Trends and Environmental Drivers of Marine Fish Landings in Cuba’s Most Productive Shelf Area
Abstract
1. Introduction
2. Materials and Methods
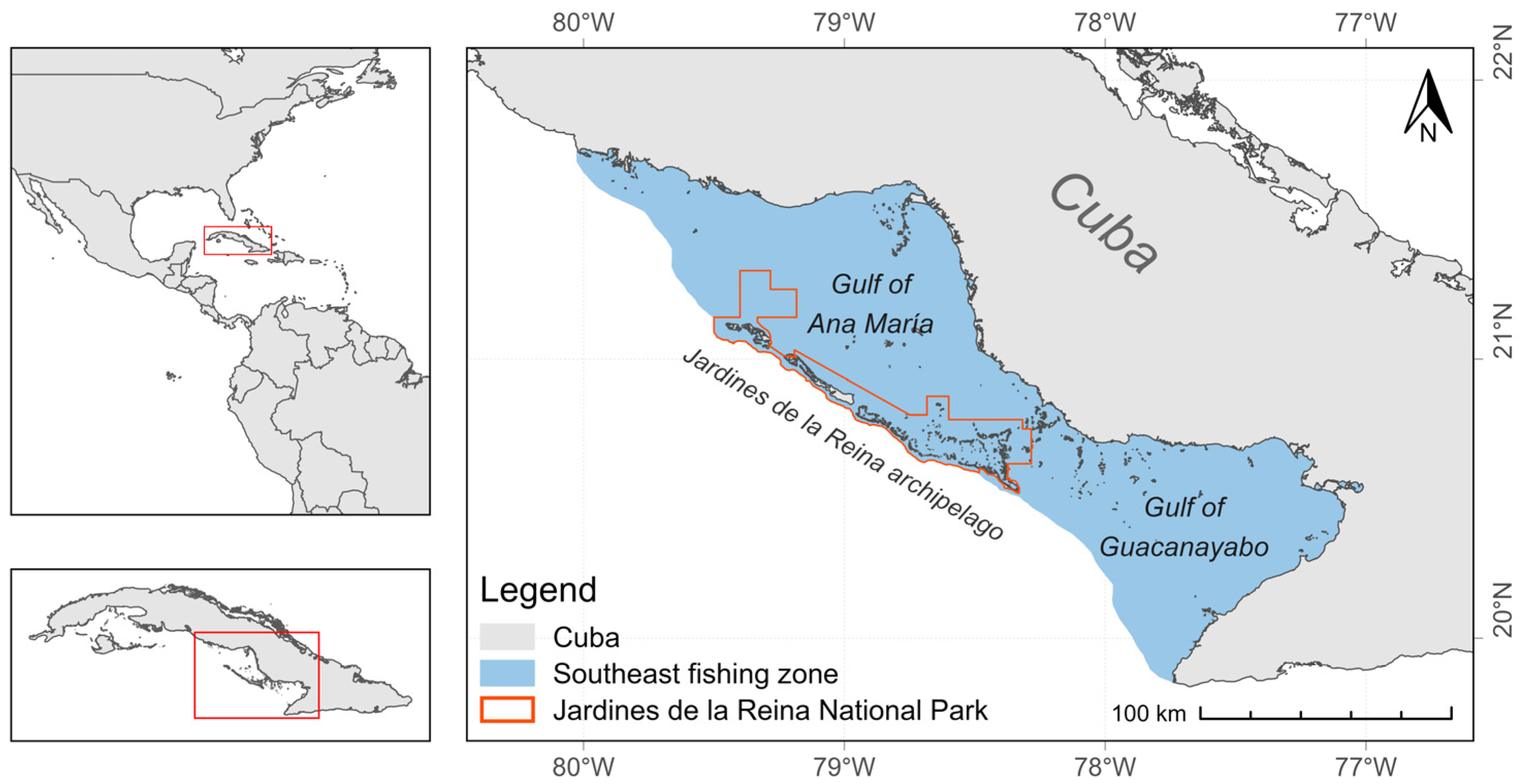
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
3. Results
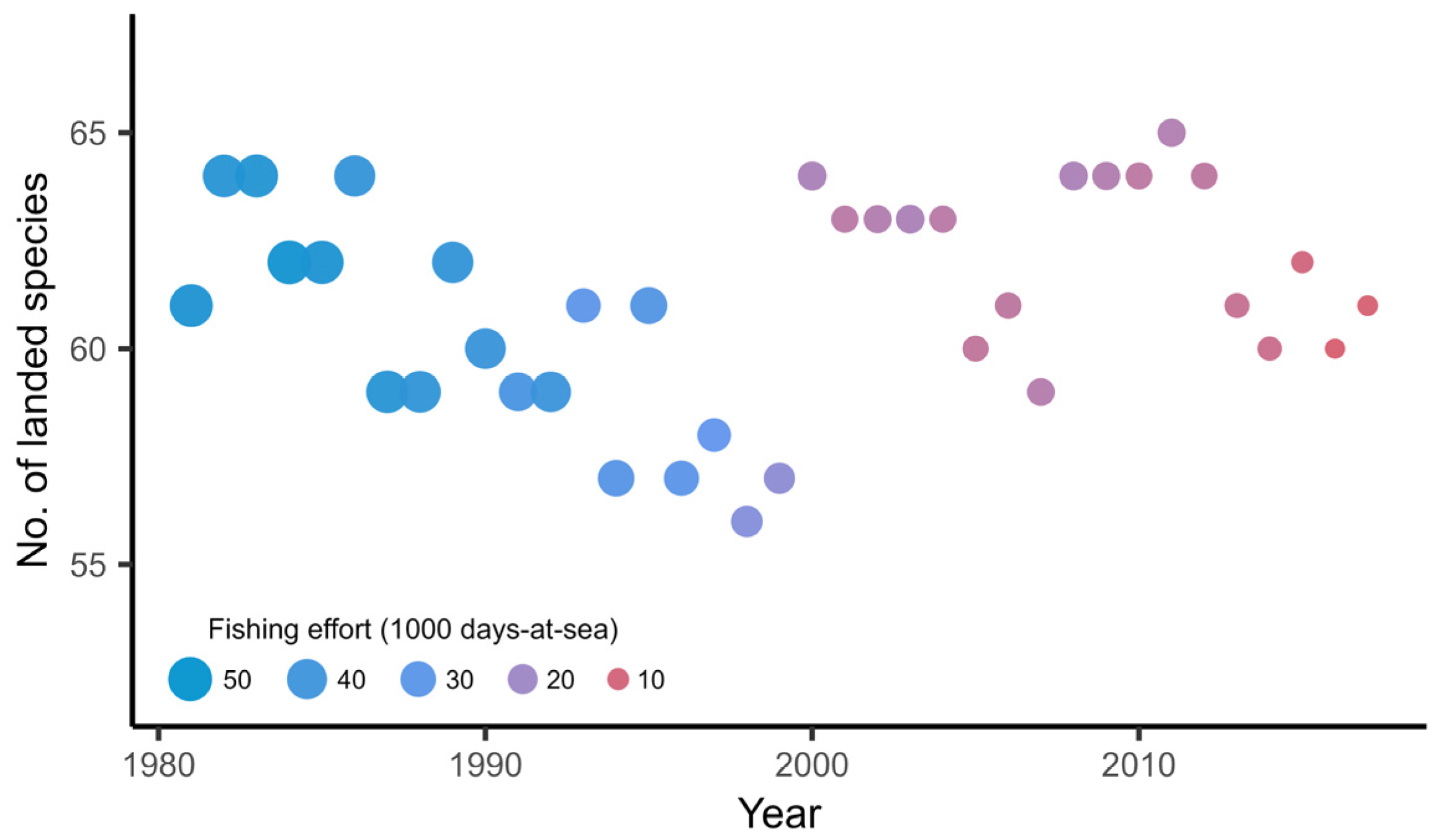
3.1. Exploratory Analysis of the Landings
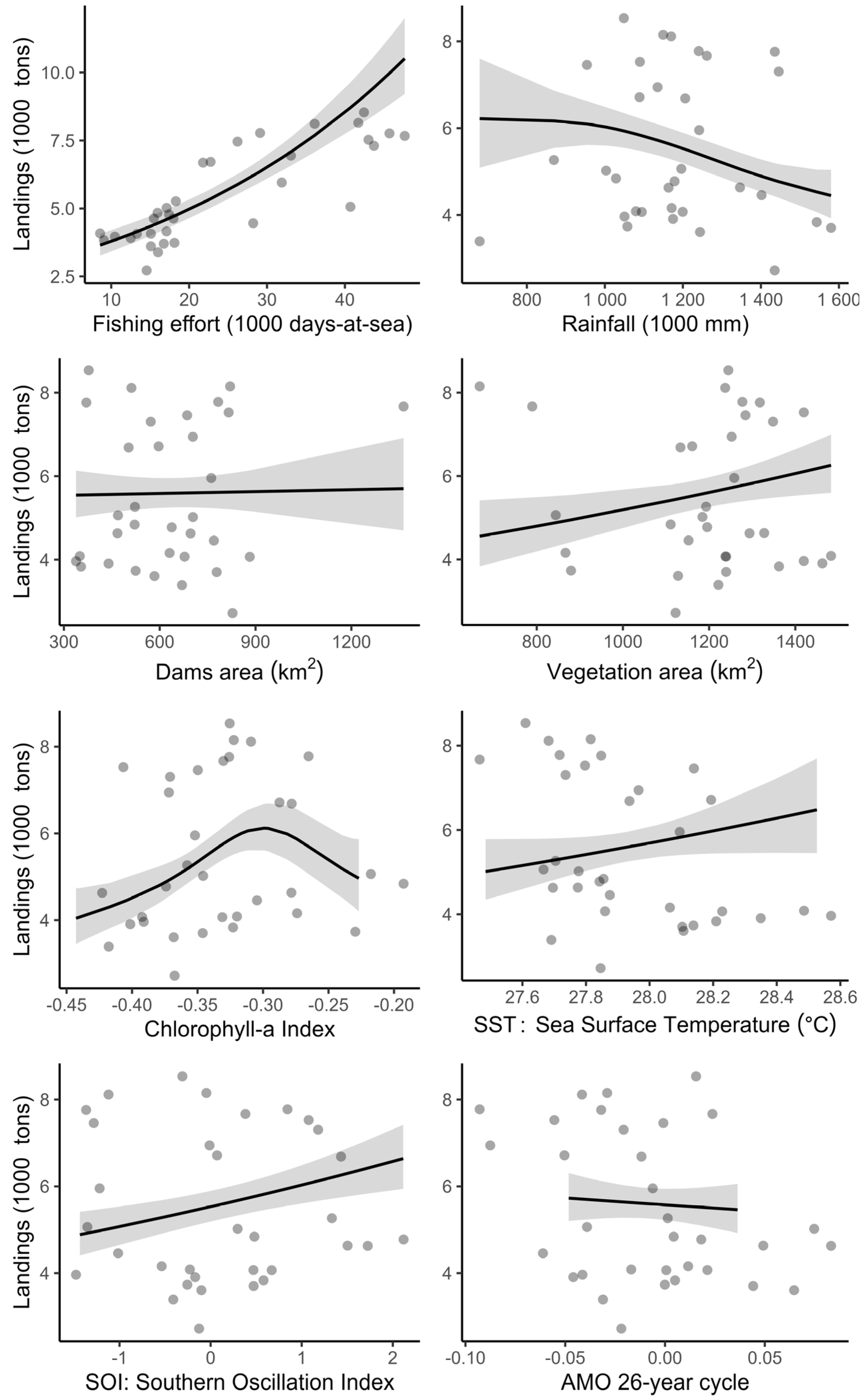
3.2. Influences of Environmental Factors on Finfish Landings
4. Discussion
4.1. Finfish Landings
4.2. Environmental Variables Affecting Finfish Landings
4.3. Management Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilborn, R.; Walters, C.J. Quantitative Fisheries Stock Assessment: Choice Dynamics and Uncertainty; Chapman and Hall: New York, NY, USA, 1992. [Google Scholar]
- Karr, K.A.; Miller, V.; Coronado, E.; Olivares-Bañuelos, N.C.; Rosales, M.; Naretto, J.; Hiriart-Bertrand, L.; Vargas-Fernández, C.; Alzugaray, R.; Puga, R.; et al. Identifying Pathways for Climate-Resilient Multispecies Fisheries. Front. Mar. Sci. 2021, 8, 721883. [Google Scholar] [CrossRef]
- Doyon, S. Fishing for the Revolution: Transformations and Adaptations in Cuban Fisheries. MAST 2007, 6, 83–108. [Google Scholar]
- Baisre, J.A. Historical Development of Cuban Fisheries: Why We Need an Integrated Approach to Fisheries Management? Proc. Gulf Caribb. Fish. Inst. 2007, 59, 49–56. [Google Scholar]
- Baisre, J.A. An Overview of Cuban Commercial Marine Fisheries: The Last 80 Years. Bull. Mar. Sci. 2018, 94, 359–375. [Google Scholar] [CrossRef]
- Puga, R.; Valle, S.; Kritzer, J.P.; Delgado, G.; Estela de León, M.; Giménez, E.; Ramos, I.; Moreno, O.; Karr, K.A. Vulnerability of Nearshore Tropical Finfish in Cuba: Implications for Scientific and Management Planning. Bull. Mar. Sci. 2018, 94, 377–392. [Google Scholar] [CrossRef]
- Valle, S.V.; Sosa, M.; Puga, R.; Font, L.; Duthit, R. Coastal Fisheries of Cuba. In Coastal Fisheries of Latin America and the Caribbean; FAO Fisheries and Aquaculture Technical Paper; Food and Agriculture Organization of the United Nations: Roma, Italy, 2011; Volume 544, pp. 155–174. ISBN 978-92-5-106722-2. [Google Scholar]
- Baisre, J.A. Chronicle of Cuban Marine Fisheries, 1935–1995: Trend Analysis and Fisheries Potential; FAO Fisheries Technical Paper; FAO: Rome, Italy, 2000; No. 394; p. 26. [Google Scholar]
- Claro, R.; Sadovy, Y.; Lindeman, K.C.; García-Cagide, A.R. Historical Analysis of Cuban Commercial Fishing Effort and the Effects of Management Interventions on Important Reef Fishes from 1960–2005. Fish. Res. 2009, 99, 7–16. [Google Scholar] [CrossRef]
- Alzugaray, R.; Puga, R.; Valle, S.; Hernández-Betancourt, A.; Boné, E.; Kleisner, K.; Manguin, T.; Karr, K.A. Situación actual y proyecciones futuras de las pesquerías multiespecíficas de peces en la región suroriental de Cuba: Situação atual e projecções futuras da pesca de peixes multiespecíficos na região sudeste de Cuba. Braz. J. Anim. Environ. Res. 2023, 6, 1950–1955. [Google Scholar] [CrossRef]
