Feed Additives Based on N. gaditana and A. platensis Blend Improve Quality Parameters of Aquacultured Gilthead Seabream (Sparus aurata) Fresh Fillets
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish Maintenance and Experimental Design
2.3. Fillet Proximate Composition and Fatty Acid Analysis
2.4. Post-Mortem Changes during Cold Storage
2.4.1. Lipid Oxidation
2.4.2. Instrumental Color Determination
2.4.3. Texture Profile Analysis (TPA)
2.4.4. pH and Water Holding Capacity (WHC)
2.5. Statistics
3. Results
3.1. Growth Performance and Body Composition
3.2. Muscle Fatty Acid Profile
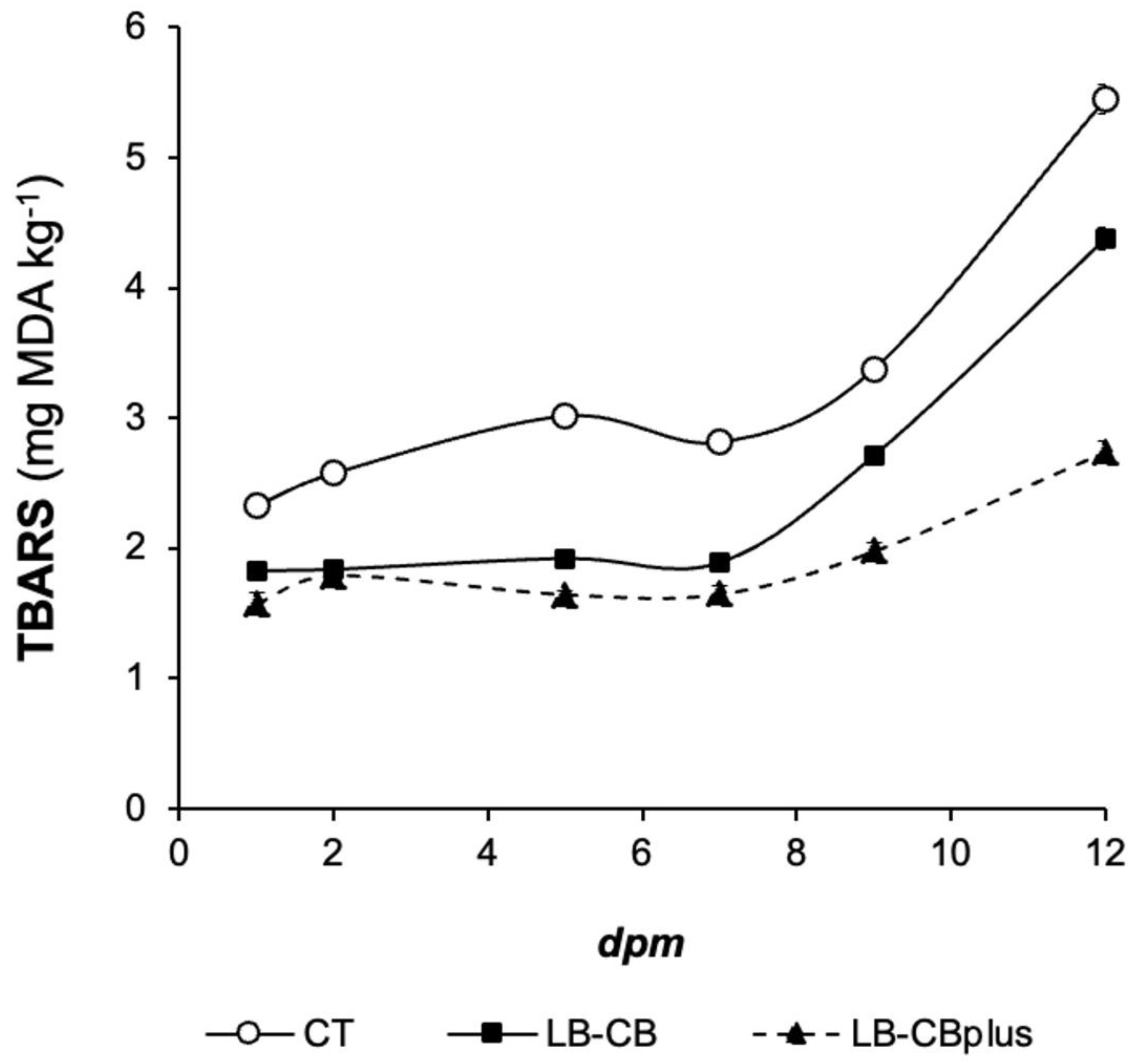
3.3. TBARS Content
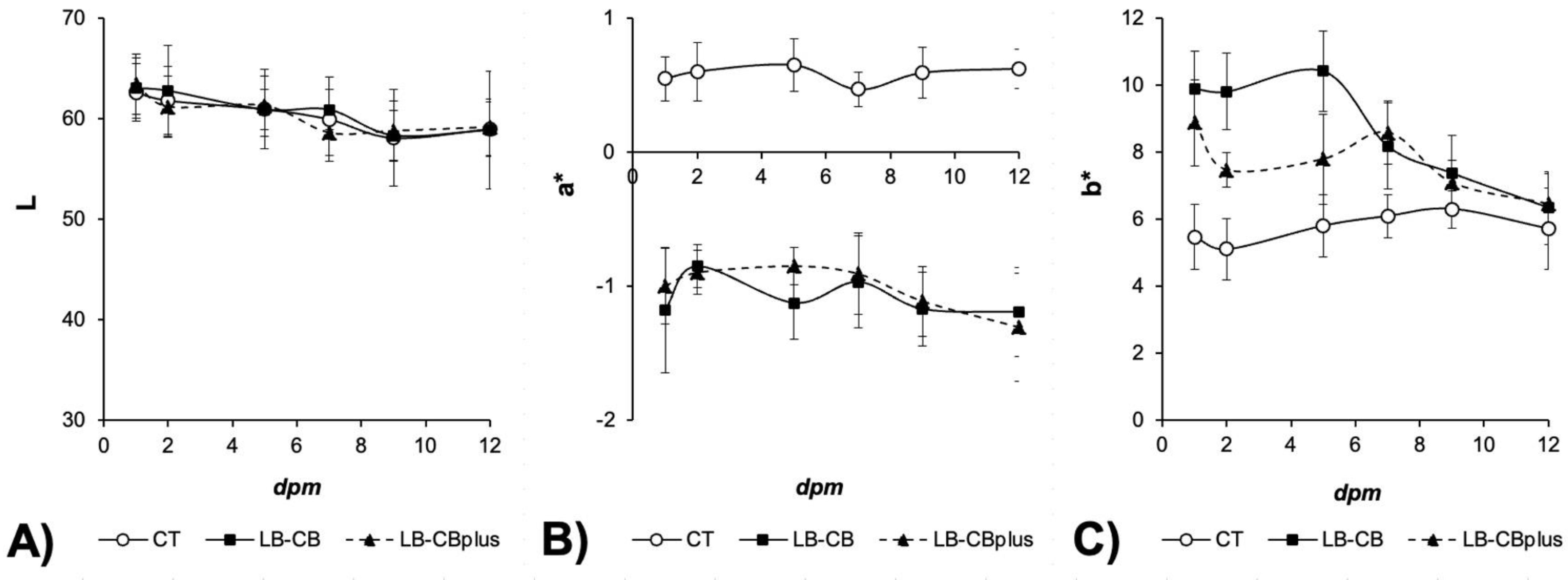
3.4. Instrumental Color Assessment
3.5. TPA Determinations
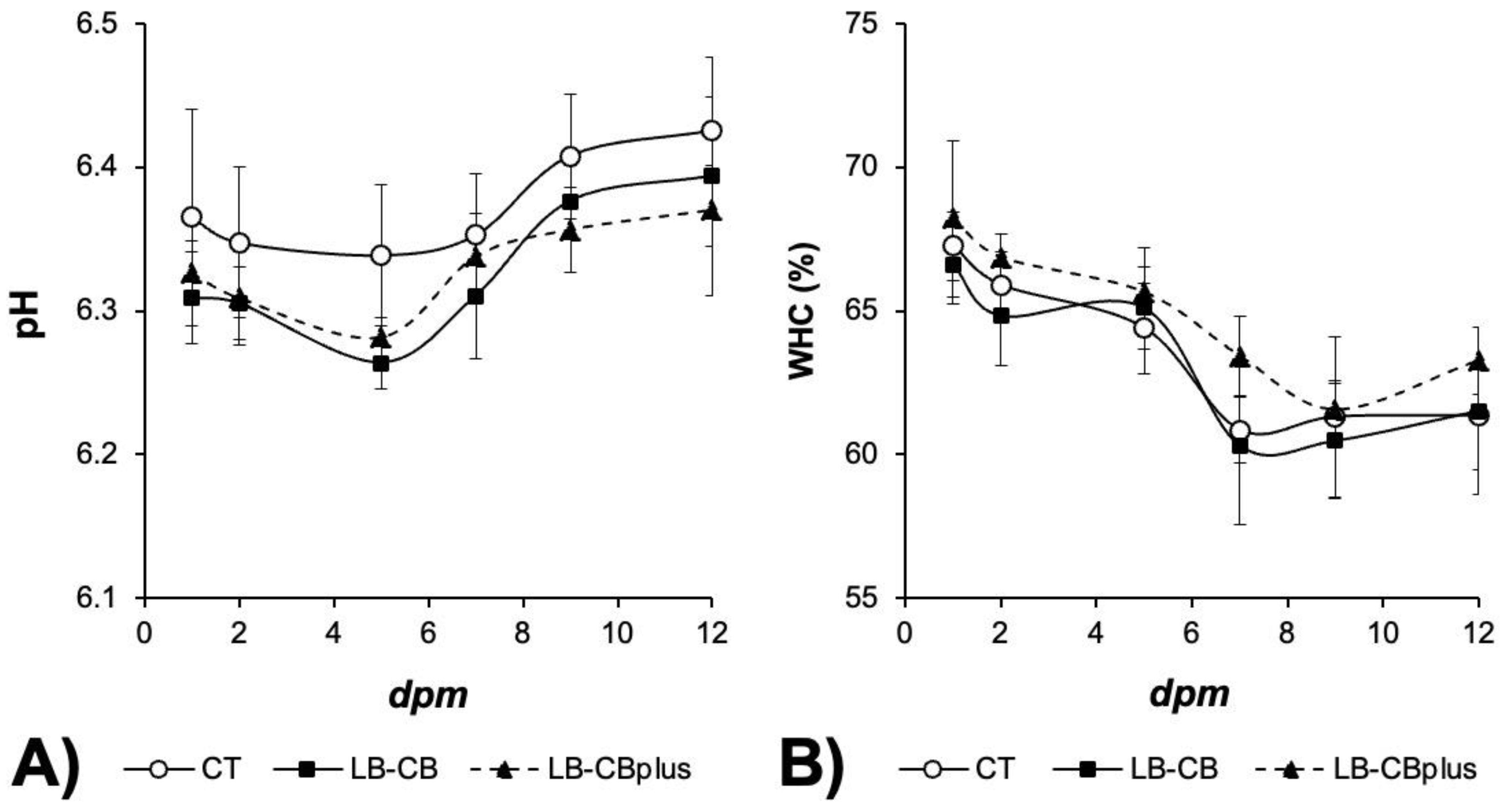
3.6. pH and WHC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Esteban, M.A. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel bioactive compounds from marine sources as a tool for functional food development. Front. Mar. Sci. 2022, 9, 10–3389. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Chowdhury, M.K.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Siddik, M.A.; Sørensen, M.; Islam, S.M.; Saha, N.; Rahman, M.A.; Francis, D.S. Expanded utilisation of microalgae in global aquafeeds. Rev. Aquac. 2024, 16, 6–33. [Google Scholar] [CrossRef]
- Magnoni, L.J.; Martos-Sitcha, J.A.; Queiroz, A.; Calduch-Giner, J.A.; Gonçalves, J.F.; Rocha, C.M.R.; Abreu, H.T.; Schrama, J.W.; Ozorio, R.O.A.; Pérez-Sánchez, J. Dietary supplementation of heat-treated Gracilaria and Ulva seaweeds enhanced acute hypoxia tolerance in gilthead seabream (Sparus aurata). Biol. Open 2017, 6, 897–908. [Google Scholar] [PubMed]
- Matos, E.; Dias, J.; Dinis, M.T.; Silva, T.S. Sustainability vs. quality in in gilthead seabream (Sparus aurata L.) farming: Are trade-offs inevitable? Rev. Aquac. 2017, 9, 388–409. [Google Scholar] [CrossRef]
- Ambati, R.R.; Siew Moi, P.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Saéz, M.I.; Galafat, A.; Martínez, T.F.; Alarcón, F.J. Chapter 10—Practical approach to the use of microalgae in aquaculture feeds. In Sustainable Industrial Processes Based on Microalgae; Lafarga, T., Acién, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 209–233. [Google Scholar] [CrossRef]
- Sáez, M.I.; Galafat, A.; Suárez, M.D.; Chaves-Pozo, E.; Arizcun, M.; Ayala, M.D.; Alarcón, F.J.; Martínez, T.F. Effects of raw and hydrolysed Nannochloropsis gaditana biomass included at low level in finishing diets for gilthead seabream (Sparus aurata) on fillet quality and shelf life. J. Appl. Phycol. 2023, 35, 1163–1181. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Soltani, M.; Heidarieh, M.; Ghorbani, M. Role of dietary microalgae on fish health and fillet quality: Recent insights and future prospects. Fishes 2024, 9, 26. [Google Scholar] [CrossRef]
- Galafat, A.; Vizcaíno, A.J.; Sáez, M.I.; Martínez, T.F.; Arizcun, M.; Chaves-Pozo, E.; Alarcón, F.J. Assessment of dietary inclusion of crude or hydrolysed Arthrospira platensis biomass in starter diets for gilthead seabream (Sparus aurata). Aquaculture 2022, 548, 737680. [Google Scholar] [CrossRef]
