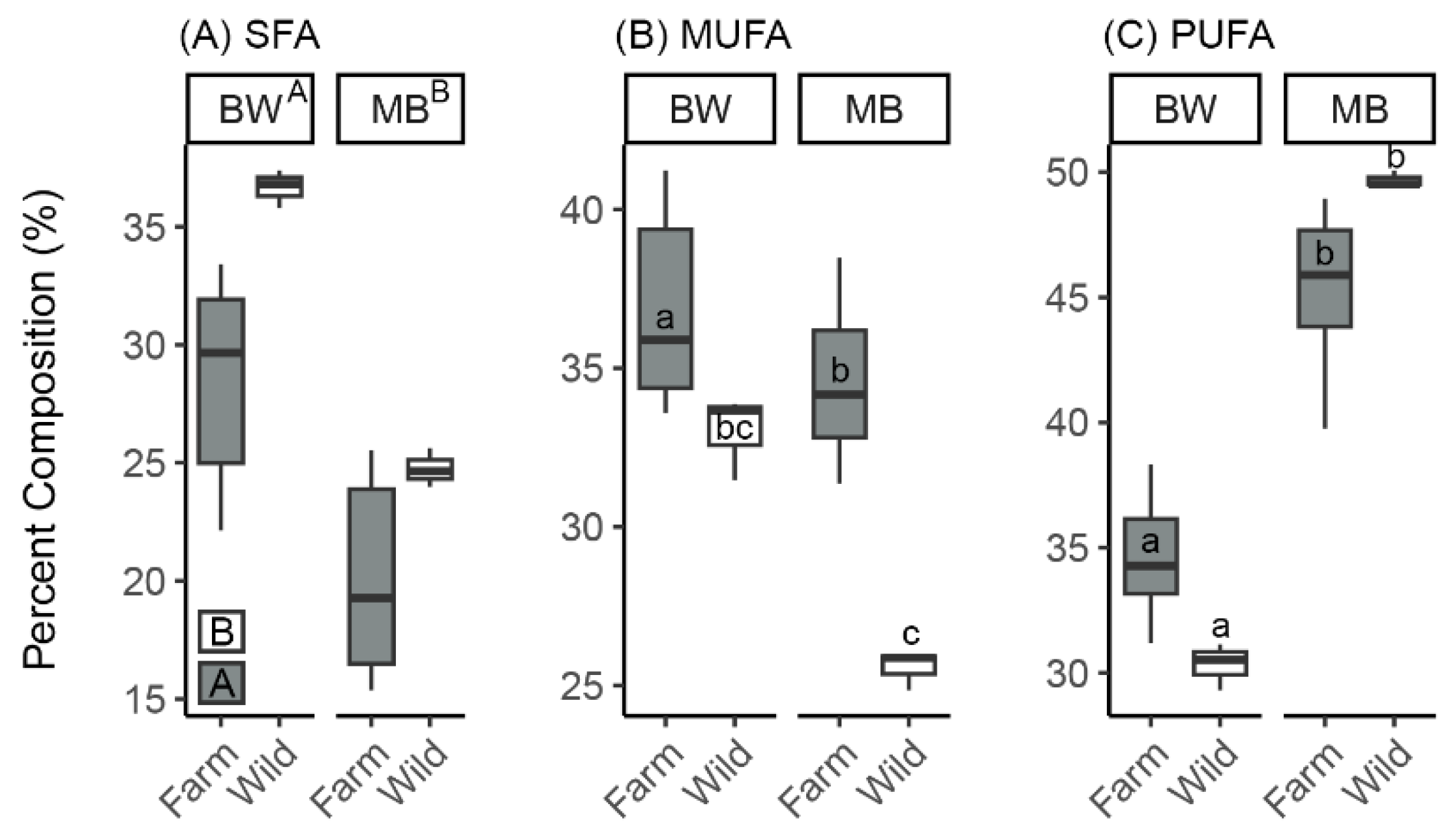
3.1. Fatty Acids
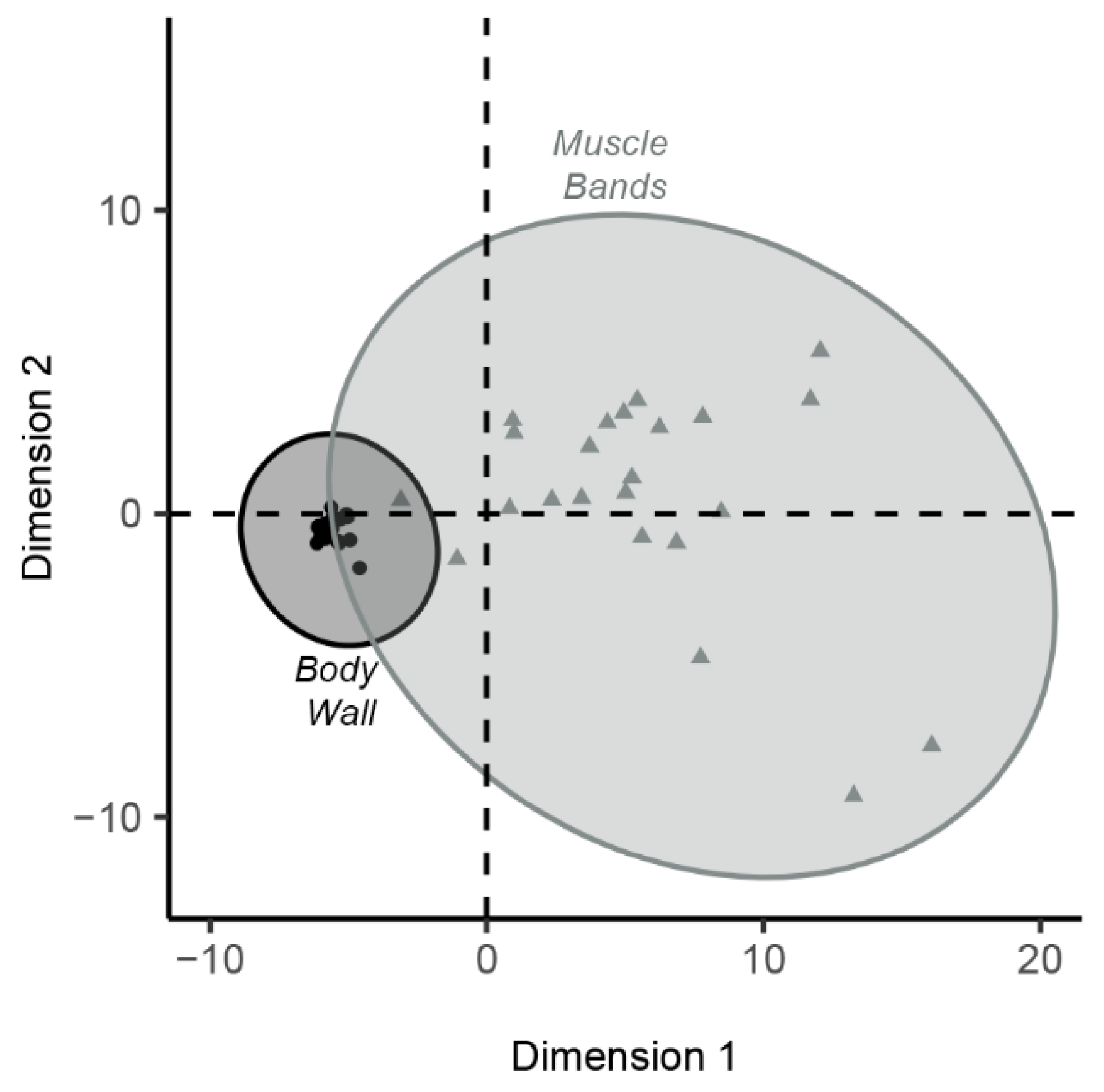
3.1.1. Effects of Sea Cucumber Treatment (Wild Versus Farmed) and Tissue Type (Body Wall Versus Muscle Band) After 3 Months of IMTA
3.1.2. Effects of Sea Cucumber Proximity to Fish During IMTA (F + S Versus S) and Length of IMTA (1, 2, or 3 Months)
3.1.3. Unsupervised Hierarchical Clustering Analysis
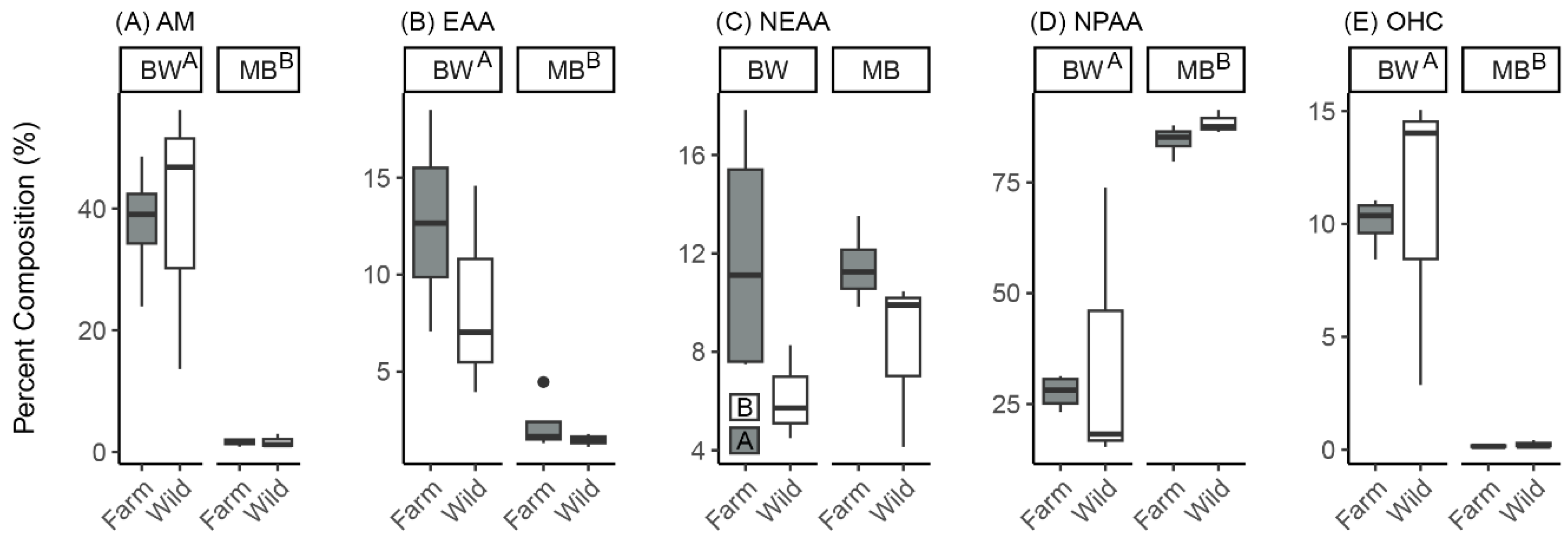
3.2. Nitrogenous Metabolites
3.2.1. Effects of Sea Cucumber Treatment (Wild Versus Farmed) and Tissue Type (Body Wall Versus Muscle Band) After 3 Months of IMTA
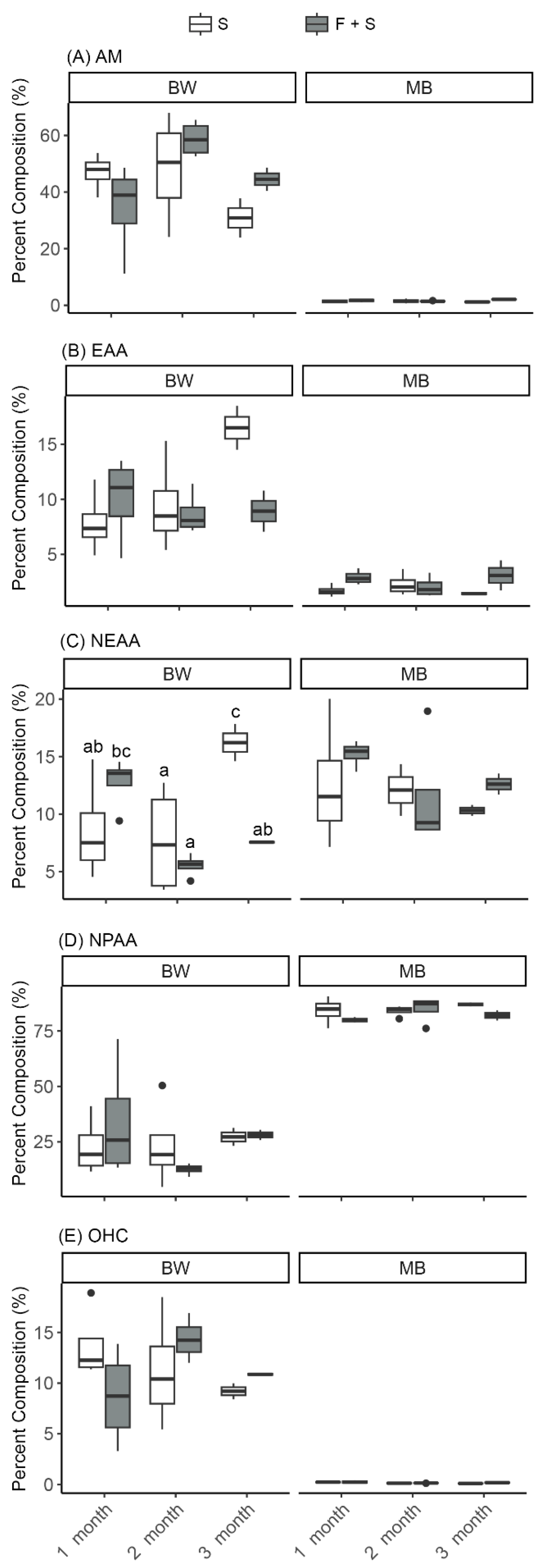
3.2.2. Effects of Sea Cucumber Proximity to Fish During IMTA (F + S Versus S) and Length of IMTA (1, 2, or 3 Months)
3.2.3. Unsupervised Hierarchical Clustering Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, A.; Senff, P.; Glaser, M. Lessons for Coastal Applications of IMTA as a Way towards Sustainable Development: A Review. Appl. Sci. 2022, 12, 11920. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Zahedi, S.; Mohammadi, A. Integrated Multitrophic Aquaculture (IMTA) as an Environmentally Friendly System for Sustainable Aquaculture: Functionality, Species, and Application of Biofloc Technology (BFT). Environ. Sci. Pollut. Res. 2022, 29, 67513–67531. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Wu, W.; Zhong, Y.; Li, H.; Wang, X.; Yang, J.; Zhang, Y. Effects of Shellfish and Macro-Algae IMTA in North China on the Environment, Inorganic Carbon System, Organic Carbon System, and Sea–Air CO2 Fluxes. Front. Mar. Sci. 2022, 9, 864306. [Google Scholar] [CrossRef]
- Joseph, S.; Ignatius, B.; Joseph, I.; Rajesh, N. Integrated Multitrophic Aquaculture (IMTA)—A Successful Diversified Farming System. In Winter School on Mariculture Technologies for Income Multiplication, Employment, Livelihood and Empowerment; ICAR-Central Marine Fisheries Research Institute: Kochi, India, 2023; pp. 53–61. [Google Scholar]
- Chopin, T.; Robinson, S.; Reid, G.; Ridler, N. Prospects for Integrated Multi-Trophic Aquaculture (IMTA) in the Open Ocean. Bull. Aquac. Assoc. Can. 2013, 111–112, 28–35. [Google Scholar]
