Potential Impact of Climate Change on Fish Reproductive Phenology: A Case Study in Gonochoric and Hermaphrodite Commercially Important Species from the Southern Gulf of Mexico
Abstract
:1. Introduction
2. Regulation of Fish Reproduction
3. Influence of Temperature in Fish Reproduction
4. Effects of Rising Temperature in Fish Reproduction
4.1. Effect on Reproductive Cycle
4.2. Effect on Sexual Determination
4.3. Effect on Sex Change
5. Case Study
5.1. Studied Species
5.2. Potential Change in Female’s Reproductive Cycle
5.3. Potential Change in Population Structure
5.3.1. Effect on Sex Determination
5.3.2. Effect on Sex Change
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Munday, P.L.; Jones, G.P.; Sheaves, M.; Williams, A.J.; Goby, G. Vulnerability of fishes of the Great Barrier Reef to climate change. In Climate Change and the Great Barrier Reef. A Vulnerability Assessment; Johnson, J.E., Marshall, P.A., Eds.; Great Barrier Marine Park Authority and Australian Greenhouse Office: Townsville, Australia, 2007; pp. 357–391. [Google Scholar]
- Pörtner, H.O.; Karl, D.M.; Boyd, P.W.; Cheung, W.W.L.; Lluch-Cota, S.E.; Nojiri, Y.; Schmidt, D.N.; Zavialov, P.O. Ocean systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 411–484. [Google Scholar]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate change and the future for coral reef fishes. Fish Fish. 2008, 9, 261–285. [Google Scholar] [CrossRef]
- Rijnsdorp, A.D.; Peck, M.A.; Engelhard, G.H.; Möllman, C.; Pinnegar, J.K. Resolving the effect of climate change on fish populations. ICES J. Mar. Sci. 2009, 66, 1570–1583. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Peck, M.A. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Farrell, A.P. Physiology and climate change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Demarée, G.R.; This Rutishauser. Origins of the word “Phenology”. Eos Trans. Am. Geophys. Union 2009, 90, 291. [Google Scholar] [CrossRef] [Green Version]
- Puppi, G. Origin and development of phenology as a science. Ital. J. Agrometeorol. 2007, 3, 24–29. [Google Scholar]
- Bruslé, J.; Quignard, J.P. Les poisons et leur environnement. In Écophysiologie et Comportement Adaptifs [Fishes and Their Environment. Ecophysiology and Adaptive Behavior]; Tec & Doc, Ed.; Lavoisier: Paris, France, 2004. [Google Scholar]
- Wootton, R.J.; Smith, C. Reproductive Biology of Teleost Fishes; Wiley Blackwell: Oxford, UK, 2015; p. 469. [Google Scholar]
- Lam, T.J. Environmental influences on gonadal activity in fish. In Fish Physiology; Hoar, W.S., Randall, D.J., Donaldson, E.M., Eds.; Academic Press: New York, NY, USA, 1983; Volume IXB, pp. 65–116. [Google Scholar]
- Pankhurst, N.W.; Porter, M.J.R. Cold and dark or warm and light: Variations on the theme of environmental control of reproduction. Fish Physiol. Biochem. 2003, 28, 385–389. [Google Scholar] [CrossRef]
- Chan, S.T.H.; Yeung, W.S.B. Sex control and sex reversal in fish under natural conditions. In Fish Physiology; Hoar, W.S., Randall, D.J., Donaldson, E.M., Eds.; Academic Press: New York, NY, USA, 1983; Volume IXB, pp. 171–222. [Google Scholar]
- Delvin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). Anuario Estadísitco de Pesca 2014 [Annual Aquaculture and Fisheries Statistics 2014]; National Commission of Aquaculture and Fishery: Mazatlán, Mexico, 2015; Available online: www.gob.mx/conapesca/documentos/anuario-estadistico-de-acuacultura-y-pesca (accessed on 1 June 2018).
- SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). Acuerdo por el que se da a conocer la actualización de la Carta Nacional Pesquera [Agreement to update the Nacional Fisheries Chart]. In Official Diary of the Federation; López-González, A., Ed.; Second Section; Secretaría de Gobernación: Mexico City, Mexico, 2012; Volume DCCVII 18, pp. 21–128. [Google Scholar]
- Burgos, R.; Defeo, O. Long-term population structure, mortality and modeling of a tropical multi-fleet fishery: The red grouper Epinephelus morio of the Campeche Bank, Gulf of Mexico. Fish. Res. 2004, 66, 325–335. [Google Scholar] [CrossRef]
- Arreguín-Sánchez, F.; del Monte Luna, P.; Zetina-Rejón, M.J.; Tripp-Valdez, A.; Albañez-Lucero, M.O.; Ruiz-Barreira, T.M. Building an ecosystems-type fisheries management approach for the Campeche Bank, subarea in the Gulf of Mexico Large Marine Ecosystem. Environ. Dev. 2017, 22, 143–149. [Google Scholar] [CrossRef]
- Nagahama, Y. Endocrine regulation of gametogenesis ion fish. Int. J. Dev. Biol. 1994, 38, 217–229. [Google Scholar] [PubMed]
- Strüssmann, C.A.; Nakamura, M. Morphology, endocrinology, and environmental modulation of gonad sex differentiation in teleost fishes. Fish Physiol. Biochem. 2002, 26, 13–29. [Google Scholar] [CrossRef]
- Frisch, A. Sex-change and gonadal steroids in sequentially-hermaphroditic teleost fish. Rev. Fish Biol. Fish 2004, 14, 481–499. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.F. Ovarian aromatase and estrogens: A pivotal role for gonad sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef]
- Miranda, L.A.; Chalde, T.; Elisio, M.; Strüssmann, C.A. Effects of global warming on fish reproductive endocrine axis, with special emphasis in pejerrey Odontesthes bonariensis. Gen. Comp. Endocrinol. 2013, 192, 45–54. [Google Scholar] [CrossRef]
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From gametogenesis to spawning: How climate-driven warming affects teleost reproductive biology. J. Fish Biol. 2020, 97, 607–632. [Google Scholar] [CrossRef]
- Dahlke, F.T.; Wohlrab, S.; Butzin, M.; Pörtner, H.O. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 2020, 369, 65–70. [Google Scholar] [CrossRef]
- Valenzuela, N.; Adams, D.C.; Janzen, F. Pattern does not equal process: Exactly when is sex environmentally determined? Am. Nat. 2003, 161, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Ospina-Álvarez, N.; Piferrer, F. Temperature-dependent sex determination in fish revisited: Prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE 2008, 3, e2837. [Google Scholar] [CrossRef] [Green Version]
- Penman, D.J.; Piferrer, F. Fish gonadogenesis. Part I: Genetic and environmental mechanisms of sex determination. Rev. Fish. Sci. 2008, 16, 16–34. [Google Scholar] [CrossRef]
- Baroiller, J.F.; Guiguen, Y.; Fostier, A. Endocrine and environmental aspects of sex differentiation in fish. Cell. Mol. Life Sci. 1999, 55, 910–931. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nagahama, Y.; Nakamura, M. Diversity and plasticity of sex determination and differentiation in fishes. Sex. Dev. 2012, 7, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kobayashi, Y.; Miura, S.; Alam, M.A.; Bhandari, R.K. Sex change in coral reef fish. Fish Physiol. Biochem. 2005, 31, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Bhandari, K.; Higa, M. The role estrogens play in sex differentiation and sex changes of fish. Fish Physiol. Biochem. 2003, 28, 113–117. [Google Scholar] [CrossRef]
- García-Cagide, A.; Claro, R.; Koshelev, B.V. Reproductive patterns of fishes of the Cuban shelf. In Ecology of the Marine Fishes of Cuba; Claro, R., Lindeman, K.C., Parenti, L.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2001; pp. 73–114. [Google Scholar]
- Claro, R.; Lindeman, K.C. Biología y Manejo de los Pargos (Lutjanidae) en el Atlántico Occidental [Biology and Management of Snapper (Lutjanidae) in the Western Atlantic]; Instituto de Oceanología, CITMAR: La Habana, Cuba, 2004; Available online: www.redciencia.cu/cdoceano (accessed on 15 June 2018).
