Effects of Dietary Supplementation with Endogenous Probiotics Bacillus subtilis on Growth Performance, Immune Response and Intestinal Histomorphology of Juvenile Rainbow Trout (Oncorhynchus mykiss)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish and Bacteria
2.2. Experimental Diets Preparation
2.3. Experimental Procedure
2.4. Growth Performance and Sample Collection
2.5. Histology Observation
2.6. Determination of Enzyme Activities
2.7. Gene Expression Analysis
2.8. Challenge Test
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Histological Observation of Intestine
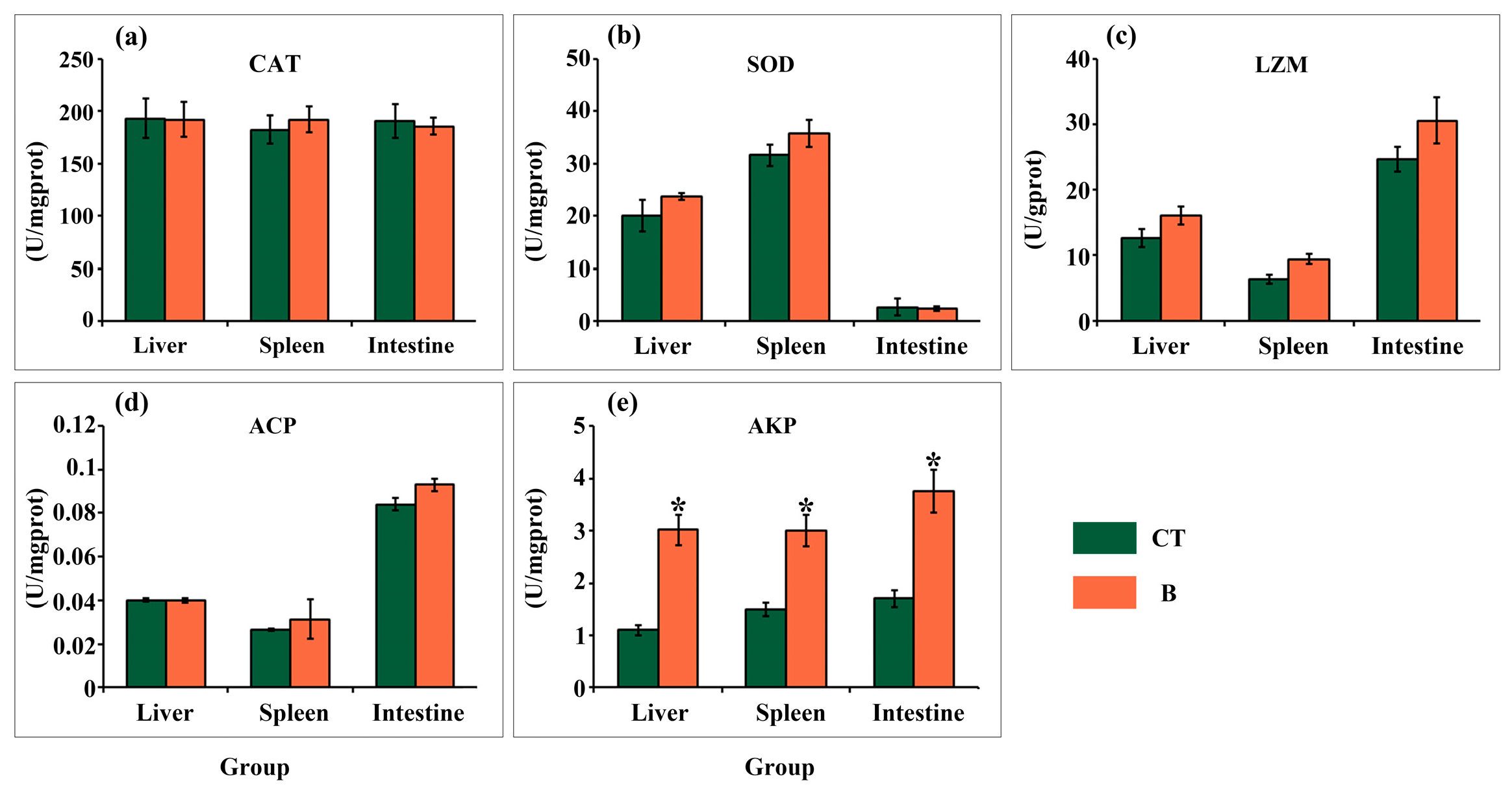
3.3. Digestive and Immune Related Enzymes Activities
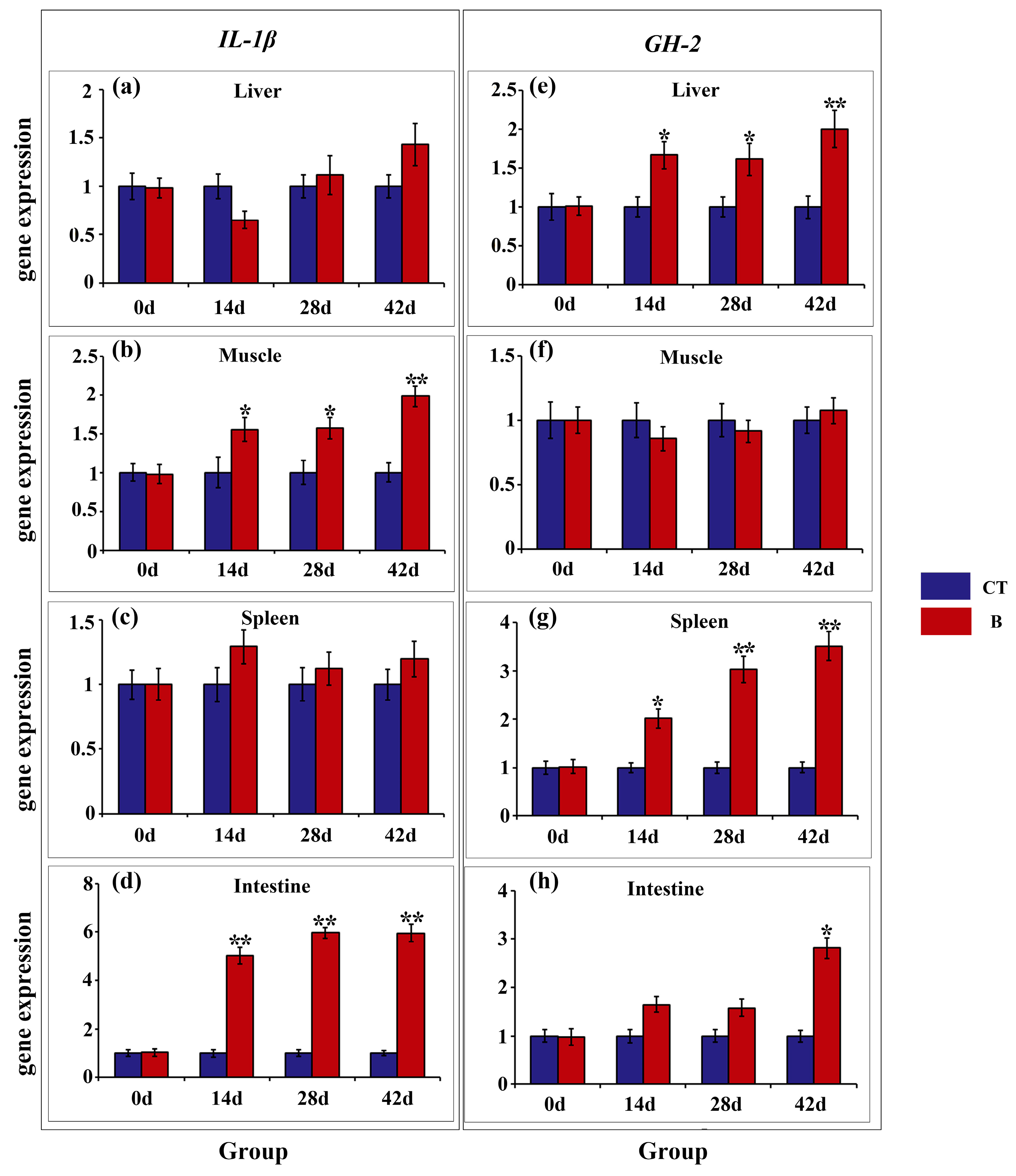
3.4. Gene Expression
3.5. Challenge Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bobe, J.; Marandel, L.; Panserat, S.; Boudinot, P.; Berthelot, C.; Quillet, E.; Volff, J.-N.; Genêt, C.; Jaillon, O.; Crollius, H.R.; et al. The rainbow trout genome, an important landmark for aquaculture and genome evolution. In Genomics in Aquaculture; Academic Press: Cambridge, MA, USA, 2016; pp. 21–43. [Google Scholar]
- Food and Agriculture Organization of the Unites Nation. The State of World Fisheries and Aquaculture: Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Xie, J.J.; Liu, Q.Q.; Liao, S.; Fang, H.H.; Yin, P.; Xie, S.W.; Tian, L.X.; Liu, Y.J.; Niu, J. Effects of dietary mixed probiotics on growth, non-specific immunity, intestinal morphology and microbiota of juvenile pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 90, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Costello, M. The global economic cost of sea lice to the salmonid farming industry. J. Fish Dis. 2009, 32, 115. [Google Scholar] [CrossRef] [PubMed]
- Kewcharoen, W.; Srisapoome, P. Probiotic effects of Bacillus spp. from Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish Shellfish Immunol. 2019, 94, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Bueno, I.; Travis, D.; Gonzalez-Rocha, G.; Alvarez, J.; Lima, C.; Benitez, C.G.; Phelps, N.B.D.; Wass, B.; Johnson, T.J.; Zhang, Q.; et al. Antibiotic Resistance Genes in Freshwater Trout Farms in a Watershed in Chile. J. Environ. Qual. 2019, 48, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, P.; Rey-Campos, M.; Figueras, A.; Novoa, B. An environmentally relevant concentration of antibiotics impairs the immune system of zebrafish (Danio rerio) and increases susceptibility to virus infection. Front. Immunol. 