- Chary, K.; Aubin, J.; Sadoul, B.; Fiandrino, A.; Covès, D.; Callier, M.D. Integrated Multi-Trophic Aquaculture of Red Drum (Sciaenops ocellatus) and Sea Cucumber (Holothuria scabra): Assessing Bioremediation and Life-Cycle Impacts. Aquaculture 2020, 516, 734621. [Google Scholar] [CrossRef]
- Cutajar, K.; Falconer, L.; Massa-Gallucci, A.; Cox, R.E.; Schenke, L.; Bardócz, T.; Sharman, A.; Deguara, S.; Telfer, T.C. Culturing the Sea Cucumber Holothuria poli in Open-Water Integrated Multi-Trophic Aquaculture at a Coastal Mediterranean Fish Farm. Aquaculture 2022, 550, 737881. [Google Scholar] [CrossRef]
- Mazón-Suástegui, J.M.; Arcos-Ortega, G.F.; Contreras-Mendoza, C.N.; Medina-Sánchez, J.R.; Chávez-Villalba, J.; Lodeiros, C.; Cruz-Flores, R.; López-Carvallo, J.A. Enhanced Growth of the Pleasure Oyster Crassostrea corteziensis Cultured under Integrated Multi-Trophic Aquaculture (IMTA) Concept, Using Farm Effluents of Shrimp Penaeus vannamei. Aquac. Res. 2022, 53, 5214–5226. [Google Scholar] [CrossRef]
- Grosso, L.; Rakaj, A.; Fianchini, A.; Morroni, L.; Cataudella, S.; Scardi, M. Integrated Multi-Trophic Aquaculture (IMTA) System Combining the Sea Urchin Paracentrotus lividus, as Primary Species, and the Sea Cucumber Holothuria tubulosa as Extractive Species. Aquaculture 2021, 534, 736268. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.G. Ecological Engineering in Aquaculture—Potential for Integrated Multi-Trophic Aquaculture (IMTA) in Marine Offshore Systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Orr, L.C.; Curtis, D.L.; Cross, S.F.; Gurney-Smith, H.; Shanks, A.; Pearce, C.M. Ingestion Rate, Absorption Efficiency, Oxygen Consumption, and Fecal Production in Green Sea Urchins (Strongylocentrotus droebachiensis) Fed Waste from Sablefish (Anoplopoma fimbria) Culture. Aquaculture 2014, 422–423, 184–192. [Google Scholar] [CrossRef]
- Fossberg, J.; Forbord, S.; Broch, O.J.; Malzahn, A.M.; Jansen, H.; Handå, A.; Førde, H.; Bergvik, M.; Fleddum, A.L.; Skjermo, J.; et al. The Potential for Upscaling Kelp (Saccharina latissima) Cultivation in Salmon-Driven Integrated Multi-Trophic Aquaculture (IMTA). Front. Mar. Sci. 2018, 9, 418. [Google Scholar] [CrossRef]
