Abstract
As an economic species widely distributed in the South China Sea (SCS), the purpleback flying squid (Sthenoteuthis oualaniensis) still has a large potential for exploitation, and the variations in its use as a resource are highly correlated with environmental and other factors. In this study, using a generalized additive model (GAM) and gradient forest analysis (GFA), in conjunction with environmental factors, the distribution of purpleback flying squid surrounding the Xisha and Zhongsha islands during the fishing moratorium period was investigated. The results indicated that catch per unit effort (CPUE) had a gradual increase from May to July 2023 in the primary fishing area surrounded the Xisha Islands during May to June, then moved southward towards 13–15° N after July. CPUE is used as an important indicator to reflect the abundance of the fishery, while the GFA results show that CPUE has a better fit than catch in this study. Therefore, the subsequent analysis focused on CPUE. Longitude and sea surface temperature (SST) were of relative higher importance, followed by sea surface salinity (SSS), latitude, chlorophyll a concentration (Chla), sea surface height (SSH), and mixed layer depth (MLD). Longitude and CPUE had a significant, positive correlation. The CPUE gradually increased with latitude within 14–16° N. The CPUE increased slowly as SST increased from 29.5 to 30.5 °C in the primary fishing area. The Chla in this fishing zone was 0–0.2 mg/m3 and displayed a significant positive association with CPUE. Conversely, SSS, SSH, and MLD had negative correlations with CPUE. These findings will promote the sustainable utilization of purpleback flying squid in the SCS.
Keywords:
purpleback flying squid; Xisha and Zhongsha islands; fishing ground; environmental factors Key Contribution:
In this study, we conducted an analysis on the distribution characteristics of purpleback flying squid in the Xisha and Nansha islands during the fishing moratorium period, as well as its correlation with environmental factors. The findings of this study contribute to a comprehensive understanding of the summer fishing grounds distribution for purpleback flying squid in the South China Sea, thereby providing valuable theoretical support for their exploitation and utilization.
1. Introduction
The South China Sea (SCS) is a closed marginal sea in the western Pacific Ocean that is characterized by an extensive continental shelf [1]. The Xisha Islands are situated in the western part of the SCS, spanning an area of more than 500,000 km2. The Zhongsha Islands are situated in the central region of the SCS, with the Xisha Islands located an additional 100 km away. The SCS is situated in the north-central northwest Pacific, serving as a transitional zone between the continental shelf and the deep sea extension. In this area, water depth ranges from 100 to 1000 m in its western part and can reach up to 3000–4000 m in its eastern section. Additionally, the sea area surrounding the Xisha and Zhongsha islands exhibits favorable climatic conditions for marine ecosystems and is predominantly influenced by the northeast monsoon during winter, which is characterized by elevated wind velocities and declining temperatures [2]. In the summer period, the dominant southerly air currents, coupled with elevated temperature and humidity levels, have a substantial influence on tropical cyclones and precipitation patterns [2]. This region also serves as the primary tropical fishing ground in China, with a rich diversity of coral reef and pelagic fish species. Additionally, it is recognized as one of the key migratory sites for purpleback flying squid (Sthenoteuthis oualaniensis) [3,4].
The purpleback flying squid is an annual warm-water oceanic cephalopod with a wide distribution throughout the SCS [5]. It has a complex population structure, a short life cycle, robust reproductive capacity, high natural mortality rates, rapid growth rate, and substantial catch [6]. It has previously been reported that the abundance and exploitable biomass of purpleback flying squid in the SCS exceed one million tons, and it is widely acknowledged that the deep-water region of the south-central SCS harbors a substantial population of purpleback flying squid with considerable developmental potential [7,8]. The stock structure of purpleback flying squid is intricate, and it is widely acknowledged that the purpleback flying squid found in the SCS can be categorized into medium and dwarf forms based on variations in dorsal luminescence [9,10]. The medium form represents the dominant stock, and is distributed extensively throughout the SCS, while the abundance of dwarf-form individuals remains relatively limited [11]. The purpleback flying squid lives in a habitat layer with a seawater temperature range of 14–31 °C and displays distinct vertical movement patterns [5]. It stays at depths below 200 m during the day but frequently ascends to the middle and upper sea regions for nocturnal feeding. Seasonal migrations of this squid are noticeable and are mainly characterized by movements from shallow to deep sea areas during winter and from deep to shallow sea areas for spawning [5,12].
Previous studies have demonstrated that migratory pelagic fish distributions exhibit a preference for feeding environments that are conducive to their growth while simultaneously seeking out optimal temperature conditions [13,14]. The distribution of purpleback flying squid in fishing grounds is significantly influenced by marine environmental factors, including sea surface temperature (SST), sea surface height (SSH), and sea surface chlorophyll a concentration (Chla) [15]. Previous studies have shown that variations in the marine environment within areas such as fishing grounds and spawning grounds can result in changes to the spatial habitat preferences of purpleback flying squid for spawning and feeding activities, consequently leading to fluctuations in its population abundance [16]. Studies of the spatiotemporal distribution of purpleback flying squid in the waters near the Xisha and Zhongsha islands have revealed a strong correlation between its abundance and environmental factors such as SST, Chla, and SSH. However, previous studies of this species have primarily focused on a limited range of environmental factors when analyzing its habitat distribution [4,17], without considering multivariate environmental factors or employing statistical models to examine their characteristics in waters, such as those near the Xisha and Zhongsha islands. It is generally believed that the waters around the islands and reefs in the SCS may be important spawning areas for purpleback flying squid. Consequently, alterations in the marine environment of these regions will likely have a substantial influence on the crucial early life history processes of the species, such as the spawning behavior, with the peak spawning period occurring from April to July [18,19]. Furthermore, the fishing moratorium period (refers to a system for the protection of fishery resources organized and implemented by the General Administration of Fisheries of China, which stipulates that certain operations are not allowed to engage in fishing operations at certain times of the year and in certain waters) in the SCS waters north of 12° N from May to August has restricted the capture of purpleback flying squid to the waters south of 12° N in the Nansha Islands. No fishing vessels are permitted in the vicinity of the Xisha and Zhongsha islands during this period. Therefore, studies of the distribution of the purpleback flying squid fishing ground in this area during the period of the fishing moratorium are relatively scarce. In this study, we utilized catch data from the fishing moratorium period in 2023, focusing on the primary fishing locations situated around the Xisha and Zhongsha islands. Using a generalized additive model (GAM) and gradient forest analysis (GFA), we analyzed the spatiotemporal variability of the purpleback flying squid resource. Additionally, we investigated the influence of various environmental factors such as SST, sea surface salinity (SSS), Chla, SSH, and mixed layer depth (MLD) on its use as a resource. This comprehensive investigation provides valuable theoretical support for sustainable utilization and management strategies to ensure the resource of purpleback flying squid in the SCS. Simultaneously, it can also offer scientific guidance for the specialized fishing operations targeting purpleback flying squid during this specific season and in this particular region.
2. Material and Methods
2.1. Data Collection
2.1.1. Fisheries Data
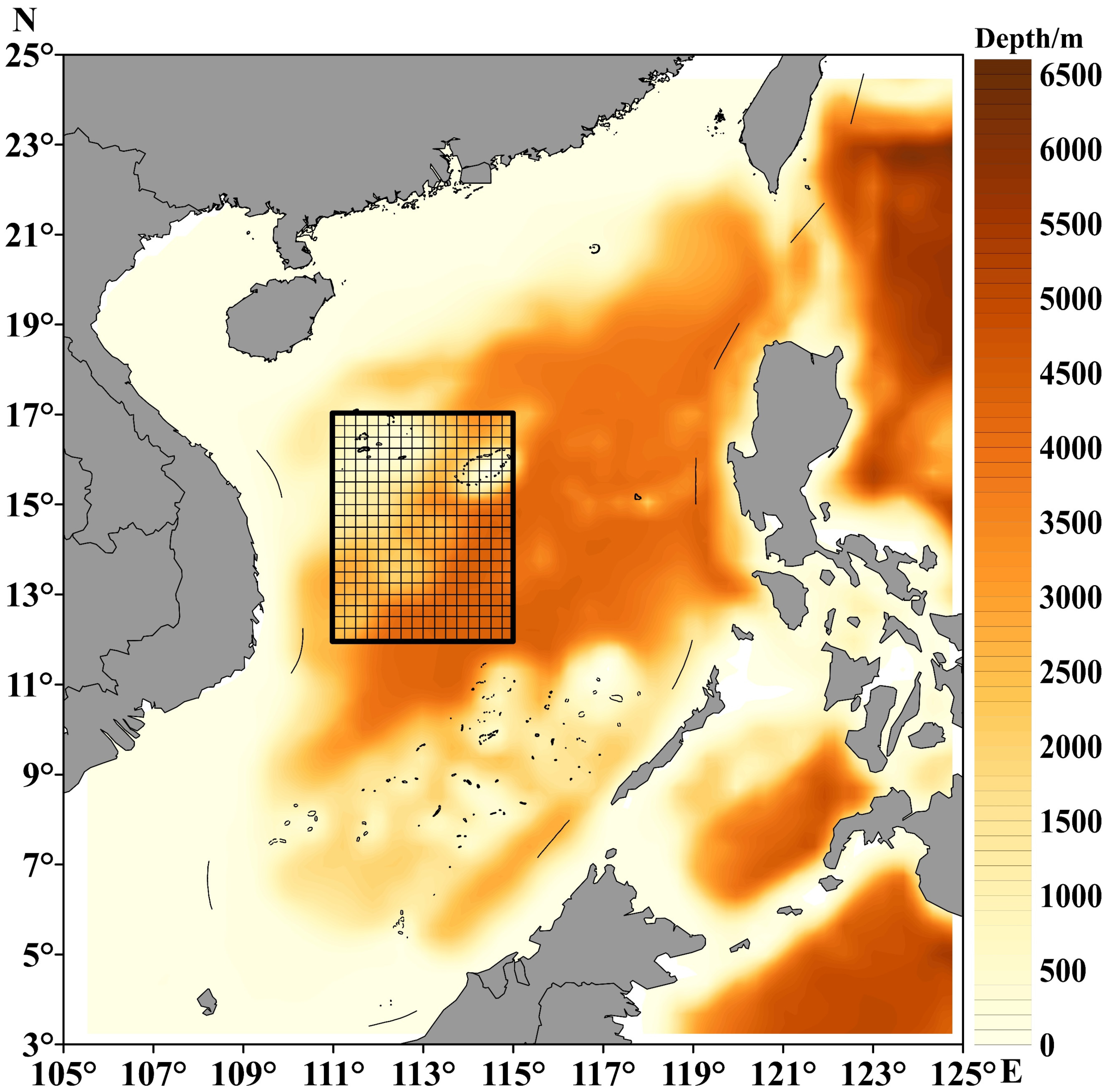
As it is a species with potential for exploitation, it is necessary to carry out rational and specialized fishing activities for the purpleback flying squid in order to rationally utilize this species. With the approval of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China, Guangxi has initiated a dedicated fishing operation targeting purpleback flying squid in the South China Sea waters during the fishing moratorium in 2023. The data utilized in this study were obtained from this specialized fishing expedition. Fishery data were obtained from 33 fishing vessels operating in the waters around the Xisha and Zhongsha islands from May to July in 2023. All fishing vessels in the study used mask nets (Figure 1). From 16 May to 23 July, a total of 33 fishing vessels sequentially departed for the capture area and subsequently returned within the boundary defined by four points: 111°00′ E, 17°00′ N; 115°00′ E, 17°00′ N; 115°00′ E, 12°00′ N; and 111°00′ E, 12°00′ N (Figure 2). The dataset constructed for the study comprised information regarding the duration of operation, longitude and latitude of operation, and catch data. The geographical coordinates of sites where fishing vessels were productive were gridded at a resolution of 0.25° (longitude × latitude). The timing of this productivity was recorded as late May, early June, middle June, late June, early July, and middle July.