2023, 13, 1100092. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.F.; Islam, S.M.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.B.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chen, S.; Wu, Y.; Ma, Y.; Qiao, H.; Fan, J.; Wu, H. Dietary supplementation with microalgae enhances the zebrafish growth performance by modulating immune status and gut microbiota. Appl. Microbiol. Biotechnol. 2022, 106, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiang, Y.; He, M.; Zhang, X.; Wang, S.; Guo, W.; Liu, C.; Cao, Z.; Zhou, Y. Evaluation of Lactococcus lactis HNL12 combined with Schizochytrium limacinum algal meal in diets for humpback grouper (Cromileptes altivelis). Fish Shellfish Immunol. 2019, 94, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of probiotics in aquaculture of China-a review of the past decade. Fish Shellfish Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Docando, F.; Nuñez-Ortiz, N.; Serra, C.R.; Arense, P.; Enes, P.; Oliva-Teles, A.; Díaz-Rosales, P.; Tafalla, C. Mucosal and systemic immune effects of Bacillus subtilis in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2022, 124, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P.; Eichenberger, P.; Driks, A. Spore Resistance Properties. In The Bacterial Spore: From Molecules to Systems; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 201–215. [Google Scholar]
- Yilmaz, M.; Soran, H.; Beyatli, Y. Antimicrobial activities of some Bacillus spp. strains isolated from the soil. Microbiol. Res. 2006, 161, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-B. Effect of probiotics on growth performance and digestive enzyme activity of the shrimp Penaeus vannamei. Aquaculture 2007, 269, 259–264. [Google Scholar] [CrossRef]
- Abbass, A.; Sharifuzzaman, S.M.; Austin, B. Cellular components of probiotics control Yersinia ruckeri infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2009, 33, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Hoseinifar, S.H.; Ringø, E.; Ángeles Esteban, M.; Dadar, M.; Dawood, M.A.O.; Faggio, C. Host-associated probiotics: A key factor in sustainable aquaculture. Rev. Fish. Sci. Aquac. 2019, 28, 16–42. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.L.; Yang, D.H.; Hao, Q.; Yang, H.W.; Meng, D.L.; Meindert de Vos, W.; Guan, L.L.; Liu, S.B.; Teame, T.; et al. Lactobacillus rhamnosus GG triggers intestinal epithelium injury in zebrafish revealing host dependent beneficial effects. iMeta 2024, 3, e181. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Deng, F.R.; Li, S.W.; Lu, T.Y.; Liu, H.B.; Wang, D. Isolation, identification and biological characterization of a Bacillus subtilis strain from rainbow trout (Oncorhynchus mykiss). Period. Ocean. Univ. China 2022, 52, 41–49. [Google Scholar]
- Cao, Y.S.; Li, S.W.; Han, S.C.; Wang, D.; Zhao, J.Z.; Xu, L.M.; Liu, H.B.; Lu, T.Y. Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 2020, 523, 735193. [Google Scholar] [CrossRef]
- Cao, Y.S.; Li, S.W.; Wang, D.; Zhao, J.Z.; Xu, L.M.; Liu, H.B.; Lu, T.Y.; Mou, Z.B. Genomic characterization of a novel virulent phage infecting the Aeromonas hydrophila isolated from rainbow trout (Oncorhynchus mykiss). Virus Res. 2019, 273, 197764. [Google Scholar] [CrossRef] [PubMed]
- Abidin, Z.; Huang, H.T.; Hu, Y.F.; Chang, J.J.; Huang, C.Y.; Wu, Y.S.; Nan, F.H. Effect of dietary supplementation with Moringa oleifera leaf extract and Lactobacillus acidophilus on growth performance, intestinal microbiota, immune response, and disease resistance in whiteleg shrimp (Penaeus vannamei). Fish Shellfish Immunol. 2022, 127, 876–890. [Google Scholar] [CrossRef]
- Hooshyar, Y.; Abedian Kenari, A.; Paknejad, H.; Gandomi, H. Effects of Lactobacillus rhamnosus ATCC 7469 on Different Parameters Related to Health Status of Rainbow Trout (Oncorhynchus mykiss) and the Protection Against Yersinia ruckeri. Probiotics Antimicrob. Proteins 2020, 12, 1370–1384. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yang, Y.; Xu, W.; Du, X.; Ye, Y.; Zhu, B.; Huang, Y.; Zhao, Y.; Li, Y. Effects of β-1,3-glucan on growth, immune responses, and intestinal microflora of the river prawn (Macrobrachium nipponense) and its resistance against Vibrio parahaemolyticus. Fish Shellfish Immunol. 2023, 142, 109142. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Hou, Y.; Feng, W.; Nomingerel, M.; Li, B.; Zhu, J. Multi-Omics Analysis to Understand the Effects of Dietary Proanthocyanidins on Antioxidant Capacity, Muscle Nutrients, Lipid Metabolism, and Intestinal Microbiota in Cyprinus carpio. Antioxidants 2023, 12, 2095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pan, Y.; Huang, J.; Li, Y.; Wu, S.; Zhao, L.; Sun, T.; Kang, Y.; Liu, Z. Dietary supplementation of Chinese herbal medicines enhances the immune response and resistance of rainbow trout (Oncorhynchus mykiss) to infectious hematopoietic necrosis virus. Front. Vet. Sci. 2024, 11, 1341920. [Google Scholar] [CrossRef] [PubMed]