- Baisre, J.A. Assessment of Nitrogen Flows into the Cuban Landscape. Biogeochemistry 2006, 79, 91–108. [Google Scholar] [CrossRef]
- Baisre, J.A.; Arboleya, Z. Going Against the Flow: Effects of River Damming in Cuban Fisheries. Fish. Res. 2006, 81, 283–292. [Google Scholar] [CrossRef]
- Claro, R.; Reshetnikov, Y.S.; Alcolado, P. Physical Attributes of Coastal Cuba. In Ecology of the Marine Fishes of Cuba; Claro, R., Lindeman, K.C., Parenti, L.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2001; pp. 1–20. [Google Scholar]
- Winton, R.S.; Calamita, E.; Wehrli, B. Reviews and Syntheses: Dams, Water Quality and Tropical Reservoir Stratification. Biogeosciences 2019, 16, 1657–1671. [Google Scholar] [CrossRef]
- Alvarez-Lajonchère, L.; Laíz-Averhoff, O.; Perigó-Arnaud, E. River Dam Effects on Cuban Fisheries and Aquaculture Development with Recommendations for Mitigation. Environ. Eng. Manag. J. 2018, 17, 491–512. [Google Scholar] [CrossRef]
- Odum, W.E.; Johannes, R.E. The Response of Mangroves to Man-Induced Environmental Stress. In Tropical Marine Pollution; Wood, E.J.F., Johannes, R.E., Eds.; Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 1975; Volume 12, pp. 52–62. [Google Scholar]
- Karnauskas, M.; Schirripa, M.J.; Craig, J.K.; Cook, G.S.; Kelble, C.R.; Agar, J.J.; Black, B.A.; Enfield, D.B.; Lindo-Atichati, D.; Muhling, B.A.; et al. Evidence of Climate-Driven Ecosystem Reorganization in the Gulf of Mexico. Glob. Chang. Biol. 2015, 21, 2554–2568. [Google Scholar] [CrossRef] [PubMed]
- Arreguín-Sánchez, F.; del Monte Luna, P.; Zetina-Rejón, M.J.; Tripp-Valdez, A.; Albañez-Lucero, M.O.; Mónica Ruiz-Barreiro, T. Building an Ecosystems-Type Fisheries Management Approach for the Campeche Bank, Subarea in the Gulf of Mexico Large Marine Ecosystem. Environ. Dev. 2017, 22, 143–149. [Google Scholar] [CrossRef]
- Arreguín-Sánchez, F. Cambio Climático y El Colapso de La Pesquería de Camarón Rosado (Farfantepenaeus duorarum) de La Sonda de Campeche. In Cambio Climático en México un Enfoque Costero-Marino; Rivera-Arriaga, E., Azuz-Adeath, I., Alpuche-Gual, L., Villalobo-Zapata, G.J., Eds.; Universidad Autónoma de Campeche Cetys-Universidad, Gobierno del Estado de Campeche: Campeche, Mexico, 2010; pp. 399–410. [Google Scholar]
- Arreguín-Sánchez, F.; del Monte-Luna, P.; Zetina-Rejón, M.J. Climate Change Effects on Aquatic Ecosystems and the Challenge for Fishery Management: Pink Shrimp of the Southern Gulf of Mexico. Fisheries 2015, 40, 15–19. [Google Scholar] [CrossRef]
- Hernández, B.; Puga, R. Influencia Del Fenómeno El Niño En La Región Occidental de Cuba y Su Impacto En La Pesquería de Langosta (Panulirus argus) Del Golfo de Batabanó. Investig. Mar. 1995, 23, 3–24. [Google Scholar] [CrossRef]
- Puga, R.; Piñeiro, R.; Alzugaray, R.; Cobas, L.S.; León, M.E.D.; Morales, O. Integrating Anthropogenic and Climatic Factors in the Assessment of the Caribbean Spiny Lobster (Panulirus argus) in Cuba: Implications for Fishery Management. Int. J. Mar. Sci. 2013, 3, 36–45. [Google Scholar] [CrossRef][Green Version]
