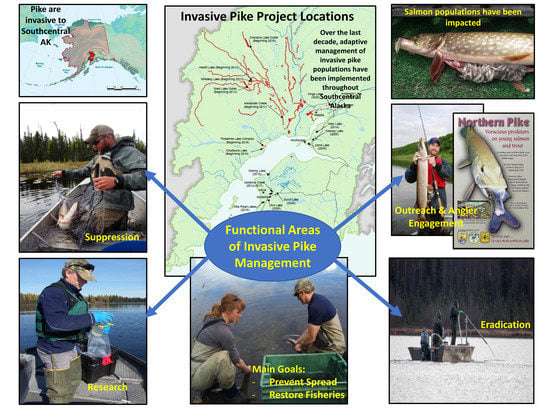
A Decade in Review: Alaska’s Adaptive Management of an Invasive Apex Predator
Abstract
:1. Introduction
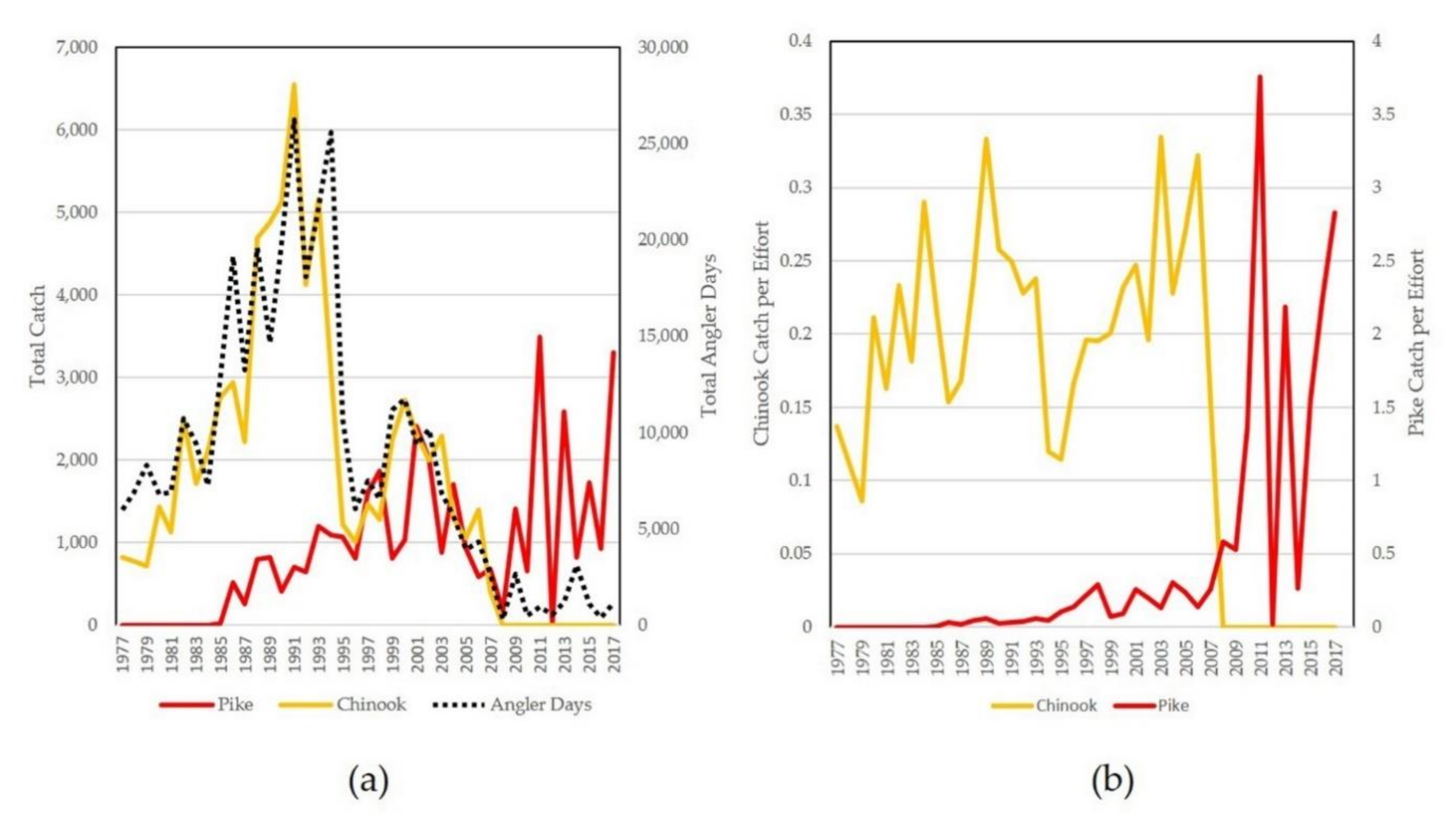
2. Ecological Role of Pike in Alaska
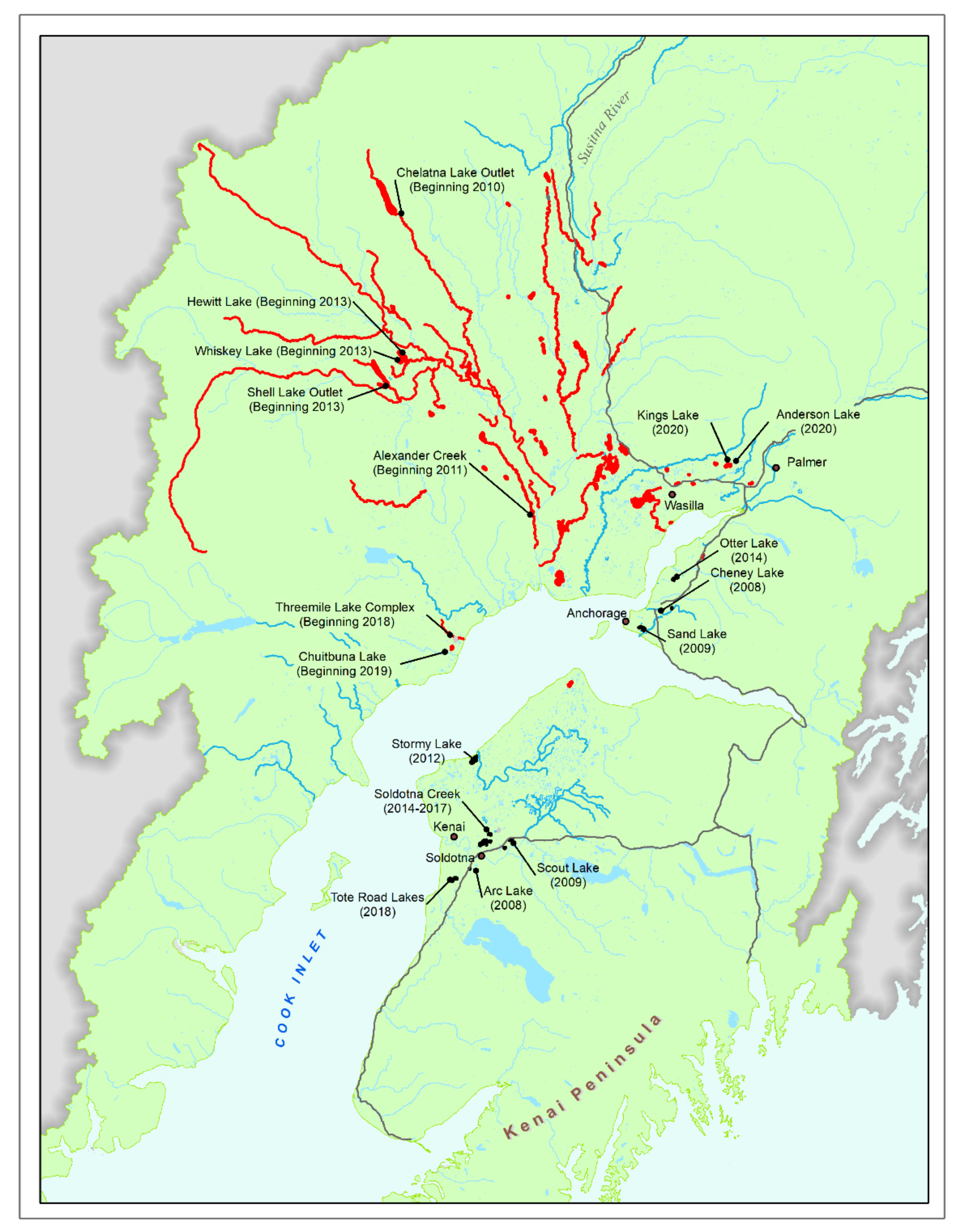
3. Management Approaches
3.1. Population Suppression
3.2. Population Eradication
3.3. Outreach and Angler Engagement
3.4. Population Monitoring and Research
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Questions/Criteria | Priority Level (Low, Medium, High) | Weighted** Score | Enter 1 or 0 (Yes = 1) (No = 0) | Score Value |
|---|---|---|---|---|
| Recreational Fisheries | ||||
| Pre-pike introduction, historic fishing level in the water body was low (<200 days) | Low | 1 | 0 | |
| Pre-pike introduction, historic fishing level in the water body was medium (>200–<1000 days) | Medium | 5 | 0 | |
| Pre-pike introduction, historic fishing level in the water body was high (>1000 days) | High | 10 | 0 | |
| Is a goal of the project to r restore opportunity for a non-pike fishery? | Medium | 5 | 0 | |
| Is the water body currently and/ or formerly stocked by ADFG? | Medium | 5 | 0 | |
| Have stocking levels in the water body been altered because of pike presence? | Medium | 5 | 0 | |
| Pike Impacts | ||||
| Do pike in the water body directly threaten a wild fishery in that water? | High | 10 | 0 | |
| Do pike in the water body threaten a wild fishery that is in close proximity (five miles or less)? | High | 10 | 0 | |
| Have regulations for wild sport fisheries exceeding 1000 angler-days been restricted because of pike in this waterbody? | High | 10 | 0 | |
| Have wild sport fisheries receiving under 1000 angler-days of effort been restricted because of pike in this waterbody? | Low | 1 | 0 | |
| Have available data indicated that a wild fish population has been eliminated associated with pike presence? | High | 10 | 0 | |
| Have available data indicated that a wild fish population has been impacted associated with pike presence? | Medium | 5 | 0 | |
| Has the public indicated concern over the pike population in this water body? | Medium | 5 | 0 | |
| Do pike represent >50% of the catch in a netting survey or from other available data? | High | 10 | 0 | |
| Do pike represent 25–50% of the catch in a netting survey or from other available data? | Medium | 5 | 0 | |
| Do pike represent <25% of the catch in a netting survey or from other available data? | Low | 1 | 0 | |
| Questions/Criteria | Priority Level (Low, Medium, High) | Weighted** Score | Enter 1 or 0 (Yes = 1) (No = 0) | Score Value |
| Does the area management biologist expect a negative impact to a sport fishery associated with pike in this waterbody? | High | 10 | 0 | |
| Does the area management biologist expect an imminent loss of a wild stock associated with pike in this drainage? | Very High | 30 | 0 | |
| Does the area management biologist associate pike with an inability to meet an escapement goal in this drainage? | High | 10 | 0 | |
| Does this water body contain a Board of Fisheries-stock of yield or management concern? | High | 10 | 0 | |
| Eliminating pike in this project area removes the pike threat in the entire management area | Very High | 30 | 0 | |
| Education and Outreach | ||||
| Are there opportunities to use this project as an educational outreach tool to increase public awareness? | High | 10 | 0 | |
| Are we demonstrating a pike control strategy to stakeholders? | Medium | 5 | 0 | |
| Does the project foster public understanding and awareness of invasive species | Medium | 5 | 0 | |
| Has there already been stakeholder input desiring a project of this nature? | High | 10 | 0 | |
| Habitat Significance | ||||