- Paltzat, D.L.; Pearce, C.M.; Barnes, P.A.; McKinley, R.S. Growth and Production of California Sea Cucumbers (Parastichopus californicus Stimpson) Co-Cultured with Suspended Pacific Oysters (Crassostrea gigas Thunberg). Aquaculture 2008, 275, 124–137. [Google Scholar] [CrossRef]
- Hannah, L.; Pearce, C.M.; Cross, S.F. Growth and Survival of California Sea Cucumbers (Parastichopus californicus) Cultivated with Sablefish (Anoplopoma fimbria) at an Integrated Multi-Trophic Aquaculture Site. Aquaculture 2013, 406–407, 34–42. [Google Scholar] [CrossRef]
- Cubillo, A.M.; Ferreira, J.G.; Robinson, S.M.C.; Pearce, C.M.; Corner, R.A.; Johansen, J. Role of Deposit Feeders in Integrated Multi-Trophic Aquaculture—A Model Analysis. Aquaculture 2016, 453, 54–66. [Google Scholar] [CrossRef]
- Zamora, L.N.; Yuan, X.; Carton, A.G.; Slater, M.J. Role of Deposit-Feeding Sea Cucumbers in Integrated Multitrophic Aquaculture: Progress, Problems, Potential and Future Challenges. Rev. Aquac. 2018, 10, 57–74. [Google Scholar] [CrossRef]
- Yang, H.; Hamel, J.-F.; Mercier, A. The Sea Cucumber Apostichopus japonicus: History, Biology and Aquaculture; Academic Press: Cambridge, MA, USA, 2015; ISBN 0128004673. [Google Scholar]
- MacDonald, C.L.E.; Stead, S.M.; Slater, M.J. Consumption and Remediation of European Seabass (Dicentrarchus labrax) Waste by the Sea Cucumber Holothuria forskali. Aquac. Int. 2013, 21, 1279–1290. [Google Scholar] [CrossRef]
- Cutajar, K.; Falconer, L.; Massa-Gallucci, A.; Cox, R.E.; Schenke, L.; Bardócz, T.; Andolina, C.; Signa, G.; Vizzini, S.; Sprague, M.; et al. Stable Isotope and Fatty Acid Analysis Reveal the Ability of Sea Cucumbers to Use Fish Farm Waste in Integrated Multi-Trophic Aquaculture. J. Environ. Manag. 2022, 318, 115511. [Google Scholar] [CrossRef]
- Sadoul, B.; Caprioli, J.-P.; Barrier-Loiseau, C.; Cimiterra, N.; Laugier, T.; Lagarde, F.; Chary, K.; Callier, M.D.; Guillermard, M.-O.; Roque d’Orbcastel, E. Is Holothuria tubulosa the Golden Goose of Ecological Aquaculture in the Mediterranean Sea? Aquaculture 2022, 554, 738149. [Google Scholar] [CrossRef]