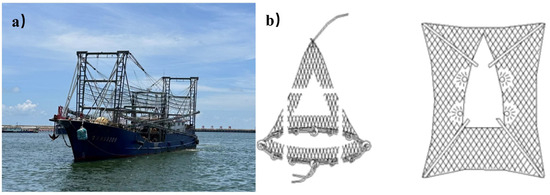
Figure 1.
Schematic diagram of masked net boat (a) and net gear (b) [19].
Figure 2.
The fishing area of purpleback flying squid.
2.1.2. Environmental Data
The selected marine environmental factors were SST, SSS, Chla, SSH, and MLD. Daily SST, Chla, and SSH data from 20 May to 20 July 2023 were obtained from the Ocean Color (http://oceanwatch.pifsc.noaa.gov/thredds/catalog.html; accessed on 1 September 2023). The daily SSS and MLD data from 20 May to 20 July 2023 were obtained from the Global ARMOR3D L4 dataset, which was downloaded from the Copernicus Marine Environment Monitoring Service (CMEMS) (https://marine.copernicus.eu/access-data; accessed on 1 September 2023). The environmental data covered the entire fishery area, and all environmental data were averaged over six time periods in late May, early June, middle June, late June, early July, and middle July and were calculated on a ten-day interval basis. The spatial resolution was converted to 0.25° (longitude × latitude) to match the fishery data.
2.2. Data Analyses
2.2.1. Calculations of the Catch per Unit Effort (CPUE) and Barycenter of the Fishing Ground
Fishery data statistics included fishing date (month and day), fishing location (longitude and latitude), daily catch (tons), and fishing effort (days). The timing of this catch was broken down into late May, early June, middle June, late June, early July, and middle July. Catch per unit effort (CPUE) can be used as an indicator to reflect the abundance of fishery resources [10,15]. In this study, CPUE was calculated by dividing the total catch of all vessels in different time periods by the total number of operating days of all fishing vessels in that time period, with the resulting value expressed in kg/day/boat. Equation (1) was calculated as follows. Purpleback flying squid catch locations were gridded separately at a resolution of 0.25° × 0.25°, with gridded CUPE data subsequently matched with environmental factors for a correlation analysis.
where is the total catch of all fishing vessels in a fishing area. is the total number of operating days of all fishing vessels in a fishing area. i, j, and m are the longitude, latitude, and month, respectively.
Additionally, to reflect the variations in the purpleback flying squid fishery at different time periods, the barycenter of the fishing ground at different time periods was calculated. Longitude and latitude were calculated as follows:
where Lon0 and Lat0 are the longitude and latitude of the barycenter of the fishing ground, i is the number of grids, Ci is the catch of the ith grid, and Loni and Lati are the longitude and latitude of each of the ith grids, respectively.
2.2.2. Catch and CPUE Fitted in Relation to Environmental Factors
We used a GFA to analyze the relationships between the purpleback flying squid catch, CPUE, and multiple environmental factors and then derived the significance and gradient response of their effects on the catch and CPUE. Gradient forests are based on random forests and are used to determine the relationships between multivariate response variables and multivariate predictor variables [20]. In the multiple regression tree, the response variables are divided into two groups at specific predictor variable values to achieve maximum homogeneity, i.e., the sum of squares of deviation (i.e., impurity) within each group is minimized. The grouping method is recursive until the group contains the least number of response variables. In contrast, random forests are collections of multiple regression trees that aggregate the results of a large number of regression trees and use a self-help method to determine the optimal grouping method [21]. The GFA builds on the above methods by providing goodness-of-fit values for each response variable, as well as the importance of the predictor variables weighted according to the goodness of fit [20]. The gradient forest provides an R2 value for each response variable and the standardized importance Ifp for each predictor variable. The standardized importance is the increase in the mean squared prediction error in the out-of-bag (OOB) samples when the predictor variables are randomly ordered or disrupted. Larger Ifp values indicate stronger predictor variables that have a more substantial impact on the model’s predictive performance. The importance of the split values along the predictor gradient reflects the relative change in the response variable. In the GFA analysis conducted in this study, the measure of resource change included catch and CPUE data, and the measure of environmental change included the environmental variable datasets (SST, SSS, Chla, SSH, and MLD). The GFA was performed for 1000 runs to obtain the mean and standard deviation (sd) of the R2 value. The results with the highest R2 were used to derive Ifp and other statistical parameters. The GFA was conducted using the “gradientForest” package in R4.1.0.
Additionally, we used a GAM to further analyze the CPUE data. The CPUE data of different periods were extracted and matched with the SST, SSS, Chla, SSH, and MLD of the corresponding time periods. The factors characterized by multicollinearity should be screened before model construction. The variance inflation factor (VIF) was applied to screen for multicollinearity characteristics among the explanatory variables added to the model. A value of VIF > 3 is generally considered to represent multicollinearity [22]. The selected factors based on the results of the VIF can all be added to the GAM for analysis (Table S1). The GAM was introduced to analyze the most influential factors affecting the distribution of the fishery with the purpleback flying squid CPUE as the response variable and SST, SSS, Chla, SSH, MLD, and season as the explanatory variables. Season was selected as the factor variable, with the final expression of the GAM model being as follows [23]:
where Y is the CPUE of the purpleback flying squid; xi is the explanatory variable; and five environmental variables (SST, SSS, Chla, SSH, and MLD) were selected. Furthermore, seasonal variables were also added to the model as factor variables, with a being the function intercept. fi(xi) is the smoothing function, and ε denotes the residuals. The error functions for the model analysis were all normally distributed, and a natural logarithmic connection function was used in the model. CPUE was log-transformed by adding a constant term before being incorporated into the model (Figure S1). The fitting and validation of the above models were conducted using the R4.1.0 software, and the construction and testing of the GAM were completed using the “mgcv” package [24,25].
