Biologically Inspired Intra-Uterine Nanofluid Flow under the Suspension of Magnetized Gold (Au) Nanoparticles: Applications in Nanomedicine
Abstract
:1. Introduction
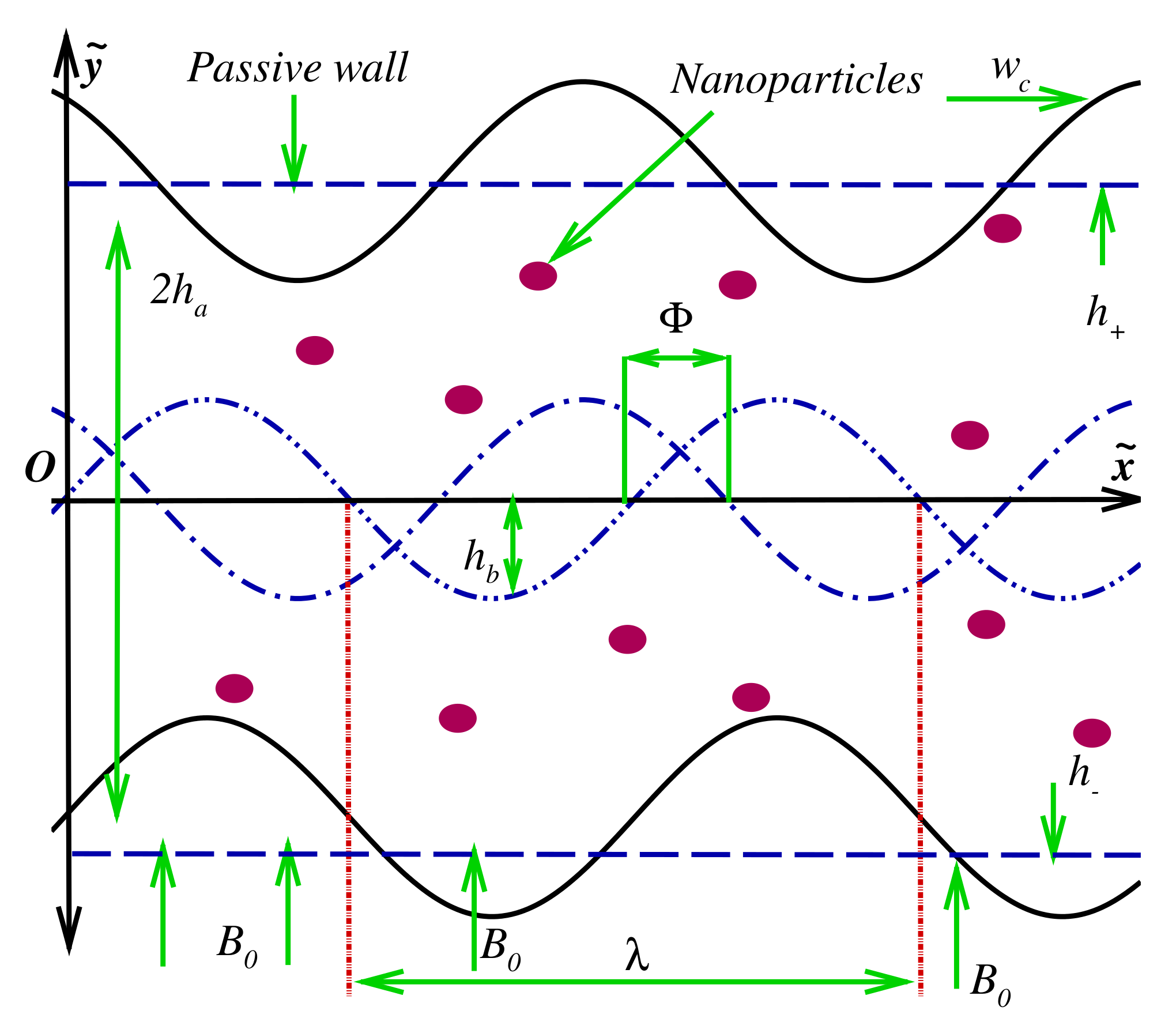
2. Modeling of Two-Dimensional Intra-Uterine Nanofluid Flow
3. Series Solutions Via Perturbation Approach
3.1. Zeroth-Order System