- Puga, R.; Piñeiro, R.; Cobas, S.; De León, M.E.; Capetillo, N.; Alzugaray, R. La Pesquería de La Langosta Espinosa, Conectividad y Cambio Climático En Cuba. In La biodiversidad en ecosistemas marinos y costeros del litoral de Iberoamérica y el cambio climático: I. Memorias del Primer Taller de la Red CYTED BIODIVMAR, Havana, Cuba; 2010; pp. 112–131. [Google Scholar]
- Alzugaray, R.; Puga, R.; Piñeiro, R.; de León, M.E.; Cobas, L.S.; Morales, O. The Caribbean Spiny Lobster (Panulirus argus) Fishery in Cuba: Current Status, Illegal Fishing, and Environmental Variability. Bull. Mar. Sci. 2018, 94, 393–408. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Cheung, W.W.; Lam, V.W.; Pauly, D.; Herrick, S. Climate Change Impacts on the Biophysics and Economics of World Fisheries. Nat. Clim. Chang. 2011, 1, 449–456. [Google Scholar] [CrossRef]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate Change and Distribution Shifts in Marine Fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- Palacios-Abrantes, J.; Frölicher, T.L.; Reygondeau, G.; Sumaila, U.R.; Tagliabue, A.; Wabnitz, C.C.C.; Cheung, W.W.L. Timing and Magnitude of Climate-Driven Range Shifts in Transboundary Fish Stocks Challenge Their Management. Glob. Chang. Biol. 2022, 28, 2312–2326. [Google Scholar] [CrossRef]
- Giménez-Hurtado, E.; Ramos-Díaz, I.; Valle, S. Análisis de La Productividad Pesquera de La Plataforma Suroriental de Cuba. Rev. Cub. Investig. Pesq. 2016, 33, 43–52. [Google Scholar]
- Ventura-Díaz, Y.; Rodríguez-Cueto, Y. Hábitats Del Golfo de Ana María Identificados Mediante El Empleo de Procesamiento Digital de Imágenes. Rev. Investig. Mar. 2012, 32, 1–8. [Google Scholar]
- Pina-Amargós, F.; Figueredo-Martín, T.; Rossi, N.A. The Ecology of Cuba’s Jardines de La Reina: A Review. Rev. Investig. Mar. 2021, 41, 2–42. [Google Scholar]
- Ramenzoni, V.C.; Borroto Escuela, D.; Rangel Rivero, A.; González-Díaz, P.; Vázquez Sánchez, V.; López-Castañeda, L.; Falcón Méndez, A.; Hernández Ramos, I.; Valentín Hernández López, N.; Besonen, M.R.; et al. Vulnerability of Fishery-Based Livelihoods to Extreme Events: Local Perceptions of Damages from Hurricane Irma and Tropical Storm Alberto in Yaguajay, Central Cuba. Coast. Manag. 2020, 48, 354–377. [Google Scholar] [CrossRef]
- Adams, C.; Alvarez, A.E.G. The Commercial Fisheries Industries of Cuba and Florida; University of Florida: Gainesville, FL, USA, 1996; p. 106. [Google Scholar]
- Bueno-Pardo, J.; Pierce, G.J.; Cabecinha, E.; Grilo, C.; Assis, J.; Valavanis, V.; Pita, C.; Dubert, J.; Leitão, F.; Queiroga, H. Trends and Drivers of Marine Fish Landings in Portugal Since Its Entrance in the European Union. ICES J. Mar. Sci. 2020, 77, 988–1001. [Google Scholar] [CrossRef]