3. Results
3.1. Variations in Catch and CPUE
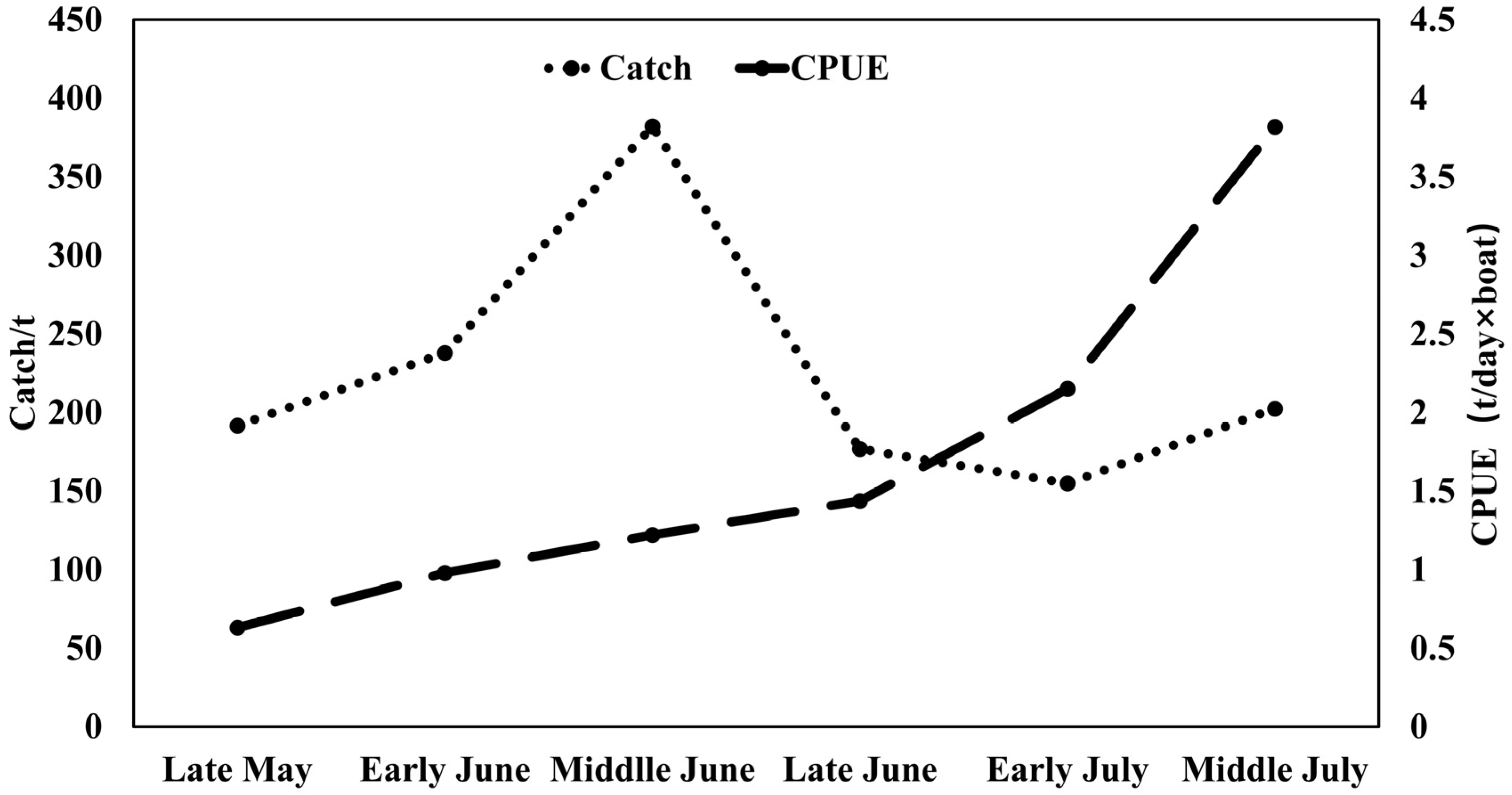
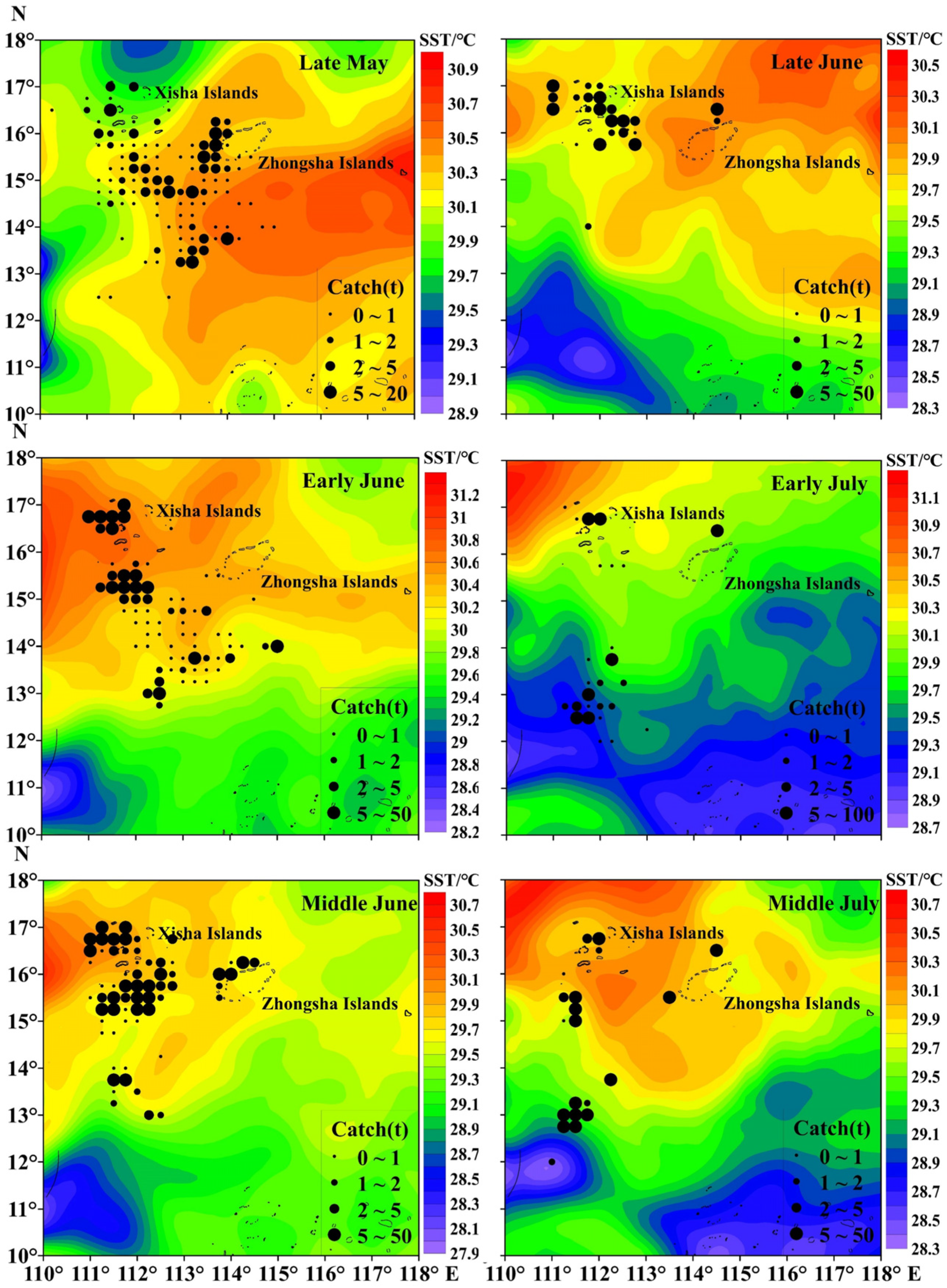
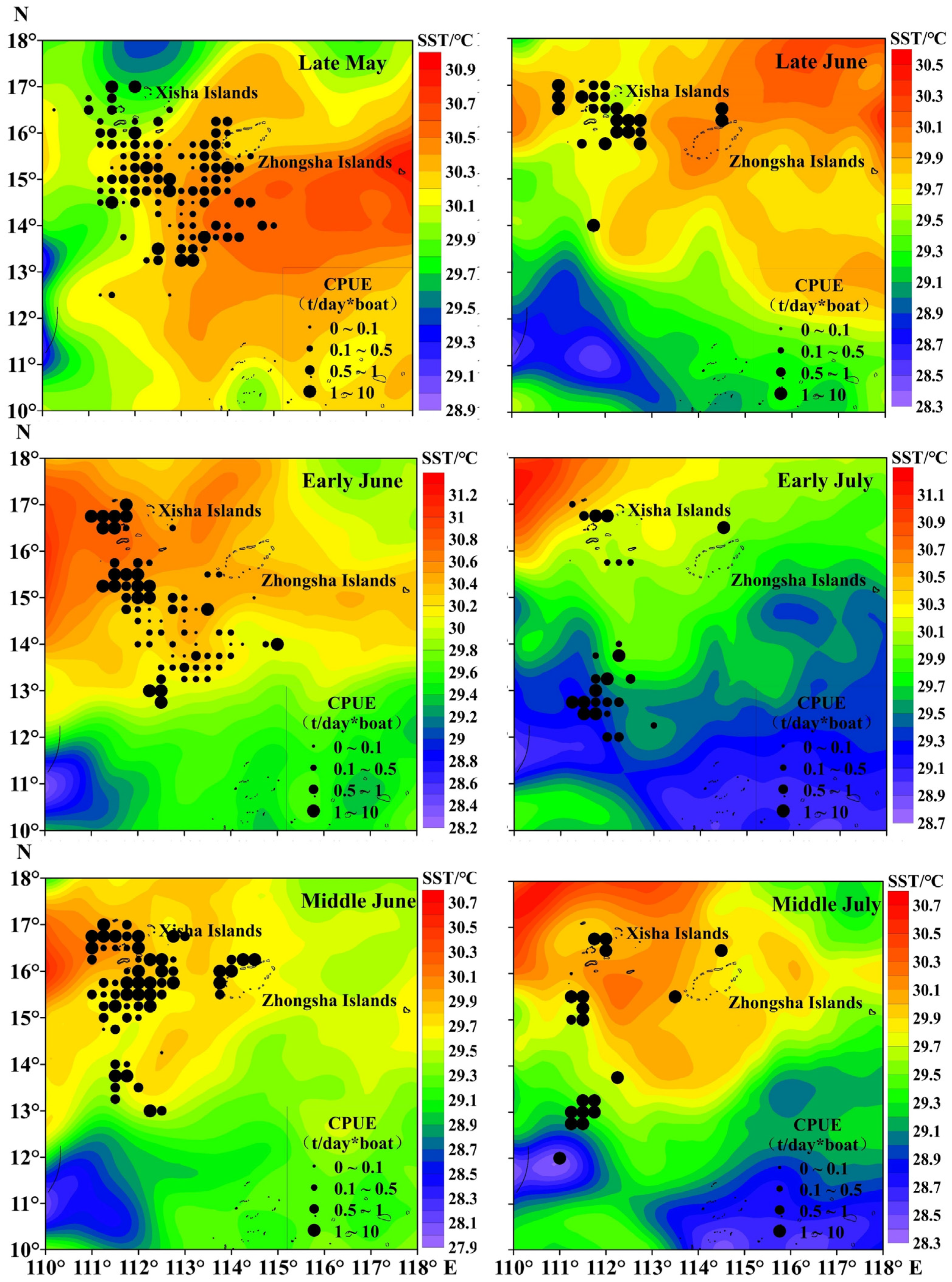
The total catch of purpleback flying squid amounted to 1345.37 tons, with the peak catch occurring in middle June at 382.02 tons, followed by 237.77 tons in early June. Subsequently, catches of 202.34, 191.64, and 176.50 tons were recorded in middle July, late May, and late June, respectively (Figure 3). The CPUE exhibited an increasing trend from late May to middle July, with an average of 1.22 t/day × boat. The highest CPUE was observed in middle July (3.82 t/day × boat), followed by 2.15 t/day × boat in early July. In late June, middle June, early June, and late May, the CPUE values were recorded as 1.44 t/day × boat, 1.22 t/day × boat, 0.98 t/day × boat, and 0.63 t/day × boat for these respective time periods (Figure 3). As shown in Figure 4 and Figure 5, distinct spatiotemporal variations were observed in catch and CPUE. In late May, a high CPUE was primarily concentrated in the western and southern waters of the Xisha Islands, indicating a well-dispersed fishing area. However, during early and middle June, the main fishing area shifted to the western and southern waters of the Zhongsha Islands, which also served as a high-productivity region, with the western and southern waters of the Xisha Islands becoming a limited productivity area by late June. Subsequently, in July, there was a significant southward shift of the production area mainly toward 12–13° N.