| Is the project area within an open system? | High | 10 | 0 | |
| If successful, can this project prevent pike distribution throughout the drainage? | Very High | 30 | 0 | |
| Is the project area within an anadromous system? | High | 10 | 0 | |
| Are wild, resident fish species present? | Medium | 5 | 0 | |
| If in a closed system, does the project have the potential to reduce native fish populations? | Medium | −5 | 0 | |
| Is the project designed to improve habitat for threatened or endangered species populations? | High | 10 | 0 | |
| Project Area Characterization (Type 1—Suitable pike habitat, Type 2—Marginally suitable habitat, Type 3—Poor pike habitat) | ||||
| (Lakes/Wetlands) | ||||
| Is it a Type 1 Lake or wetland—Eutrophic and primarily shallow (<15 feet) with abundant vegetation throughout? | High | 10 | 0 | |
| Questions/Criteria | Priority Level (Low, Medium, High) | Weighted** Score | Enter 1 or 0 (Yes = 1) (No = 0) | Score Value |
| Is it a Type 2 Lake—Mesotrophic and primarily deep (>15 feet) with vegetation covering 50% or more of the lake? | Medium | 5 | 0 | |
| Is it a Type 3 Lake—Oligotrophic and primarily deep (>15 feet) with wither sparse or no aquatic vegetation)? | Low | 1 | 0 | |
| (Rivers and Streams)*** | ||||
| Is the waterbody primarily Type 1—Low stream slope (0.0–0.5%) with abundant vegetation and is capable of supporting rearing coho (i.e., Moose River, Alexander River)? | High | 10 | 0 | |
| Is the waterbody primarily Type 2 with some type 1—Moderate stream slope (0.51–2.0%) with semi-permanent woody debris and back-waters sloughs and is capable of supporting rearing coho (i.e., Deshka River)? | Medium | 5 | 0 | |
| Is the waterbody primarily Type 2—Moderate stream slope (0.51–2.0%) with semi-permanent woody debris and is capable of supporting rearing coho (i.e., Campbell Creek)? | Medium | 5 | 0 | |
| Is the waterbody a combination of Types 2 & 3—High stream slope (>2.0%) and slow back-water sloughs capable of supporting rearing coho (i.e., Willow Creek?) | Medium | 5 | 0 | |
| Is the waterbody primarily Type 3—Clear, high stream slope (>2.0%) with few slower back-waters (i.e., the Little Susitna River)? | Low | 1 | 0 | |
| Is the waterbody exclusively Type 3—High stream slope (>2.0%) with extensive glacial turbidity (i.e., Klutina River)? | Low | 1 | 0 | |
| Cultural Significance | ||||
| Are native cultural activities (i.e., fish camps, etc.) threatened by pike in this water body? | High | 10 | 0 | |
| Does the project benefit subsistence fisheries? | High | 10 | 0 | |
| Is a goal of the project to provide economic benefits for citizens, communities, or industries? | High | 10 | 0 | |
| Does the area manager believe that user groups are negatively affected by the pike presence? | High | 10 | 0 | |
| Economic Impacts | ||||
| Questions/Criteria | Priority Level (Low, Medium, High) | Weighted** Score | Enter 1 or 0 (Yes = 1) (No = 0) | Score Value |
| Has the area manager received input from local businesses/property owners that they are experiencing a negative financial effect from pike in this water body? | High | 10 | 0 | |
| Does the project protect commercially important species? | High | 10 | 0 | |
| Research | ||||
| Will project goals, objectives, and tasks within the project plan strive to improve understanding of pike behavior or distribution in local waters? | Medium | 5 | 0 | |
| Do project goals strive to improve understanding of control or eradication techniques for pike? | High | 10 | 0 | |
| Will the project include a follow-up assessment to measure the effects of the management action? | Medium | 5 | 0 | |
| Do project goals strive to quantify fishery/economic losses resulting from invasive pike? | Medium | 5 | 0 | |