3.2. First-Order System
3.3. Second-Order System
4. Graphical Outcomes and Discussion
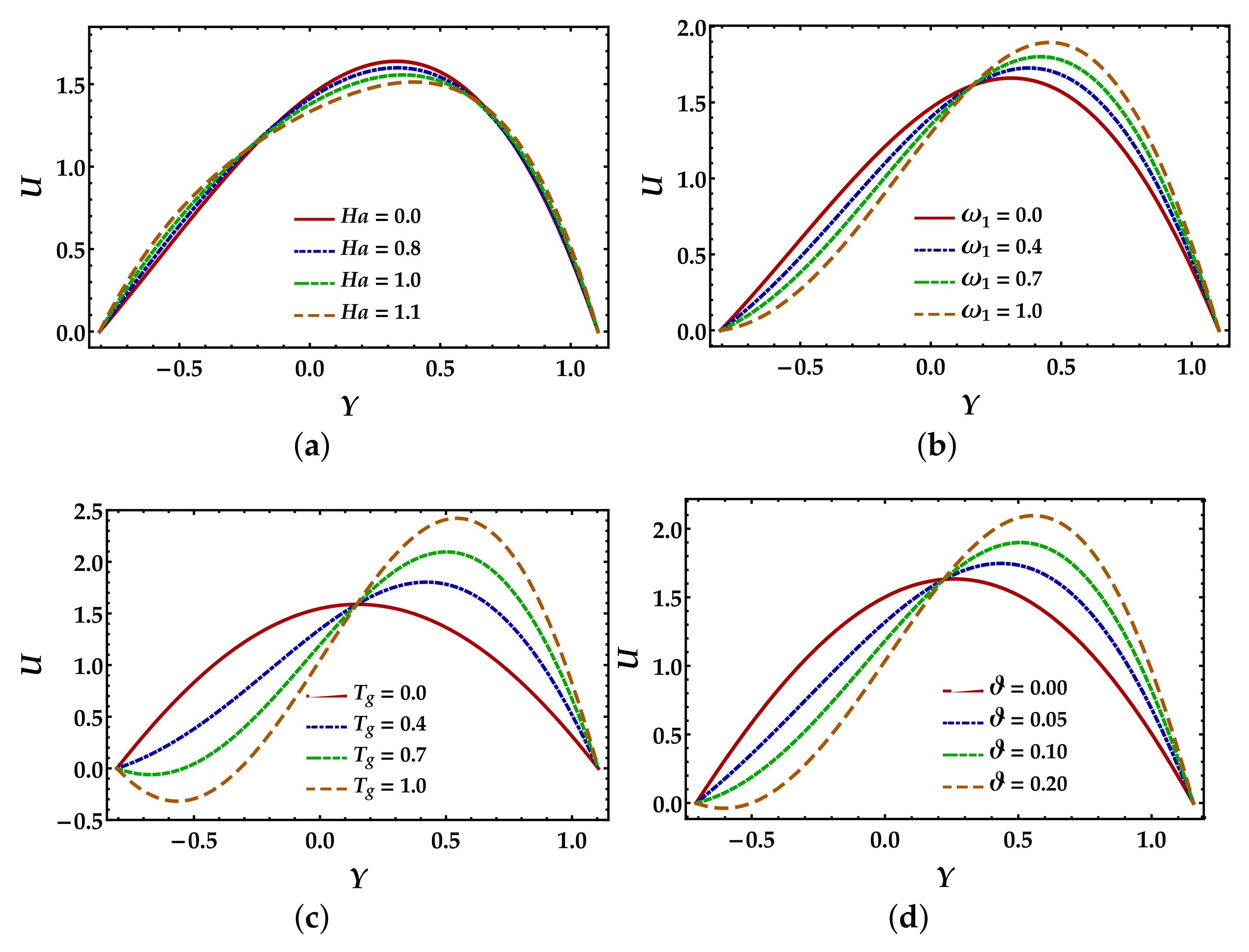
4.1. Velocity Mechanism
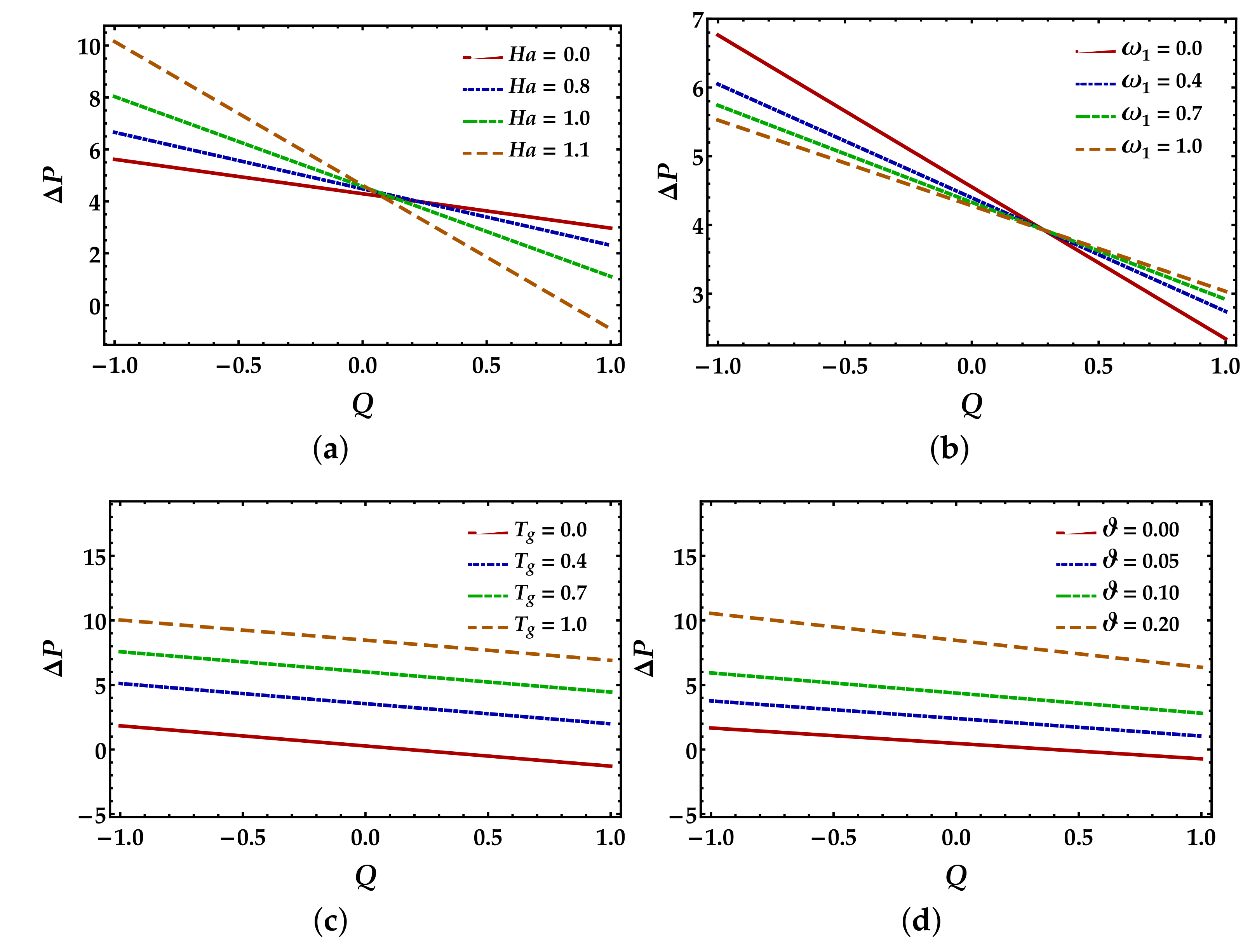
4.2. Pressure Rise Profile
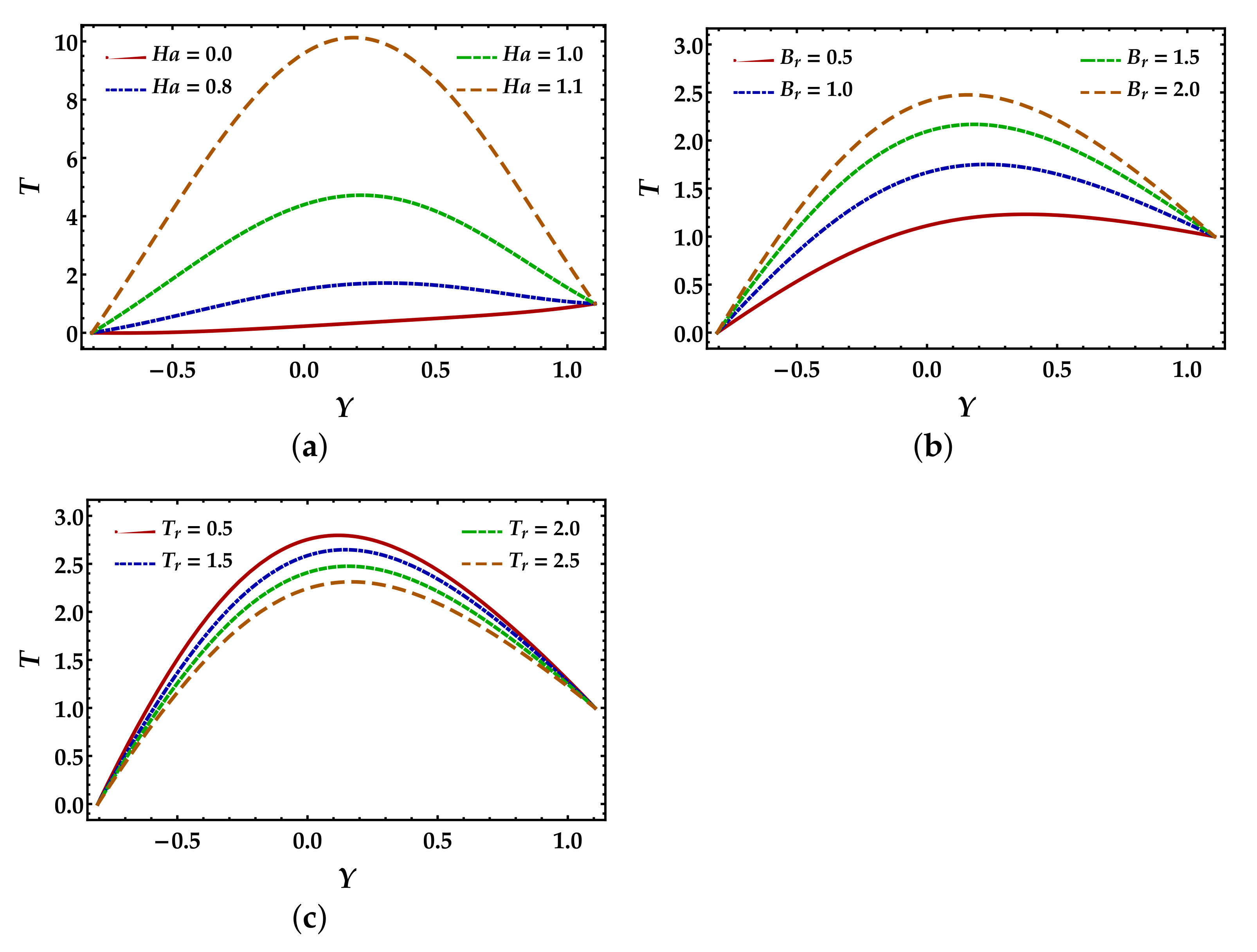
4.3. Temperature Distribution
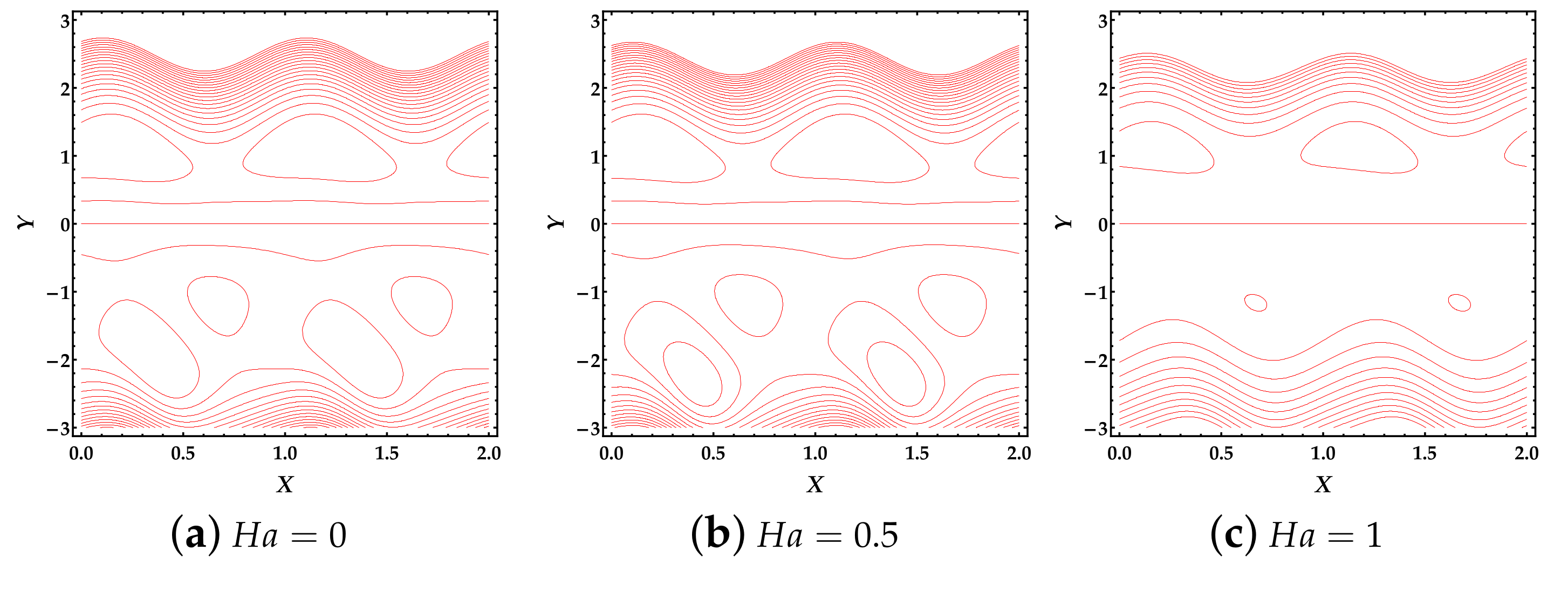
4.4. Trapping Phenomena
5. Conclusions
- (i)
- The presence of a magnetic field substantially opposes the flow in the central zone of the channel. At the same time, closer to the walls, the behavior seems to be negligible or very small.
- (ii)
- The fluid parameter, particle volume fraction, and thermal Grashof number show a reduction in the fluid motion when , whereas a significant increment is observed when .
- (iii)
- The pressure rise reveals an increasing behavior against the thermal Grashof number and particle volume fraction in the retrograde and peristaltic pumping zones. In contrast, the fluid parameter and Hartmann number show a converse process in both regions.
- (iv)
- Temperature profile remarkably rises due to strengthening in the magnetic field and Brinkmann number, while the thermal radiation opposes the increment of the temperature profile.
- (v)
- We can see that the magnetic field tends to diminish the free eddies, while the thermal Grashof number significantly affects the magnitude and number of free eddies.