- Tolon, M.T.; Emiroğlu, D.; Günay, D.; Özgül, A. Sea Cucumber (Holothuria tubulosa Gmelin, 1790) Culture under Marine Fish Net Cages for Potential Use in Integrated Multi-Trophic Aquaculture (IMTA). Indian J. Geo-Mar. Sci. 2017, 46, 749–756. [Google Scholar]
- Nelson, E.J.; MacDonald, B.A.; Robinson, S.M.C. The Absorption Efficiency of the Suspension-Feeding Sea Cucumber, Cucumaria frondosa, and its Potential as an Extractive Integrated Multi-Trophic Aquaculture (IMTA) Species. Aquaculture 2012, 370–371, 19–25. [Google Scholar] [CrossRef]
- Sun, J.; Hamel, J.-F.; Gianasi, B.L.; Graham, M.; Mercier, A. Growth, Health and Biochemical Composition of the Sea Cucumber Cucumaria frondosa after Multi-Year Holding in Effluent Waters of Land-Based Salmon Culture. Aquac. Environ. Interact. 2020, 12, 139–151. [Google Scholar] [CrossRef]
- Haddad, C.A.; Cross, S.F.; Davies, H.L.; Duff, S.D.; Fortune, A.C.; Pearce, C.M. Assessing Natural Substrates for Restricting Movement of California Sea Cucumbers Parastichopus californicus. N. Am. J. Aquac. 2018, 80, 347–354. [Google Scholar] [CrossRef]
- Montgomery, E.M.; Cannon, B.L.; Pearce, C.M. Exploring Biofouling Control by the California Sea Cucumber (Apostichopus californicus) in Integrated Multi-Trophic Aquaculture (IMTA) with Organic Chinook Salmon (Oncorhynchus tshawytscha). Fishes 2023, 8, 430. [Google Scholar] [CrossRef]
- Curtis, D.L.; Pearce, C.M.; van Dam-Bates, P.; Duprey, N.M.T.; Cross, S.F.; Cowen, L.L.E. California Sea Cucumber (Apostichopus californicus) Abundance and Movement on a Commercial Shellfish Aquaculture Farm. Diversity 2023, 15, 940. [Google Scholar] [CrossRef]
- Montgomery, E.M.; Suhrbier, A.D.; Pearce, C.M. Biology, Ecology, Aquaculture, and Commercial Products of Apostichopus californicus. In The World of Sea Cucumbers: Challenges, Advances, and Innovations; Elsevier: Amsterdam, The Netherlands, 2024; pp. 653–675. [Google Scholar]
- Yokoyama, H.; Tadokoro, D.; Miura, M. Quantification of Waste Feed and Fish Faeces in Sediments beneath Yellowtail Pens and Possibility to Reduce Waste Loading by Co-Culturing with Sea Cucumbers: An Isotopic Study. Aquac. Res. 2015, 46, 918–927. [Google Scholar] [CrossRef]