| Feasibility | ||||
| Does the water body have public access? | High | 10 | 0 | |
| Is the water body on the road system? | Low | 1 | 0 | |
| Is it technically feasible that the pike population could be permanently removed or contained if the project is implemented? | High | 10 | 0 | |
| Is there a history of reintroductions of pike in this area? | High | −10 | 0 | |
| Is the project designed to achieve program goals within the funding period? | Low | 1 | 0 | |
| Does the project achieve long term program goals within a decade of the funding period? (i.e., reestablish wild fish populations) | High | 10 | 0 | |
| Can the project begin when funding is received? | Medium | 5 | 0 | |
| The goal of the project is to have a measurable, positive outcome for fisheries. | High | 10 | 0 | |
| Permitting and Inter-Agency Cooperation | ||||
| Does the project provide opportunities to partner or collaborate with other agencies or organizations? | Low | 1 | 0 | |
| Is the NEPA process required for this project? | Low | −1 | 0 | |
| Is there reason to believe there would be a conflict with an existing coastal, watershed or restoration plan? | High | −10 | 0 | |
| ADFG Significance | ||||
| Is the project programmatically/ scientifically aligned with ADF&G’s mission in the Sport Fish Division Strategic Plan? | High | 10 | 0 | |
| SCORE |
References
- Havel, J.E.; Kovalenko, K.E.; Thmoaz, S.M.; Amalifitano, S.; Kats, L.B. Aquatic invasive species: Challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.R.; Gozlan, R.W.; Copp, G.H. Managing non-native fish in the environment. Fish Fish. 2011, 12, 256–274. [Google Scholar] [CrossRef]
- Ricciardi, A.; Hoopes, M.F.; Marchetti, M.P.; Lockwood, J.L. Progress toward understanding the ecological implacts of non-native species. Ecol. Monogr. 2013, 8, 263–282. [Google Scholar] [CrossRef] [Green Version]
- Simberloff, D. Invasive species. In Conservation Biology for All; Sodhi, N.S., Ehrich, S.R., Eds.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Dunker, K.J.; Sepulveda, A.; Massengill, R.; Rutz, D. The northern pike, a prized native but disastrous invasive. In Biology and Ecology of Pike; Skov, C., Nilsson, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 356–398. [Google Scholar]
- Lever, C. Naturalized Fishes of the World; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Welcomme, R.L. International Introductions of Inland Aquatic Species; TP No. 294; Food and Agriculture Organization of the United Nations: Rome, Italy, 1988; pp. 112–113. [Google Scholar]
- Inskip, P.D. Habitat Suitability Index Models: Northern Pike; FWS/OBS 82/10.17; United States Fish and Wildlife Service: Daphne, AL, USA, 1982.
- Persson, A.P.A.N.; Brönmark, C. Trophic interactions. In Biology and Ecology of Pike; Skov, C., Nilsson, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 185–211. [Google Scholar]
- Heins, D.C.; Knoper, H.; Baker, J.A. Consumptive and non-consumptive effects of predation by introduced northern pike on life history traits in threespine stickleback. Evol. Ecol. Res. 2016, 17, 355–372. [Google Scholar]
- Byström, P.; Karlsson, J.; Nilsson, P.A.; Van Kooten, T.; Ask, J.; Olofsson, F. Substitution of top predators:effects of pike invasion in a subarctic lake. Freshw. Biol. 2007, 52, 1271–1280. [Google Scholar] [CrossRef]
- Sepulveda, A.J.; Rutz, D.S.; Dupuis, A.W.; Shields, P.A.; Dunker, K.J. Introduced northern pike consumption of salmonids in Southcentral Alaska. Ecol. Freshw. Fish 2014. [Google Scholar] [CrossRef]
- McKinley, T. Survey of Northern Pike in Lakes of Soldotna Creek Drainage, 2002; Special Publication No. 13-02; Alaska Department of Fish and Game: Anchorage, AK, USA, 2013.