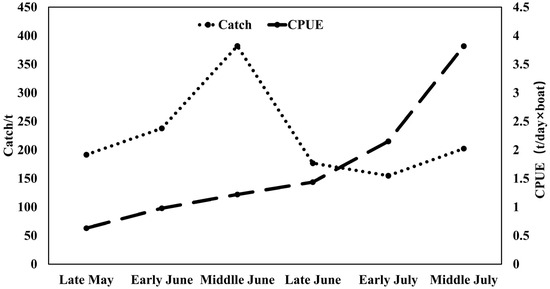
Figure 3.
Seasonal variations in the catch and CPUE of purpleback flying squid.
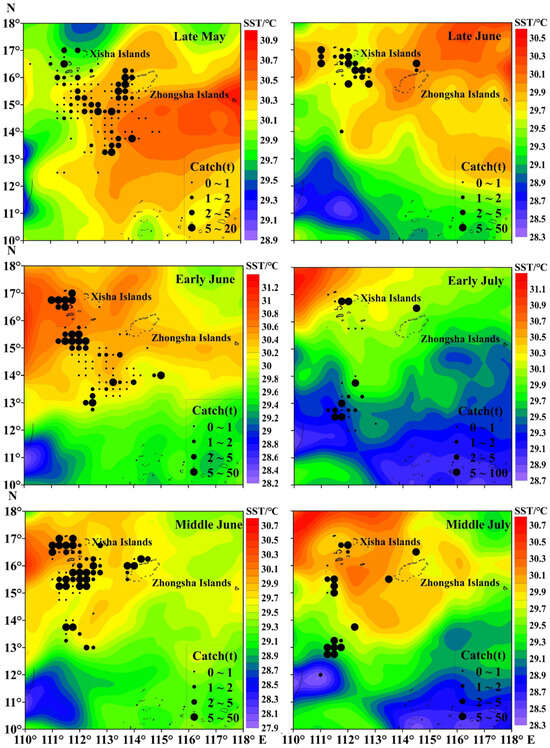
Figure 4.
Spatiotemporal distribution of the purpleback flying squid catch.
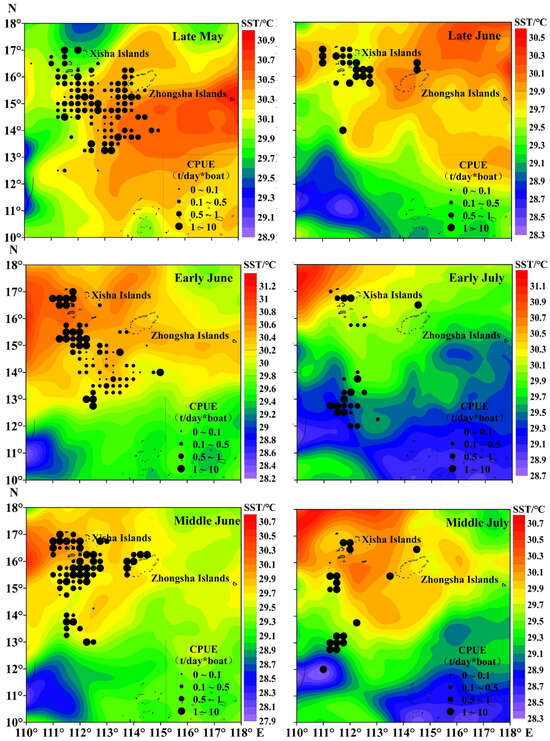
Figure 5.
Spatiotemporal distribution of the purpleback flying squid CPUE.
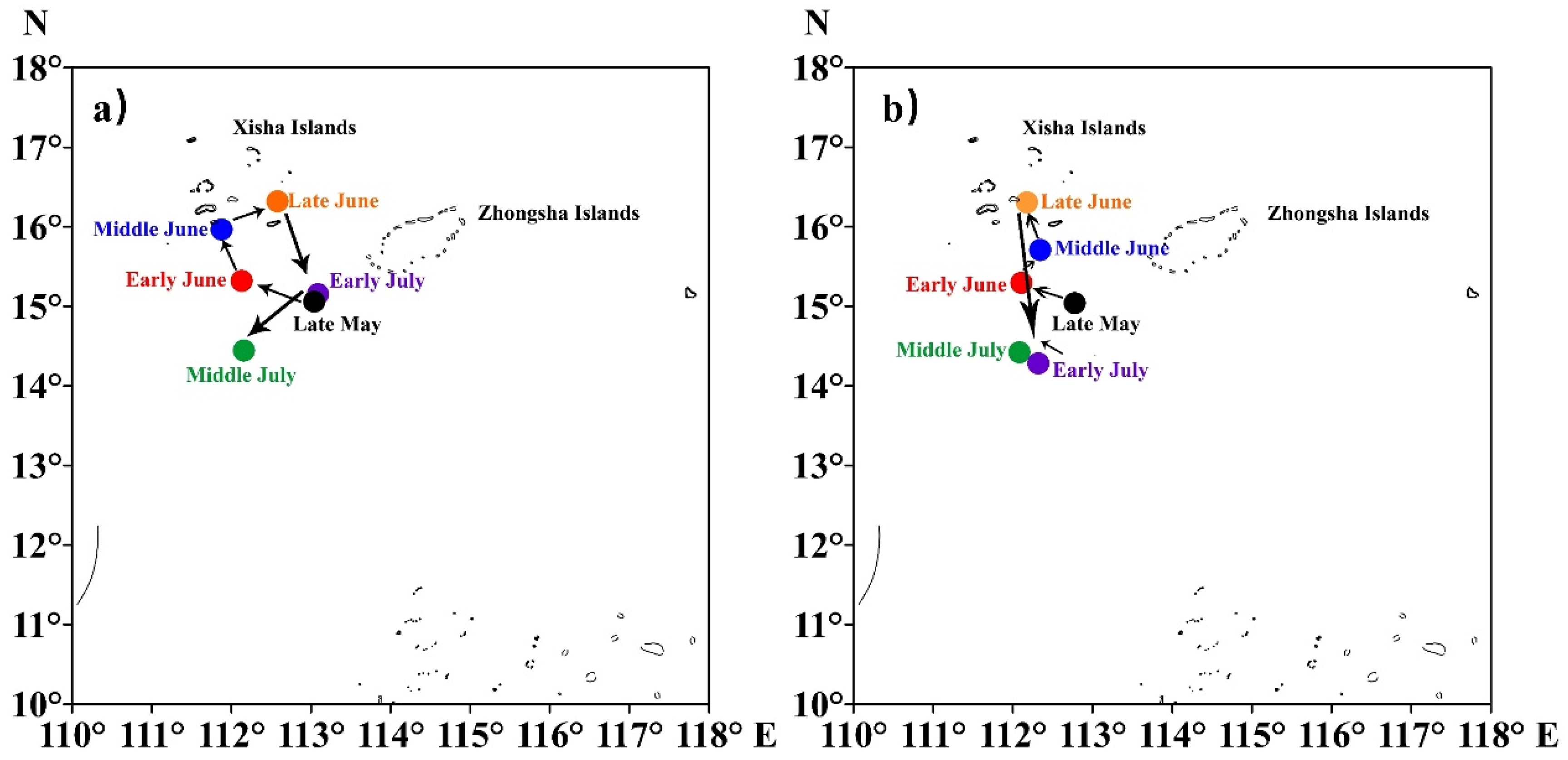
3.2. Variations in Barycenter of Fishing Ground
The fluctuations in the barycenter of the purpleback flying squid catch and CPUE indicated that the primary fishing grounds between May and July were located in the sea area encompassing the southern and western regions of the Xisha Islands. Moreover, there was a seasonal variation observed in these fishing grounds, gradually shifting northwestward and northward from the vicinity of the western part of the Xisha Islands towards late May, followed by a subsequent gradual shift southward after July (Figure 6).
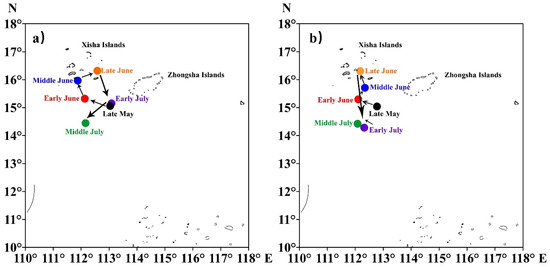
Figure 6.
