Design, Construction and Programming of a Low-Cost Pulsed High-Voltage Direct Current Power Supply for the Electrophoretic Deposition of Silicon Carbide Mixed with Graphite and/or Alumina for Thermoelectric Applications
Abstract
:1. Introduction
2. Materials and Methods
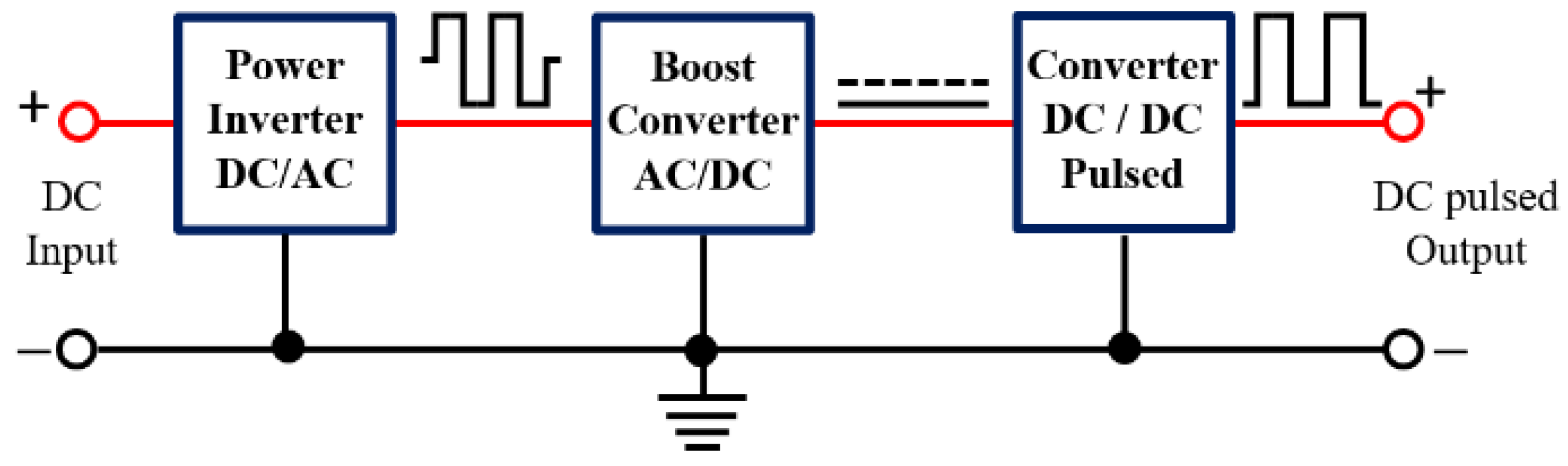
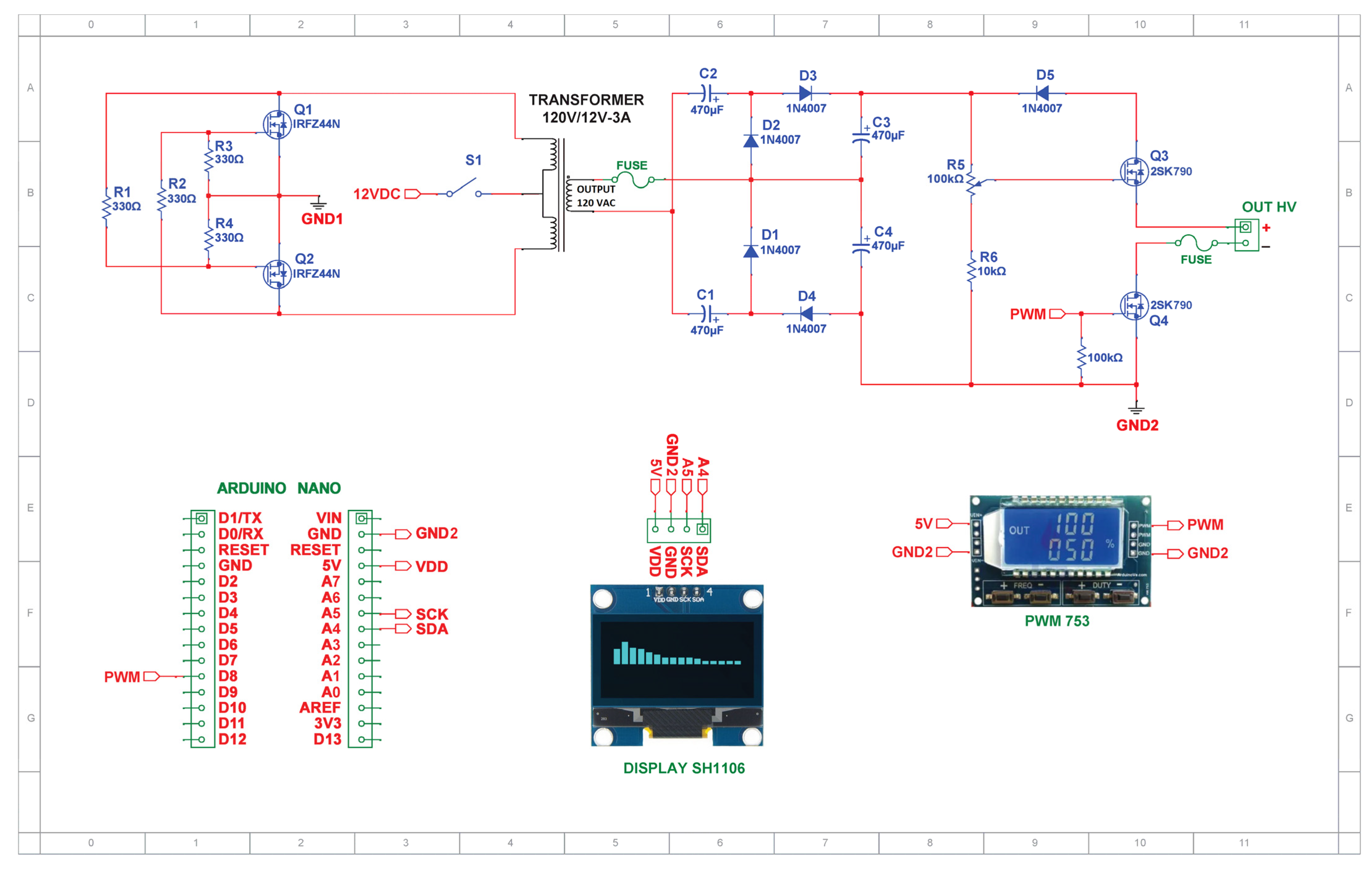
2.1. Experimental Hardware Configuration
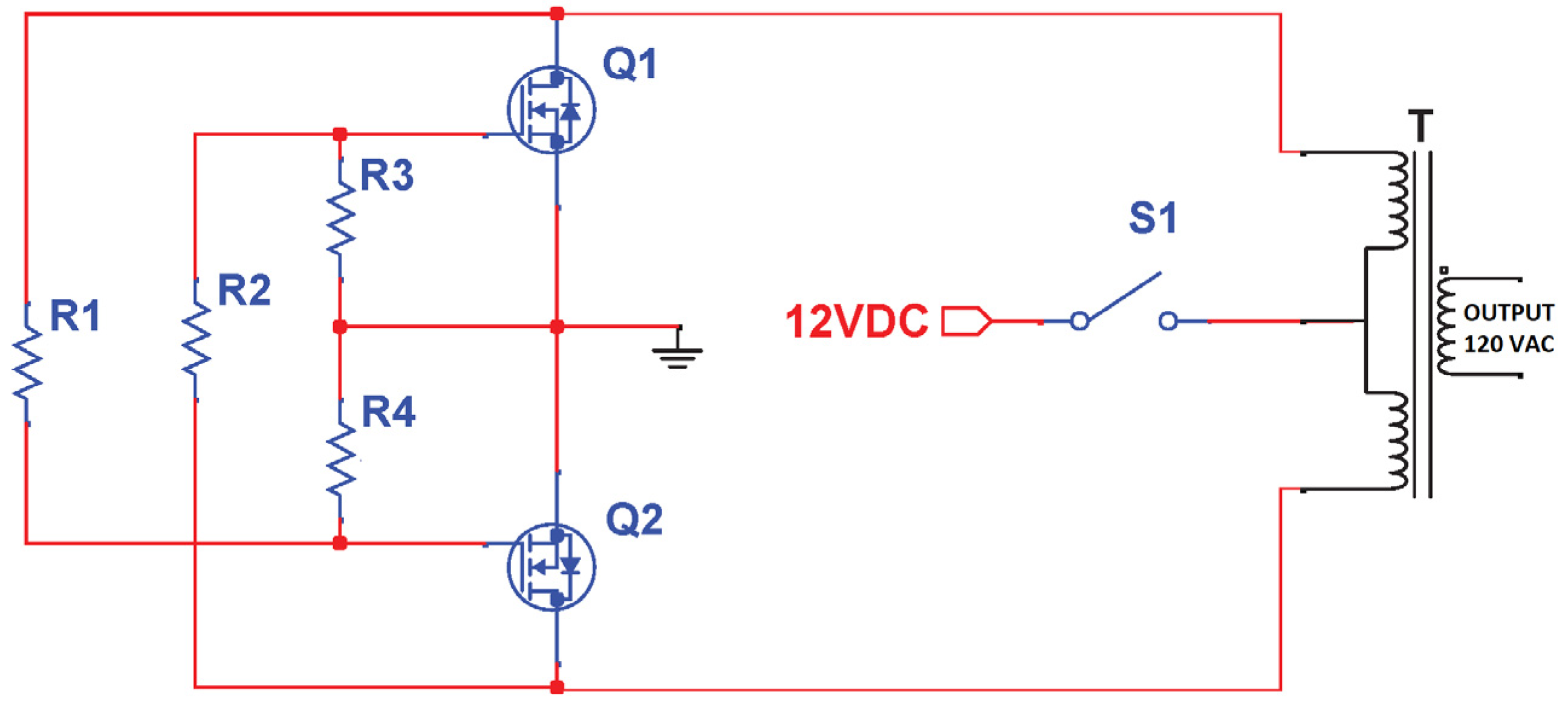
2.1.1. Voltage Inverter
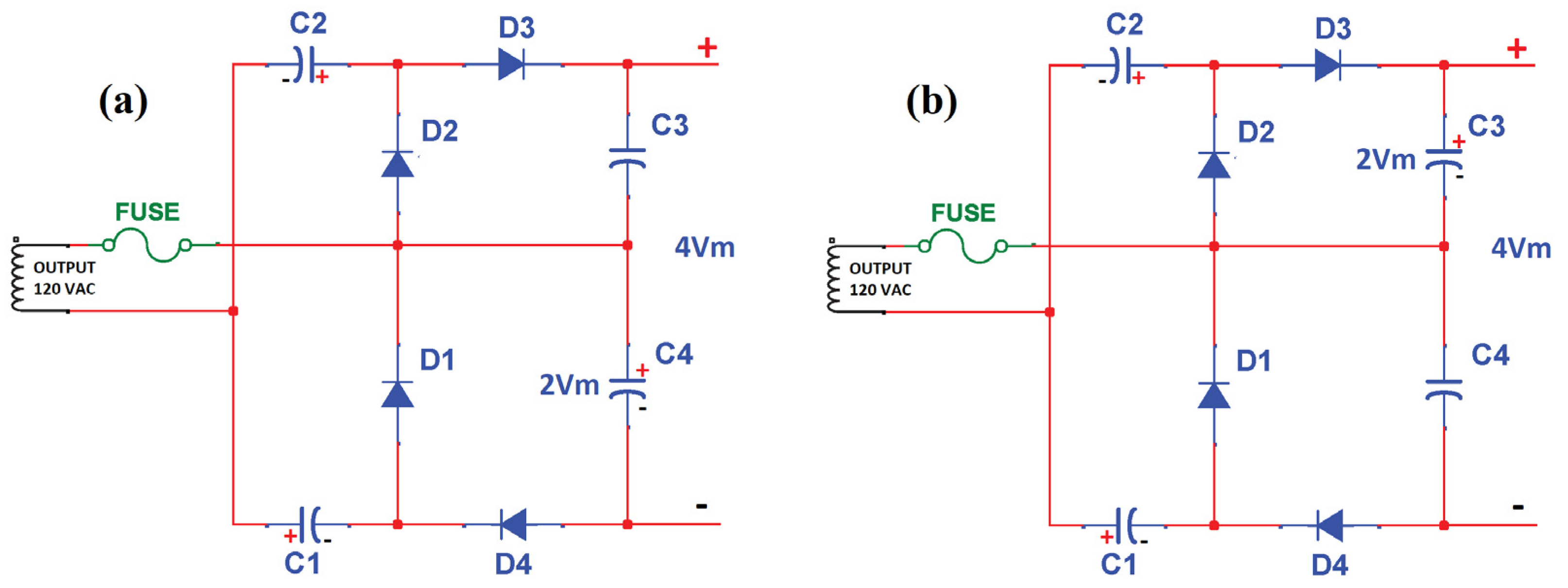
2.1.2. Voltage Quadruple
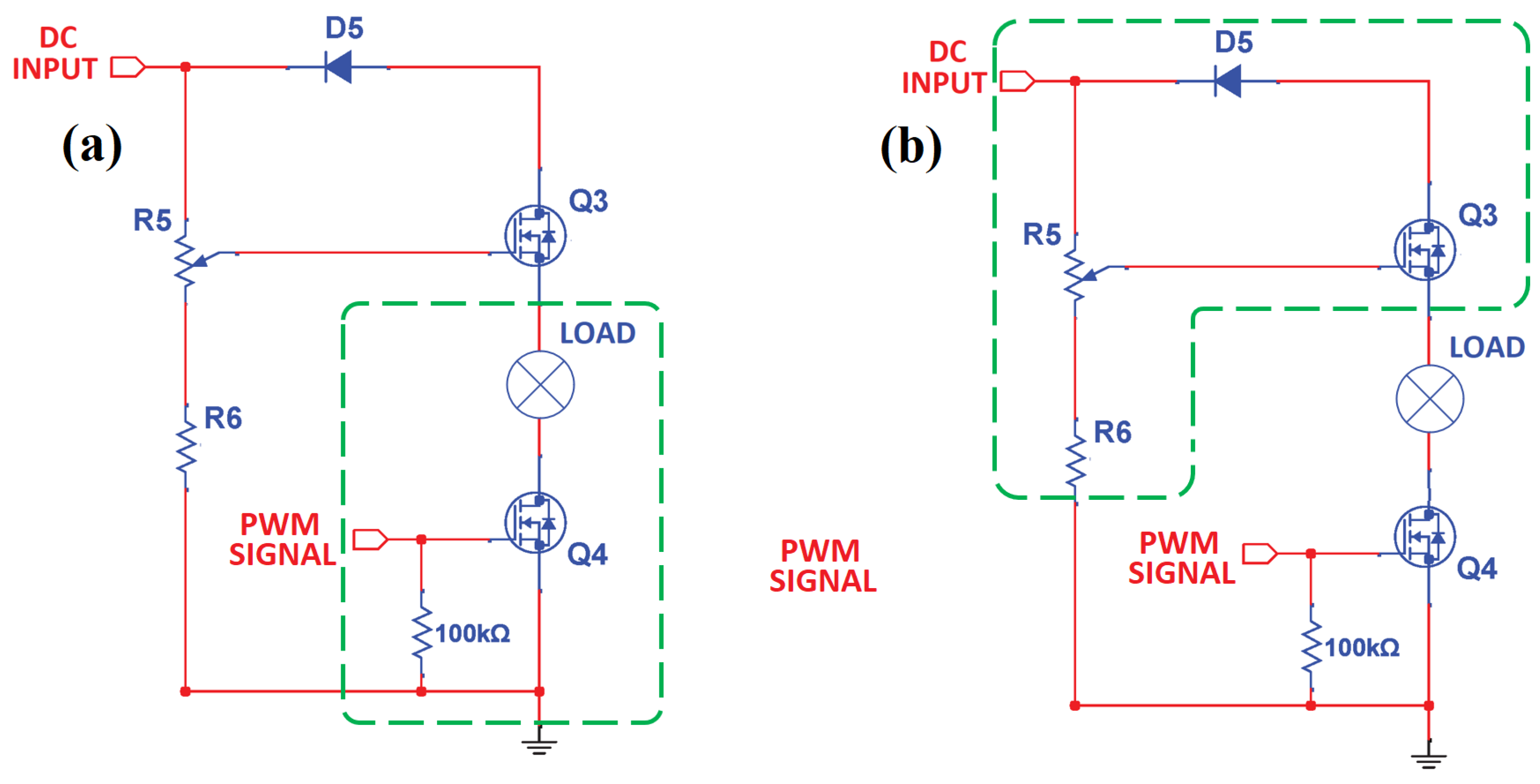
2.1.3. High-Power Drive Circuit Devices
MOSFET Gate Drive Circuit
Dimmer Circuit
2.2. Software
- #include <SPI.h>#include <Wire.h>#include <Adafruit_GFX.h>#include <Adafruit_SH1106.h>#define OLED_RESET 4Adafruit_SH1106 display (OLED_RESET);#define pulse_ip 8float duty, freq, ontime, offtime, period;void setup (){display.clearDisplay();pinMode(ledPIN, OUTPUT);pinMode(pulse_ip, INPUT);pinMode(hall_pin, INPUT);display.begin(SH1106_SWITCHCAPVCC, 0x3C);display.display();delay (3000);}void loop (){display.clearDisplay();display.setTextSize(1);display.setCursor(0,21);display.setTextColor(WHITE);display.println(“FREQ(Hz):”);display.setTextSize(1);display.setCursor(0,38);display.setTextColor(WHITE);display.println(“DUTY (%):”);display.setTextSize(1);display.setCursor(0,55);display.setTextColor(WHITE);display.println(“T ON(ms):”);ontime = pulseIn (8, HIGH);offtime = pulseIn (8, LOW);period = ontime + offtime;freq = 1000000.0/period;duty = (ontime/period) * 100;display.setTextSize(2);display.setTextColor(WHITE);display.setCursor(56,17);display.println(freq);display.setTextSize(2);display.setTextColor(WHITE);display.setCursor(56,34);display.println(duty);display.setTextSize(2);display.setTextColor(WHITE);display.setCursor(56,51);display.println(ontime/1000);display.display();}
2.3. Pulsed HVDC Power Supply Circuit
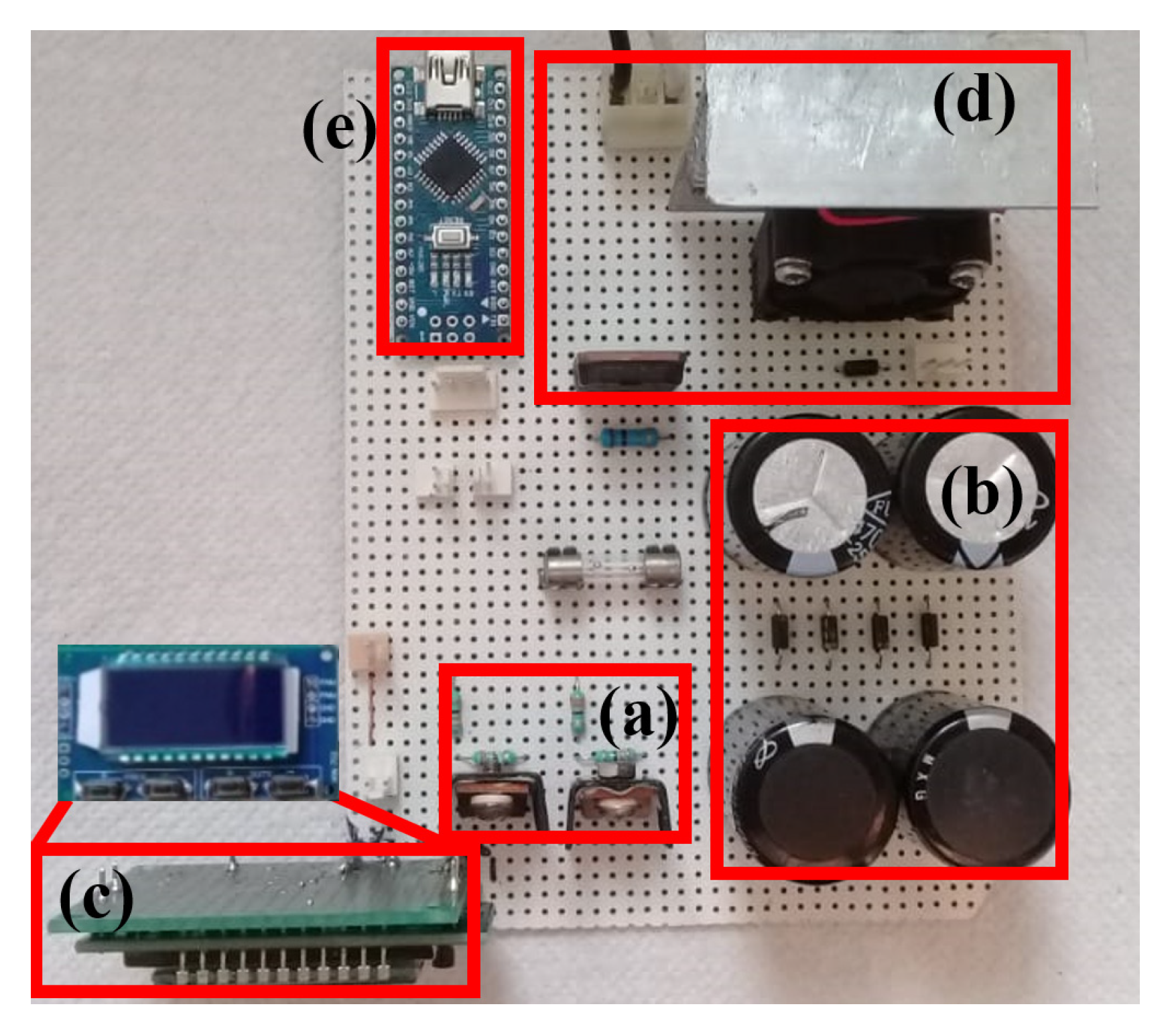
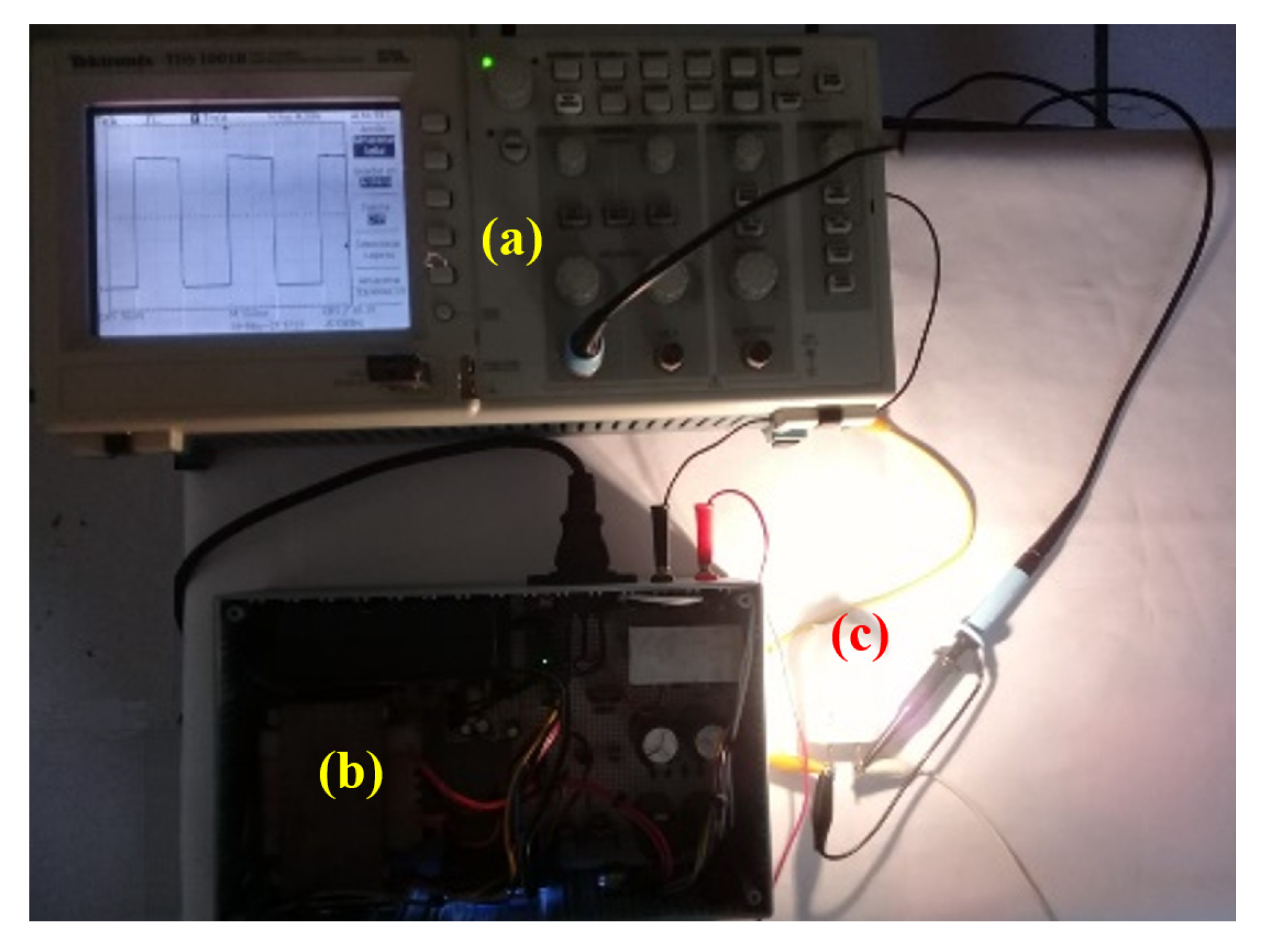