- Brulé, T.; Déniel, C.; Colás-Marrufo, T.; Sánchez-Crespo, M. Red grouper reproduction in the southern Gulf of Mexico. Trans. Am. Fish. Soc. 1999, 128, 385–402. [Google Scholar] [CrossRef]
- Brulé, T.; Renán, X.; Colás-Marrufo, T.; Hauyon, Y.; Tuz-Sulub, A.; Déniel, C. Reproduction in the protogynous grouper Mycteroperca bonaci (Poey) from the southern Gulf of Mexico. Fish. Bull. 2003, 101, 463–475. [Google Scholar]
- Brulé, T.; Déniel, C.; Colás-Marrufo, T.; Renán, X. Reproductive biology of gag in the southern Gulf of Mexico. J. Fish Biol. 2003, 63, 1505–1520. [Google Scholar] [CrossRef]
- Brulé, T.; Colás-Marrufo, T.; Pérez-Díaz, E.; Sámano-Zapata, J.C. Red snapper reproductive biology in the southern Gulf of Mexico. Trans. Am. Fish. Soc 2010, 139, 957–968. [Google Scholar] [CrossRef]
- Caballero-Arango, D. Estrategia Reproductiva de tres Especies de Mero (Epinephelus guttatus, Mycteroperca tigris y Mycteroperca venenosa) en Arrecifes Coralinos del Banco de Campeche, México [Reproductive Strategy of Three Grouper Species (Epinephelus guttatus, Mycteroperca tigris and Mycteroperca venenosa) in Campeche Bank Coral Reefs]. Ph.D. Thesis, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Unidad Mérida, Mérida, Mexico, 2013. [Google Scholar]
- Caballero-Arango, D.; Brulé, T.; Nóh-Quiñones, V.; Colás-Marrufo, T.; Pérez-Díaz, E. Reproductive biology of the tiger grouper in the southern Gulf of Mexico. Trans. Am. Fish. Soc. 2013, 142, 282–299. [Google Scholar] [CrossRef]
- Trejo-Martínez, J.; Brulé, T.; Mena-Loría, A.; Colás-Marrufo, T.; Sanchez-Crespo, M. Reproductive aspects of the yellowtail snapper Ocyurus chrysurus from the southern Gulf of Mexico. J. Fish Biol. 2011, 79, 915–936. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Martínez, J.; Brulé, T.; Morales-López, N.; Colás-Marrufo, T.; Sanchez-Crespo, M. Reproductive strategy of a continental shelf lane snapper population from the southern Gulf of Mexico. Mar. Coast. Fish 2021, 13, 140–156. [Google Scholar] [CrossRef]
- Nóh-Quiñones, V.E. Estrategia Reproductiva del Labridae de Importancia Comercial: La Doncella de Pluma Lachnolaimus maximus, en la Costa de Yucatán, México [Reproductive Strategy of the Commercial Labridae: The Hogfish Lachnolaimus maximus, from the Yucatan Coast, Mexico]. Ph.D. Thesis, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Unidad Mérida, Mérida, Mexico, 2017. [Google Scholar]
- Sadovy, Y.J. Reproduction of reef fishery species. In Reef Fisheries; Polunin, N.V.C., Roberts, C.M., Eds.; Chapman and Hall: London, UK, 1996; pp. 15–59. [Google Scholar]
- Gutiérrez, J.M.; Jones, R.G.; Narisma, G.T.; Alves, L.M.; Amjad, M.; Gorodetskaya, I.V.; Grose, M.; Klutse, N.A.B.; Krakovska, S.; Li, J.; et al. Atlas. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; Available online: http://interactive-atlas.ipcc.ch/ (accessed on 1 March 2022).