The variation in the barycenter of purpleback flying squid catch (a) and CPUE (b). The colors represent different periods.
3.3. Variations in Environmental Factors
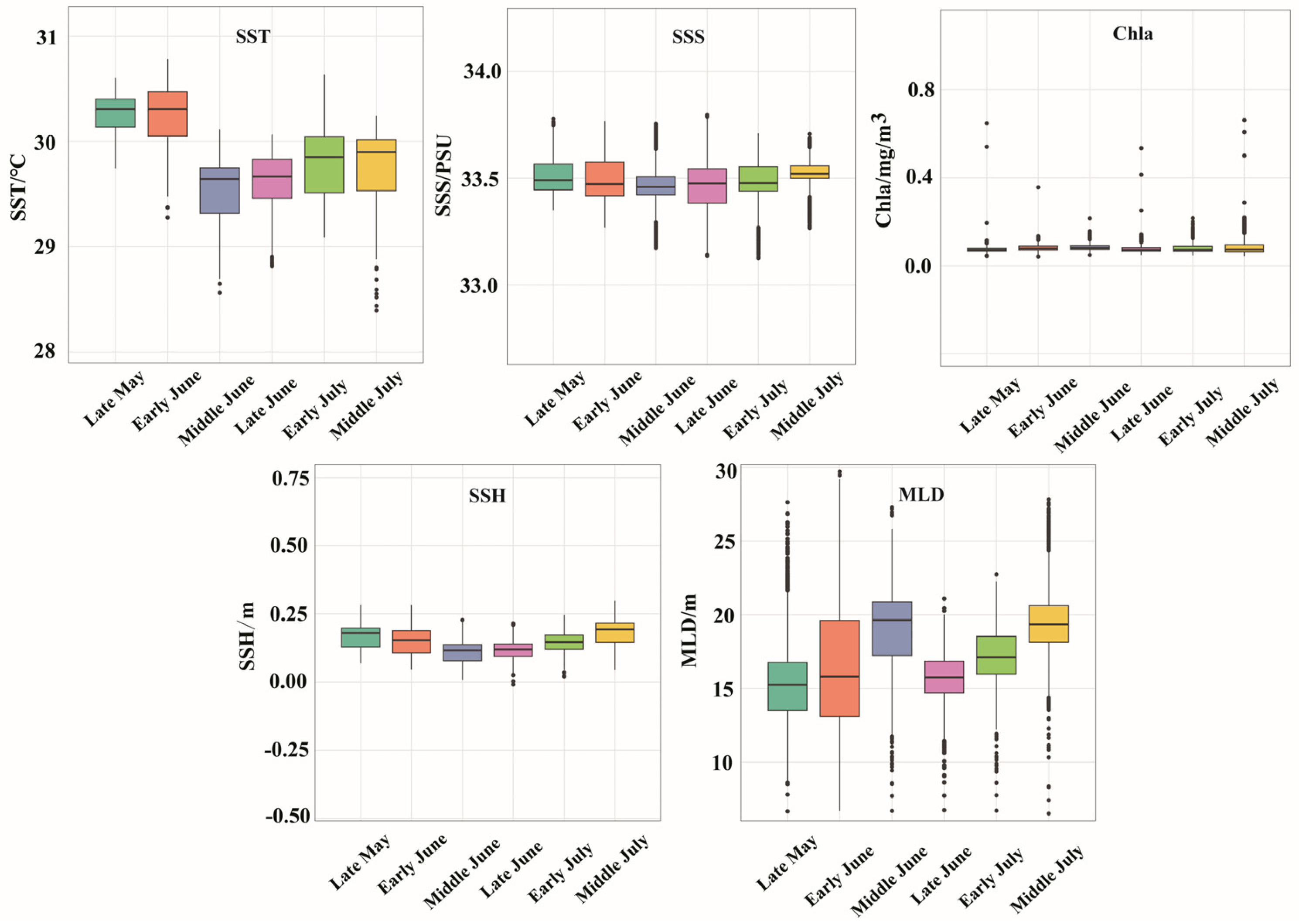
The variations in SST, SSS, Chla, SSH, and MLD for each period are presented in Figure 6. In terms of SST, the average SST was significantly higher in late May (30.26 °C) and early June (30.23 °C) than in the other periods. The mean SSS during the period from late May to middle July was 33.49, with minimal salinity fluctuations observed across the different time intervals. The average Chla for each time period was 0.08 mg/m3, indicating a consistently low level with no significant temporal or spatial variations. The mean SSH was highest in middle July at 0.18 m, followed by middle June at 0.15 m, and in terms of the spatial distribution, the sea area around the Xisha Islands was higher than the other sea areas. The MLD exhibited a sequential increase from late May (15.28 m) to late June (15.78 m), early June (16.31 m), early July (17.25 m), and middle June (18.86 m) and finally reached a peak in middle July (19.33 m). Figure 7 shows the distinct variations in the spatial distribution of the MLD.
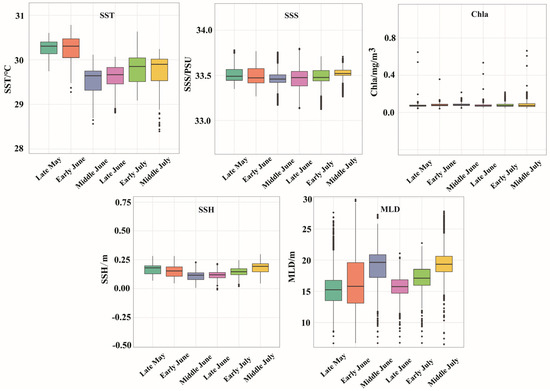
Figure 7.
Distribution of environmental factors in each season.
3.4. Relationship between the Distribution of Purpleback Flying Squid and Environmental Factors
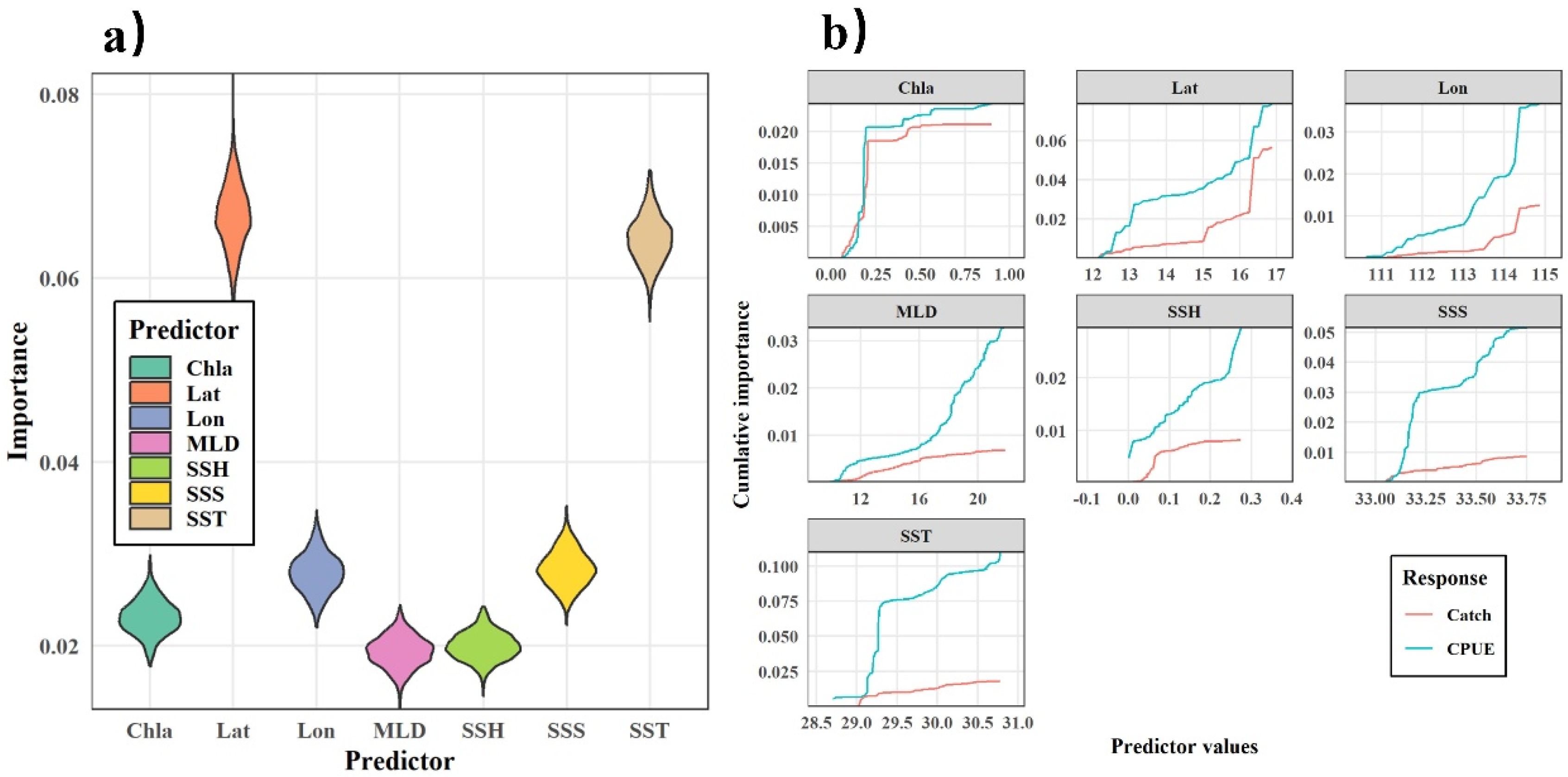
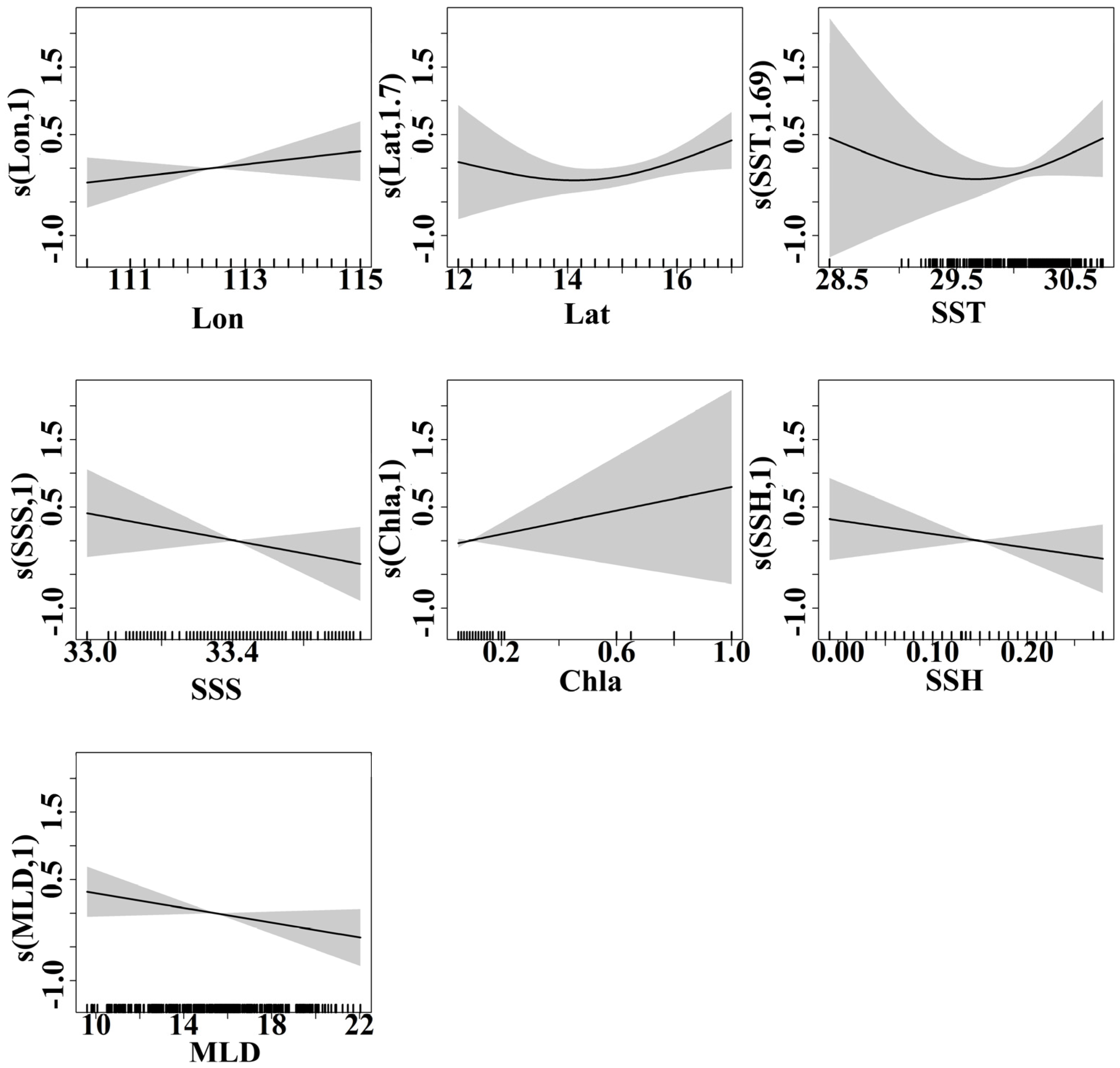
The GFA was first used to analyze the response of the purpleback flying squid catch and CPUE to SST, SSS, Chla, SSH, and MLD. Based on 1000 runs of the gradient forest, the results of 1000 simulations of the catch and CPUE have a fit of R2 = 0.018 (sd = 0.007) for catch and R2 = 0.307 (sd = 0.009) for CPUE. The GFA demonstrated that the variables had a stronger correlation with CPUE than catch alone, suggesting that the selected marine environmental factors were effective predictors of the variations in CPUE. Therefore, CPUE was selected as an indicator to represent the distribution of the purpleback flying squid resource, and the importance of each environmental factor in explaining CPUE variation was analyzed using gradient forests. After 1000 runs of the model, the results of the run with the highest R2 value were used to quantify the relationship between the marine environment and CPUE. The importance of each factor in explaining CPUE is shown in Figure 8, with longitude and SST having the highest importance, followed by SSS, latitude, Chla, SSH, and MLD. The intervals of the environmental indices with strong threshold responses to CPUE were 29.0–29.5 °C for SST, 33.0–33.2 for SSS, 0.05–0.10 m and 0.20–0.25 m for SSH, 16–20 m for MLD, and 0–0.2 mg/m3 for Chla (Figure 8).
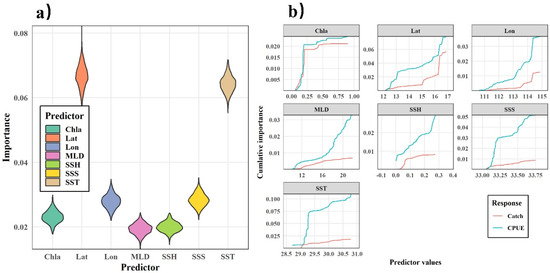
Figure 8.
Weighted importance (a) and cumulative importance (b) of environmental factors in explaining the distribution of the purpleback flying squid catch and CPUE based on a GFA.
Because a GFA solely reflects the magnitude of the marine environment’s response to CPUE variations at each gradient without indicating whether the response is positive or negative, a GAM was applied for a qualitative analysis of the trends in the responses of environmental variables to CPUE. The results of the GAM analysis are presented in Figure 8, indicating a significant and positive correlation between longitude and CPUE. The strength of this correlation gradually increased as the longitude increased. The CPUE varied least in the 12–14° N latitude region, while in the 14–16° N latitude range, the CPUE displayed a slow upward trend. The SST range within the primary fishing area of the purpleback flying squid was between 29.5 and 30.5 °C, with a gradual