- del Monte-Luna, P.; Villalobos, H.; Arreguín-Sánchez, F. Variability of Sea Surface Temperature in the Southwestern Gulf of Mexico. Cont. Shelf Res. 2015, 102, 73–79. [Google Scholar] [CrossRef]
- Landsea, C.W.; Franklin, J.L. Atlantic Hurricane Database Uncertainty and Presentation of a New Database Format. Mon. Weather Rev. 2013, 141, 3576–3592. [Google Scholar] [CrossRef]
- Hoeffding, W. A Non-Parametric Test of Independence. Ann. Math. Stat. 1948, 19, 546–557. [Google Scholar] [CrossRef]
- Makowski, D.; Wiernik, B.M.; Patil, I.; Lüdecke, D.; Ben-Shachar, M.S. Correlation: Methods for Correlation Analysis. 2023. Available online: https://CRAN.R-project.org/package=correlation (accessed on 5 April 2024).
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems: Data Exploration. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Lüdecke, D. geffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef]
- Adams, C.; Vega, P.S.; Alvarez, A.G. An Overview of the Cuban Commercial Fishing Industry and Recent Changes in Management Structure and Objectives; Electronic Data Information Source (EDIS) FE218; Department of Food of Resource Economics, University of Florida: Gainesville, FL, USA, 2000; pp. 1–12. [Google Scholar]
- Au, A.; Zylich, K.; Zeller, D. Reconstruction of Total Marine Fisheries Catches for Cuba (1950–2010). In Fisheries Catch Reconstructions: Islands, Part IV; Zylich, K., Zeller, D., Ang, M., Pauly, D., Eds.; University of British Columbia: Kelowna, BC, Canada, 2014; Volume 22, pp. 25–32. [Google Scholar]
- Joyce, I.T. The Fisheries of the Cuban Insular Shelf: Culture History and Revolutionary Performance. Ph.D. Thesis, Arts and Social Sciences: History, Simon Fraser University, Burnaby, BC, Canada, 1996. [Google Scholar]
- Moreira, A.; Barcia, S.; Cabrales, Y.; Suárez, A.M.; Fujii, M.T. El Impacto Del Huracán Dennis Sobre El Macrofitobentos de La Bahía de Cienfuegos, Cuba. Rev. Investig. Mar. 2009, 30, 175–185. [Google Scholar]
- Mitrani-Arenal, I.; Perez-Bello, A.; Cabrales-Infante, J.; Povea-Perez, Y.; Hernandez-Gonzalez, M.; Diaz-Rodriguez, O.O. Coastal Flood Forecast in Cuba, Due to Hurricanes, Using a Combination of Numerical Models. Rev. Cub. Meteorol. 2019, 25, 121–138. [Google Scholar]
- Tolmazin, D. Black Sea- Dead Sea? New Sci. 1979, 84, 767–769. [Google Scholar]
- Mohamed, N.N. Negative Impacts Of Egyptian High Aswan Dam: Lessons For Ethiopia And Sudan. Int. J. Dev. Res. 2019, 9, 28861–28874. [Google Scholar]
- Chen, Q.; Li, Q.; Lin, Y.; Zhang, J.; Xia, J.; Ni, J.; Cooke, S.J.; Best, J.; He, S.; Feng, T.; et al. River Damming Impacts on Fish Habitat and Associated Conservation Measures. Rev. Geophys. 2023, 61, e2023RG000819. [Google Scholar] [CrossRef]
- Sklar, F.H.; Browder, J.A. Coastal Environmental Impacts Brought About by Alterations to Freshwater Flow in the Gulf of Mexico. Environ. Manag. 1998, 22, 547–562. [Google Scholar] [CrossRef]
- Instituto Nacional de Recursos Hidráulicos. Boletín Hidrológico 2021-09; Instituto Nacional de Recursos Hidráulicos: Havana, Cuba, 2021; p. 17. [Google Scholar]