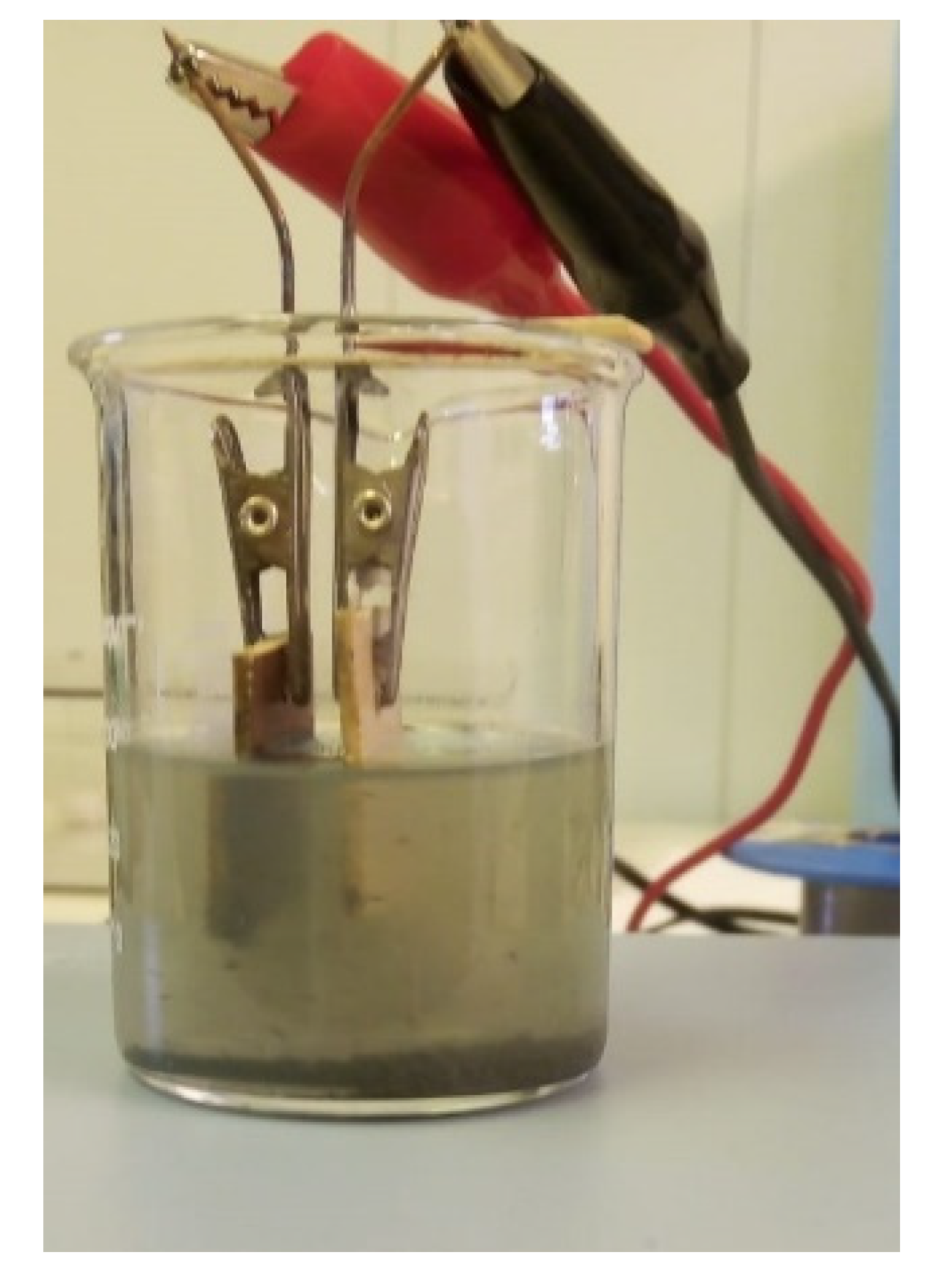
2.4. Experimental Setup
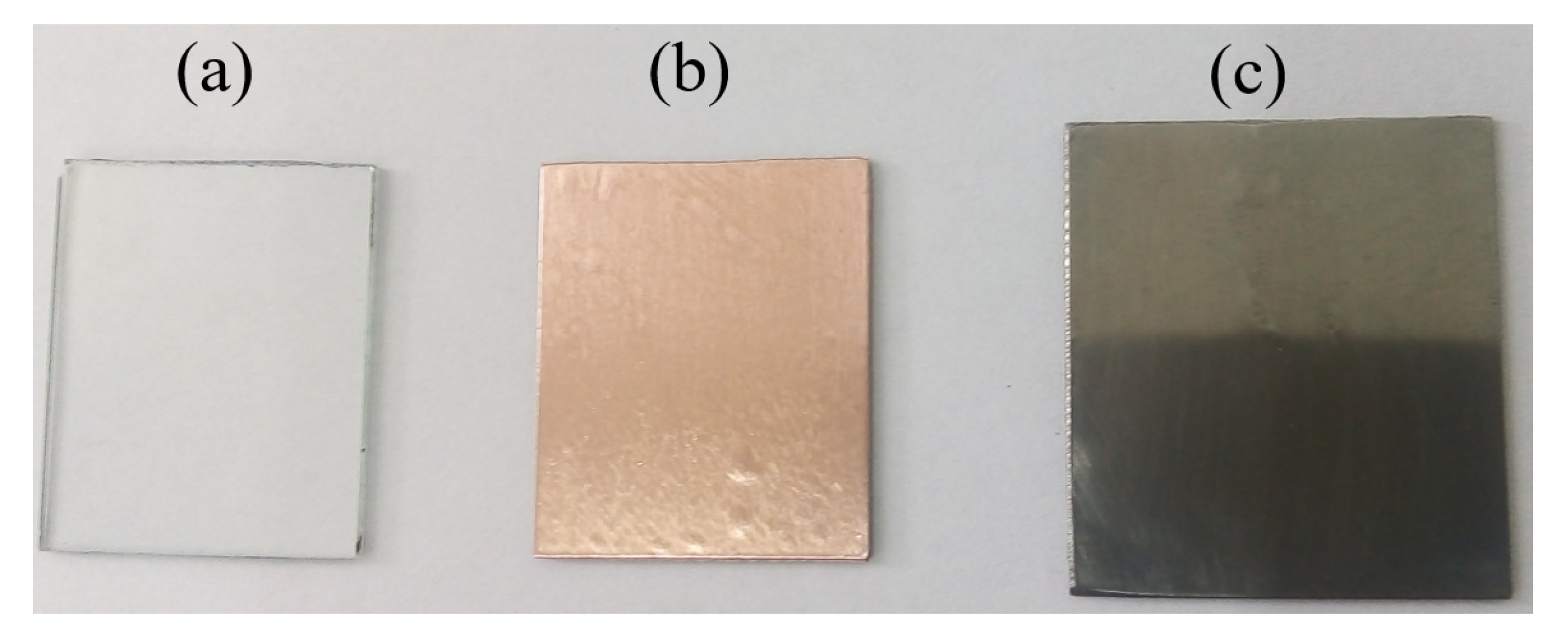
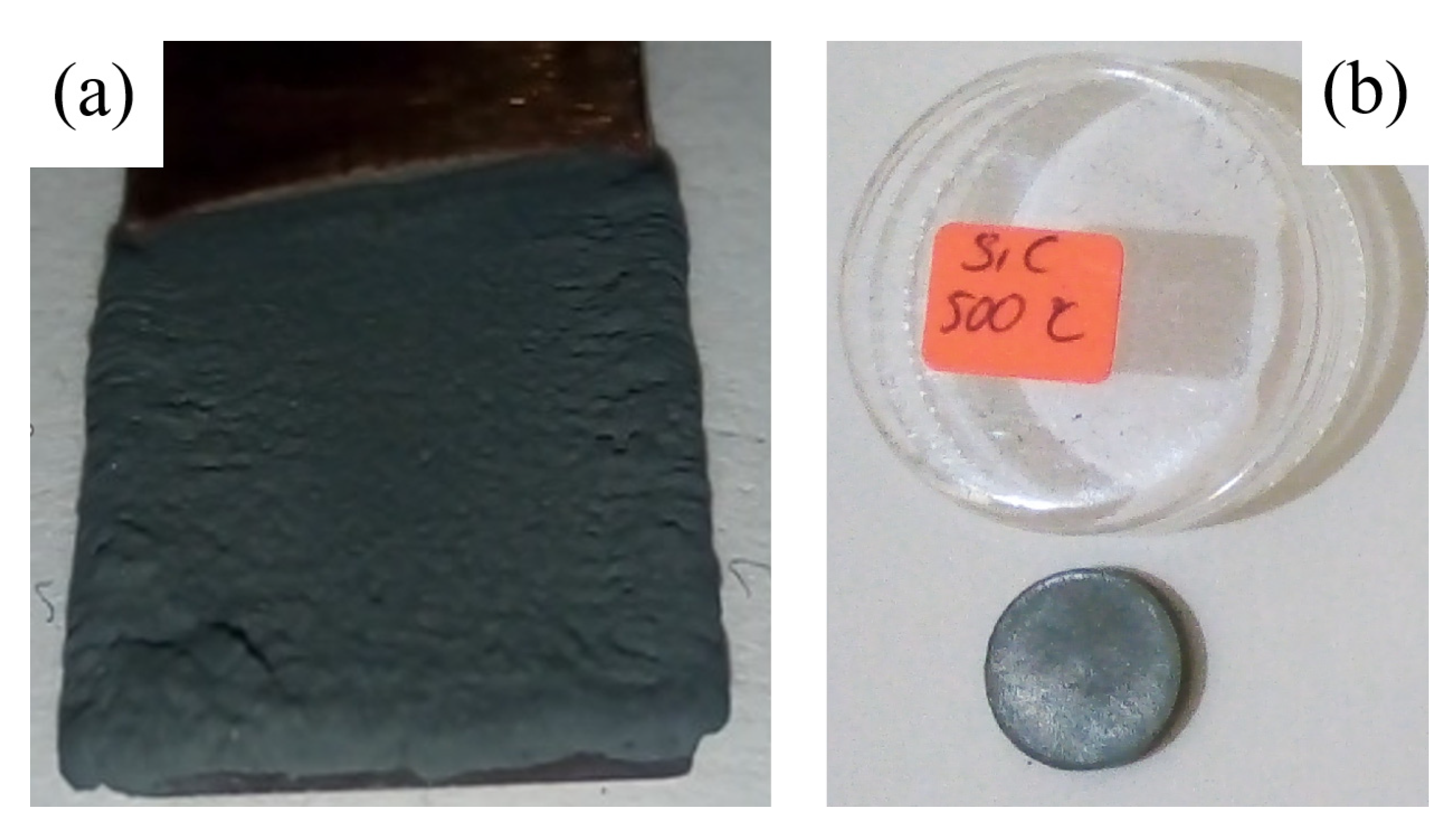
2.5. Experimental Details of SiC Coatings
3. Results
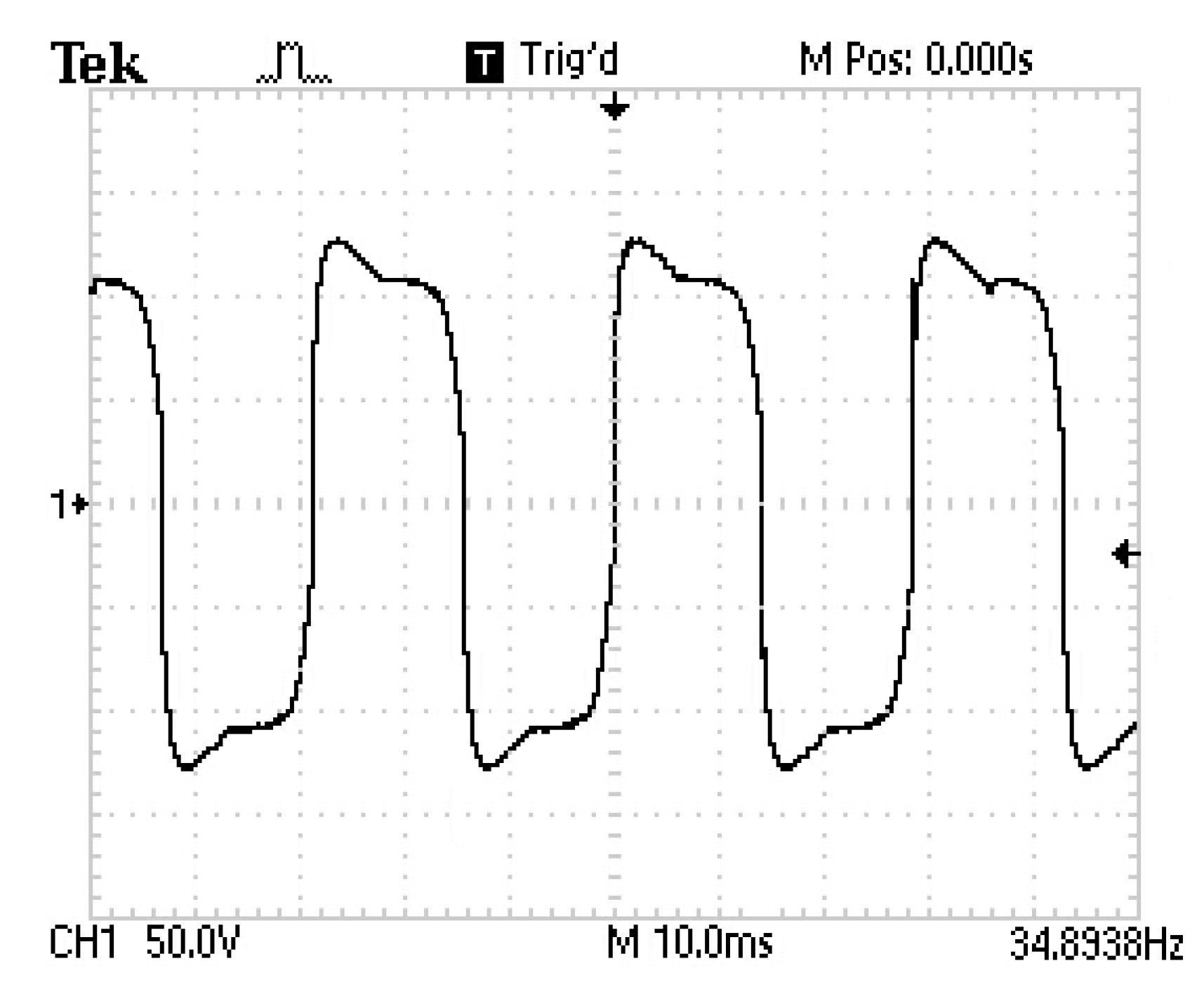
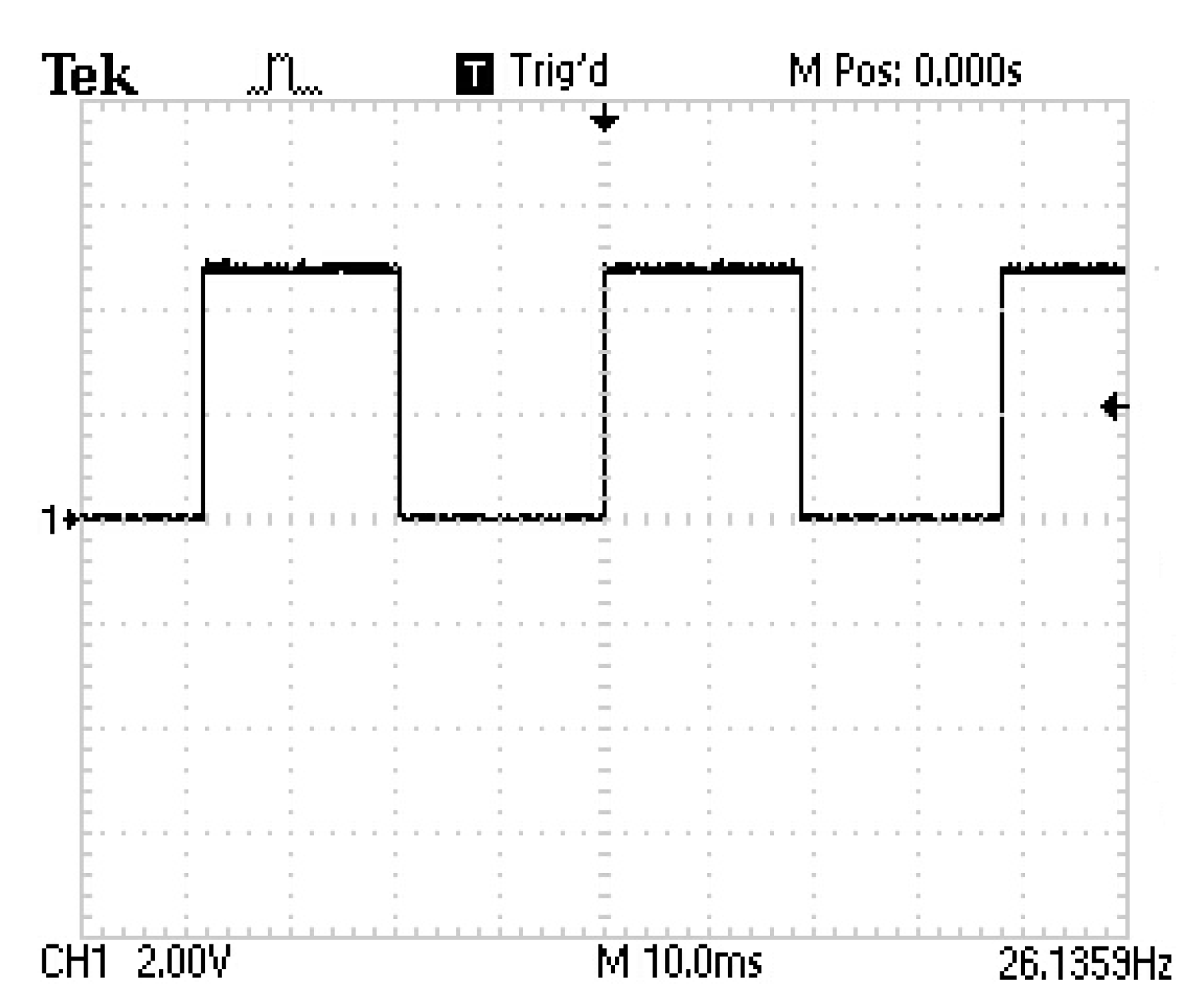
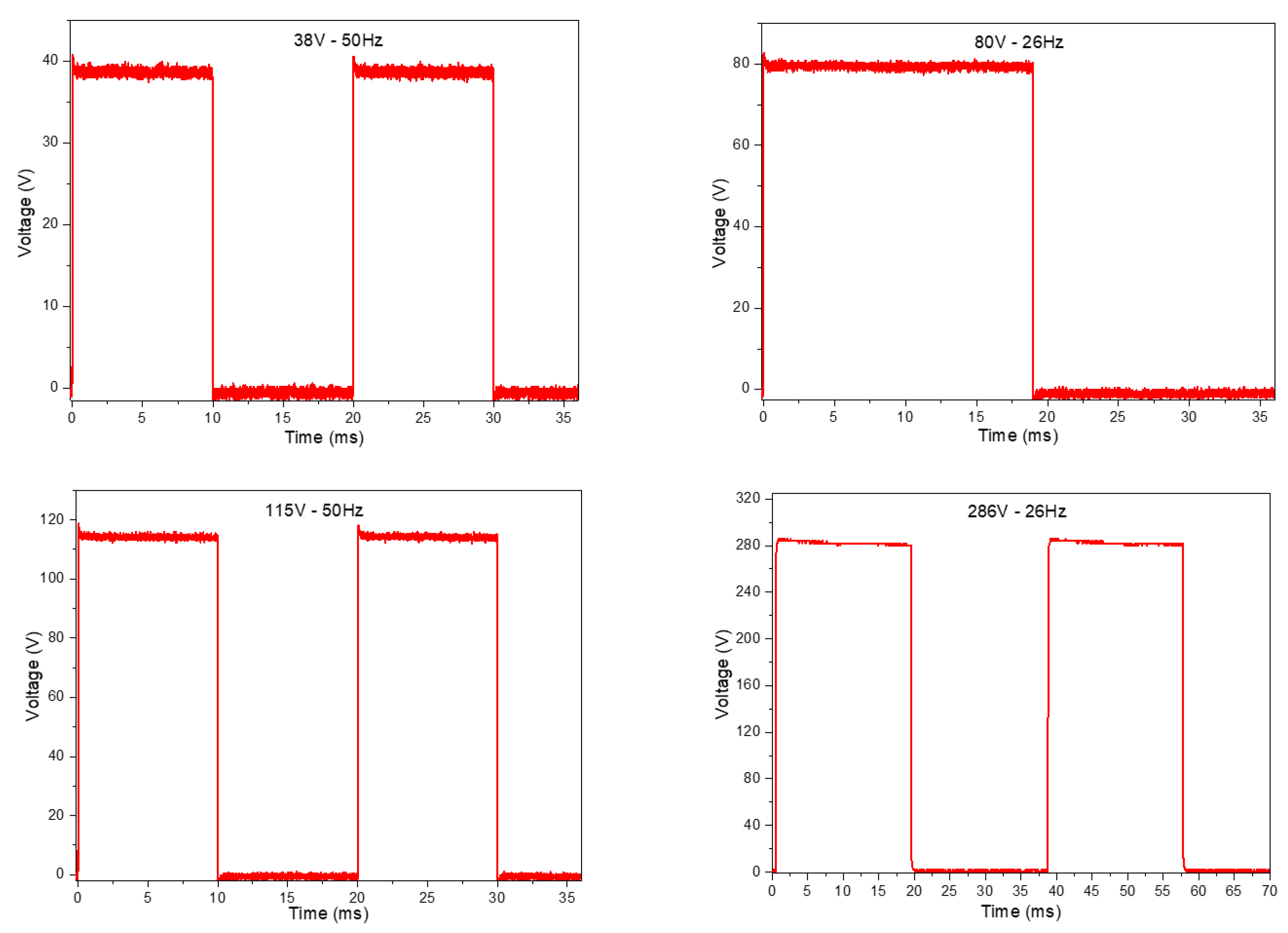
3.1. Results of the Pulsed HVDC Prototype
3.2. Results and Discussions of Bulk SiC Samples
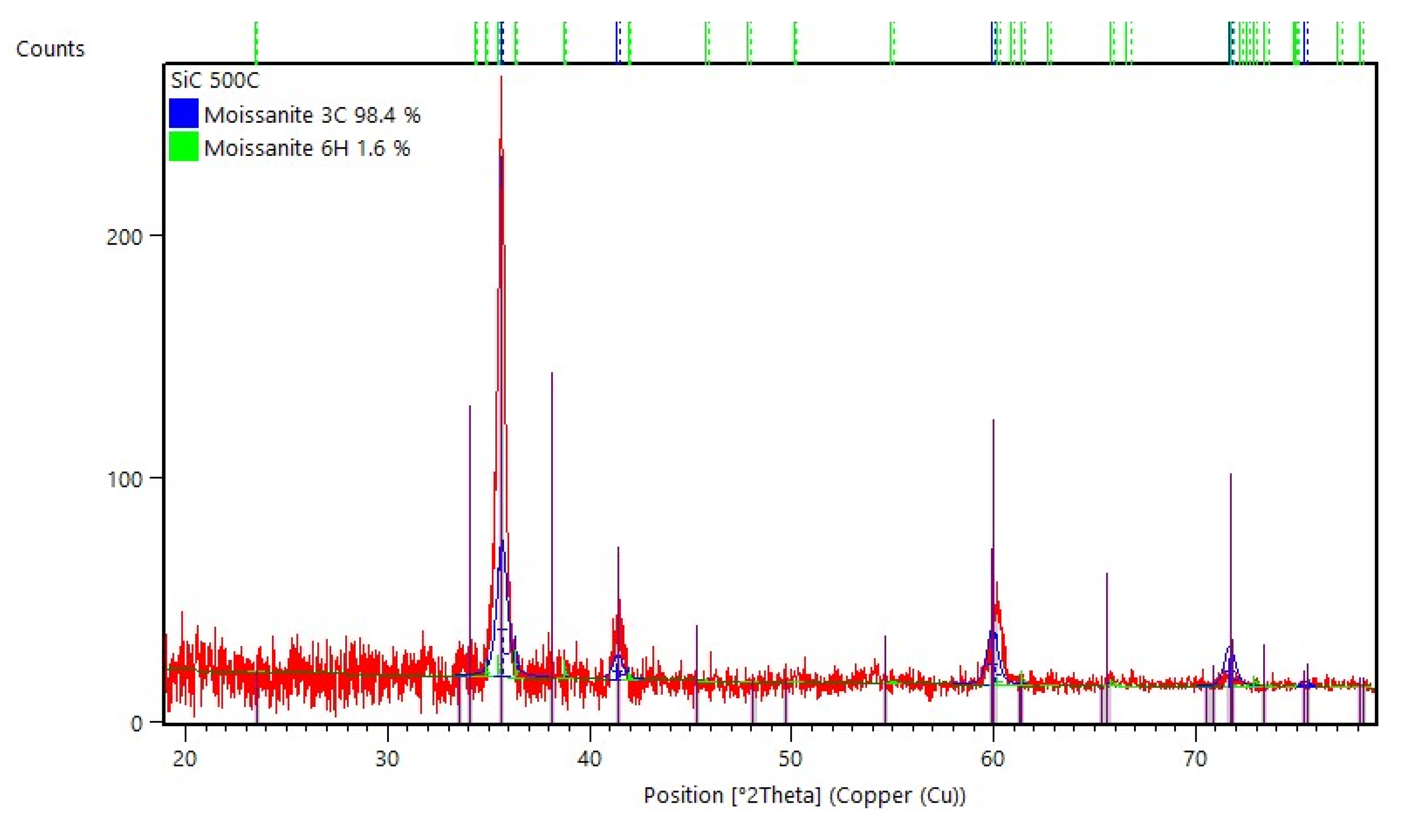
3.2.1. Structural Analysis
3.2.2. Raman Spectroscopy Analysis
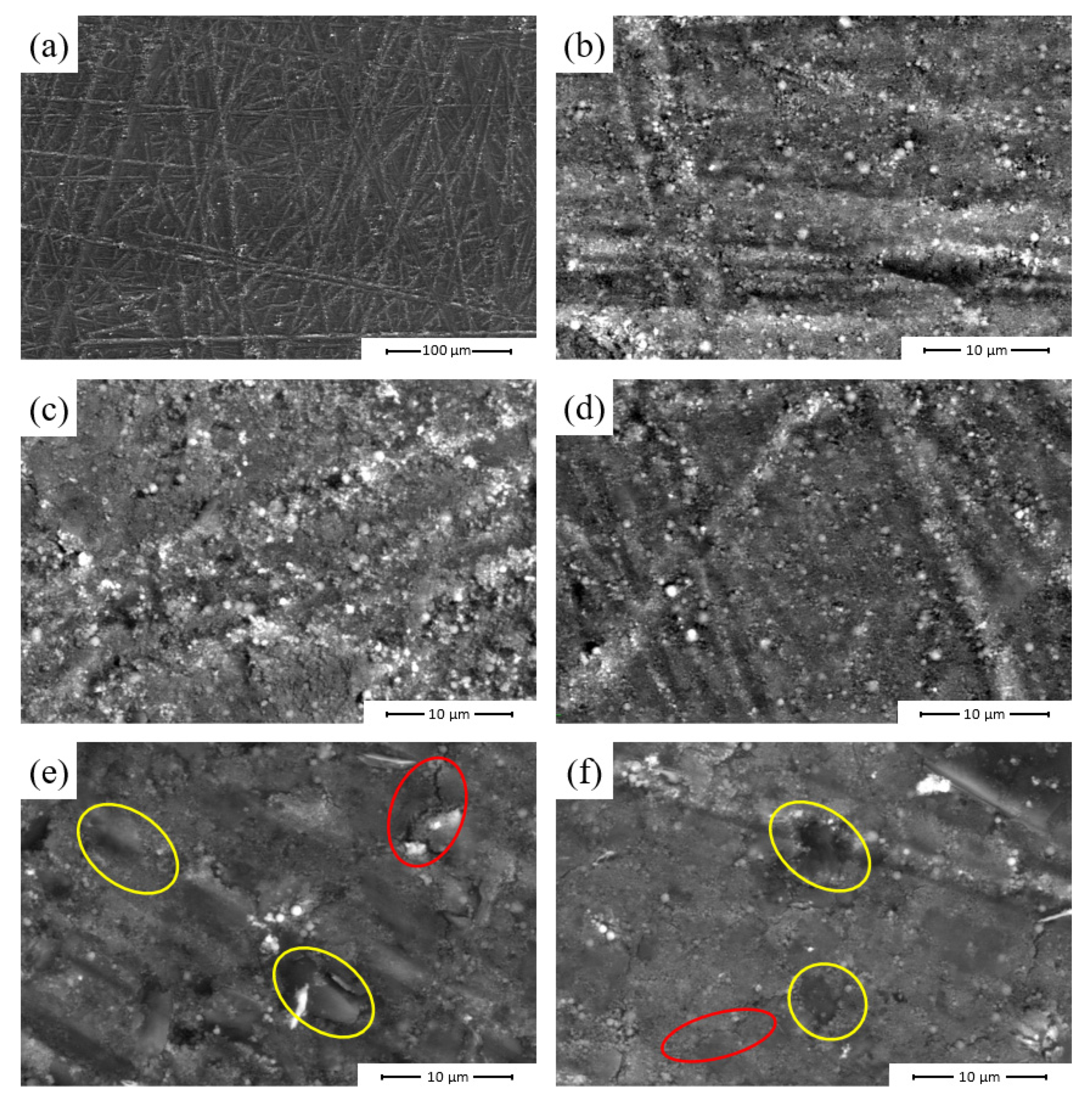
3.2.3. Scanning Electron Microscopy Analysis
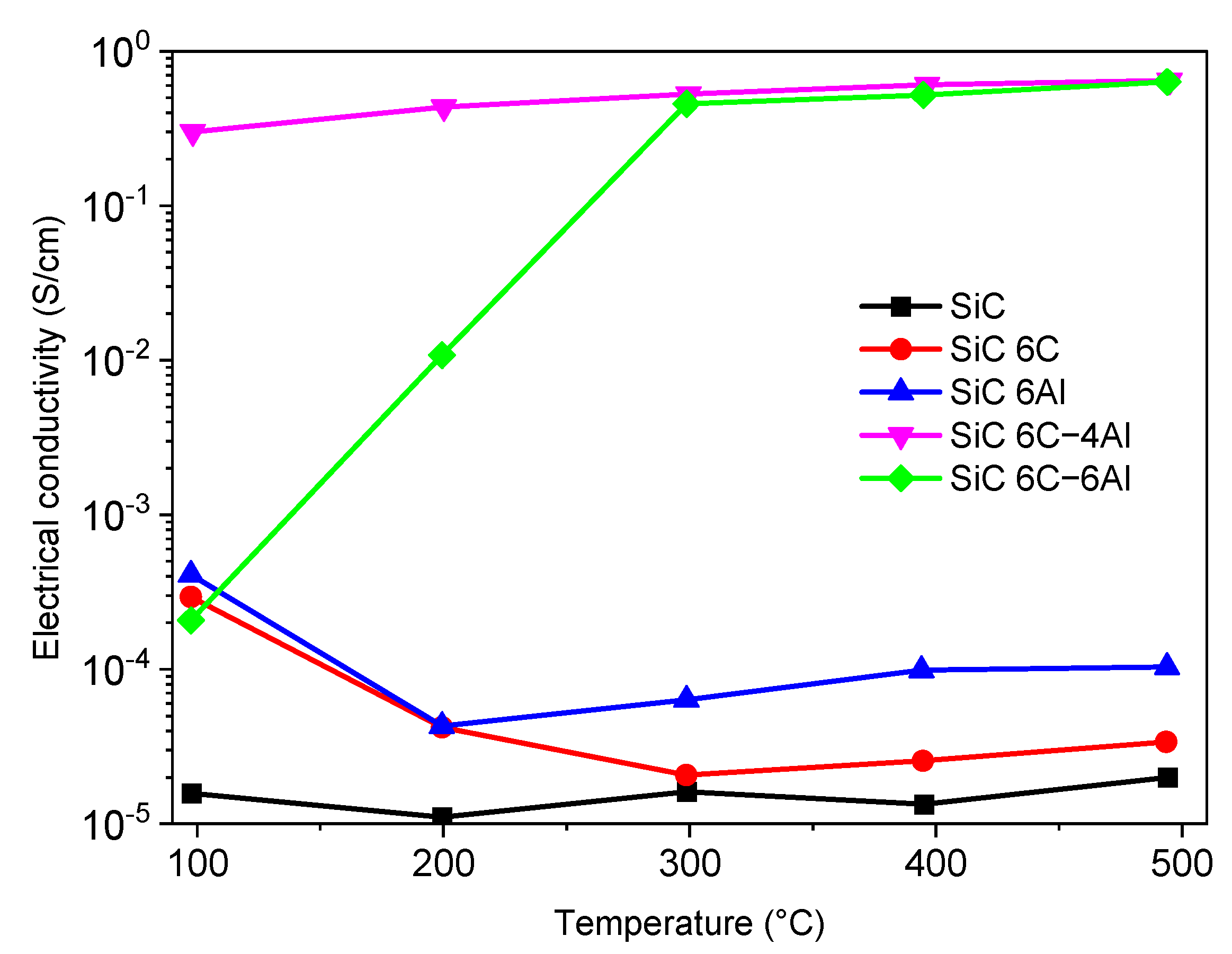
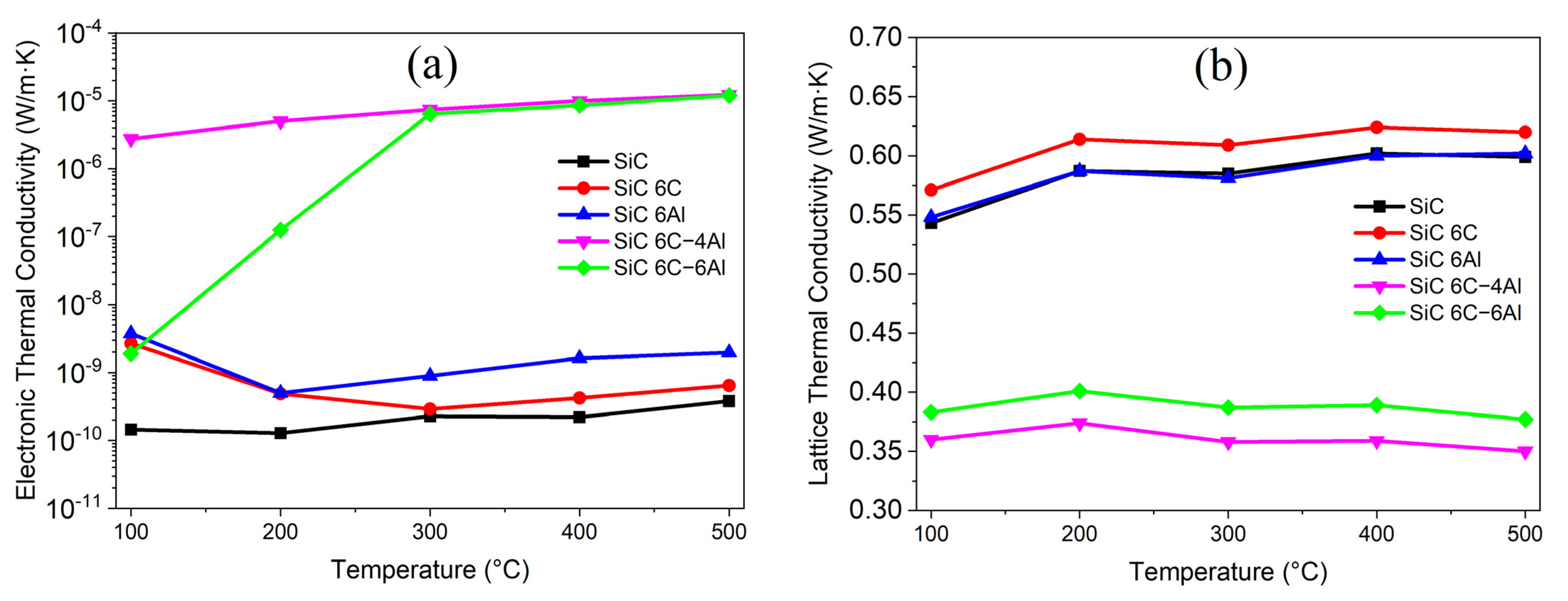
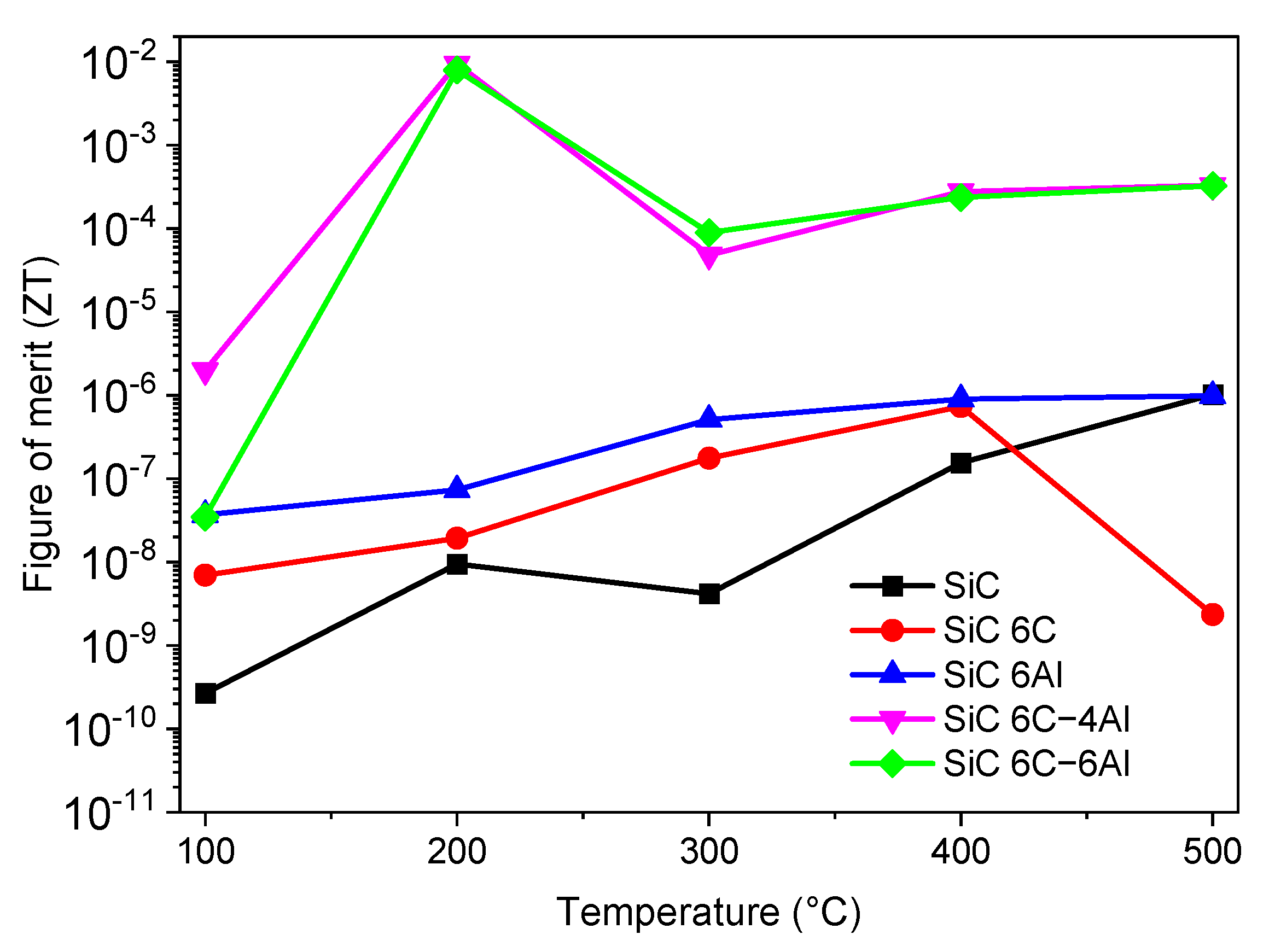
3.2.4. Thermoelectric Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaiwanglok, A.; Eguchi, K.; Julsereewong, A.; Pannil, P. Alternative of high voltage multipliers utilizing Cockcroft–Walton multiplier blocks for 220 V and 50 Hz input. Energy Rep. 2020, 6, 909–913. [Google Scholar] [CrossRef]
- Kenfack, P.; Dandoussou, A.; Nkale, E.F. Comparative study of the design and simulation of an AC to high DC voltage generation circuit. J. Electr. Syst. Inf. Technol. 2022, 9, 1–17. [Google Scholar] [CrossRef]
- Berkhout, M. Integrated Audio Amplifiers in BCD Technology; Springer Science and Business Media LLC: New York, NY, USA, 1997; ISBN 9781461377870. [Google Scholar]
- Nakashima, D.; Kino, Y.; Eguchi, K. Design of a bipolar Dickson voltage multiplier suitable for low output voltage condi-tions to provide efficient food processing. Energy Rep. 2023, 9, 207–213. [Google Scholar] [CrossRef]
- Song, H. VLSI Modulation Circuits—Signal Processing, Data Conversion, and Power Management; Lulu Press: Morrisville, NC, USA, 2014. [Google Scholar]
- Gerber, D.L.; Liou, R.; Brown, R. Energy-saving opportunities of direct-DC loads in buildings. Appl. Energy 2019, 248, 274–287. [Google Scholar] [CrossRef]