- Beck, K.G.; Zimmerman, K.; Schardt, J.D.; Stone, J.; Lukens, R.R.; Reichard, S.; Randall, J.; Cangelosi, A.A.; Cooper, D.; Thompson, J.P. Invasive species defined in a policy context: Recommendations from the federal invasive species advisory committee. Invasive Plant Sci. Manag. 2008, 1, 414–421. [Google Scholar] [CrossRef]
- Nilsson, P.A.; Eklöv, P. Finding food and staying alive. In Biology and Ecology of Pike; Skov, C., Nilsson, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 9–31. [Google Scholar]
- Sepulveda, A.J.; Rutz, D.S.; Ivey, S.S.; Dunker, K.J.; Gross, J.A. Introduced northern pike predation on salmonids in Southcentral Alaska. Ecol. Freshw. Fish 2013. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, L.; Engström-Öst, J. Coping with environments; vegetation, turbidity and abiotics. In Biology and Ecology of Pike; Skov, C., Nilsson, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 32–61. [Google Scholar]
- Craig, J.F. Pike: Biology and Exploitation; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Raat, A.J.P. Synopsis of Biological Data on the Northern Pike Esox Lucius Linneaeus, 1758; FAO Fisheries Synopsis No. 30 Rev. 2; Food and Agriculture Organization of the United Nations: Rome, Italy, 1988. [Google Scholar]
- Haugen, T.O.; Vøllestad, L.A. Pike population size and structure: Influence of density-dependent and density-independent factors. In Biology and Ecology of Pike; Skov, C., Nilsson, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 123–163. [Google Scholar]
- Mann, R.H.K. The numbers and production of pike (Esox lucius) in two Dorset rivers. J. Anim. Ecol. 1980, 49, 899–915. [Google Scholar] [CrossRef]
- Arlinghaus, R.J.A.; Beardmore, B.; Diaz, A.M.; Hühn, D.; Johnston, F.; Klefoth, T.; Kuparinen, A.; Matsumura, S.; Pagel, T.; Pieterek, T.; et al. Recreational piking—Sustainably managing pike in recreational fisheries. In Biology and Ecology of Pike; Skov, C., Nilsson, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 288–336. [Google Scholar]
- Kuparinen, A.H.L. Northern pike commercial fisheries, stock assessment and aquaculture. In Biology and Ecology of Pike; Skov, C., Nilsson, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 337–355. [Google Scholar]
- McMahon, T.E.; Bennet, D.H. Walleye and northern pike: Boost or bane to northwest fisheries. Fisheries 1996, 21, 6–13. [Google Scholar] [CrossRef]
- Zelasko, K.A.; Bestgen, K.R.; Hawkins, J.A.; White, G.C. Evaluation of a long-term predator removal program: Abundance and population synamics of invasive northern pike in the Yampa River, Colorado. Trans. Am. Fish. Soc. 2016, 145, 1153–1170. [Google Scholar] [CrossRef]
- Harvey, S.; Bean, N. Non-Native Fish Suppression Project, May 2018–April 2019 Annual Report; Project Number 2007-149-00; Kalispel Tribe of Indians: Usk, WA, USA, 2019; p. 68. [Google Scholar]
- Walrath, J.D.; Quist, M.C.; Firehammer, J.A. Trophic ecology of nonnative northern pike and thier effect on conservation of native westslope cutthroat trout. N. Am. J. Fish. Manag. 2015, 35, 158–177. [Google Scholar] [CrossRef]
- Muhlfeld, C.C.; Bennett, D.H.; Steinhorst, R.K.; Marotz, B.; Boyer, M. Using bioenergetics modeling to estiamte consumption of native juvenile salmonids by nonnative northern pike in the Upper Flathead River system, Montana. N. Am. J. Fish. Manag. 2008, 28, 636–648. [Google Scholar] [CrossRef]
- Mecklenburg, C.W.; Mecklenburg, T.A.; Thorsteinson, L.K. Fishes of Alaska; American Fisheries Society: Bethesda, MD, USA, 2002; p. 1037. [Google Scholar]
- Morrow, J.E. The Freshwater Fishes of Alaska; Northwest Publishing Company: Anchorage, AK, USA, 1980; p. 248. [Google Scholar]
- Oswood, M.W.; Reynolds, J.B.; Irons, J.G., III; Milner, A.M. Distributions of freshwater fishes in ecoregions and hydroregions of Alaska. J. N. Am. Benthol. Soc. 2000, 19, 405–418. [Google Scholar] [CrossRef]
- Seeb, J.E.; Seeb, L.W.; Oates, D.W.; Utter, F.M. Genetic nvariation and postglacial dispersal of populations of northern pike (Esox lucius) in North America. Can. J. Fish. Aquat. Sci. 1986, 44, 556–561. [Google Scholar] [CrossRef]
- Haught, S.; von Hippel, F.A. Invasive pike establishment in Cook Inlet Basin lakes, Alaska: Diet, native fish abundance and lake environment. Biol. Invasions 2011, 13, 2103–2114. [Google Scholar] [CrossRef]
- Patankar, R.; von Hippel, F.A.; Bell, M.A. Extinction of a weakly armoured threespine stickleback (Gasterosteus aculteatus) population in Prator Lake, Alaska. Ecol. Freshw. Fish 2006, 15, 482–487. [Google Scholar] [CrossRef]
- Jalbert, C.S. Impacts of a Top Predator (Esox lucius) on Salmonids in Southcentral Alaska: Genetics, Connectivity, and Vulnerability. Master’s Thesis, University of Alaska Fairbanks, School of Fisheries and Ocean Sciences, Fairbanks, AK, USA, 2018. [Google Scholar]
- Spens, J.; Ball, J.P. Salmonid or nonsalmonid lakes: Predicting the fate of northern boreal fish communities with hierarchical filters relating to a keystone piscivore. Can. J. Fish. Aquat. Sci. 2008, 65, 1945–1955. [Google Scholar] [CrossRef]
- Baker, B. Fishery Management Report for Recreational Fishereies in the Tanana River Management Area, 2017, Alaska; Fishery Management Report No. 18-33; Alaska Department of Fish and Game: Anchorage, AK, USA, 2018; p. 92.
- Stuby, L. Fishery Management Report for Sport Fisheries in the Yukon Management Area, 2017, Alaska; Fishery Management Report No. 18-30; Alaska Department of Fish and Game: Anchorage, AK, USA, 2018; p. 62.
- Rutz, D.S. Movements, Food Availability and Stomach Contents of Northern Pike Selected Susitna River Drainages, 1996–1997.; Fishery Data Series No. 99-5; Alaska Department of Fish and Game: Anchorage, AK, USA, 1999; p. 78.