- Baisre, J.A. Los Complejos Ecológicos de Pesca: Definición e Importancia En La Administración de Las Pesquerías Cubanas. FAO Fish. Rep. 1985, 327, 251–272. [Google Scholar]
- Batista-Silva, J.L. Isolíneas Del Módulo de Escurrimiento Medio Anual. Voluntad Hidráulica 1974, 32, 13–15. [Google Scholar]
- Doria-González, M.A.; Pérez-Ferro, D.; Olaya, F.C.; Payares-Varela, J.L.; Ortiz-Royero, J.C.; Doria-González, M.A.; Pérez-Ferro, D.; Olaya, F.C.; Payares-Varela, J.L.; Ortiz-Royero, J.C. Climate Variability and Small-Scale Fisheries of the Albuquerque Cays Island, Insular Colombian Caribbean. Lat. Am. J. Aquat. Res. 2022, 50, 596–609. [Google Scholar] [CrossRef]
- Power, S.B.; Kociuba, G. The Impact of Global Warming on the Southern Oscillation Index. Clim. Dyn. 2011, 37, 1745–1754. [Google Scholar] [CrossRef]
- Jury, M.R. Long-Term Variability and Trends in the Caribbean Sea. Int. J. Oceanogr. 2011, 2011, e465810. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Sun, M.; Li, J.; Zhou, X.; Chen, Z.; Zhang, K. Impacts of Strong ENSO Events on Fish Communities in an Overexploited Ecosystem in the South China Sea. Biology 2023, 12, 946. [Google Scholar] [CrossRef] [PubMed]
- Claro, R.; García Arteaga, J.P.; Gobert, B.; Cantelar Ramos, K.; Valle Gómez, S.V.; Pina Amargós, F. Situación actual de los recursos pesqueros del archipiélago Sabana-Camagüey, Cuba. BIM 2016, 33, 49–67. [Google Scholar] [CrossRef]
- Claro, R.; Parenti, L.R. The Marine Ichthyofauna of Cuba. In Ecology of the Marine Fishes of Cuba; Claro, R., Lindeman, K.C., Parenti, L.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2001; pp. 21–57. [Google Scholar]
- Pérez-Ramírez, M. Climate Change and Fisheries in the Caribbean. In Climate Change Impacts on Fisheries and Aquaculture; Phillips, B.F., Pérez-Ramírez, M., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 639–662. [Google Scholar]
- Brown, A.R.; Lilley, M.; Shutler, J.; Lowe, C.; Artioli, Y.; Torres, R.; Berdalet, E.; Tyler, C.R. Assessing Risks and Mitigating Impacts of Harmful Algal Blooms on Mariculture and Marine Fisheries. Rev. Aquac. 2020, 12, 1663–1688. [Google Scholar] [CrossRef]
- Zainuddin, M.; Nelwan, A.; Farhum, S.A.; Najamuddin; Hajar, M.A.I.; Kurnia, M.; Sudirman. Characterizing Potential Fishing Zone of Skipjack Tuna During the Southeast Monsoon in the Bone Bay-Flores Sea Using Remotely Sensed Oceanographic Data. J. Geosci. 2013, 4, 259–266. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Q.; Tang, D.; Zhao, H.; Chen, P. Response of Sthenoteuthis oualaniensis to Marine Environmental Changes in the North-Central South China Sea Based on Satellite and In Situ Observations. PLoS ONE 2019, 14, e0211474. [Google Scholar] [CrossRef] [PubMed]