- Singh, B.P.; Singh, R. Electronic Devices and Integrated Circuits; Dorling Kindersley: Noida, India, 2009. [Google Scholar]
- Thaker, U.; Vora, S.C.; Amardas, A.; Amal, S.; Saurabh, K.; Chakraborty, A.; Christian, P.; Mankani, A.; Baruah, U. Design, analysis, fabrication and testing of 100 kV, 100 mA DC full-wave voltage multiplier (FWVM) modular unit for accelerator power supply. Fusion Eng. Des. 2023, 192, 113612. [Google Scholar] [CrossRef]
- Sharma, A.K. Semiconductor Electronics; New Age International: New Delhi, India, 2008. [Google Scholar]
- Amrollahi, P.; Krasinski, J.S.; Vaidyanathan, R.; Tayebi, L.; Vashaee, D. Electrophoretic Deposition (EPD): Fundamentals and Applications from Nano- to Microscale Structures. In Handbook of Nanoelectrochemistry; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Li, C.; Li, Y.; Chen, Q.; Sun, D.; Li, H.; Chen, W.; Yan, J.; Wu, G.; Yuan, X.; Wang, S.; et al. Ti3C2Tx@polyaniline/epoxy coating based on cathodic electrophoretic deposition with effective corrosion inhibition effect for corrosion protection on P110 steel. Prog. Org. Coat. 2024, 187, 108173. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Twaha, S.; Zhu, J.; Yan, Y.; Li, B. A comprehensive review of thermoelectric technology materials, applications, modeling and performance improvement. Renew. Sustain. Energy Rev. 2016, 65, 698–726. [Google Scholar] [CrossRef]
- Gayner, C.; Kar, K.K. Recent advances in thermoelectric materials. Prog. Mater. Sci. 2016, 83, 330–382. [Google Scholar] [CrossRef]
- Dehkordi, A.M.; Zebarjadi, M.; He, J.; Tritt, T.M. Thermoelectric power factor: Enhancement mechanisms and strategies for higher performance thermoelectric materials. Mater. Sci. Eng. R Rep. 2015, 97, 1–22. [Google Scholar] [CrossRef]
- Kong, L.B.; Li, T.; Hng, H.H.; Boey, F.; Zhang, T.; Li, S. Lecture Notes in Energy. In Waste Thermal Energy Harvesting (I): Thermoelectric Effect; Springer: Berlin/Heidelberg, Germany, 2014; p. 24. [Google Scholar]