- Imbs, A.B.; Svetashev, V.I.; Rodkina, S.A. Differences in Lipid Class and Fatty Acid Composition between Wild and Cultured Sea Cucumbers, Apostichopus japonicus, Explain Modification and Deposition of Lipids. Aquac. Res. 2022, 53, 810–819. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, L.; Tang, X.; Xia, X.; Hu, W.; Zhou, P. Season and Geography Induced Variation in Sea Cucumber (Stichopus japonicus) Nutritional Composition and Gut Microbiota. J. Food Compos. Anal. 2021, 101, 103838. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Wu, P.; Tang, R.-J.; Liu, Y.; Kuang, S.-Y.; Jiang, J.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q.; et al. Nutritive Values, Flavor Amino Acids, Healthcare Fatty Acids and Flesh Quality Improved by Manganese Referring to up-Regulating the Antioxidant Capacity and Signaling Molecules TOR and Nrf2 in the Muscle of Fish. Food Res. Int. 2016, 89, 670–678. [Google Scholar]
- Peinado, I.; Miles, W.; Koutsidis, G. Odour Characteristics of Seafood Flavour Formulations Produced with Fish By-Products Incorporating EPA, DHA and Fish Oil. Food Chem. 2016, 212, 612–619. [Google Scholar]
- Hajas, W.; Hand, C.; Duprey, N.; Lochead, J.; Deault, J. Using Production Models with New and Developing Fisheries: A Case Study Using the Sea Cucumber Parastichopus californicus in British Columbia, Canada. Fish. Res. 2011, 110, 421–434. [Google Scholar] [CrossRef]
- Lochead, J.; Ridings, P.; Hansen, S.C. Ensuring Long-Term Sustainability of the Fishery for Apostichopus californicus in British Columbia (Canada) through Collaborative Science and Adaptive Management. In The World of Sea Cucumbers: Challenges, Advances, and Innovations; Elsevier: Amsterdam, The Netherlands, 2024; pp. 677–685. [Google Scholar]
- Ahlgren, M.O. Consumption and Assimilation of Salmon Net Pen Fouling Debris by the Red Sea Cucumber Parastichopus californicus: Implications for Polyculture. J. World Aquac. Soc. 1998, 29, 133–139. [Google Scholar] [CrossRef]
- Puttick, D.; Dauk, M.; Lozinsky, S.; Smith, M.A. Overexpression of a FAD3 Desaturase Increases Synthesis of a Polymethylene-Interrupted Dienoic Fatty Acid in Seeds of Arabidopsis thaliana L. Lipids 2009, 44, 753–757. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2024. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 11 March 2025).