- Sáez, M.I.; Galafat, A.; Vizcaíno, A.J.; Chaves-Pozo, E.; Ayala, M.D.; Arizcun, M.; Alarcón, F.J.; Suárez, M.D.; Martínez, T.F. Evaluation of Nannochloropsis gaditana raw and hydrolysed biomass at low inclusion level as dietary functional additive for gilthead seabream (Sparus aurata) juveniles. Aquaculture 2022, 556, 738288. [Google Scholar] [CrossRef]
- Molina-Roque, L.; Bárany, A.; Sáez, M.I.; Alarcón, F.J.; Tapia, S.T.; Fuentes, J.; Mancera, J.M.; Perera, E.; Martos-Sitcha, J.A. Biotechnological treatment of microalgae enhances growth performance, hepatic carbohydrate metabolism and intestinal physiology in gilthead seabream (Sparus aurata) juveniles close to commercial size. Aquac. Rep. 2022, 25, 101248. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Folch, J. Simple method for the isolation and purification of total lipids form animal tissue. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, J.; Belarbi, E.H.; Sánchez, J.L.G.; Alonso, D.L. Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotech. Tech. 1998, 12, 689–691. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Senso, L.; Suarez, M.D.; Ruiz-Cara, T.; Garcıa-Gallego, M. The possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata). Food Chem. 2007, 101, 298–307. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- CIE. Colorimetry Commission International de l’Eclairage, 2nd ed.; Publication CIE 15 2 Vienna; CIE: Cambridge, UK, 1986. [Google Scholar]
- Bourne, M.C. Texture Profile analysis. Food Tech. 1978, 32, 62–66. [Google Scholar]
- Suárez, M.D.; Martínez, T.F.; Sáez, M.I.; Morales, A.E.; García-Gallego, M. Effects of dietary restriction on post-mortem changes in white muscle of sea bream (Sparus aurata). Aquaculture 2010, 307, 49–55. [Google Scholar] [CrossRef]
- Tongsiri, S.; Mang-Amphan, K.; Peerapornpisal, Y. Effect of replacing fishmeal with Spirulina on growth, carcass composition and pigment of the Mekong giant catfish. Asian J. Agric. Sci. 2010, 2, 106–110. [Google Scholar]
- Teimouri, M.; Amirkolaie, A.K.; Yeganeh, S. The effects of Spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 396, 14–19. [Google Scholar] [CrossRef]
- Roy, S.S.; Pal, R. Microalgae in aquaculture: A review with special references to nutritional value and fish dietetics. Proc. Zool. Soc. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Amer, S. Effect of Spirulina platensis as feed supplement on growth performance, immune response and antioxidant status of mono-sex Nile Tilapia (Oreochromis niloticus). Benha Vet. Med. J. 2016, 30, 1–10. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Mørkøre, T.; Nengas, I.; Berge, R.K.; Sweetman, J. Microalgae and organic minerals enhance lipid retention efficiency and fillet quality in Atlantic salmon (Salmo salar L.). Aquaculture 2016, 451, 47–57. [Google Scholar] [CrossRef]
- Sáez, M.I.; Suárez, M.D.; Cárdenas, S.; Martínez, T.F. Influence of freezing and freezing-thawing cycles on textural and biochemical changes of meagre (Argyrosomus regius L.) fillets during further cold storage. Int. J. Food Prop. 2014, 18, 1635–1647. [Google Scholar] [CrossRef][Green Version]
- Batista, S.; Pintado, M.; Marques, A.; Abreu, H.; Silva, J.L.; Jessen, F.; Tulli, F.; Valente, L.M.P. Use of technological processing of seaweed and microalgae as strategy to improve their apparent digestibility coefficients in European seabass (Dicentrarchus labrax) juveniles. J. Appl. Phycol. 2020, 32, 3429–3446. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Muranaka, T.; Miki, W.; Yamaguchi, K.; Konosu, S.; Watanabe, T. Pigmentation of cultured sweet smelt fed diets supplemented with a blue-green alga Spirulina maxima. Nippon Suisan Gakk. 1987, 53, 433–438. [Google Scholar] [CrossRef][Green Version]
