Study on Fish Species Diversity in the Pingzhai Reservoir Based on Environmental DNA Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Environmental Sample Collection
2.2. DNA Extraction of Water Samples
2.3. PCR Amplification and High-Throughput Sequencing
2.4. Data Analysis
3. Results
3.1. Composition of Fish Species
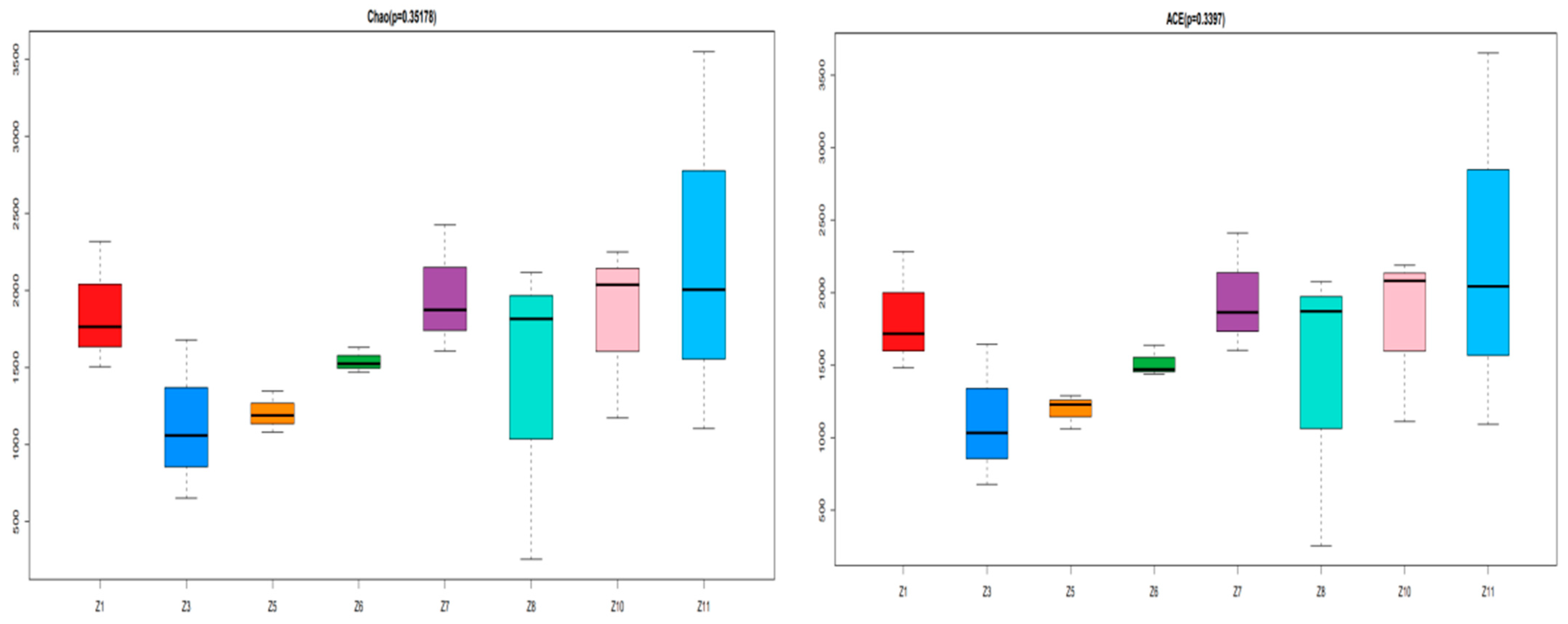
3.2. Analysis of Fish Species Diversity
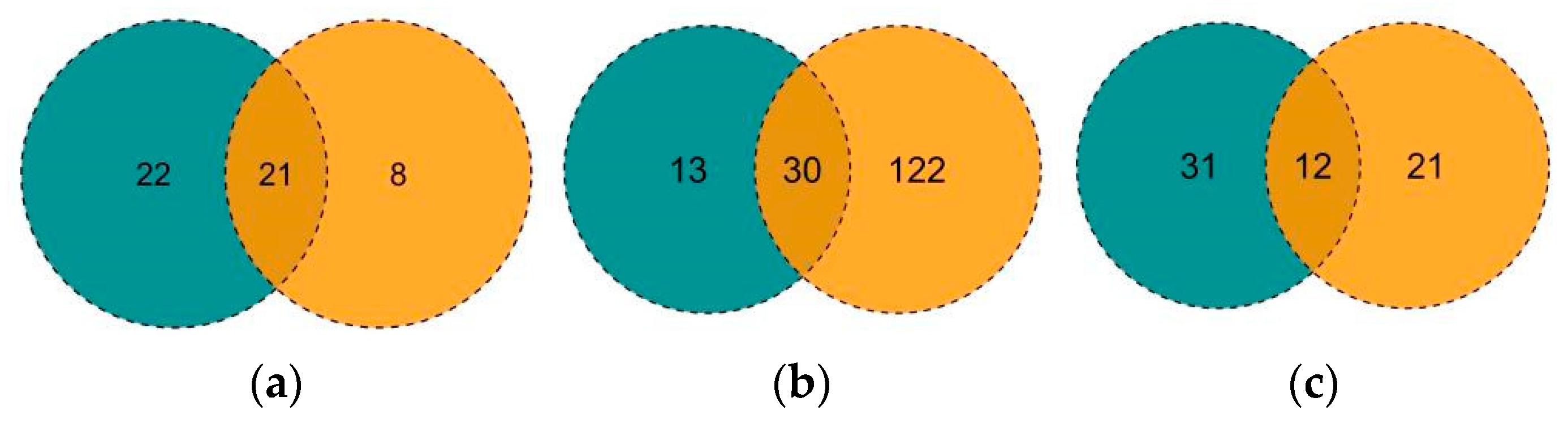
3.3. Environmental DNA Test Results and Analysis
4. Discussion
4.1. Composition of Fish in the Pingzhai Reservoir
4.2. Analysis of Fish Diversity in the Pingzhai Reservoir Based on Environmental DNA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Lundberg, J.G.; Kottelat, M.; Smith, G.R.; Stiassny, M.L.; Gill, A.C. So many fishes, so little time: An overview of recent ichthyological discovery in continental waters. Ann. Mo. Bot. Gard. 2000, 87, 26–62. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zhong, L.P.; Gao, T.X.; Zhang, H.B.; Chen, Z.; Wang, X.Y. Study on fish species diversity in the Zhongjieshan Islands based on environmental DNA metabarcoding. J. Fish. China 2023, 5, 1–11. [Google Scholar]
- Chen, Y.S.; Qu, X.; Xiong, F.Y.; Lu, Y.; Wang, L.Z.; Hughes, R.M. Challenges to saving China’s freshwater biodiversity: Fishery exploitation and landscape pressures. Ambio 2020, 49, 926–938. [Google Scholar] [CrossRef]
- Su, G.H.; Logez, M.; Xu, J.; Tao, S.L.; Villeger, S.; Brosse, S.; Brosse, S. Human impacts on global freshwater fish biodiversity. Science 2021, 371, 835–838. [Google Scholar] [CrossRef]
- Ye, S.W.; Li, Z.J.; Liu, J.S.; Zhang, T.L.; Xie, S.G. Distribution, Endemism and Conservation Status of Fishes in the Yangtze River Basin, China//Grilloo; Venora, G., Ed.; Ecosystems Biodiversity; IntechOpen: London, UK, 2011; pp. 41–66. [Google Scholar]
- Chen, J.M.; Zhao, H.T.; Miao, Z.G.; Chen, Y.X.; Zhang, F.T.; Wang, J.W. Status and conservation of fish resources in the Chishui River. Biodivers. Sci. 2010, 18, 162–168. [Google Scholar]
- Li, S.H. Characteristics and Influencing Factors of Water Eutrophication in Pingzhai Reservoir on Guizhou Plateau. Master’s Thesis, Guizhou Normal University, Guizhou, China, 2021. [Google Scholar]
