Cloning of Two HSP Genes of Eriocheir hepuensis and Their Expression under Vibrio parahaemolyticus Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. RNA Extraction and Reverse Transcription
2.3. Gene Cloning of Eh-HSP10 and Eh-HSP40
2.4. Bioinformatics Analysis
2.5. Relative Expression Levels of Eh-HSP10 and Eh-HSP40 in Different Tissues
2.6. Relative Expression Levels of Eh-HSP10 and Eh-HSP40 under V. parahaemolyticus Stress
2.7. Statistical Analysis
3. Results
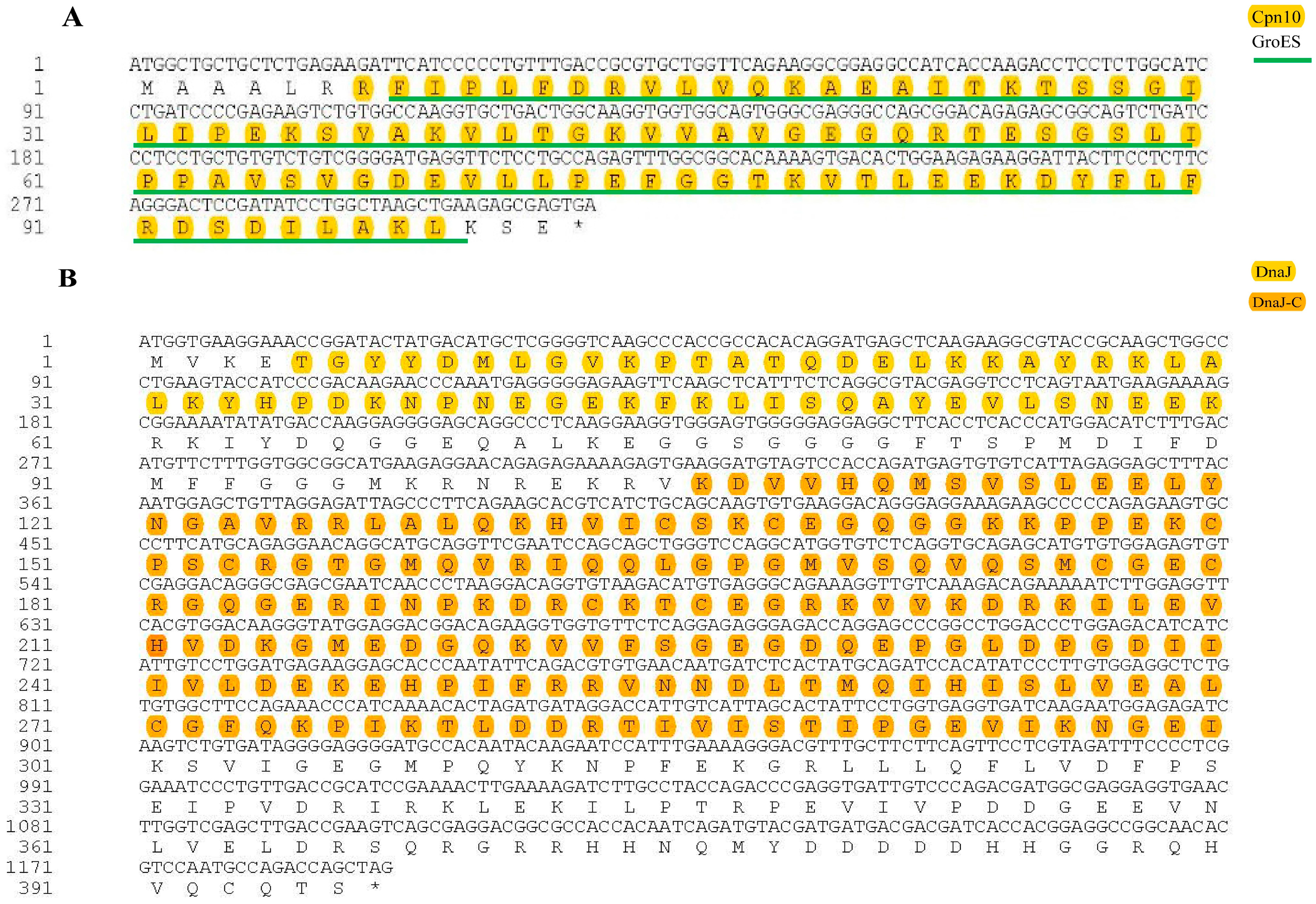
3.1. Cloning and Sequence Analysis of HSP10 and HSP40 CDS Regions of E. hepuensis
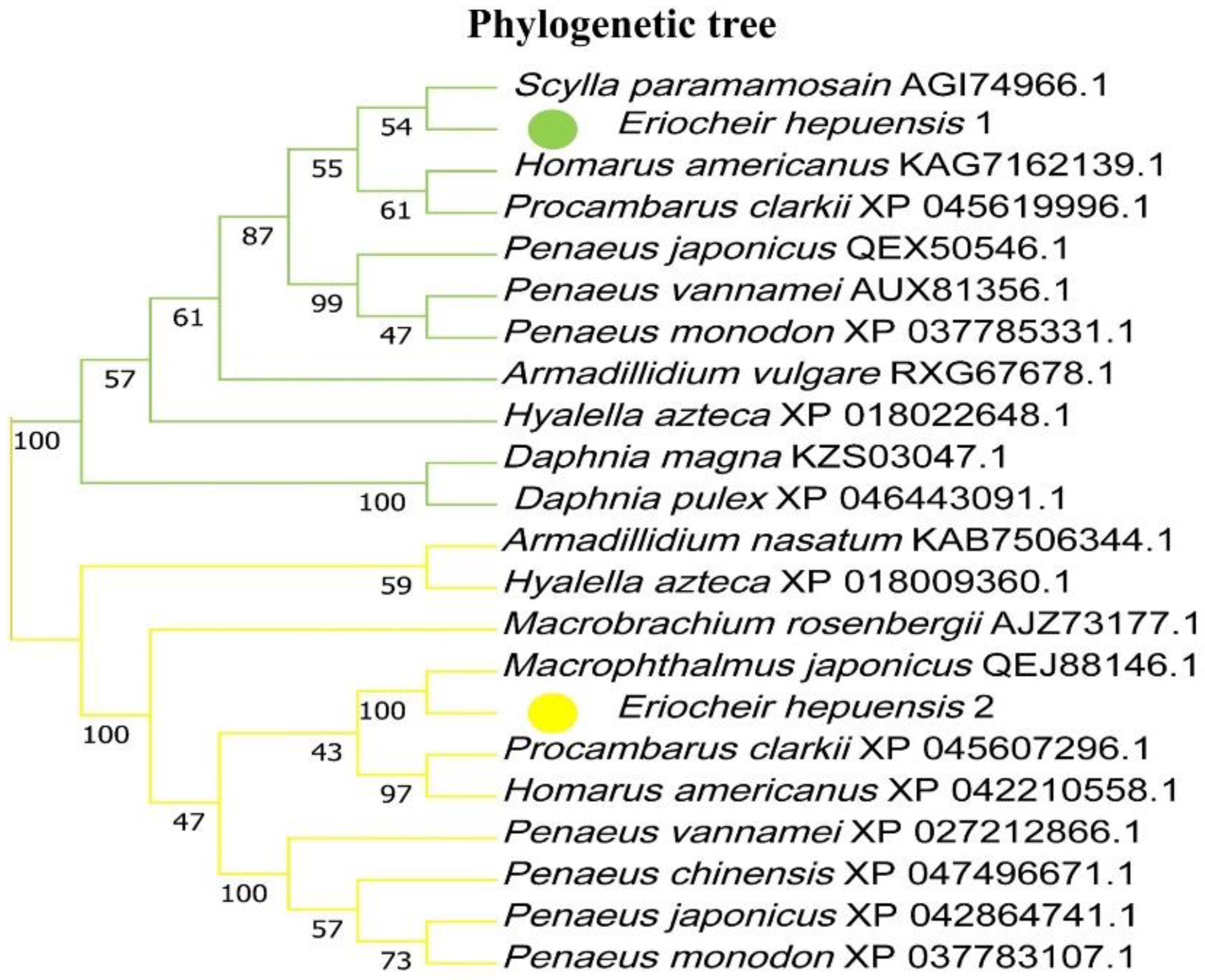
3.2. Homologous Sequence Analysis of HSP10 and HSP40 in E. hepuensis and Construction of a Phylogenetic Tree
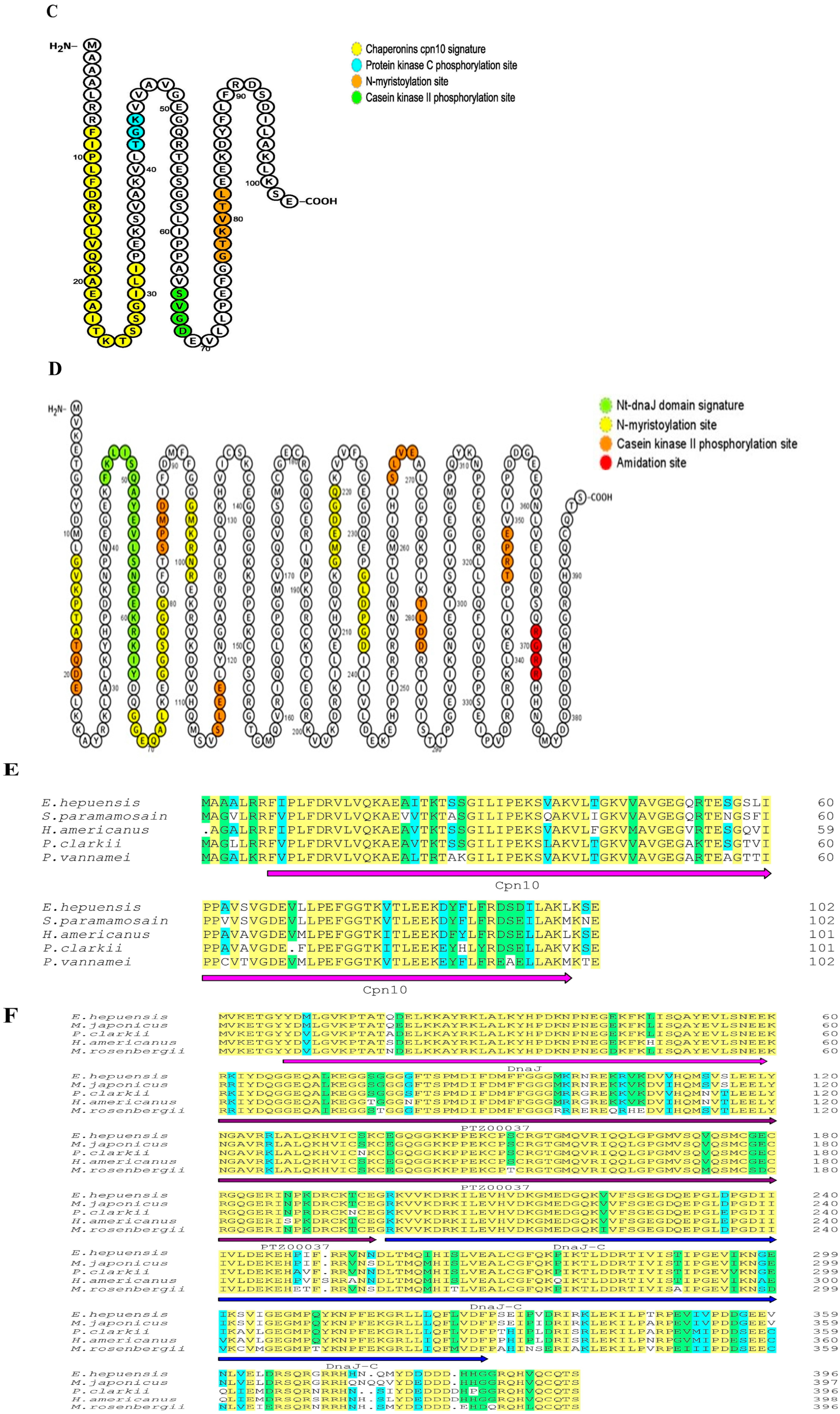
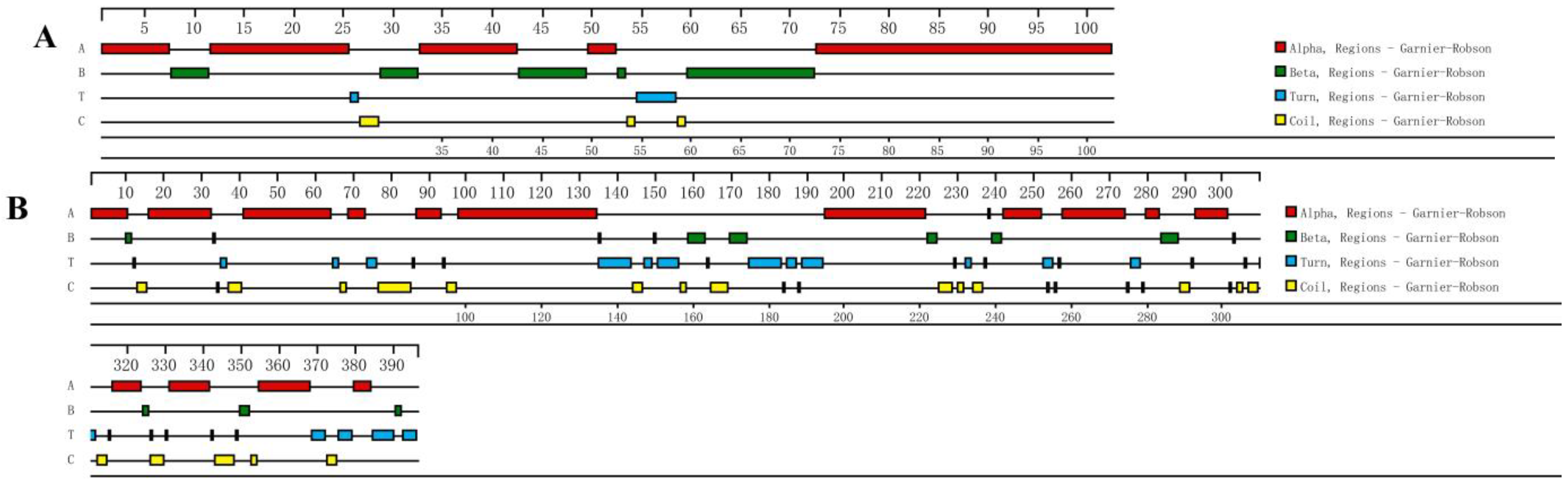
3.3. Bioinformatics Analysis of Eh-HSP10 and Eh-HSP40 Proteins
3.4. Relative Expression Levels of Eh-HSP10 and Eh-HSP40 in Different Tissues
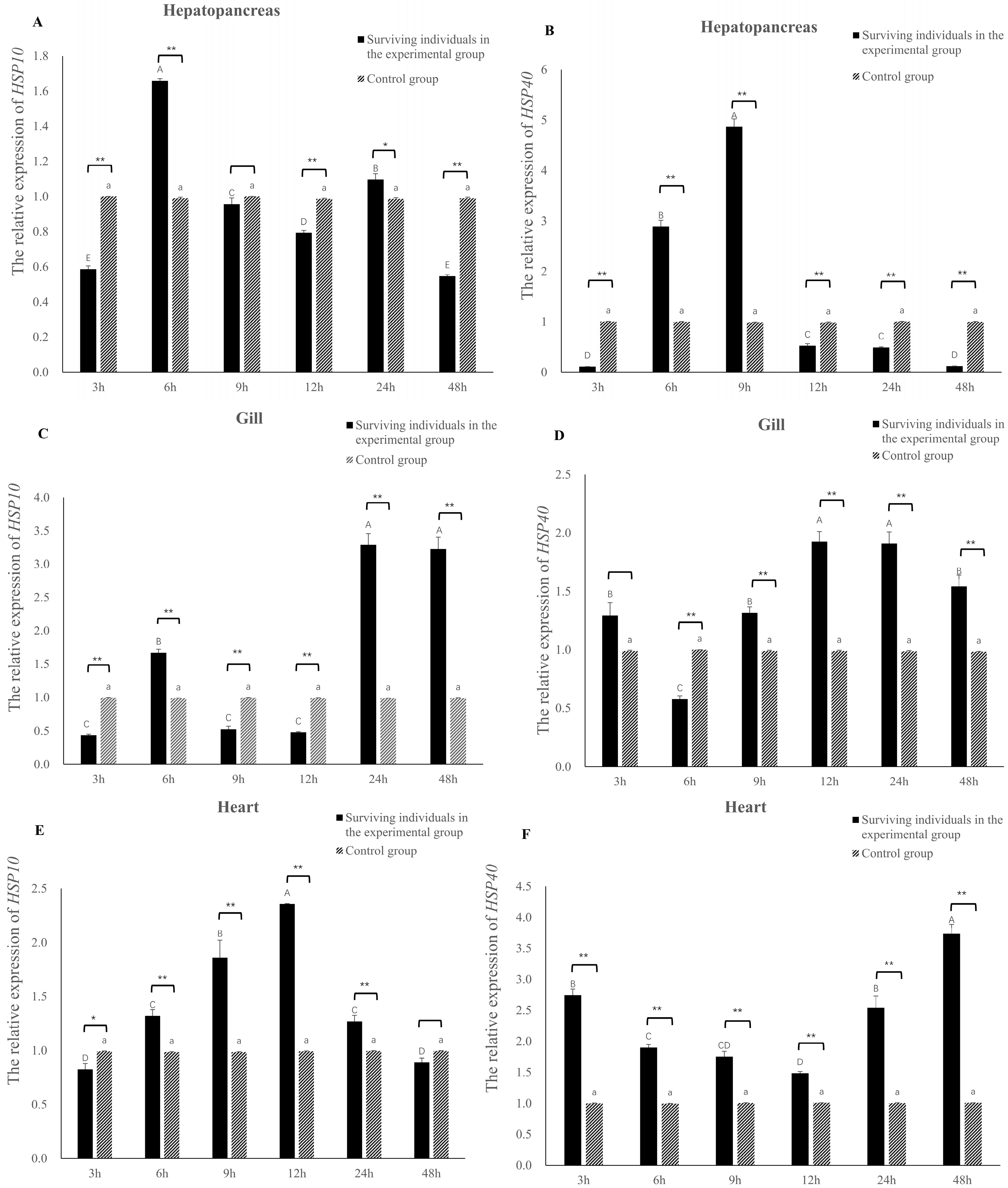
3.5. Relative Expression Levels of Eh-HSP10 and Eh-HSP40 under V. parahaemolyticus Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Liao, Y.Y.; Li, Z.G.; Giang, Y.J.; Yang, B.H. Research progress of Eriocheir hepuensis. Curr. Fish. 2021, 46, 72–74. [Google Scholar]
- Tang, B.; Zhou, K.; Song, D.; Yang, G.; Dai, A. Molecular systematics of the Asian mitten crabs, genus Eriocheir (Crustacea: Brachyura). Mol. Phylogenetics Evol. 2003, 29, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Zeng, H. Study on Morphology of Eriocheir hepuensis. J. Zhejiang Ocean. Univ. (Nat. Sci. Ed.) 2000, 19, 327–332. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Cheng, Q.X.; Lu, G.Q.; Wang, C.H. Complete mitochondrial genomes of three mitten crabs, Eriocheir sinensis, E. hepuensis, and E. japonica. Mitochondrial DNA A 2016, 27, 1175–1176. [Google Scholar] [CrossRef]
- Srivastava, P. Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2002, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.T.; Liu, B.; Xie, J.; Zhou, Q.L.; Ge, X.P.; Xu, P. Research progress of heat shock protein and its research prospect in aquatic animals. Jiangsu Agric. Sci. 2011, 39, 303–306. [Google Scholar]
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat Shock Proteins as Immunomodulants. Molecules 2018, 23, 2846. [Google Scholar] [CrossRef] [Green Version]
- Mallouk, Y.; Vayssier-Taussat, M.; Bonventre, J.V.; Polla, B.S. Heat shock protein 70 and ATP as partners in cell homeostasis (Review). Int. J. Mol. Med. 1999, 4, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.L.; Marzano, N.R.; van Oijen, A.M.; Ecroyd, H. Using Single-Molecule Approaches to Understand the Molecular Mechanisms of Heat-Shock Protein Chaperone Function. J. Mol. Biol. 2018, 430, 4525–4546. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, R.I.; Sarge, K.D.; Abravaya, K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J. Biol. Chem. 1992, 267, 21987–21990. [Google Scholar] [CrossRef]