- Rutz, D.S. Seasonal Movements, Age, and Size Statistics, and Food Habits of Upper Cook Inlet Northern Pike during 1994 and 1995; Fishery Data Series No. 96-29; Alaska Department of Fish and Game: Anchorage, AK, USA, 1996; p. 65.
- Glick, W.J.; Willette, T.M. Relative Abundance, Food Habits, Age, and Growth of Northern Pike in 5 Susitna River Drainage Lakes, 2009–2012; Fishery Data Series No. 16-34; Alaska Department of Fish and Game: Anchorage, AK, USA, 2016; p. 55.
- Eklöv, P.; Hamrin, S.F. Predatory efficiency and prey selection: Interactions between pike, Esox lucius, perch Perca fluvialtilis and rudd Scardinus erythrophthalmus. Oikos 1989, 56, 149–156. [Google Scholar] [CrossRef]
- Rutz, D.S.; Bradley, P.T.; Jacobson, C.; Dunker, K.J. Alexander Creek Northern Pike Suppression, 2011–2018, Alaska; Fishery Data Series No. 20-17; Alaska Department of Fish and Game: Anchorage, AK, USA, 2020; p. 34.
- Rutz, D.S.; Dunker, K.J.; Bradley, P.T.; Jacobson, C. Movement Patters of Northern Pike in Alexander Lake; Fishery Data Series No. 20-16; Alaska Department of Fish and Game: Anchorage, AK, USA, 2020; p. 50.
- Massengill, R. Stormy Lake Northern Pike Distribution and Movement Study to Inform Future Eradication Efforts; Special Publication No. 17-17; Alaska Department of Fish and Game: Anchorage, AK, USA, 2017; p. 58.
- Mills, M.J. Alaska Statewide Sport Fish Harvest Studies; Federal Aid in Fish Restoration, Annual Performance Report 1985–1986, Project F-10-1(27) RT-2; Alaska Department of Fish and Game: Juneau, AK, USA, 1986.
- Oslund, S.; Ivey, S.; Lescanec, D. Area Management Report for the Sport Fishereis of Northern Cook Inlet, 2017–2018; Fishery Management Report No. 20-04; Alaska Department of Fish and Game: Anchorage, AK, USA, 2020; p. 322.
- ADFG Chinook Salmon Research Team. Chinook Salmon Assessment and Research Plan; Special Publication No. 13-01; Alaska Department of Fish and Game: Anchorage, AK, USA, 2013; p. 62.
- Romberg, W.J.; Raffety, I.; Martz, M. Estimates of Participation, Catch, and Havest in Alaska Sport Fisheries; Fishery Data Series No. In Prep; Alaska Department of Fish and Game: Anchorage, AK, USA, 2020; in prep.
- Catchcart, C.N.; Dunker, K.J.; Quinn, T.P.; Sepulveda, A.J.; von Hippel, F.A.; Wizik, A.; Young, D.B.; Westley, P.A.H. Trophic plasticity and the invasion of a renowned piscivore: A diet synthesis of northern pike (Esox lucius) from the native and introduced ranges in Alaska, U.S.A. Biol. Invasions 2019, 21, 1379–1392. [Google Scholar] [CrossRef]
- Chihuly, M.B. Biology of the Northern Pike, Esox lucius Linneaus, in the Wood Lakes System of Alaska, with Emphasis on Lake Alegnagik. Master’s Thesis, University of Alaska Fairbanks, College of Environmental Sciences, Fairbanks, AK, USA, 1979. [Google Scholar]
- Dye, J.; Wallendorf, M.; Naughton, G.P.; Gryska, A.D. Stock Assessment of Northern Pike in Lake Aleknagik, 1998–1999; Fishery Data Series No. 02-14; Alaska Department of Fish and Game: Anchorage, AK, USA, 2002; p. 18.
- Wuttig, K.G. Estimated Abundance of Northern Pike in Harding Lake, 2012; Fishery Data Series No. 15-39; Alaska Department of Fish and Game: Anchorage, AK, USA, 2012; p. 32.
- Russell, R. A Fisheries Inventory of Waters in the Lake Clark National Monument Area; Alaska Department of Fish and Game: Anchorage, AK, USA, 1980; p. 207.
- Schwanke, C.J. Abundance, Length Composition, and Movement of the Northern Pike Populations in Long Lake of the Chulitna River Dainage, 2007–2010; Fishery Data Sereies No. 12-23; Alaska Department of Fish and Game: Anchorage, AK, USA, 2012; p. 32.
- Massengill, R. Control Efforts for Invasive Northern Pike on the Kenai Peninsula, 2005–2006; Fishery Data Series No. 10-05; Alaska Department of Fish and Game: Anchorage, AK, USA, 2010; p. 35.
- Massengill, R. Control Efforts for Invasive Northern Pike Esox lucius on the Kenai Peninsula, 2007; Fishery Data Series No. 11-10; Alaska Department of Fish and Game: Anchorage, AK, USA, 2011; p. 49.
- Massengill, R. Control Efforts for Invasive Northern Pike on the Kenai Peninsula, 2009; Special Publication No. 14-11; Alaska Department of Fish and Game: Anchorage, AK, USA, 2014; p. 84.
- Massengill, R. Control Efforts for Invasive Northern Pike on the Kenai Peninsula, 2008; Special Publication No. 14-12; Alaska Department of Fish and Game: Anchorage, AK, USA, 2014; p. 74.
- Massengill, R. Stormy Lake Restoration: Invasive Northern Pike Eradication, 2012; Special Publication No. 17-18; Alaska Department of Fish and Game: Anchorage, AK, USA, 2017; p. 98.
- ADFG Southcentral Northern Pike Control Committee. Management Plan for Invasive Northern Pike in Alaska; Alaska Department of Fish and Game: Anchorage, AK, USA, 2007; p. 62.
- Fay, V. Alaska Aquatic Nuisance Species Management Plan; Alaska Department of Fish and Gmae: Juneau, AK, USA, 2002; p. 116.
- Syslo, J.M.; Guy, C.S.; Bigelow, P.E.; Doepke, P.D.; Ertel, B.D.; Koel, T.M. Response of non-native lake trout (Salvelinus namaycush) to 15 years of harvest in Yellowstone Lake, Yellowstone National Park. Can. J. Fish. Aquat. Sci. 2011, 68, 2132–2145. [Google Scholar] [CrossRef] [Green Version]
- Syslo, J.M.; Guy, C.S.; Cox, B.S. Comparison of harvest scenarios for the cost-effective supression of lake trout in Swan Lake, Montana. N. Am. J. Fish. Manag. 2013, 33, 1079–1090. [Google Scholar] [CrossRef]
- Cook Inlet Staff. Alexander Creek King Salmon Stock Status and Action Plan, 2020; Report to the Board of Fisheries; Alaska Department of Fish and Game: Anchorage, AK, USA, 2020; p. 29.