- Fitriani; Ovik, R.; Long, B.; Barma, M.; Riaz, M.; Sabri, M.; Said, S.; Saidur, R. A review on nanostructures of high-temperature thermoelectric materials for waste heat recovery. Renew. Sustain. Energy Rev. 2016, 64, 635–659. [Google Scholar] [CrossRef]
- Sussardi, A.; Tanaka, T.; Khan, A.; Schlapbach, L.; Mori, T. Enhanced thermoelectric properties of samarium boride. J. Mater. 2015, 1, 196–204. [Google Scholar] [CrossRef]
- Freer, R.; Ekren, D.; Ghosh, T.; Biswas, K.; Qiu, P.; Wan, S.; Chen, L.; Han, S.; Fu, C.; Zhu, T.; et al. Key properties of inorganic thermoelectric materials. J. Phys. Energy 2022, 4, 022002. [Google Scholar] [CrossRef]
- Pai, C.H. Thermoelectric properties of aluminum compound-doped a-SiC ceramics. J. Ceram. Soc. Jpn. 2014, 122, 870–875. [Google Scholar] [CrossRef]
- Kamimura, H.; Bhattacharya, P.; Fornari, R. Comprehensive Semiconductor Science and Technology: Volume 1; Elsevier: Berkeley, CA, USA, 2011. [Google Scholar]
- Kim, K.J.; Jang, S.H.; Kim, Y.-W.; Jang, B.-K.; Nishimura, T. Conductive SiC ceramics fabricated by spark plasma sintering. Ceram. Int. 2016, 42, 17892–17896. [Google Scholar] [CrossRef]
- He, J.; Tritt, T.M. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, eaak9997. [Google Scholar] [CrossRef]
- Kim, G.-D.; Kim, Y.-W.; Song, I.-H.; Kim, K.J. Effects of carbon and silicon on electrical, thermal, and mechanical properties of porous silicon carbide ceramics. Ceram. Int. 2020, 46, 15594–15603. [Google Scholar] [CrossRef]
- Pędrak, P.; Drajewicz, M.; Dychtoń, K.; Nowotnik, A. Microstructure and thermal characteristics of SiC–Al2O3–Ni composite for high-temperature application. J. Therm. Anal. Calorim. 2016, 125, 1353–1356. [Google Scholar] [CrossRef]
- Rauber, A.; Bakker, P.D. A Comparison of Adjustable-Speed Drive Systems: Voltage Source Inverters and Load-Commutated Inverters for High-Power Applications. IEEE Ind. Appl. Mag. 2020, 26, 56–66. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, B.; Mishra, S. An Uninterruptable PV Array-Battery Based System Operating in Different Power Modes with Enhanced Power Quality. IEEE Trans. Ind. Electron. 2021, 69, 3631–3642. [Google Scholar] [CrossRef]