- Mustafa, M.G.; Umino, T.; Nakagawa, H. The effect of Spirulina feeding on muscle protein deposition in red sea bream, Pagrus major. J. Appl. Ichthyol. 1994, 10, 141–145. [Google Scholar] [CrossRef]
- Teimouri, M.; Yeganeh, S.; Amirkolaie, A.K. The effects of Spirulina platensis meal on proximate composition, fatty acid profile and lipid peroxidation of rainbow trout (Oncorhynchus mykiss) muscle. Aquac. Nutr. 2016, 22, 559–566. [Google Scholar] [CrossRef]
- Qiao, H.; Hu, D.; Ma, J.; Wang, X.; Wu, H.; Wang, J. Feeding effects of the microalga Nannochloropsis sp. on juvenile turbot (Scophthalmus maximus L.). Algal Res. 2019, 41, 101540. [Google Scholar] [CrossRef]
- Sales, R.; Galafat, A.; Vizcaíno, A.J.; Sáez, M.I.; Martínez, T.F.; Cerón-García, M.C.; Alarcón, F.J. Effects of dietary use of two lipid extracts from the microalgae Nannochloropsis gaditana (Lubián, 1982) alone and in combination on growth and muscle composition in juvenile gilthead seabream, Sparus aurata. Algal Res. 2021, 10, 2270. [Google Scholar] [CrossRef]
- Agboola, J.O.; Teuling, E.; Wierenga, P.A.; Gruppen, H.; Schrama, J.W. Cell wall disruption: An effective strategy to improve the nutritive quality of microalgae in African catfish (Clarias gariepinus). Aquac. Nutr. 2019, 25, 783–797. [Google Scholar] [CrossRef]
- Galafat, A.; Vizcaíno, A.J.; Sáez, M.I.; Martínez, T.F.; Jérez-Cepa, I.; Mancera, J.M.; Alarcón, F.J. Evaluation of Arthrospira sp. enzyme hydrolysate as dietary additive in gilthead seabream (Sparus aurata) juveniles. J. Appl. Phycol. 2020, 32, 3089–3100. [Google Scholar] [CrossRef]
- Cerezo-Ortega, I.M.; Di Zeo-Sánchez, D.E.; García-Márquez, J.; Ruiz-Jarabo, I.; Sáez-Casado, M.I.; Balebona, M.C.; Moriñigo, M.A.; Tapia-Paniagua, S.T. Microbiota composition and intestinal integrity remain unaltered after the inclusion of hydrolysed Nannochloropsis gaditana in Sparus aurata diet. Sci. Rep. 2021, 11, 18779. [Google Scholar] [CrossRef] [PubMed]
- James, R.; Sampath, K.; Thangarathinam, R.; Vasudevan, I. Effects of dietary Spirulina level on growth, fertility, coloration and leucocyte count in red swordtail, Xiphophorus helleri. Isr. J. Aquac.-Bamidgeh 2006, 58, 97–104. [Google Scholar] [CrossRef]
- Grigorakis, K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: A review. Aquaculture 2007, 272, 55–75. [Google Scholar] [CrossRef]
- Gong, Y.; Bandara, T.; Huntley, M.; Johnson, Z.I.; Días, J.; Dahle, D.; Sørensen, M.; Kiron, V. Microalgae Scenedesmus sp. as a potential ingredient in low fishmeal diets for Atlantic salmon (Salmo salar L.). Aquaculture 2019, 501, 455–464. [Google Scholar] [CrossRef]
- Harel, M.; Koven, W.; Lein, I.; Bar, Y.; Behrens, P.; Stubblefield, J.; Zohar, J.; Place, A.R. Advanced DHA, EPA and ArA enrichment materials for marine aquaculture using single cell heterotrophs. Aquaculture 2002, 213, 347–362. [Google Scholar] [CrossRef]