- Rees, H.C.; Bishop, K.; Middleditch, D.J.; Patmore, J.R.; Maddison, B.C.; Gough, K.C. The application of eDNA for monitoring of the Great Crested Newt in the UK. Ecol. Evol. 2014, 4, 4023–4032. [Google Scholar] [CrossRef]
- Rees, H.C.; Maddison, B.C.; Middleditch, D.J.; Patmore, J.R.; Gough, K.C. REVIEW: The detection of aquatic animal species using environmental DNA—A review of eDNA as a survey tool in ecology. J. Appl. Ecol. 2014, 51, 1450–1459. [Google Scholar] [CrossRef]
- Deiner, K.; Walser, J.C.; Mächler, E.; Altermatt, F. Choice of capture and extraction methods affect detection of freshwater biodiversity from environmental DNA. Biol. Conserv. 2015, 183, 53–63. [Google Scholar] [CrossRef]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17, e547. [Google Scholar] [CrossRef]
- Goldberg, C.S.; Turner, C.R.; Deiner, K.; Klymus, K.E.; Thomsen, P.F.; Murphy, M.A.; Spear, S.F.; McKee, A.; Oyler-McCance, S.J.; Cornman, R.S.; et al. Critical considerations for the application of environmental DNA methods to detect aquatic species. Methods Ecol. Evol. 2016, 7, 1299–1307. [Google Scholar] [CrossRef]
- Hänfling, B.; Lawson Handley, L.; Read, D.S.; Hahn, C.; Li, J.; Nichols, P.; Blackman, R.C.; Oliver, A.; Winfield, I.J. Environmental DNA metabarcoding of lake fish communities reflects long-term data from established survey methods. Mol. Ecol. 2016, 25, 3101–3119. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Miaud, C.; Pompanon, F.; Taberlet, P. Species detection using environmental DNA from water samples. Biol. Lett. 2008, 4, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.T.; Li, Y.; Renshaw, M.A.; Olds, B.P.; Deiner, K.; Turner, C.R.; Jerde, C.L.; Lodge, D.M.; Lamberti, G.A.; Pfrender, M.E. Fish community assessment with edna metabarcoding: Effects of sampling design and bioinformatic filtering. Can. J. Fish. Aquat. Sci. 2017, 74, 1362–1374. [Google Scholar] [CrossRef]
- Sigsgaard, E.E.; Nielsen, I.B.; Carl, H.; Krag, M.A.; Knudsen, S.W.; Xing, Y.; Holm-Hansen, T.H.; Møller, P.R.; Thomsen, P.F. Seawater environmental DNA reflects seasonality of a coastal fish community. Mar. Biol. 2017, 164, 128. [Google Scholar] [CrossRef]
- Ling, J.Z.; Jiang, Y.Z.; Sun, P. Application and evaluation of environmental DNA technology in fish diversity research in Xiangshan Bay. J. Fish. Sci. China 2021, 28, 205–214. [Google Scholar]