- Saengrung, J.; Bunnoy, A.; Du, X.; Huang, L.; An, R.; Liang, X.; Srisapoome, P. Effects of ribonucleotide supplementation in modulating the growth of probiotic Bacillus subtilis and the synergistic benefits for improving the health performance of Asian seabass (Lates calcarifer). Fish Shellfish Immunol. 2023, 140, 108983. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, D.; Cao, Y.S.; Lu, T.Y.; Liu, H.B.; Li, S.W. Effects of propolis on the immune enhancement of the formalin-inactivated Aeromonas salmonicida vaccine. Aquac. Res. 2020, 51, 4759–4770. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Biao, G. The Effect of Acute Temperature Stress on Blood Components and Key Metabolism Mechanisms in Rainbow Trout (Oncorhynchus mykiss); Ocean University of China: Qingdao, China, 2014. [Google Scholar]
- Van Doan, H.; Wangkahart, E.; Thaimuangphol, W.; Panase, P.; Sutthi, N. Effects of Bacillus spp. mixture on growth, immune responses, expression of immune-related genes, and resistance of Nile Tilapia against Streptococcus agalactiae infection. In Probiotics and Antimicrobial Proteins; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–16. [Google Scholar]
- Liao, J.; Cai, Y.; Wang, X.; Shang, C.; Zhang, Q.; Shi, H.; Wang, S.; Zhang, D.; Zhou, Y. Effects of a potential host gut-derived probiotic, Bacillus subtilis 6-3-1, on the growth, non-specific immune response and disease resistance of hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Probiotics Antimicrob. Proteins 2021, 13, 1119–1137. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Zhang, H.; Chen, J.; Zhu, J.; He, J.; Luo, L.; Lin, S.; Chen, Y. Effects of dietary Bacillus subtilis DSM 32315 supplementation on the growth, immunity and intestinal morphology, microbiota and inflammatory response of juvenile largemouth bass Micropterus salmoides. Aquac. Nutr. 2021, 27, 2119–2131. [Google Scholar] [CrossRef]