- Liu, C.; Palihawadana, A.M.; Nadanasabesan, N.; Vasanth, G.K.; Vatsos, I.N.; Días, J.; Valente, L.M.P.; Micallef, G.; Sørensen, M.; Kiron, V. Utilization of Nannochloropsis oceanica in plant-based feeds by Atlantic salmon (Salmo salar). Aquaculture 2022, 561, 738651. [Google Scholar] [CrossRef]
- Grigorakis, K.; Alexis, M.N.; Taylor, A.; Hole, M. Comparison of wild and cultured gilthead sea bream, (Sparus aurata): Composition, appearance and seasonal variations. Int. J. Food Sci. Tech. 2002, 37, 477–484. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Montero, D.; Robaina, L.; Caballero, M.J.; Rosenlund, G.; Gines, R. Alterations in fillet fatty acid profile and flesh quality in gilthead seabream (Sparus aurata) fed vegetable oils for a long term period. Recovery of fatty acid profiles by fish oil feeding. Aquaculture 2005, 250, 431–444. [Google Scholar] [CrossRef]
- Rodríguez, C.; Acosta, C.; Badía, P.; Cejas, J.R.; Santamaría, F.J.; Lorenzo, A. Assessment of lipid and essential fatty acids requirements of black seabream (Spondyliosoma cantharus) by comparison of lipid composition in muscle and liver of wild and captive adult fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Bronner, L.; Willett, W.C.; Stampfer, M.J.; Rexrode, K.M.; Christine, M.; Albert, C.M.; Hunter, D.; Manson, J.E. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002, 287, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Celik, M. Seasonal changes in the proximate chemical compositions and fatty acids of chub mackerel (Scomber japonicus) andhorse mackerel (Trachurus trachurus) from the northeastern Mediterranean Sea. Int. J. Food Sci. Technol. 2008, 43, 933–938. [Google Scholar] [CrossRef]
- Ghaeni, M.; Ghahfarokhi, K.N.; Zaheri, L. Fatty acids profile, Atherogenic (IA) and thrombogenic (IT) health lipid indices in Leiognathusbindus and Upeneussulphureus. J. Mar. Sci. Res. Dev. 2013, 3, 138. [Google Scholar] [CrossRef]
- Archile-Contreras, A.C.; Purslow, P.P. Oxidative stress may affect meat quality by interfering with collagen turnover by muscle fibroblasts. Food Res. Int. 2011, 44, 582–588. [Google Scholar] [CrossRef]
- Hosseini, S.V.; Abedian-Kenari, A.; Rezaei, M.; Nazari, R.M. Influence of the in vivo addition of alpha-tocopherol acetate with three lipid sources on the lipid oxidation and fatty acid composition of Beluga sturgeon, Huso huso, during frozen storage. Food Chem. 2010, 118, 341–348. [Google Scholar] [CrossRef]
- Secci, G.; Parisi, G. From farm to fork: Lipid oxidation in fish products. A review. Ital. J. Anim. Sci. 2016, 15, 124–136. [Google Scholar] [CrossRef]
- Almendinger, M.; Saalfrank, F.; Rohn, S.; Kurth, E.; Springer, M.; Pleissner, D. Characterization of selected microalgae and cyanobacteria as sources of compounds with antioxidant capacity. Algal Res. 2021, 53, 102168. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Xu, W.; Han, D.; Xie, S.; Jin, J.; Zhu, X. Effects of dietary Arthrospira platensis supplementation on the growth, pigmentation, and antioxidation in yellow catfish (Pelteobagrus fulvidraco). Aquaculture 2019, 510, 267–275. [Google Scholar] [CrossRef]
- Sefc, K.M.; Brown, A.C.; Clotfelter, E.D. Carotenoid-based coloration in cichlid fishes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 173, 42–51. [Google Scholar] [CrossRef]