- Iturbide, M.; Fernández, J.; Gutiérrez, J.M.; Bedia, J.; Cimadevilla, E.; Díez-Sierra, J.; Manzanas, R.; Casanueva, A.; Baño-Medina, J.; Milovac, J.; et al. Repository Supporting the Implementation of FAIR Principles in the IPCC-WG1 Atlas (v2.0); Zenodo: Geneve, Switzerland, 2021. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef] [Green Version]
- Brown-Peterson, N.J.; Wyansky, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A Standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Tucker, J.W., Jr. Marine Fish Culture; Kluwer Academic Publishers: Norwell, MA, USA, 1998; p. 750. [Google Scholar]
- Nemeth, R.S.; Blondeau, J.; Herzlieb, S.; Kadison, E. Spatial and temporal patterns of movement and migration at spawning aggregations of red hind, Epinephelus guttatus, in the U.S. Virgin Islands. Environ. Biol. Fishes 2007, 78, 365–381. [Google Scholar] [CrossRef]
- Sedberry, G.R.; Pashuk, O.; Wyansky, D.M.; Stephen, J.A.; Weinbach, P. Spawning Locations for Atlantic Reef Fishes off the Southeastern, U.S. Proc. Gulf Caribb. Fish. Inst. 2006, 57, 464–514. [Google Scholar]
- Sadovy, Y.; Colin, P.L.; Domeier, M.L. Aggregation and spawning in the tiger grouper, Mycteroperca tigris (Pisces: Serranidae). Copeia 1994, 2, 511–516. [Google Scholar] [CrossRef]
- Tuz-Sulub, A.; Brulé, T. Spawning aggregations of three protogynous groupers in the southern Gulf of Mexico. J. Fish Biol. 2015, 86, 162–185. [Google Scholar] [CrossRef]
- Arnold, C.R.; Wakeman, J.M.; Williams, T.D.; Treece, G.D. Spawning of red snapper (Lutjanus campechanus) in captivity. Aquaculture 1978, 15, 301–302. [Google Scholar] [CrossRef]
- Minton, R.V.; Hawke, J.P.; Tatum, W.M. Hormone induced spawning of red snapper Lutjanus campechanus. Aquaculture 1983, 30, 363–368. [Google Scholar] [CrossRef]
- Collins, L.A.; Fitzhugh, G.R.; Mourand, L.; Lombardi-Carlson, L. Preliminary results from a continuing study of spawning and fecundity of the red snapper (Lutjanidae: Lutjanus campechanus) from the Gulf of Mexico, 1998–1990. Proc. Gulf Caribb. Fish. Inst. 2001, 52, 34–47. [Google Scholar]