- Shu, L.; Lin, J.Y.; Xu, Y. Investigating the fish diversity in erhai lake based on environmental dna metabarcoding. Acta Hydrobiol. Sin. 2020, 44, 1080–1086. [Google Scholar]
- Wang, X.; Lu, G.; Zhao, L.; Yang, Q.; Gao, T. Assessment of fishery resources using environmental DNA: Small yellow croaker (Larimichthys polyactis) in East China Sea. PLoS ONE 2020, 15, 0244495. [Google Scholar] [CrossRef]
- Pilliod, D.S.; Goldberg, C.S.; Arkle, R.S.; Waits, L.P. Estimating occupancy and abundance of stream amphibians using environmental DNA from filtered water samples. Can. J. Fish. Aquat. Sci. 2013, 70, 1123–1130. [Google Scholar] [CrossRef]
- Taberlet, P.; Bonin, A.; Zinger, L.; Coissac, E. Environmental DNA-for Biodiversity Research and Monitoring; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Deiner, K.; Fronhofer, E.A.; Mächler, E.; Walser, J.C.; Altermatt, F. Environmental DNA reveals that rivers are conveyer belts of biodiversity information. Nat. Commun. 2016, 7, 12544. [Google Scholar] [CrossRef]
- Gillet, B.; Cottet, M.; Destanque, T.; Kue, K.; Descloux, S.; Chanudet, V.; Hughes, S. Direct fishing and eDNA metabarcoding for biomonitoring during a 3-year survey significantly improves number of fish detected around a South East Asian Reservoir. PLoS ONE 2018, 13, e0208592. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, S. Species composition and diversity of fish in the Wujiang River. J. Fish. China 2023, 47, 143–153. [Google Scholar]
- Xioa, Q.; Yang, Z.; Tang, H.Y. Species diversity of fish and its conservation in the mainstream of the lower reaches of Wu River. Biodivers. Sci. 2015, 23, 499–506. [Google Scholar] [CrossRef]
- Shan, X.J.; Li, M.; Wang, W.J. Application of Environmental DNA Technology in Aquatic Ecosystem. Prog. Fish. Sci. 2018, 39, 23–29. [Google Scholar]
- Dejean, T.; Valentini, A.; Miquel, C.; Taberlet, P.; Bellemain, E.; Miaud, C. Improved detection of an alien invasive species through environmental DNA barcoding: The example of the American bullfrog Lithobates catesbeianus. J. Appl. Ecol. 2012, 49, 953–959. [Google Scholar] [CrossRef]
- Wang, R.X.; Yang, G.; Geng, Z. Application of environmental DNA technology in fish diversity analysis in the YANGTZE river estuary. Acta Hydrobiol. Sin. 2023, 47, 365–375. [Google Scholar]