- (vi)
- We also found that the particle volume fraction and fluid parameter markedly change the shape and the number of free eddies.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, D. Numerical and analytical simulation of peristaltic flows of generalized Oldroyd-B fluids. Int. J. Numer. Methods Fluids 2011, 67, 1932–1943. [Google Scholar] [CrossRef]
- Tripathi, D.; Bég, O.A. A study on peristaltic flow of nanofluids: Application in drug delivery systems. Int. J. Heat Mass Transf. 2014, 70, 61–70. [Google Scholar] [CrossRef]
- Akbar, N.S.; Raza, M.; Ellahi, R. Influence of induced magnetic field and heat flux with the suspension of carbon nanotubes for the peristaltic flow in a permeable channel. J. Magn. Magn. Mater. 2015, 381, 405–415. [Google Scholar] [CrossRef]
- Tripathi, D.; Bhushan, S.; Bég, O.A. Transverse magnetic field driven modification in unsteady peristaltic transport with electrical double layer effects. Colloids Surf. Physicochem. Eng. Asp. 2016, 506, 32–39. [Google Scholar] [CrossRef]
- Fusi, L.; Farina, A. Peristaltic axisymmetric flow of a Bingham fluid. Appl. Math. Comput. 2018, 320, 1–15. [Google Scholar] [CrossRef]
- Alokaily, S.; Feigl, K.; Tanner, F.X. Characterization of peristaltic flow during the mixing process in a model human stomach. Phys. Fluids 2019, 31, 103105. [Google Scholar] [CrossRef]
- Rashid, M.; Ansar, K.; Nadeem, S. Effects of induced magnetic field for peristaltic flow of Williamson fluid in a curved channel. Phys. Stat. Mech. Appl. 2020, 553, 123979. [Google Scholar] [CrossRef]
- Romanò, F.; Suresh, V.; Galie, P.A.; Grotberg, J.B. Peristaltic flow in the glymphatic system. Sci. Rep. 2020, 10, 21065. [Google Scholar]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [Green Version]
- Orive, G.; Gascon, A.R.; Hernández, R.M.; Domínguez-Gil, A.; Pedraz, J.L. Techniques: New approaches to the delivery of biopharmaceuticals. Trends Pharmacol. Sci. 2004, 25, 382–387. [Google Scholar] [CrossRef]
- Razzacki, S.Z.; Thwar, P.K.; Yang, M.; Ugaz, V.M.; Burns, M.A. Integrated microsystems for controlled drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 185–198. [Google Scholar] [CrossRef] [PubMed]
- M Saeed, A.; Najma, S.; Faiza, Q. Nanoparticles in delivery of cardiovascular drugs. Pak. J. Pharm. Sci. 2007, 20, 340–348. [Google Scholar]
- Patra, J.K.; Baek, K.H. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef] [Green Version]
- Joseph, R.R.; Venkatraman, S.S. Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine 2017, 12, 683–702. [Google Scholar] [CrossRef] [Green Version]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Hirst, D.; O’Sullivan, J. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef]
- Hussain, F.; Ellahi, R.; Zeeshan, A.; Vafai, K. Modelling study on heated couple stress fluid peristaltically conveying gold nanoparticles through coaxial tubes: A remedy for gland tumors and arthritis. J. Mol. Liq. 2018, 268, 149–155. [Google Scholar] [CrossRef]
- Abd Elmaboud, Y.; Mekheimer, K.S.; Emam, T.G. Numerical examination of gold nanoparticles as a drug carrier on peristaltic blood flow through physiological vessels: Cancer therapy treatment. BioNanoScience 2019, 9, 952–965. [Google Scholar] [CrossRef]
- Rogers, N.J.; Jeffery, H.C.; Claire, S.; Lewis, D.J.; Zikeli, G.; Hodges, N.J.; Egginton, S.; Nash, G.B.; Pikramenou, Z. Tailoring iridium luminescence and gold nanoparticle size for imaging of microvascular blood flow. Nanomedicine 2017, 12, 2725–2740. [Google Scholar] [CrossRef] [Green Version]