- Watanabe, W.O.; Benetti, D.D.; Feeley, M.W.; Davis, D.A.; Phelps, R.P. Status of Artificial Propagation of mutton, yellowtail, and red snapper (family Lutjanidae) in the Southeastern United States. Am. Fish. Soc. Symp. 2005, 46, 517–540. [Google Scholar]
- Papanikos, N.; Phelps, R.P.; Davis, D.A.; Ferry, A.; Maus, D. Spontaneous spawning of captive red snapper, Lutjanus campechanus, and dietary lipid effect on reproductive performance. J. World Aquac. Soc. 2008, 39, 324–338. [Google Scholar] [CrossRef]
- Luckhurst, B.L.; Dean, J.M.; Reichert, M. Age, growth and reproduction of the lane snapper Lutjanus synagris (Pisces: Lutjanidae) at Bermuda. Mar. Ecol. Prog. Ser. 2000, 203, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Claro, R.; Lindeman, K.C. Spawning aggregation sites of snapper and grouper species (Lutjanidae and Serranidae) on the insular shelf of Cuba. Gulf Caribb. Res. 2003, 14, 91–106. [Google Scholar] [CrossRef]
- Wallace, R.K., Jr. Thermal acclimation, upper temperature tolerance, and preferred temperature of juvenile yellowtail snappers, Ocyurus chrysurus (Bloch) (Pisces: Lutjanidae). Bull. Mar. Sci. 1977, 27, 292–298. [Google Scholar]
- Turano, M.J.; Davis, D.A.; Arnold, C.R. Observations and techniques for maturation, spawning, and larval rearing of the yellowtail snapper Ocyurus chrysurus. J. World Aquac. Soc. 2000, 31, 59–68. [Google Scholar] [CrossRef]
- Colin, P.L. Spawning and larval development of the hogfish, Lacholaimus maximus (Pisces: Labridae). Bull Fish. 1982, 80, 853–862. [Google Scholar]
- Grimes, C.B. Reproductive biology of the Lutjanidae: A review. In Tropical Snappers and Groupers: Biology and Fisheries Management; Polovina, J.J., Ralston, S., Eds.; Westview Press: Boulder, CO, USA, 1987; pp. 239–294. [Google Scholar]
- Sadovy, Y.; Shapiro, D.Y. Criteria for the diagnosis of hermaphroditism in fishes. Copeia 1987, 1, 136–156. [Google Scholar] [CrossRef]
- Hausfather, Z.; Peters, G.P. Emissions—The ‘business as usual’ story is misleading. Nature 2020, 577, 618–620. [Google Scholar] [CrossRef]