- Nagappan, S.; Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Mahata, C.; Kumar, G. Potential of microalgae as a sustainable feed ingredient for aquaculture. J. Biotechnol. 2021, 341, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chang, Y.; Ye, Y.; Ma, Z.; Liang, Y.; Li, T.; Luo, L. The effect of dietary pigments on the coloration of Japanese ornamental carp koi (Cyprinus carpio L.). Aquaculture 2012, 342, 62–68. [Google Scholar] [CrossRef]
- Ansarifard, F.; Rajabi Islami, H.; Shamsaie Mehrjan, M.; Soltani, M. Effects of Arthrospira platensis on growth, skin color and digestive enzymes of Koi, Cyprinus carpio. Iran. J. Fish. Sci. 2018, 17, 381–393. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–nutritious, sustainable aqua-and animal feed source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Chen, Z.; Yuan, L.; Liu, H.; Han, D.; Xie, S. Effects of dietary whole and defatted Arthrospira platensis (Cyanobacterium) on growth, body composition and pigmentation of the yellow catfish Pelteobagrus fulvidraco. J. Appl. Phycol. 2021, 33, 2251–2259. [Google Scholar] [CrossRef]
- Cerón-García, M.C.; González-López, C.; Camacho-Rodríguez, J.; López-Rosales, L.; García-Camacho, F.; Molina-Grima, E. Maximizing carotenoid extraction from microalgae used as food additives and determined by liquid chromatography (HPLC). Food Chem. 2018, 257, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Cardinaletti, G.; Messina, M.; Bruno, M.; Tulli, F.; Poli, B.M.; Giorgi, G.; Chini-Zittelli, G.; Tredici, M.; Tibaldi, E. Effects of graded levels of a blend of Tisochrysis lutea and Tetraselmis suecica dried biomass on growth and muscle tissue composition of European sea bass (Dicentrarchus labrax) fed diets low in fish meal and oil. Aquaculture 2018, 85, 173–182. [Google Scholar] [CrossRef]
- Mukherjee, P.; Nandi, C.; Khatoon, N.; Pal, R. Mixed algal diet for skin colour enhancement of ornamental fishes. J. Algal Biomass Util. 2015, 6, 35–46. [Google Scholar]
- Silva-Brito, F.; Alexandrino, D.A.M.; Jia, Z.; Mo, Y.; Kijjoa, A.; Abreu, H.; Carvalho, M.F.; Ozorio, R.; Magnoni, L. Fish performance, intestinal bacterial community, digestive function and skin and fillet attributes during cold storage of gilthead seabream (Sparus aurata) fed diets supplemented with Gracilaria by-products. Aquaculture 2021, 541, 736808. [Google Scholar] [CrossRef]
- Ayala, M.D.; Galián, C.; Fernández, V.; Chaves-Pozo, E.; García de la Serrana, D.; Sáez, M.I.; Galafat, A.; Alarcón, F.J.; Martínez, T.F.; Arizcun, M. Influence of low dietary inclusion of the microalga Nannochloropsis gaditana (Lubián 1982) on performance, fish morphology, and muscle growth in juvenile gilthead seabream (Sparus aurata). Animals 2020, 10, 2270. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-H.; Qu, J.-H.; Sun, D.-W.; Zeng, X.-A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, F.; Cardinal, M.; Bugeon, J.; Labbe, L.; Medale, F.; Quillet, E. Selection for muscle fat content and triploidy affect flesh quality in pan-size rainbow trout, Oncorhynchus mykiss. Aquaculture 2015, 448, 569–577. [Google Scholar] [CrossRef]
- El-Sayed, A.M. Evaluation of soybean meal, spirulina meal and chicken offtal meal as protein sources for silver seabream (Rhabdosargus sarba) fingerlings. Aquaculture 1994, 127, 169–176. [Google Scholar] [CrossRef]
- Liao, W.L.; Takeuchi, T.; Watanabe, T.; Yamaguchi, K. Effect of dietary Spirulina supplementation on extractive nitrogenous constituents and sensory test of cultured striped jack. J. Tokyo Univ. Fish. 1990, 77, 241–246. [Google Scholar]