- Georgopoulos, C.P.; Lundquist-Heil, A.; Yochem, J.; Feiss, M. Identification of the E. coli dnaJ gene product. Mol. Gen. Genet. 1980, 178, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Zylicz, M.; Yamamoto, T.; Mckittrick, N.; Sell, S.; Georgopoulos, C. Purification and properties of the dnaJ replication protein of Escherichia coli. J. Biol. Chem. 1985, 260, 7591–7598. [Google Scholar] [CrossRef] [PubMed]
- Kästle, M.; Grune, T. Chapter 4—Interactions of the Proteasomal System with Chaperones: Protein Triage and Protein Quality control. In Progress in Molecular Biology and Translational Science; Grune, T., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 113–160. [Google Scholar]
- Li, J.; Zhang, Y.H.; Liu, Y.; Zhang, Y.; Xiao, S.; Yu, Z.N. Co-expression of heat shock protein (HSP) 40 and HSP70 in Pinctada martensii response to thermal, low salinity and bacterial challenges. Fish Shellfish. Immunol. 2016, 48, 239–243. [Google Scholar] [CrossRef]
- Knox, C.; Luke, G.A.; Blatch, G.L.; Pesce, E.R. Heat shock protein 40 (Hsp40) plays a key role in the virus life cycle. Virus Res. 2011, 160, 15–24. [Google Scholar] [CrossRef]
- Cheetham, M.E.; Caplan, A.J. Structure, function and evolution of DnaJ: Conservation and adaptation of chaperone function. Cell Stress Chaperones 1998, 3, 28–36. [Google Scholar] [CrossRef]
- Cappello, F. HSP60 and HSP10 as diagnostic and prognostic tools in the management of exocervical carcinoma. Gynecol. Oncol. 2003, 91, 661. [Google Scholar] [CrossRef] [Green Version]