- Wang, M.; Yang, X.; Wang, W. Fish diversity in ChongQing section of the national nature reserve for rare and endemic fish in the upper YANGTZE river based on eDNA technology. Acta Hydrobiol. Sin. 2022, 46, 2–16. [Google Scholar]
- Ba, J.W.; Chen, D.Q. Invasive fishes in Three Gorges Reservoir area and preliminary study on effects of fish invasion owing to impoundment. J. Lake Sci. 2012, 24, 185–189. [Google Scholar]
- Yan, T.M.; He, J.Y.; Luo, J. Assessment of ecosystem health on the upper and middle reaches of Nanhe River with the fish-based biotic integrity index. Freshw. Fish. 2021, 51, 3–12. [Google Scholar]
- Chen, Z.; Song, N.; Yuan, L.W. The eDNA collection method of Zhoushan coastal waters. Acta Hydrobiol. Sin. 2020, 44, 50–58. [Google Scholar]
- Strickler, K.M.; Fremier, A.K.; Goldberg, C.S. Quantifying effects of UV-B, temperature, and pH on eDNA degradation in aquatic microcosms. Biol. Conserv. 2015, 183, 85–92. [Google Scholar] [CrossRef]


| Order | Family | Genus | Species |
|---|---|---|---|
| Cypriniforms | Cyprinidae | Ctenopharyngodon | C. idella |
| Cyprinidae | Megalobrama | M. terminalis | |
| Cyprinidae | Megalobrama | M. amblycephala | |
| Cyprinidae | Hypophthalmichthys | H. molitrix | |
| Cyprinidae | Hypophthalmichthys | H. nobilis | |
| Cyprinidae | Procypris | P. rabaudi | |
| Cyprinidae | Percocypris | P. pingi | |
| Cyprinidae | Cyprinus | C. carpio | |
| Cyprinidae | Carassius | C. auratus | |
| Cyprinidae | Schizothorax | S. prenanti | |
| Cyprinidae | Schizothorax | S. kozlovi | |
| Cyprinidae | Schizothorax | S. davidi | |
| Cyprinidae | Acrossocheilus | A. longipinnis | |
| Cyprinidae | Acrossocheilus | A. yunnanensis | |
| Cyprinidae | Elopichthys | E. bambusa | |
| Cyprinidae | Squaliobarbus | S. curriculus | |
| Cyprinidae | Hemiculter | H. leucisculus | |
| Cyprinidae | Zacco | Z. platypus | |
| Cyprinidae | Opsariichthys | O. bidens | |
| Cyprinidae | Chanodichthys | C. mongolicus | |
| Cyprinidae | Rhodeus | R. ocellatus | |
| Cyprinidae | Hemiculter | H. leucisculus | |
| Cyprinidae | Zacco | Z. platypus | |
| Cyprinidae | Opsariichthys | O. bidens | |
| Cyprinidae | Chanodichthys | C. mongolicus | |
| Cyprinidae | Bangana | B. rendahli | |
| Cyprinidae | Spinibarbus | S. denticulatus | |
| Cyprinidae | Spinibarbus | S. sinensis | |
| Cyprinidae | Onychostoma | O. lini | |
| Cyprinidae | Varicorhinus | V. barbatulus | |
| Cyprinidae | Microphyogobio | M. kiatingensis | |