- Bai, H.K.; Costinett, D.; Tolbert, L.M.; Qin, R.; Zhu, L.; Liang, Z.; Huang, Y. Charging Electric Vehicle Batteries: Wired and Wireless Power Transfer: Exploring EV charging technologies. IEEE Power Electron. Mag. 2022, 9, 14–29. [Google Scholar] [CrossRef]
- Tuckey, A.; Round, S. Grid-Forming Inverters for Grid-Connected Microgrids: Developing “good citizens” to ensure the continued flow of stable, reliable power. IEEE Electrif. Mag. 2022, 10, 39–51. [Google Scholar] [CrossRef]
- Gottlieb, I.M. Power Supplies, Switching Regulators, Inverters, & Converters; McGraw-Hill: New York, NY, USA, 1994. [Google Scholar]
- Niu, M. Advanced Electronic Circuits: Principles, Architectures and Applications on Emerging Technologies; IntechOpen: London, UK, 2018. [Google Scholar]
- Emadi, A. Advanced Electric Drive Vehicles; Taylor & Francis Group: Abingdon, UK, 2015. [Google Scholar]
- Barrett, S.F. Arduino I: Getting Started; Morgan & Claypool: San Rafael, CA, USA, 2020. [Google Scholar]
- Ramshaw, R.S. Power Electronics Semiconductor Switches; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering an Introduction; John Wiley & Son: Tokyo, Japan, 2020. [Google Scholar]
- Pankratz, L.B.; Stuve, J.M.; Gokcen, N.A. Thermodynamic Data for Mineral Technology; U.S. Department of the Interior: Washington, DC, USA, 1984. [Google Scholar]
- Kim, H.-S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
- Dey, P.P.; Khare, A. Tailoring of stoichiometry and band-tail emission in PLD a-SiC thin films by varying He deposition pressure. SN Appl. Sci. 2020, 2, 1059. [Google Scholar] [CrossRef]