- Watanabe, T.; Liao, W.L.; Takeuchi, T.; Yamamoto, H. Effect of dietary Spirulina supplementation on growth performance and flesh lipids of cultured striped jack. J. Tokyo Univ. Fish. 1990, 77, 231–239. [Google Scholar]
- Watanabe, T.; Takeuchi, T.; Okamoto, N.; Satoh, S.; Iso, N.; Sakamoto, H.; Arakawa, T. Improvement of flesh quality of cultured striped jack with a newly developed soft-dry pellet. J. Tokyo Univ. Fish. 1993, 80, 19–29. [Google Scholar]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; FAO Fisheries Technical Paper No. 348; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 1995. [Google Scholar]
- Goulas, A.E.; Kontominas, M.G. Combined effect of light salting, modified atmosphere packaging and oregano essential oil on the shelf-life of sea bream (Sparus aurata): Biochemical and sensory attributes. Food Chem. 2007, 100, 287–296. [Google Scholar] [CrossRef]
- Duun, A.S.; Rustad, T. Quality changes during superchilled storage of cod (Gadus morhua) fillets. Food Chem. 2007, 105, 1067–1107. [Google Scholar] [CrossRef]


| Diets | |||
|---|---|---|---|
| Ingredient Composition (g 100 g−1 Dry Matter) | CT | LB-CB | LB-CBplus |
| Fish meal LT94 1 | 15.0 | 15.0 | 15.0 |
| Lysine 2 | 1.2 | 1.2 | 1.2 |
| Methionine 2 | 0.5 | 0.5 | 0.5 |
| Squid meal 3 | 1.0 | 1.0 | 1.0 |
| Fish meal hydrolysate 4 | 0.5 | 0.5 | 0.5 |
| LB-ChromaBream 5 | - | 10 | - |
| LB-ChromaBream-plus 5 | - | - | 10 |
| Wheat gluten 6 | 15.0 | 13.0 | 13.0 |
| Soybean protein concentrate 7 | 35.0 | 33.0 | 33.0 |
| Fish oil 8 | 5.0 | 4.5 | 4.5 |
| Soybean oil 9 | 8.0 | 7.2 | 7.2 |
| Soybean lecithin 10 | 1.0 | 1.0 | 1.0 |
| Wheat meal 11 | 12.7 | 8.0 | 8.0 |
| Choline chloride 12 | 0.5 | 0.5 | 0.5 |
| Betain 13 | 0.5 | 0.5 | 0.5 |
| Vitamin and mineral premix 14 | 2.1 | 2.1 | 2.1 |
| Vitamin C 15 | 0.1 | 0.1 | 0.1 |
| Guar gum 16 | 2.0 | 2.0 | 2.0 |
| Crude protein | 42.5 ± 0.8 | 42.1 ± 1.2 | 43.0 ± 1.7 |
| Crude lipid | 14.2 ± 0.3 | 14.5± 0.2 | 13.8 ± 0.9 |
| Ash | 6.0 ± 0.5 | 5.8 ± 0.1 | 5.9 ± 0.0 |
| Experimental Diets | ||||
|---|---|---|---|---|
| CT | LB-CB | LB-CBplus | p | |
| 14:0 | 1.43 ± 0.01 a | 1.62 ± 0.01 b | 1.58 ± 0.02 b | 0.001 |
| 16:0 | 14.09 ± 0.05 a | 14.79 ± 0.06 b | 14.66 ± 0.08 b | 0.003 |
| 18:00 | 4.44 ± 0.04 b | 4.3 ± 0.01 a | 4.35 ± 0.02 ab | 0.027 |
| 16:1n-7 | 2.14 ± 0.04 | 1.76 ± 0.67 | 2.26 ± 0.02 | n.s. |
| 18:1n-7 | 1.23 ± 0.04 | 1.25 ± 0.01 | 1.26 ± 0.01 | n.s. |
| 18:1n-9 | 21.03 ± 0.06 b | 20.42 ± 0.09 a | 20.5 ± 0.06a | 0.006 |
| 20:1n-9 | 0.05 ± 0.07 | 0.05 ± 0.07 | 0.05 ± 0.07 | n.s. |
| 18:2n-6 | 37.86 ± 0.12 b | 36.53 ± 0.09 a | 36.57 ± 0.26 a | 0.008 |
| 18:3n-3 | 0.96 ± 0.14 a | 1.32 ± 0.01b | 1.24 ± 0.00 ab | 0.043 |
| 16:2n4 | 0.29 ± 0.06 | 0.3 ± 0.06 | 0.29 ± 0.06 | n.s. |
| 16:3n4 | 0.44 ± 0.06 | 0.44 ± 0.06 | 0.44 ± 0.06 | n.s. |
| 18:4n-3 | 0.86 ± 0.02 | 0.82 ± 0.01 | 0.85 ± 0.01 | n.s. |
| 20:4n-6 | 0.73 ± 0.22 | 0.56 ± 0.01 | 0.57 ± 0.02 | n.s. |
| 20:4n-3 | 0.15 ± 0.01 | 0.14 ± 0.02 | 0.14 ± 0.01 | n.s. |
| 20:5n-3 (EPA 1) | 2.99 ± 0.01 | 2.98 ± 0.01 | 2.85 ± 0.20 | n.s. |
| 22:5n-3 | 0.38 ± 0.01 | 0.38 ± 0.01 | 0.38 ± 0.01 | n.s. |
| 22:6n-3 (DHA 2) | 7.49 ± 0.12 | 7.37 ± 0.03 | 7.44 ± 0.07 | n.s. |