- IUCN (International Union for Conservation of Nature). The IUCN Red List of Threatened Species. Version 2017-3. 2018. Available online: www.iucnredlist.org (accessed on 22 June 2018).




| Family | Species | Sexual Pattern | |
|---|---|---|---|
| Scientific Name | Common Name | ||
| Epinephelidae (grouper) | Epinephelus guttatus | Red hind | MPH a |
| Epinephelus morio | Red grouper | MPH a | |
| Mycteroperca bonaci | Black grouper | MPH a | |
| Mycteroperca microlepis | Gag | MPH a | |
| Mycteroperca tigris | Tiger grouper | MPH a | |
| Mycteroperca venenosa | Yellowfin grouper | MPH a | |
| Lutjanidae (snapper) | Lutjanus campechanus | Red snapper | G b |
| Lutjanus synagris | Lane snapper | G b | |
| Ocyurus chrysurus | Yellowtail snapper | G b | |
| Labridae (wrasse) | Lachnolaimus maximus | Hogfish | MPH a |
| Species | Spawning Temperature Range (°C) | Reference |
|---|---|---|
| Grouper | ||
| Epinephelus guttatus | 22–26 1 26–28 2 | [50] [51] |
| Epinephelus morio | 20–23 1 17–24 2 | [50] [52] |
| Mycteroperca bonaci | 26 1 | [50] |
| Mycteroperca microlepis | 26 1 17 2 | [50] [52] |
| Mycteroperca tigris | ~25 2 21–24 2 | [53] [54] |
| Mycteroperca venenosa | 28–30 1 17–24 2 | [50] [54] |
| Snapper | ||
| Lutjanus campechanus | 23–26 1 25–28 1 21–29 2 >24 1 19–28 2 21–29 2 | [55] [56] [57] [58] [52] [59] |
| Lutjanus synagris | 22–28 2 26–27 2 | [60] [61] |
| Ocyurus chrysurus | 24–30 1 22–27 1 24–26 1 | [62] [63] [58] |
| Wrasse | ||
| Lachnolaimus maximus | 24–26 2 | [64] |
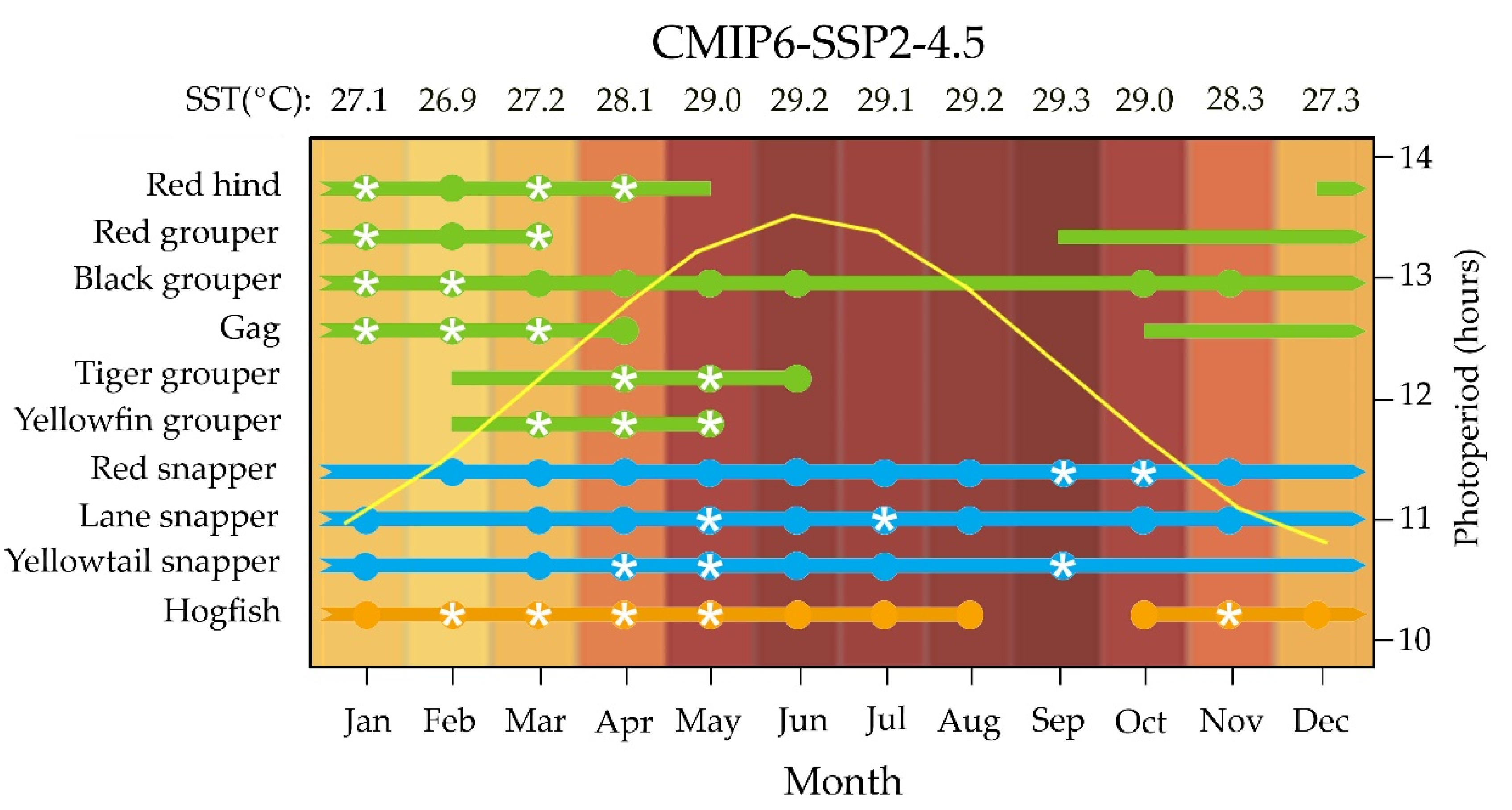
| Year | Mean Monthly Sea Surface Temperature (°C) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan. | Feb. | Mar. | Apr. | May | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | Dec. | |
| Scenario CMIP6 SSP2-4.5 | ||||||||||||
| 2040 | 27.3 | 27.2 | 27.5 | 28.4 | 29.3 | 29.5 | 29.5 | 29.7 | 29.7 | 29.5 | 28.8 | 28.1 |
| 2060 | 27.9 | 27.7 | 28.1 | 28.9 | 29.8 | 30.0 | 29.9 | 20.1 | 30.2 | 30.3 | 29.2 | 28.4 |
| 2080 | 28.2 | 28.0 | 28.3 | 29.1 | 29.1 | 30.2 | 30.2 | 30.4 | 30.5 | 30.3 | 29.6 | 28.7 |
| 2100 | 28.3 | 28.1 | 28.4 | 29.3 | 30.2 | 30.5 | 30.4 | 30.7 | 30.9 | 30.6 | 29.9 | 29.0 |