- Beveridge, J.S.; Stephens, J.R.; Williams, M.E. The use of magnetic nanoparticles in analytical chemistry. Annu. Rev. Anal. Chem. 2011, 4, 251–273. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Wang, L.; Wei, J.; Yang, H.; Yang, S.; Zhao, J. CoFe2O4@ MnFe2O4/polypyrrole nanocomposites for in vitro photothermal/magnetothermal combined therapy. RSC Adv. 2015, 5, 7349–7355. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [Green Version]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, M.; Sarma, T.K.; Cabral, H.; Kaida, S.; Sekino, M.; Herlambang, N.; Osada, K.; Kano, M.R.; Nishiyama, N.; Kataoka, K. Enhanced in vivo Magnetic Resonance Imaging of Tumors by PEGylated Iron-Oxide–Gold Core–Shell Nanoparticles with Prolonged Blood Circulation Properties. Macromol. Rapid Commun. 2010, 31, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Bae, H.; Rhee, I.; Chang, Y.; Jin, S.U.; Hong, S. Gold-coated iron oxide nanoparticles as a T 2 contrast agent in magnetic resonance imaging. J. Nanosci. Nanotechnol. 2012, 12, 5132–5137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehizadeh, H.; Hekmatian, E.; Sadeghi, M.; Kennedy, K. Synthesis and characterization of core-shell Fe3O4-gold-chitosan nanostructure. J. Nanobiotechnol. 2012, 10, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayal, S.; Ramanujan, R.V. Anti-cancer drug loaded iron–gold core–shell nanoparticles (Fe@ Au) for magnetic drug targeting. J. Nanosci. Nanotechnol. 2010, 10, 5527–5539. [Google Scholar] [CrossRef] [PubMed]
- Eldabe, N.; Moatimid, G.; El-Shekhipy, A.; Aballah, N.F. Peristaltic blood flow with gold nanoparticles on a carreau nanofluid through a non-darcian porous medium. J. Biomater. Nanobiotechnol. 2018, 9, 290–306. [Google Scholar] [CrossRef] [Green Version]
- Prakash, J.; Tripathi, D.; Tiwari, A.K.; Sait, S.M.; Ellahi, R. Peristaltic pumping of nanofluids through a tapered channel in a porous environment: Applications in blood flow. Symmetry 2019, 11, 868. [Google Scholar] [CrossRef] [Green Version]
- Ellahi, R.; Zeeshan, A.; Hussain, F.; Asadollahi, A. Peristaltic blood flow of couple stress fluid suspended with nanoparticles under the influence of chemical reaction and activation energy. Symmetry 2019, 11, 276. [Google Scholar] [CrossRef] [Green Version]
- Mekheimer, K.S.; Abo-Elkhair, R.; Moawad, A. Electrothermal transport via gold nanoparticles as antimicrobials of blood flow through an electro-osmosis artery with overlapping stenosis. Int. J. Fluid Mech. Res. 2020, 47, 135–152. [Google Scholar] [CrossRef]
- Bibi, A.; Xu, H. Peristaltic channel flow and heat transfer of Carreau magneto hybrid nanofluid in the presence of homogeneous/heterogeneous reactions. Sci. Rep. 2020, 10, 11499. [Google Scholar]
- Sadaf, H.; Abdelsalam, S.I. Adverse effects of a hybrid nanofluid in a wavy non-uniform annulus with convective boundary conditions. RSC Adv. 2020, 10, 15035–15043. [Google Scholar] [CrossRef]
- Asproulis, N.; Drikakis, D. An artificial neural network-based multiscale method for hybrid atomistic-continuum simulations. Microfluid. Nanofluid. 2013, 15, 559–574. [Google Scholar] [CrossRef]
- Rahbari, A.; Fakour, M.; Hamzehnezhad, A.; Vakilabadi, M.A.; Ganji, D. Heat transfer and fluid flow of blood with nanoparticles through porous vessels in a magnetic field: A quasi-one dimensional analytical approach. Math. Biosci. 2017, 283, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Mekheimer, K.S.; Hasona, W.; Abo-Elkhair, R.; Zaher, A. Peristaltic blood flow with gold nanoparticles as a third grade nanofluid in catheter: Application of cancer therapy. Phys. Lett. 2018, 382, 85–93. [Google Scholar] [CrossRef]
- Sunitha, G. Influence of thermal radiation on peristaltic blood flow of a Jeffrey fluid with double diffusion in the presence of gold nanoparticles. Inform. Med. Unlocked 2019, 17, 100272. [Google Scholar]