- Wiryawan, B.; Loneragan, N.; Mardhiah, U.; Kleinertz, S.; Wahyuningrum, P.I.; Pingkan, J.; Wildan; Timur, P.S.; Duggan, D.; Yulianto, I. Catch Per Unit Effort Dynamic of Yellowfin Tuna Related to Sea Surface Temperature and Chlorophyll in Southern Indonesia. Fishes 2020, 5, 28. [Google Scholar] [CrossRef]
- Serafy, J.E.; Shideler, G.S.; Araújo, R.J.; Nagelkerken, I. Mangroves Enhance Reef Fish Abundance at the Caribbean Regional Scale. PLoS ONE 2015, 10, e0142022. [Google Scholar] [CrossRef]
- Carrasquila-Henao, M.; Juanes, F. Mangroves Enhance Local Fisheries Catches: A Global Meta-Analysis. Fish Fish. 2017, 18, 79–93. [Google Scholar] [CrossRef]
- Sandoval-Londoño, L.A.; Leal-Flórez, J.; Blanco-Libreros, J.F. Linking Mangroves and Fish Catch: A Correlational Study in the Southern Caribbean Sea (Colombia). Bull. Mar. Sci. 2020, 96, 415–430. [Google Scholar] [CrossRef]
- Barbier, E.B. The Protective Service of Mangrove Ecosystems: A Review of Valuation Methods. Mar. Pollut. Bull. 2016, 109, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Sunkur, R.; Kantamaneni, K.; Bokhoree, C.; Ravan, S. Mangroves’ Role in Supporting Ecosystem-Based Techniques to Reduce Disaster Risk and Adapt to Climate Change: A Review. J. Sea Res. 2023, 196, 102449. [Google Scholar] [CrossRef]
- Hutchison, J.; Spalding, M.; Zu Ermgassen, P. The Role of Mangroves in Fisheries Enhancement. Nat. Conserv. 2014, 54, 434. [Google Scholar]
- Sathicq, M.B.; Bauer, D.E.; Gómez, N. Influence of El Niño Southern Oscillation Phenomenon on Coastal Phytoplankton in a Mixohaline Ecosystem on the Southeastern of South America: Río de La Plata Estuary. Mar. Pollut. Bull. 2015, 98, 26–33. [Google Scholar] [CrossRef]
- Melo-González, N.; Müller-Karger, F.E.; Estrada, S.C.; Pérez de los Reyes, R.; del Río, I.V.; Pérez, P.C.; Arenal, I.M. Near-Surface Phytoplankton Distribution in the Western Intra-Americas Sea: The Influence of El Niño and Weather Events. J. Geophys. Res. 2000, 105, 14029–14043. [Google Scholar] [CrossRef]
- Bayarre-Vea, H.D.; Álvarez-Lauzarique, M.E.; Pérez-Piñero, J.S.; Almenares-Rodríguez, K.; Rodríguez-Cabrera, A.; Pría Barros, M.D.C.; Rodríguez Rivera, L.; Fernández Seco, A.; Corral Martín, A. Enfoques, Evolución y Afrontamiento Del Envejecimiento Demográfico En Cuba. Rev. Panam. Salud Publica 2018, 42, e21. [Google Scholar] [CrossRef]
- Destremau, B. Population Aging in Cuba: Coping with Social Care Deficit. In Contextualizing Health and Aging in the Americas; Vega, W.A., Angel, J.L., Gutiérrez Robledo, L.M.F., Markides, K.S., Eds.; Springer: Cham, Switzerland, 2018; pp. 311–336. [Google Scholar]
- Delaney, A.; Yagi, N. Implementing the Small-Scale Fisheries Guidelines: Lessons from Japan. In The Small-Scale Fisheries Guidelines: Global Implementation; Jentoft, S., Chuenpagdee, R., Barragán-Paladines, M.J., Franz, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 313–332. [Google Scholar]
- Teh, L.C.L.; Teh, L.S.L.; Abe, K.; Ishimura, G.; Roman, R. Small-Scale Fisheries in Developed Countries: Looking Beyond Developing Country Narratives Through Japan’s Perspective. Mar. Policy 2020, 122, 104274. [Google Scholar] [CrossRef]