- Raida, M.K.; Larsen, J.L.; Nielsen, M.E.; Buchmann, K. Enhanced resistance of rainbow trout, Oncorhynchus mykiss (Walbaum), against Yersinia ruckeri challenge following oral administration of Bacillus subtilis and B. licheniformis (BioPlus2B). J. Fish Dis. 2003, 26, 495–498. [Google Scholar] [CrossRef]
- Villumsen, K.R.; Ohtani, M.; Forberg, T.; Aasum, E.; Tinsley, J.; Bojesen, A.M. Synbiotic feed supplementation significantly improves lipid utilization and shows discrete effects on disease resistance in rainbow trout (Oncorhynchus mykiss). Sci. Rep. 2020, 10, 16993. [Google Scholar] [CrossRef] [PubMed]
- Hisar, O.; Yilmaz, S.; Yigit, M. Effects of different probiotic bacteria on growth, body composition, immune response and hematological parameters of rainbow trout (Oncorhynchus mykiss) under sublethal water temperature. Mar. Sci. Technol. Bull. 2015, 4, 21–28. [Google Scholar]
- Cláudia, R.S.; Eduarda, M.A.; Inês, G.; Rafaela, S.; Merrifield, D.L.; Tavares, F.; Oliva-Teles, A.; Enes, P. Selection of carbohydrate-active probiotics from the gut of carnivorous fish fed plant-based diets. Sci. Rep. 2019, 9, 6384. [Google Scholar]
- Xia, X.; Liang, N.; Ma, X.; Qin, L.; Chang, Z.; Zhang, X. Effect of dietary supplementation with Leuconostoc mesenteroides DH on the antimicrobial capacity and overall health of juvenile loach (Misgurnus anguillicaudatus). Aquaculture 2024, 579, 740208. [Google Scholar] [CrossRef]
- Caspary, W.F. Physiology and pathophysiology of intestinal absorption. Am. J. Clin. Nutr. 1992, 55, 299S–308S. [Google Scholar] [CrossRef] [PubMed]
- Standen, B.T.; Peggs, D.L.; Rawling, M.D.; Foey, A.; Davies, S.J.; Santos, G.A.; Merrifield, D.L. Dietary administration of a commercial mixed-species probiotic improves growth performance and modulates the intestinal immunity of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 49, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.M.W.; Merrifield, D.L.; Harper, G.M.; Rawling, M.D.; Mustafa, S.; Picchietti, S.; Balcázar, J.L.; Davies, S.J. The effect of Pediococcus acidilactici on the gut microbiota and immune status of on-growing red tilapia (Oreochromis niloticus). J. Appl. Microbiol. 2010, 109, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Kühlwein, H.; Merrifield, D.L.; Rawling, M.D.; Foey, A.D.; Davies, S.J. Effects of dietary β-(1,3)(1,6)-D-glucan supplementation on growth performance, intestinal morphology and haemato-immunological profile of mirror carp (Cyprinus carpio L.). J. Anim. Physiol. Anim. Nutr. 2013, 98, 279–289. [Google Scholar] [CrossRef]