increase in CPUE corresponding to higher SST values within this specific range. The CPUE had a significant negative correlation with SSS within the range of 33.0–33.5. Additionally, the Chla levels in the primary fishing area of the purpleback flying squid ranged from 0 to 0.2 mg/m3 and displayed a significant positive correlation with CPUE. The SSH in the production area of the purpleback flying squid exhibited a significant negative correlation with CPUE in the range of 0–0.20 m, while the MLD within the range of 10–20 m had a significant negative correlation with CPUE. Additionally, there was a significant negative correlation between the MLD within the range of 10–20 m and CPUE (Figure 9).
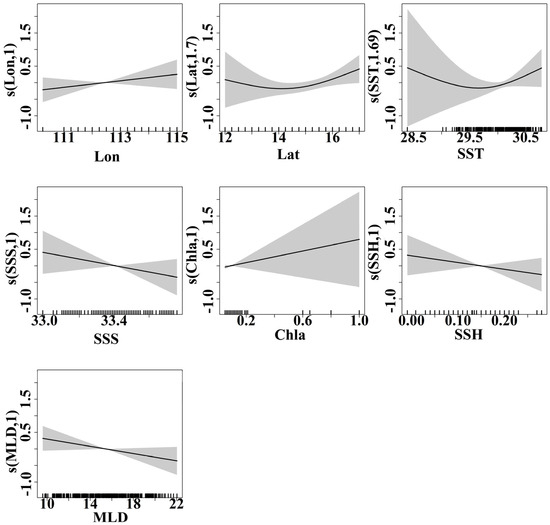
Figure 9.
Effects of various factors on the CPUE of purpleback flying squid during the period from late May to early July in 2023 are depicted, with shaded areas representing the 95% confidence intervals.
4. Discussion
4.1. Spatiotemporal Distribution of Purpleback Flying Squid Fishing Ground
The CPUE is commonly assumed to be positively correlated with the abundance of fishery resources and is therefore a crucial indicator for assessing biomass [26]. In this study, our focus was on determining the distribution of CPUE for purpleback flying squid. The average CPUE for this species was 1.22 t/day × boat, with a gradual increase observed from May to July (Figure 2). The highest CPUE recorded was 3.82 t/day × boat in middle July, while the lowest CPUE was 0.63 t/day × boat in late May. It was found that the northeastern part of the study area exhibited a high level of fishing activity, particularly during the period from late May to June. Subsequently, after July, the focal point of intensive fishing shifted towards the western area within the 13–15° N latitude range. By analyzing the barycenter shift in the purpleback flying squid fishing ground, it was evident that the primary fishery area for this species during May to July was located between the southern Xisha Islands and western Zhongsha Islands. Moreover, there was a noticeable seasonal variation in its distribution, with a gradual northwestern and northern movement from middle May towards the western part of the Zhongsha Islands, followed by a subsequent southward shift after July. The distribution of the purpleback flying squid fishery varied seasonally [15]. The overall distribution of the fishery in the SCS exhibited a distinct north-to-south shift, characterized by patchy and sporadic patterns. During spring (March–June), the fishery was predominantly concentrated in the northern waters of the SCS. In summer (July–August), it was mainly located in the southwestern SCS waters. In fall (September–November), it was distributed in the south-central SCS waters, particularly along coastal areas where fishing conditions were favorable. In winter (December–February), there was a limited presence of resources within the SCS [19]. Based on the resource survey conducted by the South China Sea Fisheries Research Institute of the Chinese Academy of Fisheries Sciences in the SCS, it was found that during summer, the area with a high-density of purpleback flying squid was primarily situated in the area between 17–18° N, 113.5–115.5° E and 12.5–15.5° N, 115.5–117° E [19]. This observation revealed a significant overlap between the central fishing ground area and the area covered by our study. Furthermore, Yu et al. [4] investigated the spatial distribution in the sea area surrounding the Xisha and Zhongsha islands during spring, revealing the area’s significance as a crucial fishing ground for purpleback flying squid. The resource of this species gradually reached its peak in May, with the primary fishing ground located at 15–17° N, 110–112° E [27].