| Cyprinidae | Squalidus | S. wolterstorffi | |
| Cyprinidae | Abbottina | A. rivularis | |
| Cyprinidae | Pseudorasbora | P. parva | |
| Cobitis | Leptobotia | L. pellegrini | |
| Cobitis | Syncrossus | S. hymenophysa | |
| Cobitidae | Paramisgurnus | P. dabryanus | |
| Balitoridae | Beaufortia | B. kweichowensis | |
| Peciformes | Percichthyidae | Siniperca | S. undulata |
| Gobiidae | Rhinogobius | R. giurinus | |
| Cichlaidae | Oreochromis | O. niloticus | |
| Centrarchidae | Micropterus | M. salmoides | |
| Siluriformes | Bagridae | Pseudobagrus | P. emarginatus |
| Bagridae | Leiocassis | L. longirostris | |
| Bagridae | Hemibagrus | H. guttatus | |
| Cyprinodontiformes | Adrianichthyida | Oryzias | O. latipes |
| Synbranchiformes | Mastacembelidae | Sinobdella | S. sinensis |
| Sample | Chao1 | Shannon | ACE | Pielou | Simpson | Coverage |
|---|---|---|---|---|---|---|
| Z1 | 1862.410 | 4.488 | 1827.371 | 0.4253 | 0.1067 | 0.999460 |
| Z2 | 1130.423 | 2.6241 | 1118.466 | 0.2653 | 0.4473 | 0.999641 |
| Z3 | 1206.049 | 3.2862 | 1192.871 | 0.3322 | 0.3015 | 0.999621 |
| Z4 | 1542.363 | 4.0764 | 1515.621 | 0.3989 | 0.142 | 0.999461 |
| Z5 | 1969.734 | 4.8649 | 1958.943 | 0.4573 | 0.0822 | 0.999367 |
| Z6 | 2220.166 | 4.8164 | 2262.968 | 0.4446 | 0.0920 | 0.999486 |
| Z7 | 1396.617 | 4.2260 | 1400.638 | 0.4400 | 0.1261 | 0.999539 |
| Z8 | 1820.673 | 3.8412 | 1794.476 | 0.3658 | 0.2213 | 0.999374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Wen, J.; Kong, Q.; Zhou, X. Study on Fish Species Diversity in the Pingzhai Reservoir Based on Environmental DNA Technology. Fishes 2024, 9, 382. https://doi.org/10.3390/fishes9100382
Yuan J, Wen J, Kong Q, Zhou X. Study on Fish Species Diversity in the Pingzhai Reservoir Based on Environmental DNA Technology. Fishes. 2024; 9(10):382. https://doi.org/10.3390/fishes9100382
Chicago/Turabian StyleYuan, Jingjing, Jing Wen, Qiuhong Kong, and Xianjun Zhou. 2024. "Study on Fish Species Diversity in the Pingzhai Reservoir Based on Environmental DNA Technology" Fishes 9, no. 10: 382. https://doi.org/10.3390/fishes9100382
APA StyleYuan, J., Wen, J., Kong, Q., & Zhou, X. (2024). Study on Fish Species Diversity in the Pingzhai Reservoir Based on Environmental DNA Technology. Fishes, 9(10), 382. https://doi.org/10.3390/fishes9100382