- Wickham, H. Ggplot2; Use R! Springer International Publishing: London, UK, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Leys, C.; Schumann, S. A Nonparametric Method to Analyze Interactions: The Adjusted Rank Transform Test. J. Exp. Soc. Psychol. 2010, 46, 684–688. [Google Scholar] [CrossRef]
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only ANOVA Procedures. In Proceedings of the Conference on Human Factors in Computing Systems—Proceedings 2011, Vancouver, BC, Canada, 7–12 May 2011; pp. 143–146. [Google Scholar] [CrossRef]
- Khoomrung, S.; Raber, G.; Laoteng, K.; Francesconi, K.A. Identification and Characterization of Fish Oil Supplements Based on Fatty Acid Analysis Combined with a Hierarchical Clustering Algorithm. Eur. J. Lipid Sci. Technol. 2014, 116, 795–804. [Google Scholar] [CrossRef]
- Husson, F.; Josse, J.; Le, S.; Mazet, J.; Husson, M.F. Package ‘Factominer’. R. Package 2016, 96, 698. [Google Scholar]
- Elvines, D.M.; MacLeod, C.K.; Ross, D.J.; Hopkins, G.A.; White, C.A. Fate and Effects of Fish Farm Organic Waste in Marine Systems: Advances in Understanding Using Biochemical Approaches with Implications for Environmental Management. Rev. Aquac. 2023, 16, 66–85. [Google Scholar] [CrossRef]
- Sissener, N.H. Are We What We Eat? Changes to the Feed Fatty Acid Composition of Farmed Salmon and its Effects through the Food Chain. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb161521. [Google Scholar]
- Orr, L.C. Co-Culture of Invertebrates with Sablefish (Anoplopoma fimbria) in IMTA in British Columbia: Use of Laboratory Feeding Trials to Assess the Organic Extractive Potential of Various Candidate Species. MSc Thesis, University of Victoria, Victoria, BC, Canada, 2012. [Google Scholar]
- Robinson, S.M.C.; Martin, J.D.; Cooper, J.A.; Lander, T.R.; Reid, G.K.; Powell, F.; Griffin, R. The Role of Three Dimensional Habitats in the Establishment of Integrated Multi-Trophic Aquaculture (IMTA) Systems. Bull. Aquac. Assoc. Can. 2011, 109, 23–29. [Google Scholar]
- Reid, G.K.; Lefebvre, S.; Filgueira, R.; Robinson, S.M.C.; Broch, O.J.; Dumas, A.; Chopin, T.B.R. Performance Measures and Models for Open-Water Integrated Multi-Trophic Aquaculture. Rev. Aquac. 2020, 12, 47–75. [Google Scholar] [CrossRef]
- Irisarri, J.; Fernández-Reiriz, M.J.; Labarta, U.; Cranford, P.J.; Robinson, S.M.C. Availability and Utilization of Waste Fish Feed by Mussels Mytilus edulis in a Commercial Integrated Multi-Trophic Aquaculture (IMTA) System: A Multi-Indicator Assessment Approach. Ecol. Indic. 2015, 48, 673–686. [Google Scholar] [CrossRef]