- Stanishevsky, A.; Li, H.; Badzian, A.; Badzian, T.; Drawl, W.; Khriachtchev, L.; McDaniel, E. B–C–N coatings prepared by microwave chemical vapor deposition. Thin Solid Films 2001, 398–399, 270–274. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Y.; Lin, Z.-M.; Li, J.-T.; Du, J.-S. Mechanical-activation-assisted combustion synthesis of SiC powders with polytetrafluoroethylene as promoter. Mater. Res. Bull. 2007, 42, 1625–1632. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, W.; Su, X.; Luo, F.; Zhu, D.; Liu, P. Preparation and Characterization of Aluminum-Doped Silicon Carbide by Combustion Synthesis. J. Am. Ceram. Soc. 2008, 91, 2607–2610. [Google Scholar] [CrossRef]
- Charguia, R.; Hcini, S.; Boudard, M.; Dhahri, A. Microstructural properties, conduction mechanism, dielectric behavior, impedance and electrical modulus of La0.6Sr0.2Na0.2MnO3 manganite. J. Mater. Sci. Mater. Electron. 2019, 30, 2975–2984. [Google Scholar] [CrossRef]
- Li, G.; Yang, J.; Xiao, Y.; Fu, L.; Luo, Y.; Zhang, D.; Liu, M.; Li, W.; Zhang, M. Effect of TiC Nanoinclusions on Thermoelectric and Mechanical Performance of Polycrystalline In4Se2.65. J. Am. Ceram. Soc. 2015, 98, 3813–3817. [Google Scholar] [CrossRef]
- Masuda, H.; Mabuchi, H.; Tsuda, H.; Matsui, T.; Morii, K. Thermoelectric properties of 3C-SiC produced by silicon carbonization. Mater. Sci. Forum 2002, 389–393, 763–766. [Google Scholar] [CrossRef]
- Cramer, C.L.; Farnell, C.C.; Farnell, C.C.; Geiss, R.H.; Williams, J.D. Thermoelectric Properties and Morphology of Si/SiC Thin-Film Multilayers Grown by Ion Beam Sputtering. Coatings 2018, 8, 109. [Google Scholar] [CrossRef]
- Wan, P.; Wang, J. Highly porous nano-SiC with very low thermal conductivity and excellent high temperature behavior. J. Am. Ceram. Soc. 2018, 38, 463–467. [Google Scholar] [CrossRef]
- Hong, S.-N.; Yu, C.-J.; Hwang, U.-S.; Kim, C.-H.; Ri, B.-H. Effect of porosity and temperature on thermal conductivity of jennite: A molecular dynamics study. Mater. Chem. Phys. 2020, 250, 123146. [Google Scholar] [CrossRef]
- Kinoshita, T.; Munekawa, S. Effect of grain boundary segregation on thermal conductivity of hot-pressed silicon carbide. Acta Mater. 1997, 45, 2001–2012. [Google Scholar] [CrossRef]