- Magen, D.; Georgopoulos, C.; Bross, P.; Ang, D.; Segev, Y.; Goldsher, D.; Nemirovski, A.; Shahar, E.; Ravid, S.; Luder, A.; et al. Mitochondrial hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am. J. Hum. Genet. 2008, 83, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Zhang, Y.; Huo, Z.J.; Zhou, X.R.; Pang, B.P. Molecular cloning of heat shock protein 10 (Hsp10) and 60 (Hsp60) cDNAs from Galeruca daurica (Coleoptera: Chrysomelidae) and their expression analysis. Bull. Entomol. Res. 2018, 108, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.K.; Cui, Y.G.; Liu, J.Y. Research Progress of Heat Shock Protein 10. Med. Recapitul. 2018, 108, 510–522. [Google Scholar]
- Corrao, S.; Campanella, C.; Anzalone, R.; Farina, F.; Zummo, G.; Conway de Macario, E.; Macario, A.J.; Cappello, F.; La Rocca, G. Human Hsp10 and Early Pregnancy Factor (EPF) and their relationship and involvement in cancer and immunity: Current knowledge and perspectives. Life Sci. 2010, 86, 145–152. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Jiang, K.J.; Sun, M.N.; Zhang, D.; Ma, L.B. Multiplex immune- related genes expression analysis response to bacterial challenge in mud crab, Scylla paramamosain. Fish Shellfish. Immunol. 2013, 34, 712–716. [Google Scholar] [CrossRef]
- Lu, S.J.; Jiao, J.B.; Yuan, X.M.; Yu, Z.; Zhang, H.Q.; Hang, X.Y.; Shi, W.D.; Wu, Y.L. Research progress of Eriocheir sinensis immunostimulants. Jiangsu Agric. Sci. 2021, 49, 19–23. [Google Scholar]
- Luo, J.M.; Luo, J.X. Research Progress of the Immunity of Heat Shock Proteins. Med. Recapitul. 2013, 19, 1179–1181. [Google Scholar]