| ΣSFAs 3 | 19.96 ± 0.02 a | 20.71 ± 0.07 b | 20.59 ± 0.07 b | 0.002 |
| ΣMUFAs 4 | 24.45 ± 0.01 | 23.48 ± 0.64 | 24.07 ± 0.02 | n.s. |
| ΣPUFAs 5 | 56.3 ± 0.09 b | 54.84 ± 0.09 a | 54.84 ± 0.53 a | 0.029 |
| ΣPUFAs n-3 | 12.83 ± 0.27 | 13.02 ± 0.06 | 12.91 ± 0.27 | n.s. |
| ΣPUFAs n-6 | 38.6 ± 0.34 b | 37.08 ± 0.1 a | 37.15 ± 0.29 a | n.s. |
| n-3/n-6 | 0.33 ± 0.01 | 0.35 ± 0.00 | 0.35 ± 0.00 | n.s. |
| EPA/DHA | 0.40 ± 0.01 | 0.40 ± 0.00 | 0.38 ± 0.02 | n.s. |
| PUFA/SFA | 2.82 ± 0.01 b | 2.64 ± 0.01 a | 2.66 ± 0.01 a | 0.006 |
| Parameters | CT | LB-CB | LB-CBplus | p |
|---|---|---|---|---|
| Initial weight (g) | 182.50 ± 0.16 | 182.60 ± 0.30 | 182.60 ± 0.17 | n.s. |
| Final weight (g) | 233.0 ± 0.66 a | 240.8 ± 3.20 ab | 247.7 ± 2.42 b | 0.013 |
| SGR * (%) | 0.59 ± 0.01 a | 0.67 ± 0.03 ab | 0.74 ± 0.02 b | 0.008 |
| Fillet yield (%) | 60.41 ± 2.43 | 59.96 ± 1.64 | 59.12 ± 2.50 | n.s. |
| CT | LB-CB | LB-CBplus | p | |
|---|---|---|---|---|
| Crude protein | 19.82 ± 0.26 a | 20.13 ± 0.12 a | 20.95 ± 0.20 b | 0.012 |
| Crude lipid | 3.26 ± 0.03 b | 3.01 ± 0.06 a | 2.88 ± 0.03 a | <0.001 |
| Ash | 6.51 ± 0.02 | 6.53 ± 0.07 | 6.51 ± 0.13 | n.s. |
| Moisture | 71.91 ± 0.56 | 71.60 ± 0.72 | 70.86 ± 0.52 | n.s. |
| Fatty Acids (%) | CT | LB-CB | LB-CBplus | p |
|---|---|---|---|---|
| 14:0 | 2.84 ± 0.02 c | 2.77 ± 0.01 b | 2.64 ± 0.01 a | <0.001 |
| 16:0 | 15.58 ± 0.06 a | 15.85 ± 0.11 b | 15.90 ± 0.02 b | 0.043 |
| 18:00 | 3.91 ± 0.01 ab | 3.88 ± 0.04 a | 3.99 ± 0.00 b | 0.049 |
| 16:1n-7 | 5.30 ± 0.05 | 5.01 ± 0.24 | 4.95 ± 0.01 | n.s. |
| 18:1n-7 | 2.92 ± 0.00 b | 2.89 ± 0.02 b | 2.78 ± 0.00 a | 0.003 |
| 18:1n-9 | 25.36 ± 0.12 b | 25.32 ± 0.09 b | 24.44 ± 0.06 a | 0.003 |
| 20:1n-9 | 2.16 ± 0.01 | 1.20 ± 0.84 | 1.77 ± 0.10 | n.s. |
| 18:2n-6 | 14.64 ± 0.07 a | 14.98 ± 0.05 b | 15.94 ± 0.00 c | <0.001 |
| 18:3n-3 | 2.14 ± 0.05 ab | 2.20 ± 0.04 b | 2.04 ± 0.01 a | 0.043 |
| 16:2n4 | 0.42 ± 0.22 | 0.55 ± 0.01 | 0.53 ± 0.02 | n.s. |
| 16:3n4 | 0.53 ± 0.01 | 0.54 ± 0.00 | 0.52 ± 0.00 | n.s. |
| 18:4n-3 | 0.73 ± 0.01 | 1.30 ± 0.70 | 0.72 ± 0.00 | n.s. |
| 20:4n-6 | 0.96 ± 0.01 | 0.92 ± 0.07 | 0.94 ± 0.02 | n.s. |
| 20:4n-3 | 4.80 ± 0.01 a | 5.01 ± 0.02 b | 5.04 ± 0.03 b | 0.003 |
| 20:5n-3 (EPA 1) | 1.25 ± 0.08 a | 1.63 ± 0.07 b | 1.76 ± 0.14 b | 0.027 |
| 22:5n-3 | 2.48 ± 0.01 c | 2.34 ± 0.02 b | 2.26 ± 0.00 a | <0.001 |
| 22:6n-3 (DHA 2) | 10.24 ± 0.02 a | 10.17 ± 0.11 a | 10.99 ± 0.03 b | 0.002 |
| ΣSFAs 3 | 22.34 ± 0.09 | 22.51 ± 0.15 | 22.53 ± 0.03 | n.s. |
| ΣMUFAs 4 | 35.73 ± 0.18 | 34.42 ± 0.97 | 33.94 ± 0.03 | n.s. |
| ΣPUFAs 5 | 39.4 ± 0.15 a | 39.74 ± 0.03 b | 41.46 ± 0.04 c | <0.001 |
| ΣPUFAs n3 | 21.65 ± 0.07 a | 22.64 ± 0.09 b | 22.82 ± 0.12 b | <0.001 |
| ΣPUFAs n6 | 15.6 ± 0.08 a | 15.90 ± 0.02 b | 16.88 ± 0.02 c | <0.001 |
| n3/n6 | 1.39 ± 0 | 1.42 ± 0.06 | 1.35 ± 0.01 | n.s. |
| EPA/DHA | 0.12 ± 0.01 a | 0.16 ± 0.01 b | 0.16 ± 0.01 b | 0.035 |
| AI 6 | 0.38 ± 0.01 b | 0.38 ± 0.01 b | 0.36 ± 0.01 a | 0.027 |
| TI 7 | 0.24 ± 0.01 | 0.24 ± 0.01 | 0.24 ± 0.01 | n.s. |
| dpm | CT | LB-CB | LB-CBplus | p | |
|---|---|---|---|---|---|
| Springiness (mm) | 1 | 0.78 ± 0.07 | 0.79 ± 0.04 | 0.84 ± 0.04 2 | n.s. |
| 2 | 0.81 ± 0.04 | 0.77 ± 0.04 | 0.84 ± 0.05 12 | n.s. | |
| 5 | 0.75 ± 0.04 | 0.77 ± 0.04 | 0.78 ± 0.01 1 | n.s. | |
| 7 | 0.75 ± 0.03 | 0.76 ± 0.05 | 0.77 ± 0.04 12 | n.s. | |