- Cutler, M.; Silva, A.; Gentile, L.; Colburn, L. Tracking Shifts in the Vulnerability and Resiliency of Commercial Fishing Vessel Crew and Hired Captains in New England and the Mid-Atlantic. Mar. Policy 2022, 138, 104980. [Google Scholar] [CrossRef]
- Kritzer, J.P.; Hicks, C.C.; Mapstone, B.D.; Pina-Amargos, F.; Sale, P.F.; Fogarty, M.J.; McCarthy, J.J. Ecosystem-Based Management of Coral Reefs and Interconnected Nearshore Tropical Habitats. Sea 2014, 16, 369–419. [Google Scholar]
- Karr, K.A.; Fujita, R.; Carcamo, R.; Epstein, L.; Foley, J.R.; Fraire-Cervantes, J.A.; Gongora, M.; Gonzalez-Cuellar, O.T.; Granados-Dieseldorff, P.; Guirjen, J.; et al. Integrating Science-Based Co-Management, Partnerships, Participatory Processes and Stewardship Incentives to Improve the Performance of Small-Scale Fisheries. Front. Mar. Sci. 2017, 4, 345. [Google Scholar] [CrossRef]
- Miller, V.; Mirabal-Patterson, A.; García-Rodríguez, E.; Karr, K.A.; Whittle, D. The SOS Pesca Project: A Multinational and Intersectoral Collaboration for Sustainable Fisheries, Marine Conservation and Improved Quality of Life in Coastal Communities. MEDICC Rev. 2018, 20, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Alzugaray, R.; Puga, R.; Valle, S.; Morales, O.; Grovas, A.; López, L.; Kleisner, K.; Boné, E.; Mangin, T.; Kritzer, J.; et al. Un enfoque multiinstitutional para modelar el beneficio bioeconómico de perspectivas de manejo pesquero en Cuba. Rev. Cub. Investig. Pesq. 2019, 36, 52–61. [Google Scholar]
- Claro, R.; Baisre, J.A.; Lindeman, K.C.; García-Arteaga, J.P. Cuban Fisheries: Historical Trends and Current Status. In Ecology of Marine Fishes of Cuba; Claro, R., Lindeman, K.C., Parenti, L.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2001; pp. 194–219. [Google Scholar]
- McGilliard, C.R.; Hilborn, R.; MacCall, A.; Punt, A.E.; Field, J.C. Can Information from Marine Protected Areas Be Used to Inform Control-Rule-Based Management of Small-Scale, Data-Poor Stocks? ICES J. Mar. Sci. 2011, 68, 201–211. [Google Scholar] [CrossRef]
- Punt, A.E.; Thomson, R.; Little, L.R.; Bessell-Browne, P.; Burch, P.; Bravington, M. Including Close-Kin Mark-Recapture Data in Statistical Catch-at-Age Stock Assessments and Management Strategies. Fish. Res. 2024, 276, 107057. [Google Scholar] [CrossRef]
- Dorta, C.; Martín-Sosa, P. Fishery Essentiality: A Short-Term Decision-Making Method Based on Economic Viability as a Tool to Understand and Manage Data-Limited Small-Scale Fisheries. Fish. Res. 2022, 246, 106171. [Google Scholar] [CrossRef]
- Costello, C.; Ovando, D.; Clavelle, T.; Strauss, C.K.; Hilborn, R.; Melnychuk, M.C.; Branch, T.A.; Gaines, S.D.; Szuwalski, C.S.; Cabral, R.B.; et al. Global Fishery Prospects Under Contrasting Management Regimes. Proc. Natl. Acad. Sci. USA 2016, 113, 5125–5129. [Google Scholar] [CrossRef]