- Taki, Y.; Kitiwan, M.; Katsui, H.; Goto, T. Electrical and thermal properties of off-stoichiometric SiC prepared by spark plasma sintering. J. Asian Ceram. Soc. 2018, 6, 95–101. [Google Scholar] [CrossRef]
- Cao, L.; Liu, Y.; Zhang, Y.; Cao, Y.; Li, J.; Chen, J.; Zhang, L.; Qi, Z. Thermal conductivity and bending strength of SiC composites reinforced by pitch-based carbon fibers. J. Adv. Ceram. 2022, 11, 247–262. [Google Scholar] [CrossRef]
- Okamoto, Y.; Aruga, A.; Kasai, H.; Morimoto, J.; Miyakawa, T.; Fujimoto, S. Temperature dependence of thermoelectric properties of SiC/B4C. AIP Conf. Proc. 1994, 316, 92–95. [Google Scholar]
- Qiao, G.J.; Shi, Y.; Gao, J.Q.; Wang, H.J.; Yang, J.F.; Jin, Z.H. Study on Si Dependence of RB-SiC Thermoelectric Properties. Key Eng. Mater. 2005, 280–283, 401–405. [Google Scholar]
- Ivanova, L.M.; Aleksandrov, P.A.; Demakov, K.D. Thermoelectric properties of vapor-grown polycrystalline cubic SiC. Inorg. Mater. 2006, 42, 1205–1209. [Google Scholar] [CrossRef]
- Koumoto, K.; Shimohigoshi, M.; Takeda, S.; Yanagida, H. Thermoelectric energy conversion by porous SiC ceramics. J. Mater. Sci. Lett. 1987, 6, 1453–1455. [Google Scholar] [CrossRef]
- Ozer, S.C.; Arslan, K.; Turan, S. Improved thermoelectric properties of SiC with TiC segregated. J. Eur. Ceram. Soc. 2024, 43, 6154–6161. [Google Scholar] [CrossRef]
- Mori, T. Perspectives of High-Temperature Thermoelectric Applications and p-type and n-type Aluminoborides. JOM 2016, 68, 2673–2679. [Google Scholar] [CrossRef]


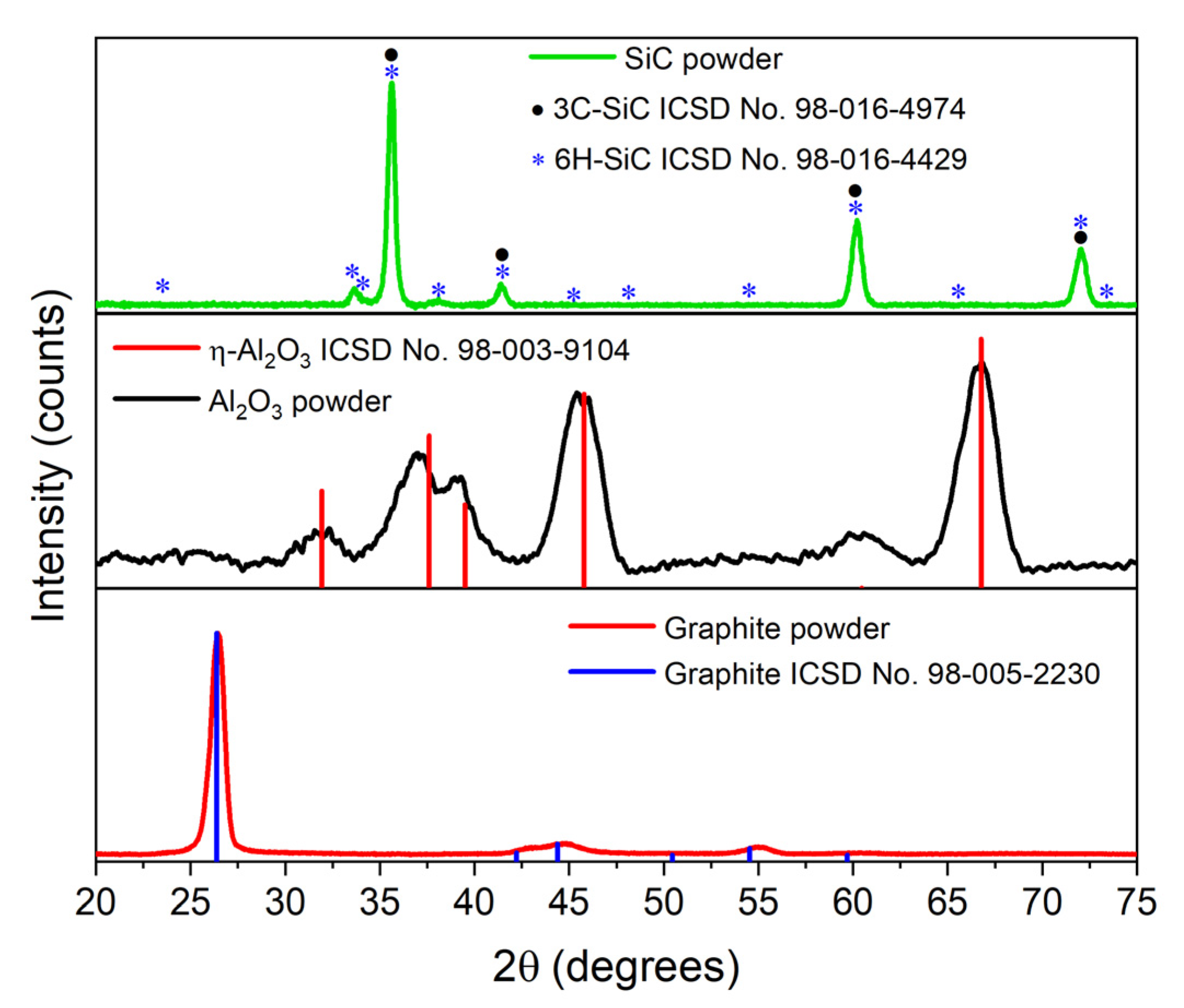
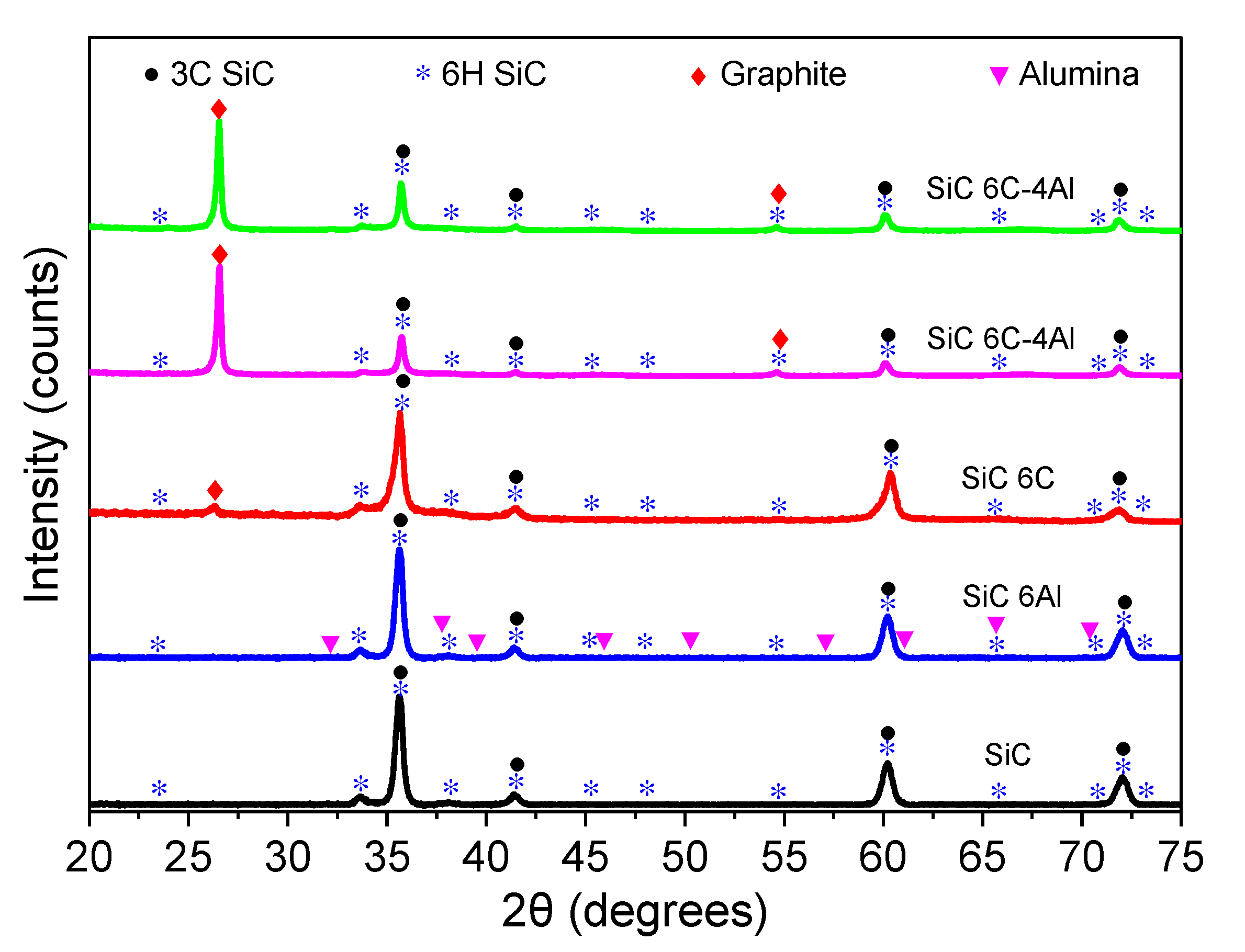





| Sample Nomenclature | Composition (% wt.) | ||
|---|---|---|---|
| SiC | C | Al2O3 | |
| SiC | 100 | - | - |
| SiC 6C | 100 | 6 | - |
| SiC 6Al | 100 | - | 6 |
| SiC 6C-4Al | 100 | 6 | 4 |
| SiC 6C-6Al | 100 | 6 | 6 |
| Parameter | Value |
|---|---|
| Input Voltage (VDC) | 12 |