- Nahon, S.; de Brito, G.V.; Quental-Ferreira, H.; Aubin, J.; Jaeger, C.; Menniti, C.; Kerhervé, P.; Larroquet, L.; Cunha, M.E. Food Web in Mediterranean Coastal Integrated Multi-Trophic Aquaculture Ponds: Learnings from Fatty Acids and Stable Isotope Tracers. Aquaculture 2023, 567, 739292. [Google Scholar] [CrossRef]
- Bechtel, P.J.; Oliveira, A.C.M.; Demir, N.; Smiley, S. Chemical Composition of the Giant Red Sea Cucumber, Parastichopus californicus, Commercially Harvested in Alaska. Food Sci. Nutr. 2013, 1, 63–73. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Q.; Yang, H. Seasonal Biochemical Changes in Composition of Body Wall Tissues of Sea Cucumber Apostichopus japonicus. Chinese J. Oceanol. Limnol. 2011, 29, 252–260. [Google Scholar] [CrossRef]
- Künili, I.E.; Çolakoğlu, F.A. Chemical and Nutritional Characteristics of Holothuria tubulosa (Gmelin, 1788); A Seasonally Comparative Study. J. Aquat. Food Prod. Technol. 2019, 28, 716–728. [Google Scholar] [CrossRef]
- Hafezieh, M.; Jamili, S.; Dadgar, S. Changes of Sea Cucumber (Holothuria leucospilota) Proximate Composition during Geographical-Seasonal Variation in the Persian Gulf and Oman Sea Waters. Iran. Sci. Fish. J. 2018, 27, 13–24. [Google Scholar]
- Abuzaytoun, R.; Budge, S.M.; Xia, W.; Mackinnon, S. Unusual Ether Lipids and Branched Chain Fatty Acids in Sea Cucumber (Cucumaria frondosa) Viscera and Their Seasonal Variation. Mar. Drugs 2022, 20, 435. [Google Scholar] [CrossRef]
- Fankboner, P.V. Seasonal Visceral Atrophy and Response to Salinity by Parastichopus californicus. SPC Beche-de-mer Inf. Bull. 2002, 17, 22–26. [Google Scholar]
- Fankboner, P.V.; Cameron, J.L. Seasonal Atrophy of the Visceral Organs in a Sea Cucumber. Can. J. Zool. 1985, 63, 2888–2892. [Google Scholar] [CrossRef]
- Sveier, H.; Raae, A.J.; Lied, E. Growth and Protein Turnover in Atlantic Salmon (Salmo salar L.); the Effect of Dietary Protein Level and Protein Particle Size. Aquaculture 2000, 185, 101–120. [Google Scholar] [CrossRef]
- Morais, S.; Koven, W.; Rønnestad, I.; Dinis, M.T.; Conceição, L.E.C. Dietary Protein/Lipid Ratio Affects Growth and Amino Acid and Fatty Acid Absorption and Metabolism in Senegalese Sole (Solea senegalensis Kaup 1858) Larvae. Aquaculture 2005, 246, 347–357. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S. Value, Market Preferences and Trade of Beche-De-Mer from Pacific Island Sea Cucumbers. PLoS ONE 2014, 9, e95075. [Google Scholar] [CrossRef]
- Purcell, S.; Conand, C.; Uthicke, S.; Byrne, M. Ecological Roles of Exploited Sea Cucumbers. Oceanogr. Mar. Biol. Ann. Rev. 2016, 54, 367–386. [Google Scholar]