- Akram, J.; Akbar, N.S.; Maraj, E. A comparative study on the role of nanoparticle dispersion in electroosmosis regulated peristaltic flow of water. Alex. Eng. J. 2020, 59, 943–956. [Google Scholar] [CrossRef]
- Ashraf, M.U.; Qasim, M.; Wakif, A.; Afridi, M.I.; Animasaun, I.L. A generalized differential quadrature algorithm for simulating magnetohydrodynamic peristaltic flow of blood-based nanofluid containing magnetite nanoparticles: A physiological application. Numer. Methods Part. Differ. Equ. 2020. [Google Scholar] [CrossRef]
- Zhang, L.; Bhatti, M.M.; Marin, M.; Mekheimer, K.S. Entropy analysis on the blood flow through anisotropically tapered arteries filled with magnetic zinc-oxide (ZnO) nanoparticles. Entropy 2020, 22, 1070. [Google Scholar] [CrossRef]
- Seikh, A.H.; Akinshilo, A.; Taheri, M.; Rahimi-Gorji, M.; Alharthi, N.; Khan, I.; Khan, A.R. Influence of the nanoparticles and uniform magnetic field on the slip blood flows in arterial vessels. Phys. Scr. 2019, 94, 125218. [Google Scholar] [CrossRef]
- Imran, M.; Shaheen, A.; Sherif, E.S.M.; Rahimi-Gorji, M.; Seikh, A.H. Analysis of peristaltic flow of Jeffrey six constant nano fluid in a vertical non-uniform tube. Chin. J. Phys. 2020, 66, 60–73. [Google Scholar] [CrossRef]
- Kahshan, M.; Lu, D.; Abu-Hamdeh, N.H.; Golmohammadzadeh, A.; Farooq, A.; Rahimi-Gorji, M. Darcy-Brinkman flow of a viscous fluid through a porous duct: Application in blood filtration process. J. Taiwan Inst. Chem. Eng. 2020, 117, 223–230. [Google Scholar] [CrossRef]
- He, J.H. Homotopy perturbation method: A new nonlinear analytical technique. Appl. Math. Comput. 2003, 135, 73–79. [Google Scholar] [CrossRef]
- Abbas, M.A.; Hussain, I. Statistical Analysis of the Mathematical Model of Entropy Generation of Magnetized Nanofluid. Inventions 2019, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.A.; Bhatti, M.M.; Sheikholeslami, M. Peristaltic propulsion of Jeffrey nanofluid with thermal radiation and chemical reaction effects. Inventions 2019, 4, 68. [Google Scholar] [CrossRef] [Green Version]
- Abo-Elkhair, R.; Bhatti, M.M.; Mekheimer, K.S. Magnetic force effects on peristaltic transport of hybrid bio-nanofluid (AuCu nanoparticles) with moderate Reynolds number: An expanding horizon. Int. Commun. Heat Mass Transf. 2021, 123, 105228. [Google Scholar] [CrossRef]
- Ellahi, R.; Bhatti, M.M.; Riaz, A.; Sheikholeslami, M. Effects of magnetohydrodynamics on peristaltic flow of Jeffrey fluid in a rectangular duct through a porous medium. J. Porous Media 2014, 17, 143–157. [Google Scholar] [CrossRef]



| Physical Properties | (J/Kg·K) | (Kg/m3) | (W/mK) | (S/m) |
|---|---|---|---|---|
| Blood | 1050 | 3617 | 0.52 | 1.33 |
| Gold (Au) | 129.1 | 19300 | 320 | 4.5 × 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatti, M.M. Biologically Inspired Intra-Uterine Nanofluid Flow under the Suspension of Magnetized Gold (Au) Nanoparticles: Applications in Nanomedicine. Inventions 2021, 6, 28. https://doi.org/10.3390/inventions6020028
Bhatti MM. Biologically Inspired Intra-Uterine Nanofluid Flow under the Suspension of Magnetized Gold (Au) Nanoparticles: Applications in Nanomedicine. Inventions. 2021; 6(2):28. https://doi.org/10.3390/inventions6020028
Chicago/Turabian StyleBhatti, Muhammad Mubashir. 2021. "Biologically Inspired Intra-Uterine Nanofluid Flow under the Suspension of Magnetized Gold (Au) Nanoparticles: Applications in Nanomedicine" Inventions 6, no. 2: 28. https://doi.org/10.3390/inventions6020028
APA StyleBhatti, M. M. (2021). Biologically Inspired Intra-Uterine Nanofluid Flow under the Suspension of Magnetized Gold (Au) Nanoparticles: Applications in Nanomedicine. Inventions, 6(2), 28. https://doi.org/10.3390/inventions6020028