- Bradley, P.; Jacobson, C.; Dunker, K. Operational Plan: Alexander Creeknorthern Pike Suppression 2019–2021; Regional Operational Plan ROF.SF.2A2019.04; Alaska Department of Fish and Game: Anchorage, AK, USA, 2019; p. 31.
- King, B.E.; Walker, S.C. Susitna River Sockeye Salmon Fry Studies, 1994 and 1995; Alaska Department of Fish and Game: Anchorage, AK, USA, 1997; p. 58.
- Courtney, M.B.; Schoen, E.R.; Wizik, A.; Westley, P.A.H. Quantifying the net benefits of suppression: Truncated size structure and consuption of native salmonids by invasive northern pike in an Alaska lake. N. Am. J. Fish. Manag. 2018, 38, 1306–1315. [Google Scholar] [CrossRef]
- DeCino, R.; Willette, T.M. Chelatna Lake Northern Pike Suppression Project 2017–2019; Regional Operational Plan ROP.CF.4K2017.11; Alaska Department of Fish and Game: Soldotna, AK, USA, 2017; p. 22.
- Tarbox, K.E.; Kyle, G.B. An Estimate of Adult Sockeye Salmon (Oncorhynchus Nerka) Production, Based on Euphotic Volume, for the Susitna River Drainage, Alaska; Alaska Department of Fish and Game: Anchorage, AK, USA, 1989; p. 59.
- Wizik, A. Shell Lake Sockeye Salmon Progress Report 2015; Cook Inlet Aquaculture Association: Soldotna, AK, USA, 2015; p. 49. [Google Scholar]
- Wizik, A.; Schoen, E.; Courtney, M.; Westley, P. Shell Lake Sockeye Salmon Progress Report 2017; Cook Inlet Aquaculture Association: Soldotna, AK, USA, 2017; p. 63. [Google Scholar]
- Wizik, A. Shell Lake Sockeye Salmon Progress Report 2018; Cook Inlet Aquaculture Association: Soldotna, AK, USA, 2018; p. 59. [Google Scholar]
- DeCino, R.; Willette, T.M. Chelatna Lake Pike Suppression; Fishery Data Series No. In Prep; Alaska Department of Fish and Game: Anchorage, AK, USA, 2020; in prep.
- Yanusz, R.J.; Merizon, R.A.; Willette, T.M.; Evans, D.G.; Spencer, T.R.; Raborn, S. Inriver Abundance and Spawning Distribution of Susitna River Sockeye Salmon Oncorhynchus Nerka, 2006; Fishery Data Series 07-83; Alaska Department of Fish and Game: Anchorage, AK, USA, 2007; p. 68.
- Yanusz, R.J.; Merizon, R.A.; Willette, T.M.; Evans, D.G.; Spencer, T.R. Inriver Abundance and Istribution of Spawning Susitna River Sockeye Salmon Oncorhynchus Nerka, 2007; Fishery Data Series No. 11-19; Alaska Department of Fish and Game: Anchorage, AK, USA, 2011; p. 50.
- Yanusz, R.J.; Merizon, R.A.; Willette, T.M.; Evans, D.G.; Spencer, T.R. Inriver Abundance and Spawning Distribution of Spawning Susitina River Sockeye Salmon Onchorhynchus Nerka, 2008; Fishery Data Series No. 11-12; Alaska Department of Fish and Game: Anchorage, AK, USA, 2011; p. 44.
- DeCino, R.; Willette, T.M. Susitna River Drainage Lakes Pelagic Fish Estimates, Using Split-Beam Hydroacoustic and Midwater Trawl Sampling Techniques, 2005–2008; Fishery Data Series No. 14-47; Alaska Department of Fish and Game: Anchorage, AK, USA, 2014; p. 58.
- Smukall, M. Whiskey Lake Salmon Smolt Progress Report, 2014; Cook Inlet Aquaculture Association: Soldotna, AK, USA, 2015; p. 33. [Google Scholar]
- Smukall, M. Northern Pike Investigations Final Report (2012–2014); Cook Inlet Aquaculture Association: Soldotna, AK, USA, 2015; p. 69. [Google Scholar]
- Dunker, K.; Bradley, P.; Jacobson, C. Threemile Lake Invasive Northern Pike Population Assessment; Regional Operational Plan SF.2A.2018.11; Alaska Department of Fish and Game: Anchorage, AK, USA, 2018; p. 38.
- Bradley, P.; Jacobson, C.; Dunker, K. Operational Plan: Threemile Lake Invasive Northern Pike Suppression and Chuitbuna Lake Population Assessment; Regional Operational Plan SF.2A.2020.01; Alaska Department of Fish and Game: Anchorage, AK, USA, 2019; p. 28.
- Larsson, P.; Tibblin, P.; Koch-Schmidt, P.; Engstedt, O.; Nilsson, J.; Nordahl, O.; Forsman, A. Ecology, evolution, and management strategies of northern pike populations in the Baltic Sea. Ambio 2015, 44, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, B.D.; Limburg, K.E. The use of otolith chemistry to characterize diadrmous migrations. J. Fish Biol. 2012, 81, 796–825. [Google Scholar] [CrossRef]
- Britton, J.R.; Brazier, M.; Davies, G.D.; Chare, S.I. Case studies on eradicating the Asiatic cyprinid Pseudorasbora parva from fishing lakes in England to prevent their riverine dispersal. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 867–876. [Google Scholar] [CrossRef]
- Knapp, R.A.; Matthews, K.R. Eradication of nonnative fish by gillnetting from a small mountain lake in California. Restor. Ecol. 1998, 6, 207–213. [Google Scholar] [CrossRef]
- Massengill, R. Soldotna Creek Drainage Restoration: Invasive Northern Pike Eradication, 2014–2018; Special Publication No. In Prep; Alaska Department of Fish and Game: Anchorage, AK, USA, 2020; in prep.
- Finlayson, B.; Skaar, D.; Anderson, J.; Carter, J.; Duffield, D.; Flammang, M.; Jackson, C.; Overlock, J.; Steinkjer, J.; Wilson, R. Planning and Standard Operating Procedures for the Use of Rotenone in Fish Management—Rotenone SOP Manual, 2nd ed.; American Fisheries Society: Bethesda, MD, USA, 2018; p. 176. [Google Scholar]
- USEPA. Reregistration Eligibility Decision for Rotenone; Document EPA 738-R-07-005; United States Environmental Protection Agency: Washington, DC, USA, 2007; p. 45.