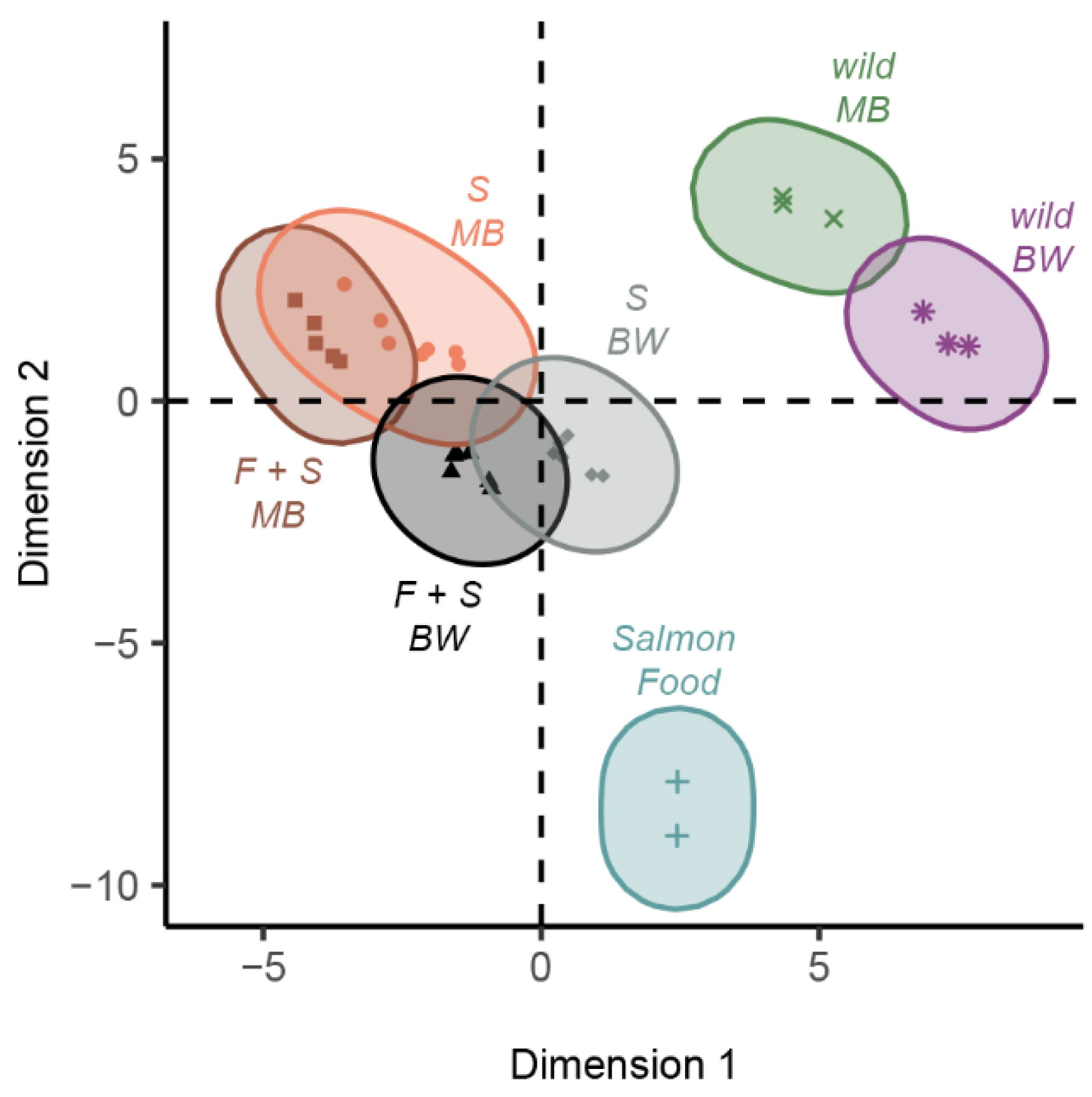
| HCPC Cluster | Enriched Fatty Acids Relative to Overall Mean | p-Value |
|---|---|---|
| F + S muscle bands | SFAs (none) MUFAs (20:1n9, 22:1n-9, 24:1n-9) PUFAs (20:2n-6, 20:4n-6, 22:5n-3, 22:5n-6, 22:6n-3) | All < 0.01 |
| S muscle bands | SFAs (none) MUFAs (20:1n-9, 20:1n-11, 22:1n-11, 24:1n-9) PUFAs (20:4n-6, 20:5n-3, 22:5n-6) | All < 0.01 |
| Wild muscle bands | SFAs (20:0, 21:0, 22:0) MUFAs (none) PUFAs (16:4n-1, 22:4n-6) | All < 0.01 |
| F + S body walls | SFAs (none) MUFAs (18:1n-9) PUFAs (18:2n-6, 22:5n-3, 22:6n-3) | All < 0.01 |
| S body walls | SFAs (15:0, 16:0, 17:0) MUFAs (16:1n-7, 20:1n-11) PUFAs (16:3n-4) | All < 0.01 |
| Wild body walls | SFAs (15:0, 20:0, 21:0, 22:0) MUFAs (none) PUFAs (16:2n-4, 18:3n-6) | All < 0.01 |
| Commercial salmon food | SFAs (14:0) MUFAs (17:1, 18:1n-7) PUFAs (18:2n-6, 18:3n-3, 18:4n-3) | All < 0.01 |
| HCPC Cluster | Enriched Metabolites Relative to Overall Mean | Metabolite Type | p-Value |
|---|---|---|---|
| Body walls | Ethanolamine Trigonelline | Amine Organoheterocyclic | Both < 0.05 |
| Muscle bands | Alanine Arginine Betaine Glutamic acid Phenylalanine | Non-essential amino acid Non-essential amino acid Non-proteinogenic amino acid Non-essential amino acid Essential amino acid | All < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montgomery, E.M.; Cannon, B.L.; Nomura, M.; Leme, R.B.; Forster, I.P.; Pearce, C.M. You Are What You Eat: California Sea Cucumbers Become “Fishier” After Integrated Multi-Trophic Aquaculture with Chinook Salmon. Fishes 2025, 10, 154. https://doi.org/10.3390/fishes10040154
Montgomery EM, Cannon BL, Nomura M, Leme RB, Forster IP, Pearce CM. You Are What You Eat: California Sea Cucumbers Become “Fishier” After Integrated Multi-Trophic Aquaculture with Chinook Salmon. Fishes. 2025; 10(4):154. https://doi.org/10.3390/fishes10040154
Chicago/Turabian StyleMontgomery, Emaline M., Barb L. Cannon, Miki Nomura, Rodrigo B. Leme, Ian P. Forster, and Christopher M. Pearce. 2025. "You Are What You Eat: California Sea Cucumbers Become “Fishier” After Integrated Multi-Trophic Aquaculture with Chinook Salmon" Fishes 10, no. 4: 154. https://doi.org/10.3390/fishes10040154
APA StyleMontgomery, E. M., Cannon, B. L., Nomura, M., Leme, R. B., Forster, I. P., & Pearce, C. M. (2025). You Are What You Eat: California Sea Cucumbers Become “Fishier” After Integrated Multi-Trophic Aquaculture with Chinook Salmon. Fishes, 10(4), 154. https://doi.org/10.3390/fishes10040154