- Jung, H.; Lyons, R.E.; Li, Y.; Thanh, N.M.; Dinh, H.; Hurwood, D.A.; Salin, K.R.; Mather, P.B. A candidate gene association study for growth performance in an improved giant freshwater prawn (Macrobrachium rosenbergii) culture line. Mar. Biotechnol. 2014, 16, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, J.X.; Ren, T.J.; Xu, Y.H.; Lu, M.; Fang, H.Y.; Zhang, Y.; Liao, Y.Y.; Zhu, P. Cloning and analysis of three glutathione S-transferases in Eriocheir hepuensis and their expression in response to azadirachtin stress. Aquac. Rep. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.; Pang, Y.Y.; Yang, H.; Yang, Z.G. Cloning and Expression Analysis of Aquaporin 11 Gene in Eriocheir sinensis. Genom. Appl. Biol. 2021, 40, 1497–1504. [Google Scholar]
- Liao, Y.Y.; Liu, K.; Ren, T.J.; Zhang, Z.N.; Ma, Z.H.; Dan, S.F.; Lan, Z.Y.; Lu, M.; Fang, H.Y.; Zhang, Y.; et al. The characterization, expression and activity analysis of three superoxide dismutases in Eriocheir hepuensis under azadirachtin stress. Fish Shellfish. Immunol. 2021, 117, 228–239. [Google Scholar] [CrossRef]
- Liang, H.Y.; Wang, Z.X.; Lei, Q.N.; Huang, R.L.; Deng, Y.W.; Wang, Q.H.; Jiao, Y.; Du, X.D. Molecular cloning and expression analysis of a pearl oyster (Pinctada martensii) heat shock protein 90 (HSP90). Genet. Mol. Res. 2015, 14, 18778–18791. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Jin, Z.X.; Huang, H.Y.; Ye, H.H.; Li, S.J. Cloning and expression analysis of the regulatory subunit B gene of PP2A in the mud crab Scylla paramamosain. Oceanol. Limnol. Sin. 2012, 43, 932–937. [Google Scholar]