| Input Current (A) | 1.25 |
| Output Voltage (VDC) | 0–430 |
| Output Steady Current During Pulse (mA) | 0.3–60 |
| Pulse Frequency (kHz) | 0–150 |
| Cycle Duty (%) | 0–100 |
| Phase | wt.% | χ2 | Rexp | Rp | Rwp | a (Å) | c (Å) | Vol. (Å3) | ρ (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|
| 3C-SiC | 98.4 | 1.18 | 20.82 | 24.32 | 22.64 | 4.37202 | 83.5694 | 3.19 | |
| 6H SiC | 1.6 | 3.02414 | 15.23072 | 120.6325 | 3.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes Valdez, J.J.; De la Cruz Terrazas, E.C.; Rodríguez González, E. Design, Construction and Programming of a Low-Cost Pulsed High-Voltage Direct Current Power Supply for the Electrophoretic Deposition of Silicon Carbide Mixed with Graphite and/or Alumina for Thermoelectric Applications. Inventions 2024, 9, 40. https://doi.org/10.3390/inventions9020040
Reyes Valdez JJ, De la Cruz Terrazas EC, Rodríguez González E. Design, Construction and Programming of a Low-Cost Pulsed High-Voltage Direct Current Power Supply for the Electrophoretic Deposition of Silicon Carbide Mixed with Graphite and/or Alumina for Thermoelectric Applications. Inventions. 2024; 9(2):40. https://doi.org/10.3390/inventions9020040
Chicago/Turabian StyleReyes Valdez, Juan Jesús, Edna Carina De la Cruz Terrazas, and Eugenio Rodríguez González. 2024. "Design, Construction and Programming of a Low-Cost Pulsed High-Voltage Direct Current Power Supply for the Electrophoretic Deposition of Silicon Carbide Mixed with Graphite and/or Alumina for Thermoelectric Applications" Inventions 9, no. 2: 40. https://doi.org/10.3390/inventions9020040
APA StyleReyes Valdez, J. J., De la Cruz Terrazas, E. C., & Rodríguez González, E. (2024). Design, Construction and Programming of a Low-Cost Pulsed High-Voltage Direct Current Power Supply for the Electrophoretic Deposition of Silicon Carbide Mixed with Graphite and/or Alumina for Thermoelectric Applications. Inventions, 9(2), 40. https://doi.org/10.3390/inventions9020040