4.2. Response of Purpleback Flying Squid Fishing Ground Distribution to Environmental Change
The SCS is a vast expanse of water characterized by an intricate topography and dynamic ocean currents. The distribution of purpleback flying squid is directly influenced by the physical factors inherent to this marine environment [16]. The life history, distribution of forage species, and their relationships with environmental factors play crucial roles in the formation of the purpleback flying squid fishing ground [17,28,29]. The formation of these fishing grounds in the waters surrounding the Xisha and Zhongsha islands in the SCS is influenced by various factors, including the marine environment, availability of forage species, and human activities. These fishing grounds have characteristics in common with other areas but also have several unique features [3,30,31]. The findings of this study demonstrated that environmental factors have predictive capabilities for determining CPUE. Additionally, the GFA results revealed pronounced seasonal and spatial variations in purpleback flying squid CPUE, with SST emerging as the most influential factor governing its distribution (Figure 8). Temperature is a crucial environmental factor that directly or indirectly constrains fish activity, including schooling, migration, and distribution [14,32]. As it is a warm-water pelagic species, for the purpleback flying squid, there is a close relationship between the formation of fishing grounds and SST. Its life habits are influenced by a specific range of SST suitability, with noticeable seasonal variations [3,16]. The GAM results revealed that the optimal temperature range for the purpleback flying squid was between 29.5 and 30.5 °C. This finding agreed with the results of Fan et al. [3] regarding the seasonal distribution of the purpleback flying squid fishery in the waters surrounding the Xisha and Zhongsha islands.
Phytoplankton serve as an essential food source for zooplankton and play a vital role in marine primary productivity [33]. The formation of purpleback flying squid fisheries is closely linked to the abundance of zooplankton; however, there is a temporal delay in the relationship between the Chla (as an indicator of plankton abundance and productivity) and the purpleback flying squid population. This delay can be attributed to the behavioral patterns of the purpleback flying squid [34]. Previous studies have revealed a stronger association between the purpleback flying squid fishery and the Chla during the summer and autumn, which coincides with the peak feeding activity for this species; thus, the Chla exerts a further influence on the distribution of purpleback flying squid by impacting their food organisms [3,27]. Previous studies have shown that the suitable Chla range for purpleback flying squid fishing grounds in the SCS is 0.05–0.27 mg/m3 [15]. Another study indicated that the optimal Chla for purpleback flying squid in the central and western Nansha Islands in the SCS falls within the range of 0.1–0.13 mg/m3 [4]. Additionally, it has been observed that purpleback flying squid in the Nansha Islands mainly form schools when the Chla ranges from 0.05 to 0.10 mg/m3 [10]. Our results revealed a primary Chla in the purpleback flying squid fishing area ranging from 0 to 0.20 mg/m3, which was similar to results from previous studies.
Unlike the other environmental factors, the SSH data obtained from satellite remote sensing does not directly reflect the distribution of fish. However, this data can help to identify variations in ocean current patterns and seawater flow rates, which are complex dynamic processes. A value of SSH less than the mean sea surface is conducive to the irradiation or uplift of seawater, leading to the continuous replenishment of nutrients and forage species in the bottom layer upwards. Consequently, this process facilitates the growth and spawning of pelagic fish species [35,36]. The SCS, due to the perennial monsoon climate and the influence of tropical cyclones, generates a strong upwelling region, with a large change in SSH, which is prone to the formation of fronts and eddies in the current field, thus carrying a large amount of nutrients and forming a fishing ground [37]. Consequently, SSH has a significant influence on the resource of purpleback flying squid. Furthermore, the MLD had an influence not only on the vertical distribution of nutrients but also on the vertical distribution of water temperature. Above the mixed layer, there is minimal variation in water temperature and more stable water mass properties, while below the mixed layer, there is greater variability in water temperature [38]. Wang et al. [39] identified a strong correlation between a chub mackerel fishery and vertical water temperature structure. Chub mackerel tend to avoid diving through the thermocline into deeper waters, facilitating the formation of temperature-based fisheries. Light trapping methods are commonly used to capture chub mackerel, with higher catch rates expected when the thermocline is shallower and fish remain active above it. However, when the water temperature below the thermocline is suitable for fish, it becomes difficult for light to penetrate the thermocline and reach the upper water column. Moreover, a deeper MLD will result in an expanded range of depths that are conducive for fish habitats, consequently impacting the efficacy of light-induced fish trapping. The GAM results revealed that the MLD within the fishery area was relatively shallow, with a significant negative correlation observed between the CPUE of purpleback flying squid and MLD (Figure 9). Purpleback flying squid can be captured through light trapping methods, and its distribution may therefore be correlated with the MLD.
4.3. Future Work
Oceanographic conditions exert a direct influence on the survival, distribution, recruitment, and migration patterns of cephalopods. Understanding the impact of the marine environment on the resource of purpleback flying squid is essential for effective and sustainable management of this marine resource. Remote sensing of the marine environment is widely used in studies of sustainable fisheries development and fishing efficiency. Under appropriate conditions, satellite remote sensing can provide extensive and high-resolution information regarding the sea surface environment. This is conducive to studying fisheries oceanography, identifying central fishing grounds, and facilitating the work of scientific researchers, fisheries production workers, and fisheries administrators. Marine remote sensing inversion data for SST and Chla have been extensively applied in fisheries, while environmental conditions such as SSH anomalies, SSS, photosynthetically active radiation, and seawater surface wind speed have gradually gained prominence in recent years, providing a more comprehensive understanding of the impact of the marine environment on fishery resources. In this study, we exclusively utilized data on the distribution of purpleback flying squid during a fishing moratorium of one voyage, which entails certain limitations. Subsequent research should aim to continue the specialized fishing efforts in order to enhance our understanding of the purpleback flying squid’s distribution during this specific period. As it is a pelagic fish species, the distribution of the purpleback flying squid is closely related to the environmental factors in the upper ocean, however. It is a species with obvious vertical movement habits, and there is indeed a certain limitation in considering only the effects of surface environmental factors, and the effects of deeper environmental factors may be considered in further analysis. Further improvements are also needed in model selection for the application of catch data, perhaps taking into account the effect of fishing vessels or fishermen as a random effect on the distribution of yields. Additionally, it is crucial to continuously monitor the frequent occurrence of unusual weather events (e.g., the El Niño–Southern Oscillation (ENSO)). By making these improvements, it may be possible to explore the distribution of the purpleback flying squid more comprehensively, which may provide a reasonable theoretical basis for accurate prediction of the purpleback flying squid fishery.
5. Conclusions
In this study, we employed both GFA and GAM to investigate the distribution patterns of purpleback flying squid in the Xisha and Zhongsha Islands during the fishing moratorium. The findings revealed distinct seasonal variations in the distribution of purpleback flying squid, with temperature and longitude identified as significant influencing factors. Over the past few decades, increased fishing pressure has been observed in northern waters of the SCS, resulting in a significant decline in traditional economic fish resources. However, despite an estimated resource potential exceeding one million tons for purpleback flying squid off the SCS, current catches remain far below sustainable levels. Given its short life cycle and vulnerability to climate and marine environmental changes, it is crucial to integrate multiple environmental variables for accurate prediction of purpleback flying squid fisheries’ distribution characteristics, which is a key consideration for sustainable exploitation of this valuable resource.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fishes9070253/s1: Table S1. Variance inflation factor values for each explanatory variable. Figure S1. Q-Q residual plot of GAM. Figure S2. Spatial-temporal distribution of SST in the SCS. Figure S3. Spatial-temporal distribution of SSS in the SCS. Figure S4. Spatial-temporal distribution of Chla in the SCS. Figure S5. Spatial-temporal distribution of SSH in the SCS. Figure S6 Spatial-temporal distribution of MLD in the SCS.
Author Contributions
Conceptualization, L.W. and J.Z.; data curation, L.W. and C.Y.; formal analysis, L.W., C.Y. and B.S.; funding acquisition, J.Z., D.S. and T.G.; investigation, C.Y., Y.L. and B.S.; methodology, L.W.; project administration, J.Z., D.S. and T.G.; resources, C.Y. and B.S.; software, L.W. and B.S.; supervision, C.Y., J.Z. and D.S.; validation, L.W.; visualization, D.S.; writing—original draft preparation, L.W. and D.S.; writing—review and editing, L.W. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work is partially supported by the Guangxi Fishery Resources Survey Project (GXZC2022-G3-001062-ZHZB), the Research Project on the Current Status of Exploitation and Utilization of Purpleback Flying Squid off the South China Sea, the Central Public-interest Scientific Institution Basal Research Fund, CAFS (No. 2023TD93), Biodiversity, Germplasm Resources Bank and Information Database Construction of the South China Sea Project (No. HNDW2020-112), the Hainan Provincial Natural Science Foundation of China under contract No. 324QN367, and the National Natural Science Foundation of China (No. 42206109).