- Zhou, S.-M.; Tao, Z.; Shen, C.; Qian, D.; Wang, C.-L.; Zhou, Q.-C.; Jin, S. β-actin gene expression is variable among individuals and not suitable for normalizing mRNA levels in Portunus trituberculatus. Fish Shellfish. Immunol. 2018, 81, 338–342. [Google Scholar] [CrossRef]
- Nitnavare, R.B.; Yeshvekar, R.K.; Sharma, K.K.; Vadez, V.; Reddy, M.K.; Reddy, P.S. Molecular cloning, characterization and expression analysis of a heat shock protein 10 (Hsp10) from Pennisetum glaucum (L.), a C-4 cereal plant from the semi-arid tropics. Mol. Biol. Rep. 2016, 43, 861–870. [Google Scholar] [CrossRef] [Green Version]
- David, S.; Bucchieri, F.; Corrao, S.; Czarnecka, A.M.; Campanella, C.; Farina, F.; Peri, G.; Tomasello, G.; Sciumè, C.; Modica, G.; et al. Hsp10: Anatomic distribution, functions, and involvement in human disease. Front. Biosci. 2013, 5, 768–778. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Yamout, M.; Legge, G.B.; Zhang, O.; Wright, P.E.; Dyson, H.J. Solution structure of the cysteine-rich domain of the Escherichia coli chaperone protein DnaJ. J. Mol. Biol. 2000, 300, 805–818. [Google Scholar] [CrossRef]
- Lu, Z.; Cyr, D.M. The conserved carboxyl terminus and zinc finger-like domain of the co-chaperone Ydj1 assist Hsp70 in protein folding. J. Biol. Chem. 1998, 273, 5970–5978. [Google Scholar] [CrossRef] [Green Version]