- Ling, N. Rotenone—A Review of Its Toxicity and Use for Fisheries Management; Science for Conservation 211; New Zealand Department of Conseration: Wellington, New Zealand, 2003; p. 40.
- Dalu, T.; Wasserman, R.J.; Jordaan, M.; Froneman, W.P.; Wey, O.L.F. An assessment of the effect of rotenone on selected non-target aquatic fauna. PLoS ONE 2015, 10(11), e0142140. [Google Scholar] [CrossRef] [Green Version]
- Dunker, K.J.; Sepulveda, A.J.; Massengill, R.L.; Olsen, J.B.; Russ, O.L.; Wenburg, J.K.; Antonovich, A. Potential of environmental DNA to evaluate northern pike (Esox lucius) eradication efforts: An experimental test and case study. PLoS ONE 2016, 11(9), e0162277. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda, A.J.; Hutchins, P.R.; Massengill, R.L.; Dunker, K.J. Tradeoffs of a portable, field-based environmental DNA platform for detecting invasive northern pike (Esox lucius) in Alaska. Manag. Biol. Invasions 2018, 9, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Massengill, R.; Begich, L.R.; Dunker, K. Kenai Peninsula Invasive Northern Pike Monitoring and Native Fish Restoration; Regional Operational Plan SF.2A.2020.02; Alaska Department of Fish and Game: Anchorage, AK, USA, 2018.
- ADF&G. Statewide Stocking Plan for Sport Fish, 2020–2024; Alaska Deparment of Fish and Game: Anchorage, AK, USA, 2020; p. 26.
- Bell, M.A.; Heins, D.C.; Wund, M.A.; von Hippel, F.A.; Massengill, R.; Dunker, K.; Bristow, G.A.; Aquirre, W.E. Reintroduction of threespine stickleback into Cheney and Scout Lakes, Alaska. Evol. Ecol. Res. 2016, 17, 157–178. [Google Scholar]
- Wund, M.A.; Singh, O.D.; Geiselman, A.; Bell, M.A. Morphological evolution of an anadromous threespine stickleback population within one generation after reintroduction to Cheney Lake, Alaska. Evol. Ecol. Res. 2016, 17, 203–224. [Google Scholar]
- Kurz, M.L.; Heins, D.C.; Bell, M.A.; von Hippel, F.A. Shifts in life-history traits of two introduced populations of threespine stickleback. Evol. Ecol. Res. 2016, 17, 225–242. [Google Scholar]
- Parkes, J.P.; Panetta, F.D. Eradication of invasive species: Progress and emerging issues in the 21st century. In Invasive Speces Management: A Handbook of Principles and Techniques; Oxford University Press: Oxford, UK, 2009; pp. 44–72. [Google Scholar]
- Post, J.R.; Sullivan, M.; Cox, S.; Lester, N.P.; Walters, C.J.; Parkinson, E.A.; Paul, A.J.; Jackson, J.; Shuter, B.J. Canada’s recreational fishereis: The invisible collapse? Fisheries 2002, 27, 6–17. [Google Scholar] [CrossRef]
- Pierce, R.B.; Tomcko, C.M.; Schupp, D.H. Exploitation of northern pike in seven small north-central Minnesota lakes. N. Am. J. Fish. Manag. 1995, 15, 601–609. [Google Scholar] [CrossRef]
- Pansko, S.; Goldberg, J. Review of harvest incentives to control invasive species. Manag. Biol. Invasions 2014, 5, 263–277. [Google Scholar] [CrossRef] [Green Version]
- Olsen, J.B.; Lewis, C.J.; Massengill, R.L.; Dunker, K.J.; Wenburg, J.K. An evaluation of target specificity and sensitivity of three qPCR assays for detecting environmental DNA from Northern Pike (Esox lucius). Conserv. Genet. Resour. 2015. [Google Scholar] [CrossRef]
- Schill, D.J.; Heindel, J.A.; Campbell, M.R.; Meyer, K.A.; Mamer, E.R.J.M. Production of a YY male brook trout broodstock for potential eradication of undesired brook trout populations. N. Am. J. Aquac. 2016, 78, 72–83. [Google Scholar] [CrossRef]
- Schill, D.J.; Meyer, K.A.; Hansen, M.J. Simulated effects of YY-male stocking and manual suppression for eradicating nonnative brook trout populations. N. Am. J. Aquac. 2017, 37, 1054–1066. [Google Scholar] [CrossRef]
- Thresher, R.E. Autocideal technoogy for the control of invasive fish. Fisheries 2008, 33, 114–121. [Google Scholar] [CrossRef]
- Thresher, R.W.; Hayes, K.; Bax, N.J.; Teem, J.; Benfey, T.J.; Gould, F. Genetic control of invasive fish: Technological options and its role in integrated pest management. Biol. Invasions 2014, 16, 1201–1216. [Google Scholar] [CrossRef]







| Year | Water Body | Location | Volume (ha-m) a | Quantity b | Rotenone Concentration mg/L (Target /Actual) | Detoxification Time | Application Method | Species Reintroduced c |
|---|---|---|---|---|---|---|---|---|
| 2008 | Arc Lake | Soldotna | 17.8 | 181.7 L CFT | 0.050/0.035 | 8 months | Boat | SS, ST |
| 2008 | Cheney Lake | Anchorage | 21.6 | 219.6 L CFT | 0.050/0.030 | 5 months | Boat, Backpack Spray | RT, GR, SS, ST |
| 2009 | Scout Lake | Sterling | 103.0 | 700.3 L CFT + 499.0 kg Powder | 0.070/0.030 | 8 months | Boat, Backpack Spray | RT, GR, SS, ST |
| 2009 | Sand Lake | Anchorage | 140.4 | 1348 L CFT | 0.050/0.030 | 7 months | Boat | RT, GR, SS, AC |