Institutional Review Board Statement
As squid is an invertebrate, the data involved in this study are from the production of special fishing boats in Guangxi, so the approval of the Ethics Committee is not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We are grateful to the entire crew of the Guangxi 2023 Specialized Fishing Fleet for their invaluable assistance in facilitating data collection.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Qiu, Y.S.; Zeng, X.G.; Chen, T.; Yuan, W.W. Fishery Resources and Fisheries Management in the South China Sea; China Ocean Press: Beijing, China, 2008. [Google Scholar]
- Zhao, F. Numerical Study and Case Forecast of Mesoscale Eddies in the Northern South China Sea; National Marine Environmental Forecasting Center: Beijing, China, 2017. [Google Scholar]
- Fan, J.T.; Chen, Z.Z.; Zhang, J.; Feng, X. Sthenoteuthis oualaniensis fishing grounds analysis based on marine environmental factors and different weight coefficients in the Zhongxisha and Xisha Islands, South China Sea. South China Fish. Sci. 2016, 12, 57–63. [Google Scholar]
- Yu, J.; Hu, Q.W.; Li, C.H.; Zhang, P.; Mao, J.M. Relationship between the Sthenoteuthis oualaniensis resource and environmental factors in the Xisha-Zhongsha waters in spring. Acta Oceanol. Sin. 2017, 39, 62–73. [Google Scholar]
- Chen, X.J.; Liu, B.L.; Wang, Y.G. Cephalopods of the World; China Ocean Press: Beijing, China, 2009. [Google Scholar]
- Fan, J.T.; Qiu, Y.S.; Chen, Z.Z.; Feng, X.; Zhang, J. Morphological difference of the beak between two stock of Sthenoteuthis oualaniensis inhabiting South China Sea. Period. Ocean Univ. China 2015, 10, 42–49. [Google Scholar]
- Zhang, P.; Yang, L.; Zhang, X.F.; Tan, Y.G. The present status and prospect on exploitation of tuna and squid fishery resources in South China Sea. South China Fish. Sci. 2010, 6, 68–74. [Google Scholar]
- Yan, L.; Zhang, P.; Yang, B.Z.; Chen, S.; Li, Y.N.; Li, Y.; Song, P.Q.; Lin, L.S. Relationship between the catch of Symplectoteuthis oualaniensis and surface temperature and the vertical temperature structure in the South China Sea. J. Fish. China 2016, 23, 469–477. [Google Scholar]
- Fan, J.T.; Fang, Z.; Ma, S.W.; Zhang, P.; Chen, Z.Z. Age, growth, and population characteristics of Sthenoteuthis oualaniensis in the South China Sea. Reg. Stud. Mar. Sci. 2022, 55, 102517. [Google Scholar] [CrossRef]
- Zhao, C.X.; Shen, C.Y.; Andrew, B.; Yan, Y.R.; Kang, B. Purpleback flying squid Sthenoteuthis oualaniensis in the South China sea: Growth, resources and association with the environment. Water 2021, 13, 65. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, L.; Yang, B.Z.; Tan, Y.G.; Zhang, X.F.; Chen, S.; Li, J. Population structure of purpleback flying squid (Sthenoteuthis oualaniensis) in Nansha area in spring. South China Fish. Sci. 2015, 11, 11–19. [Google Scholar]
- Zhao, C.X. Biology and Habitat Characteristics of the Purpleback Flying Squid Sthenoteuthis oualaniensis in the South China Sea. Ph.D. Thesis, Jimei University, Xiamen, China, 2021. [Google Scholar]
- Liu, S.G.; Liu, Y.; Fu, C.H.; Yan, L.X.; Xu, Y.; Wan, R.; Li, J.C.; Tian, Y.J. Using novel spawning ground indices to analyze the effects of climate change on pacific saury abundance. J. Marine Syst. 2019, 191, 13–23. [Google Scholar] [CrossRef]
- Wang, L.M.; Ma, S.Y.; Liu, Y.; Li, J.C.; Sun, D.R. Climate-induced variation in a temperature suitability index of chub mackerel in the spawning season and its effect on the abundance. Front. Mar. Sci. 2022, 9, 996626. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Xie, E.G.; Wu, Q.E.; Feng, F. The relationship between the resources of Sthenoteuthis oualaniensis in the open South China Sea and the marine environment. Acta Oceanol. Sin. 2021, 43, 38–48. [Google Scholar]
- Fan, J.T.; Chen, Z.Z.; Feng, X.; Yu, W. Climate-related changes in seasonal habitat pattern of Sthenoteuthis oualaniensis in the South China Sea. Ecosyst. Health Sust. 2021, 7, 1926338. [Google Scholar] [CrossRef]
- Xu, H.Y.; Cui, X.S.; Zhou, W.F.; Chen, G.B.; Li, A.Z. Analysis on optimal habitats of purpleback flying squid in the open South China Sea based on remote sensing data. Chin. J. Ecol. 2016, 35, 3080–3085. [Google Scholar]
- Su, L.; Chen, Z.Z.; Zhang, P. Reproductive biology of purplrback flying squid (Sthenoteuthis oualaniensis) in the south-central South China Sea in Spring and autumn. South China Fish. Sci. 2016, 12, 96–102. [Google Scholar]
- Jiang, M. The Current Situation and Countermoves on Development and Utilization of Sthenoteuthis oualaniensis in South China Sea. Master’s Thesis, Tianjin Agricultural University, Tianjin, China, 2018. [Google Scholar]
- Ellis, N.; Smith, S.J.; Pitcher, C.R. Gradient forests: Calculating importance gradients on physical predictors. Ecology 2012, 93, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Sagarese, S.R.; Frisk, M.G.; Cerrat, R.M.; Sosebee, K.A.; Musick, J.A.; Rago, P.J. Application of generalized additive models to examine ontogenetic and seasonal distributions of spiny dogfish (Squalus acanthias) in the northeast (US) shelf large marine ecosystem. Can. J. Fish. Aquat. Sci. 2014, 71, 847–877. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; Chapman and Hall: London, UK; New York, NY, USA, 1990. [Google Scholar]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Wood, S.N. Thin-plate regression splines. J. R. Stat. Soc. B 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Zhu, G.P.; Wang, R. Catch per unit effort of Antarctic krill (Euphausia superba) fishery and its suitability to abundance estimation. J. Fish. China 2016, 40, 1072–1079. [Google Scholar]
- Hu, Q.W. Remote Sensing of Environmental Effects on the Spatiotemporal Distribution of Symplectoteuthis oualaniensis in the Xisha-Zhongsha Waters. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2018. [Google Scholar]
- Chen, X.J.; Liu, B.L.; Tian, S.Q.; Qian, W.G.; Zhao, X.H. Fishery biology of purpleback squid, Sthenoteuthis oualaniensis, in the northwest Indian Ocean. Fish. Res. 2007, 83, 98–104. [Google Scholar]
- Yan, Y.R.; Feng, B.; Lu, H.S.; Lai, J.Y.; Du, S.Q. Fishery biology of purpleback flying squid Sthenoteuthis oualaniensis in northern sea areas around Nansha Islands in summer. Oceanol. Limnol. Sin. 2012, 43, 1177–1186. [Google Scholar]
- Takagi, K.; Kitahara, T.; Suzuki, N.; Mori, J.; Yatsu, A. The age and growth of Sthenoteuthis oualaniensis (Cephalopoda: Ommastrephidae) in the Pacific Ocean. Bull. Mar. Sci. 2002, 71, 1105–1108. [Google Scholar]
- Mohamed, K.S.; Sajikumar, K.; Ragesh, N.; Amvrose, T.V.; Jayasabkar, J.; Said Koya, K.P.; Sasikumar, G. Relating abundance of purpleback flying squid Sthenoteuthis oualaniensis (Cephalopoda: Ommastrephidae) to environmental parameters using GIS and GAM in south-eastern Arabian Sea. J. Nat. Hist. 2018, 52, 1869–1882. [Google Scholar] [CrossRef]
- Wang, L.M.; Ma, S.Y.; Liu, Y.; Li, J.C.; Liu, S.G.; Lin, L.S.; Tian, Y.J. Fluctuations in the abundance of chub mackerel in relation to climatic/oceanic regime shifts in the northwest Pacific Ocean since the 1970s. J. Mar. Syst. 2021, 218, 103541. [Google Scholar] [CrossRef]
- Liu, K.K.; Chao, S.Y.; Shaw, P.T.; Gong, G.C.; Tang, T.Y. Monsoon-forced chlorophyll distribution and primary production in the South China Sea: Observation and a numerical study. Deep-Sea Res. Part I 2002, 49, 1387–1412. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Zhan, F.P.; Yang, Y.T.; Zhang, P.; Kon, X.L.; Jiang, Y.E.; Chen, Z.Z. Feeding habits of Symplectoteuthis oualaniensis in the South China Sea. South China Fish. Sci. 2016, 12, 80–87. [Google Scholar]
- Shao, F.; Chen, X.J. Relationship between fishing ground of Symlectoteuthis oualaniensis and sea surface height in the northwest Indian ocean. Mar. Sci. 2008, 32, 88–92. [Google Scholar]
- Song, T. Relationship between Fishing Grounds of Ommastrephes bartrami and Satellite Altimeter Data in Northwestern Pacific. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2014. [Google Scholar]
- Wang, X.X.; Yu, J.; Li, Y.Z.; Chen, G.B.; Huang, M.F. The relationship between major upwelling and the upwelling fishing grounds in the South China Sea. Mar. Sci. 2015, 39, 131–137. [Google Scholar]
- Yang, H.Y.; Mao, X.Y.; Guo, X.Y. A preliminary study on nutrients concentration within the mixed layer in the Northwest Pacific based on WOD data. Period. Ocean Univ. China 2018, 48, 1–9. [Google Scholar]
- Wang, L.M.; Li, Y.; Zhang, R.; Tian, Y.J.; Zhang, J.; Lin, L.S. Relationship between the resource distribution of Scomber japnicus and seawater temperature vertical structure of Northwestern Pacific Ocean. Period. Ocean Univ. China 2019, 49, 29–38. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).