- Cunnea, P.M.; Miranda-Vizuete, A.; Bertoli, G.; Simmen, T.; Damdimopoulos, A.E.; Hermann, S.; Leinonen, S.; Huikko, M.P.; Gustafsson, J.A.; Sitia, R.; et al. ERdj5, an endoplasmic reticulum (ER)-resident protein containing DnaJ and thioredoxin domains, is expressed in secretory cells or following ER stress. J. Biol. Chem. 2003, 278, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Kiyun, P.; Ihn-Sil, K. Salinity and bisphenol A alter cellular homeostasis and immune defense by heat shock proteins in the intertidal crab Macrophthalmus japonicus—ScienceDirect. Estuar. Coast. Shelf Sci. 2019, 229, 106381. [Google Scholar]
- Ding, J.; Chen, F.Y.; Ren, S.Y.; Qiao, K.; Chen, B.; Wang, K.J. Molecular characterization and promoter analysis of crustacean heat shock protein 10 in Scylla paramemosain. Genome 2013, 56, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Söderhäll, K.; Cerenius, L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998, 10, 23–28. [Google Scholar] [CrossRef]
- Shi, J.X.; Fu, M.J.; Zhao, C.; Zhou, F.L.; Yang, Q.B.; Qiu, L.B. Characterization and function analysis of Hsp60 and Hsp10 under different acute stresses in black tiger shrimp, Penaeus monodon. Cell Stress Chaperones 2016, 21, 295–312. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.X.; Wei, B.Y.; Yin, Q.; Jiang, X.Z.; Li, M.; Chen, X.H. Sequence of HSP10 gene in Litopenaeus vannmei and its low temperature expression analysis. J. South. Agric. 2013, 44, 838–843. [Google Scholar]
- Shi, H.N.; Liu, Z.; Zhang, J.P.; Kang, Y.J.; Wang, J.F.; Huang, J.Q.; Wang, W.M. Short Communication: Effect of heat stress on heat-shock protein (Hsp60) mRNA expression in rainbow trout Oncorhynchus mykiss. Genet. Molecilar Res. 2015, 14, 5280–5286. [Google Scholar] [CrossRef] [PubMed]