| 9 | 0.72 ± 0.04 a | 0.73 ± 0.05 ab | 0.81 ± 0.02 b,12 | 0.045 | |
| 12 | 0.77 ± 0.03 | 0.77 ± 0.02 | 0.76 ± 0.04 12 | n.s. | |
| P | n.s. | n.s. | 0.017 | ||
| Cohesiveness | 1 | 0.42 ± 0.02 2 | 0.41 ± 0.03 2 | 0.45 ± 0.02 3 | n.s. |
| 2 | 0.42 ± 0.02 2 | 0.40 ± 0.02 2 | 0.42 ± 0.02 23 | n.s. | |
| 5 | 0.39 ± 0.04 12 | 0.39 ± 0.00 12 | 0.39 ± 0.03 12 | n.s. | |
| 7 | 0.38 ± 0.01 12 | 0.37 ± 0.02 1 | 0.37 ± 0.02 1 | n.s. | |
| 9 | 0.38 ± 0.01 12 | 0.37 ± 0.03 1 | 0.37 ± 0.06 1 | n.s. | |
| 12 | 0.36 ± 0.01 1 | 0.37 ± 0.02 1 | 0.37 ± 0.02 1 | n.s. | |
| P | 0.015 | 0.024 | 0.009 | ||
| Gumminess (N/mm2) | 1 | 9.08 ± 0.44 a,3 | 10.19 ± 0.74 b,2 | 12.63 ± 0.86 b,3 | <0.001 |
| 2 | 8.20 ± 0.41 a,23 | 8.83 ± 0.38 ab,12 | 10.41 ± 0.83 b,23 | 0.001 | |
| 5 | 7.40 ±1.13 12 | 7.04 ± 0.41 12 | 7.42 ±0.82 12 | n.s. | |
| 7 | 7.11 ± 1.34 1 | 6.49 ± 0.99 12 | 6.80 ± 0.90 1 | n.s. | |
| 9 | 6.79 ± 0.67 1 | 6.28 ± 0.95 12 | 6.30 ± 0.95 1 | n.s. | |
| 12 | 6.34 ± 1.48 1 | 5.95 ± 0.78 1 | 6.43 ± 0.54 1 | n.s. | |
| P | 0.003 | 0.014 | <0.001 | ||
| Chewiness (N.mm) | 1 | 7.30 ± 0.35 a,2 | 8.30 ± 0.39 b,2 | 11.13 ± 0.95 c,3 | <0.001 |
| 2 | 6.67 ± 0.59 a,2 | 7.12 ± 0.46 a,12 | 8.85 ± 0.50 b,2 | 0.001 | |
| 5 | 5.51 ± 0.78 1 | 5.44 ± 0.40 1 | 5.76 ± 0.60 1 | n.s. | |
| 7 | 5.30 ± 1.00 1 | 4.93 ± 0.81 1 | 5.23 ± 0.61 1 | n.s. | |
| 9 | 4.89 ± 0.62 1 | 4.63 ± 0.92 1 | 5.28 ± 0.99 1 | n.s. | |
| 12 | 4.88 ± 1.21 1 | 4.88 ± 0.36 1 | 4.89 ± 0.55 1 | n.s. | |
| P | 0.001 | 0.036 | <0.001 | ||
| Resilience (N/mm) | 1 | 0.17 ± 0.02 | 0.17 ± 0.02 | 0.19 ± 0.03 | n.s. |
| 2 | 0.17 ± 0.04 | 0.16 ± 0.02 | 0.19 ± 0.04 | n.s. | |
| 5 | 0.17 ± 0.02 | 0.16 ± 0.01 | 0.15 ± 0.02 | n.s. | |
| 7 | 0.17 ± 0.05 | 0.15 ± 0.01 | 0.15 ± 0.01 | n.s. | |
| 9 | 0.15 ± 0.02 | 0.15 ± 0.02 | 0.14 ± 0.02 | n.s. | |
| 12 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 | n.s. | |
| P | n.s. | n.s. | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez, M.I.; Galafat, A.; Tapia Paniagua, S.T.; Martos-Sitcha, J.A.; Alarcón-López, F.J.; Martínez Moya, T.F. Feed Additives Based on N. gaditana and A. platensis Blend Improve Quality Parameters of Aquacultured Gilthead Seabream (Sparus aurata) Fresh Fillets. Fishes 2024, 9, 205. https://doi.org/10.3390/fishes9060205
Sáez MI, Galafat A, Tapia Paniagua ST, Martos-Sitcha JA, Alarcón-López FJ, Martínez Moya TF. Feed Additives Based on N. gaditana and A. platensis Blend Improve Quality Parameters of Aquacultured Gilthead Seabream (Sparus aurata) Fresh Fillets. Fishes. 2024; 9(6):205. https://doi.org/10.3390/fishes9060205
Chicago/Turabian StyleSáez, María Isabel, Alba Galafat, Silvana Teresa Tapia Paniagua, Juan Antonio Martos-Sitcha, Francisco Javier Alarcón-López, and Tomás Francisco Martínez Moya. 2024. "Feed Additives Based on N. gaditana and A. platensis Blend Improve Quality Parameters of Aquacultured Gilthead Seabream (Sparus aurata) Fresh Fillets" Fishes 9, no. 6: 205. https://doi.org/10.3390/fishes9060205
APA StyleSáez, M. I., Galafat, A., Tapia Paniagua, S. T., Martos-Sitcha, J. A., Alarcón-López, F. J., & Martínez Moya, T. F. (2024). Feed Additives Based on N. gaditana and A. platensis Blend Improve Quality Parameters of Aquacultured Gilthead Seabream (Sparus aurata) Fresh Fillets. Fishes, 9(6), 205. https://doi.org/10.3390/fishes9060205