- Kwaku, A.; Huang, Q.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Dong, X. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 796–808. [Google Scholar]
- Vazquez-Duhalt, R.; Callet, T.; Médale, F.; Larroquet, L.; Surget, A.; Aguirre, P.; Kerneis, T.; Labbé, L.; Quillet, E.; Geurden, I.; et al. Successful selection of rainbow trout (Oncorhynchus mykiss) on their ability to grow with a diet completely devoid of fishmeal and fish oil, and correlated changes in nutritional traits. PLoS ONE 2017, 12, e0186705. [Google Scholar]
- Zhang, Y.; Wen, B.; Meng, L.J.; Gao, J.Z.; Chen, Z.Z. Dynamic changes of gut microbiota of discus fish (Symphysodon haraldi) at different feeding stages. Aquaculture 2021, 531, 735912. [Google Scholar] [CrossRef]
- Dai, C.; Lin, A.; Mo, A.; Yuan, Y.; Yuan, J.; Gu, Z.; Wang, J. Effect of dietary Bacillus subtilis supplement on Cd toxicokinetics and Cd-induced immune and antioxidant impairment of Procambarus clarkii. Environ. Sci. Pollut. Res. 2023, 30, 43914–43926. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Sun, Y.; Zhou, Y.; Men, X.; Wang, B.; Li, B.; Ren, Y. Effects of probiotics on growth, the toll-like receptor mediated immune response and susceptibility to Aeromonas salmonicida infection in rainbow trout Oncorhynchus mykiss. Aquaculture 2022, 561, 738668. [Google Scholar] [CrossRef]
- Panase, A.; Thirabunyanon, M.; Promya, J.; Chitmanat, C. Influences of Bacillus subtilis and fructooligosaccharide on growth performances, immune responses, and disease resistance of Nile tilapia, Oreochromis niloticus. Front. Vet. Sci. 2023, 9, 1094681. [Google Scholar] [CrossRef]
- Yun, S.W.; Kim, J.K.; Lee, K.E.; Oh, Y.J.; Choi, H.J.; Han, M.J.; Kim, D.H. A probiotic Lactobacillus gasseri alleviates Escherichia coli-induced cognitive impairment and depression in mice by regulating IL-1β expression and gut microbiota. Nutrients 2020, 12, 3441. [Google Scholar] [CrossRef]


| Gene | Primer (5′-3′) | Product Size (bp) |
|---|---|---|
| GH-2 | F: TGCGTCCTAACCCTGACTTC R: AAGCCTCTCTCTCCACACACA | 104 |
| IL-1β | F: CGAGCTGGACATGGAGGAGC R: TCGTCCCAGTTGGTGACGAT | 103 |
| β-actin | F: GATGGGCCAGAAAGACAGCTA R: TCGTCCCAGTTGGTGACGAT | 119 |
| Growth Index | Control Group (CT) | Experimental Group (B) | ||||