| 2012 | Stormy Lake | Nikiski | 858.3 | 3444.7 L CFT + 3500 kg Powder | 0.050/0.048 | 4 months | Boat (Weighted Hose), Airboat, Backpack Spray, Deactivation | RT, AC, SS, LS |
| 2014 | Union Lake | Soldotna | 88.7 | 327.1 L CFT + 504.8 kg Powder | 0.050/0.024 | 8 months | Boat, Backpack Spray | RT, SS, DV, SC, ST |
| 2014 | East Mackey Lake | Soldotna | 115.6 | 424 L CFT + 655.4 kg Powder | 0.050/0.026 | 6 months | Boat, Backpack Spray | RT, SS, DV, SC, ST |
| 2014 | West Mackey Lake | Soldotna | 150.5 | 564 L CFT + 902.6 kg Powder | 0.050/0.024 | 8 months | Boat, Backpack Spray | RT, SS, DV, SC ST |
| 2014 | Derks Lake | Soldotna | 56.4 | 206.3 L CFT + 226.8 kg Powder | 0.050/0.024 | 6 months | Boat, Airboat, Backpack Spray, Deactivation | RT, SS, DV, SC, ST |
| 2015 | Otter Lake | JBER | 110.2 | 1400.6 L. CFT + 22.7 kg Powder | 0.050/0.024 | 4 months | Boat, Airboat, Backpack Spray, Drip Stations | RT |
| 2016/17 | Sevena Lake | Soldotna | 74.0 | 605.7 L CFT | 0.040/0.036 | 10 days | Boat, Airboat, Backpack Spray | RT, SS, DVSC, ST |
| 2016 | Soldotna Creek | Soldotna | - | 191.5 L. CFT | 0.040/0.036 | 5 days | Helicopter, Drip Stations, Backpack Sprayers, ATV, Deactivation | All Species Recolonized from Kenai River |
| 2016 | Loon Lake | Soldotna | 24.4 | 111.7 L CFT + 65.8 kg Powder | 0.040/0.028 | 8 months | Boat | RT |
| 2018 | Hope Lake | Soldotna | 50.1 | 418.3 L CFT | 0.040/0.018 | 3 months | Boat, Backpack Spray | RT, SS, ST |
| 2018 | G Lake | Soldotna | 35.0 | 288.4 L CFT | 0.040/0.028 | 3 months | Boat | RT, SS, ST |
| 2018 | Crystal Lake | Soldotna | 34.2 | 279.7 L CFT | 0.040/0.040 | 3 months | Boat | RT, SS, ST |
| 2018 | Leisure Lake | Soldotna | 15.2 | 124.2 L CFT | 0.040/0.040 | 3 months | Boat | RT, SS, ST |
| 2018 | Leisure Pond | Soldotna | 1.4 | 13.6 L CFT | 0.040/0.024 | 3 months | Boat, Backpack Spray | RT, SS, ST |
| 2018 | Fred’s Lake | Soldotna | 1.9 | 57.5 L CFT | 0.040/0.011 | 3 months | Boat, Backpack Spray | RT, SS, ST |
| 2018 | Ranchero Lake | Soldotna | 5.1 | 42.0 L CFT | 0.040/0.024 | 3 months | Boat, Backpack Spray | RT, SS, ST |
| 2018 | CC Lake | Soldotna | 33.0 | 26.9 L CFT | 0.040/0.026 | 3 months | Boat | RT, SS, ST |
| 2020 | Anderson Lake d | Wasilla | 118.4 | 1211.3 L CFT | 0.040/TBD | TBD | Boat | RT |
| 2020 | King’s Lake d | Wasilla | 107.6 | 1100.0 L CFT | 0.040/TBD | TBD | Boat | RT |
| Year | Lake | Rainbow Trout a | Dolly Varden b | Threespine Stickleback c | Sculpin Spp. d | Coho Salmon e | All Species | Salmonids/Hectare f,g |
|---|---|---|---|---|---|---|---|---|
| 2015 | Derks | 30 | 161 | 950 | 3 | 6107 | 7251 | 68 |
| 2016 | 199 | 217 | 3386 | 229 | 2452 | 6483 | 31 | |
| 2017 | 0 | 0 | 0 | 0 | 38 | 38 | 1 | |
| Total | 229 | 378 | 4,336 | 232 | 8597 | 13,772 | 100 | |
| 2015 | East Mackey | 355 | 366 | 5362 | 960 | 1396 | 8439 | 8 |
| 2016 | 696 | 484 | 4103 | 439 | 6564 | 12,286 | 31 | |
| 2017 | 176 | 436 | 0 | 0 | 2506 | 3118 | 13 | |
| 2018 | 220 | 436 | 0 | 0 | 2506 | 3162 | 13 | |
| Total | 1447 | 1722 | 9465 | 1399 | 12,972 | 27,005 | 65 | |
| 2015 | Union | 195 | 173 | 3532 | 183 | 2173 | 6256 | 12 |
| 2016 | 277 | 407 | 3563 | 419 | 7259 | 11,925 | 38 | |
| 2017 | 38 | 130 | 0 | 0 | 604 | 772 | 4 | |
| 2018 | 35 | 0 | 0 | 0 | 0 | 35 | 1 | |
| Total | 545 | 710 | 7095 | 602 | 10,036 | 18,988 | 55 | |
| 2015 | West Mackey | 354 | 437 | 5553 | 399 | 904 | 7647 | 4 |
| 2016 | 1088 | 1034 | 6401 | 1062 | 13,388 | 22,973 | 34 | |
| 2017 | 203 | 556 | 0 | 0 | 3374 | 4,133 | 9 | |
| 2018 | 679 | 0 | 0 | 0 | 0 | 679 | 2 | |
| Total | 2324 | 2027 | 11,954 | 1461 | 17,666 | 35,432 | 49 | |
| Grand Total | 4545 | 4837 | 32,850 | 3694 | 49,271 | 95,197 | 269 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunker, K.; Massengill, R.; Bradley, P.; Jacobson, C.; Swenson, N.; Wizik, A.; DeCino, R. A Decade in Review: Alaska’s Adaptive Management of an Invasive Apex Predator. Fishes 2020, 5, 12. https://doi.org/10.3390/fishes5020012
Dunker K, Massengill R, Bradley P, Jacobson C, Swenson N, Wizik A, DeCino R. A Decade in Review: Alaska’s Adaptive Management of an Invasive Apex Predator. Fishes. 2020; 5(2):12. https://doi.org/10.3390/fishes5020012
Chicago/Turabian StyleDunker, Kristine, Robert Massengill, Parker Bradley, Cody Jacobson, Nicole Swenson, Andy Wizik, and Robert DeCino. 2020. "A Decade in Review: Alaska’s Adaptive Management of an Invasive Apex Predator" Fishes 5, no. 2: 12. https://doi.org/10.3390/fishes5020012
APA StyleDunker, K., Massengill, R., Bradley, P., Jacobson, C., Swenson, N., Wizik, A., & DeCino, R. (2020). A Decade in Review: Alaska’s Adaptive Management of an Invasive Apex Predator. Fishes, 5(2), 12. https://doi.org/10.3390/fishes5020012