| Scenario CMIP6 SSP3-7.0 | ||||||||||||
| 2040 | 27.4 | 27.3 | 27.7 | 28.4 | 29.2 | 29.5 | 29.4 | 29.6 | 29.7 | 29.5 | 28.8 | 28.0 |
| 2060 | 28.1 | 27.9 | 28.2 | 29.0 | 29.9 | 30.2 | 30.1 | 30.4 | 30.6 | 30.3 | 29.6 | 28.8 |
| 2080 | 28.7 | 28.5 | 28.9 | 29.7 | 30.5 | 30.8 | 30.7 | 30.1 | 31.2 | 31.0 | 30.3 | 29.5 |
| 2100 | 29.5 | 29.3 | 29.7 | 30.5 | 31.3 | 31.7 | 31.7 | 32.0 | 32.2 | 32.0 | 31.3 | 30.4 |
| Scenario CMIP6 SSP5-8.5 | ||||||||||||
| 2040 | 27.4 | 27.3 | 27.7 | 28.6 | 29.4 | 29.7 | 29.7 | 29.9 | 30.0 | 29.7 | 29.1 | 28.2 |
| 2060 | 28.3 | 28.2 | 28.6 | 29.3 | 30.3 | 30.5 | 30.5 | 30.7 | 30.9 | 30.5 | 29.8 | 29.0 |
| 2080 | 29.2 | 29.1 | 29.3 | 30.1 | 31.0 | 31.3 | 31.3 | 31.6 | 31.7 | 31.4 | 30.7 | 29.8 |
| 2100 | 30.0 | 29.8 | 30.2 | 31.1 | 31.9 | 32.2 | 32.2 | 32.5 | 32.6 | 32.5 | 31.8 | 30.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brulé, T.; Renán, X.; Colás-Marrufo, T. Potential Impact of Climate Change on Fish Reproductive Phenology: A Case Study in Gonochoric and Hermaphrodite Commercially Important Species from the Southern Gulf of Mexico. Fishes 2022, 7, 156. https://doi.org/10.3390/fishes7040156
Brulé T, Renán X, Colás-Marrufo T. Potential Impact of Climate Change on Fish Reproductive Phenology: A Case Study in Gonochoric and Hermaphrodite Commercially Important Species from the Southern Gulf of Mexico. Fishes. 2022; 7(4):156. https://doi.org/10.3390/fishes7040156
Chicago/Turabian StyleBrulé, Thierry, Ximena Renán, and Teresa Colás-Marrufo. 2022. "Potential Impact of Climate Change on Fish Reproductive Phenology: A Case Study in Gonochoric and Hermaphrodite Commercially Important Species from the Southern Gulf of Mexico" Fishes 7, no. 4: 156. https://doi.org/10.3390/fishes7040156
APA StyleBrulé, T., Renán, X., & Colás-Marrufo, T. (2022). Potential Impact of Climate Change on Fish Reproductive Phenology: A Case Study in Gonochoric and Hermaphrodite Commercially Important Species from the Southern Gulf of Mexico. Fishes, 7(4), 156. https://doi.org/10.3390/fishes7040156