|---|---|---|---|---|---|---|
| 0 d | 28 d | 42 d | 0 d | 28 d | 42 d | |
| BWG (g) | - | 8.67 ± 3.20 | 19.37 ± 5.39 | - | 21.55 ± 2.53 ** | 43.36 ± 5.16 ** |
| WGR (%) | - | 24.57 ± 1.94 | 54.86 ± 3.78 | - | 58.73 ± 1.77 ** | 118.20 ± 6.97 ** |
| SGR (%) | - | 0.78 ± 0.03 | 1.04 ± 0.14 | - | 1.65 ± 0.17 * | 1.86 ± 0.19 ** |
| FCR | - | 0.91 ± 0.03 | 0.92 ± 0.05 | - | 0.84 ± 0.04 ** | 0.83 ± 0.06 * |
| HSI (%) | 0.99 ± 0.13 | 1.11 ± 0.85 | 1.55 ± 0.85 | 1.04 ± 0.51 | 1.15 ± 0.69 | 1.56 ± 0.68 |
| SSI (%) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.02 | 0.08 ± 0.01 | 0.10 ± 0.02 | 0.09 ± 0.02 |
| ILI (%) | 30.10 ± 0.99 | 32.71 ± 1.12 | 36.54 ± 1.56 | 29.82 ± 1.03 | 33.41 ± 1.15 | 39.22 ± 1.56 |
| Survival rate (%) | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Items | Control Group (CT) | Experimental Group (B) |
|---|---|---|
| Villus height (μm) | 378.5 ± 21.4 | 428.9 ± 14.3 * |
| Villus width (μm) | 95.7 ± 14.3 | 76.6 ± 8.25 |
| Goblet cell number (per 100 μm) | 9.9 ± 0.4 | 19.3 ± 1.5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Fan, D.; Zhao, R.; Lu, T.; Li, S.; Wang, D. Effects of Dietary Supplementation with Endogenous Probiotics Bacillus subtilis on Growth Performance, Immune Response and Intestinal Histomorphology of Juvenile Rainbow Trout (Oncorhynchus mykiss). Fishes 2024, 9, 229. https://doi.org/10.3390/fishes9060229
Wang J, Fan D, Zhao R, Lu T, Li S, Wang D. Effects of Dietary Supplementation with Endogenous Probiotics Bacillus subtilis on Growth Performance, Immune Response and Intestinal Histomorphology of Juvenile Rainbow Trout (Oncorhynchus mykiss). Fishes. 2024; 9(6):229. https://doi.org/10.3390/fishes9060229
Chicago/Turabian StyleWang, Jing, Dan Fan, Ran Zhao, Tongyan Lu, Shaowu Li, and Di Wang. 2024. "Effects of Dietary Supplementation with Endogenous Probiotics Bacillus subtilis on Growth Performance, Immune Response and Intestinal Histomorphology of Juvenile Rainbow Trout (Oncorhynchus mykiss)" Fishes 9, no. 6: 229. https://doi.org/10.3390/fishes9060229
APA StyleWang, J., Fan, D., Zhao, R., Lu, T., Li, S., & Wang, D. (2024). Effects of Dietary Supplementation with Endogenous Probiotics Bacillus subtilis on Growth Performance, Immune Response and Intestinal Histomorphology of Juvenile Rainbow Trout (Oncorhynchus mykiss). Fishes, 9(6), 229. https://doi.org/10.3390/fishes9060229