- Watanabe, F.; Alcantara, E.; Rodrigues, T.; Rotta, L.; Bernardo, N.; Imai, N. Remote Sensing of the Chlorophyll-a Based on OLI/Landsat-8 and MSI/Sentinel-2A (Barra Bonita Reservoir, Brazil). An. Acad. Bras. Cienc. 2017, 90, 1987–2000. [Google Scholar] [CrossRef]

| Variable | Extension | Source |
|---|---|---|
| North Atlantic multidecadal oscillation index (AMO) | Regional | NOAA |
| AMO 9-, 26-, and 67-year cycles | Regional | del Monte-Luna et al. [34] |
| Southern Oscillation Index (SOI) | Regional | NOAA |
| Caribbean Sea Surface Temperature (SST) | Regional | NOAA |
| Cuba’s southeast zone SST | Local | Landsat image time series |
| Southeast zone’s accumulated cyclonic energy (ACE) | Local | HURDAT; Landsea and Franklin [35] |
| Natural coastal vegetation cover | Local | Landsat image time series |
| Chlorophyll-a concentration | Local | Landsat image time series |
| Area of water reservoirs over land | Local | Landsat image time series |
| Precipitations over land | Local | ERA5 |
| Total nutrient nitrogen per area of cropland | Local | FAO |
| Fishing effort | Local | Cuba’s Fisheries Research Center |
| Component | Term | Estimate | Standard Error | t-Value | p-Value |
|---|---|---|---|---|---|
| Parametric coefficient | (Intercept) | 8.558 | 0.017 | 507.379 | <0.001 |
| Component | Term | edf | Ref. df | F-value | p-value |
| Smooth terms | Effort | 1.00 | 1.00 | 114.37 | <0.001 |
| Rainfall | 2.09 | 2.59 | 5.17 | 0.02 | |
| Dams | 1.00 | 1.00 | 0.05 | 0.828 | |
| Vegetation | 1.00 | 1.00 | 6.81 | 0.017 | |
| Chlorophyll-a | 3.42 | 4.11 | 7.38 | 0.001 | |
| SST | 1.00 | 1.00 | 3.42 | 0.079 | |
| SOI | 1.00 | 1.00 | 13.64 | 0.001 | |
| AMO 26 | 1.00 | 1.00 | 0.4 | 0.533 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivera-Espinosa, Y.; Rodríguez-Cueto, Y.; Pina-Amargós, F.; Arreguín-Sánchez, F.; Zetina-Rejón, M.J.; Karr, K.; del Monte-Luna, P. Trends and Environmental Drivers of Marine Fish Landings in Cuba’s Most Productive Shelf Area. Fishes 2024, 9, 246. https://doi.org/10.3390/fishes9070246
Olivera-Espinosa Y, Rodríguez-Cueto Y, Pina-Amargós F, Arreguín-Sánchez F, Zetina-Rejón MJ, Karr K, del Monte-Luna P. Trends and Environmental Drivers of Marine Fish Landings in Cuba’s Most Productive Shelf Area. Fishes. 2024; 9(7):246. https://doi.org/10.3390/fishes9070246
Chicago/Turabian StyleOlivera-Espinosa, Yunier, Yandy Rodríguez-Cueto, Fabián Pina-Amargós, Francisco Arreguín-Sánchez, Manuel J. Zetina-Rejón, Kendra Karr, and Pablo del Monte-Luna. 2024. "Trends and Environmental Drivers of Marine Fish Landings in Cuba’s Most Productive Shelf Area" Fishes 9, no. 7: 246. https://doi.org/10.3390/fishes9070246
APA StyleOlivera-Espinosa, Y., Rodríguez-Cueto, Y., Pina-Amargós, F., Arreguín-Sánchez, F., Zetina-Rejón, M. J., Karr, K., & del Monte-Luna, P. (2024). Trends and Environmental Drivers of Marine Fish Landings in Cuba’s Most Productive Shelf Area. Fishes, 9(7), 246. https://doi.org/10.3390/fishes9070246