| Primer Sequence | Forward | Reverse |
|---|---|---|
| HSP10CDS | CACCACGACCACACGACG | CAATGCCTCTATGGGAACACG |
| HSP10DL | TTGACCGCGTGCTGGTTCAGA | CCACCACCTTGCCAGTCAGCA |
| HSP40CDS | TCCAGTCCACACTCAACCAAG | TGAACGGGGAAGAAGGTTTGC |
| HSP40DL | GACCGAAGTCAGCGAGGACGG | CTGGCATTGGACGTGTTGCCG |
| Ef1-DL | TCTGACTCCAAGAACGACCC | CAGGCAATGTGAGCAGTGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Q.; Liu, J.; Ren, T.; Zhang, Z.; Ma, Z.; Lan, Z.; Duan, Y.; Liang, Z.; Chen, B.; Zhang, Y.; et al. Cloning of Two HSP Genes of Eriocheir hepuensis and Their Expression under Vibrio parahaemolyticus Stress. Fishes 2022, 7, 372. https://doi.org/10.3390/fishes7060372
Fu Q, Liu J, Ren T, Zhang Z, Ma Z, Lan Z, Duan Y, Liang Z, Chen B, Zhang Y, et al. Cloning of Two HSP Genes of Eriocheir hepuensis and Their Expression under Vibrio parahaemolyticus Stress. Fishes. 2022; 7(6):372. https://doi.org/10.3390/fishes7060372
Chicago/Turabian StyleFu, Qianni, Jinxia Liu, Tianjiao Ren, Zining Zhang, Zihang Ma, Zhenyu Lan, Yitao Duan, Ziwei Liang, Boyu Chen, Yan Zhang, and et al. 2022. "Cloning of Two HSP Genes of Eriocheir hepuensis and Their Expression under Vibrio parahaemolyticus Stress" Fishes 7, no. 6: 372. https://doi.org/10.3390/fishes7060372
APA StyleFu, Q., Liu, J., Ren, T., Zhang, Z., Ma, Z., Lan, Z., Duan, Y., Liang, Z., Chen, B., Zhang, Y., Zhu, P., & Liao, Y. (2022). Cloning of Two HSP Genes of Eriocheir hepuensis and Their Expression under Vibrio parahaemolyticus Stress. Fishes, 7(6), 372. https://doi.org/10.3390/fishes7060372