Analysis of a Newly Developed Afterburner System Employing Hydrogen–Methane Blends
Abstract
1. Introduction
2. Materials and Methods
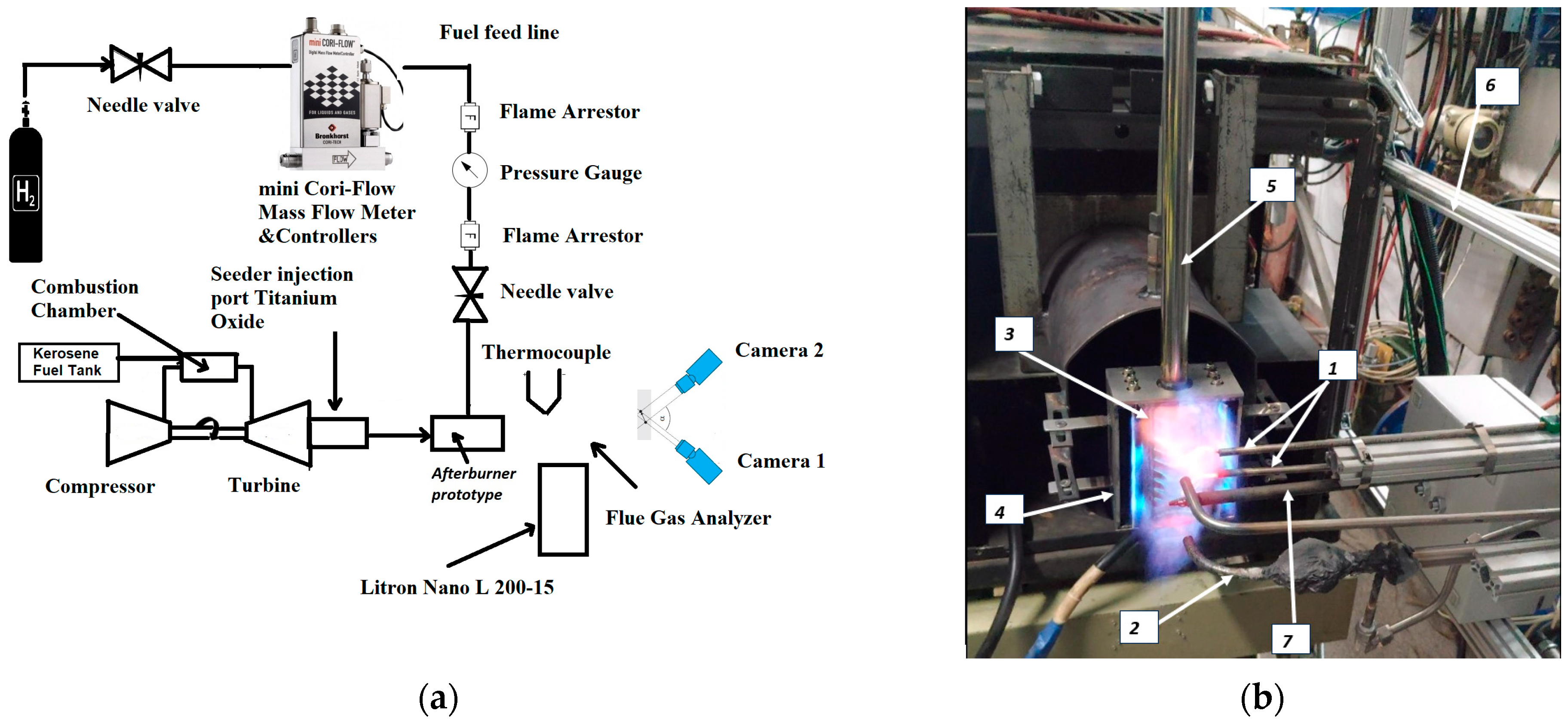
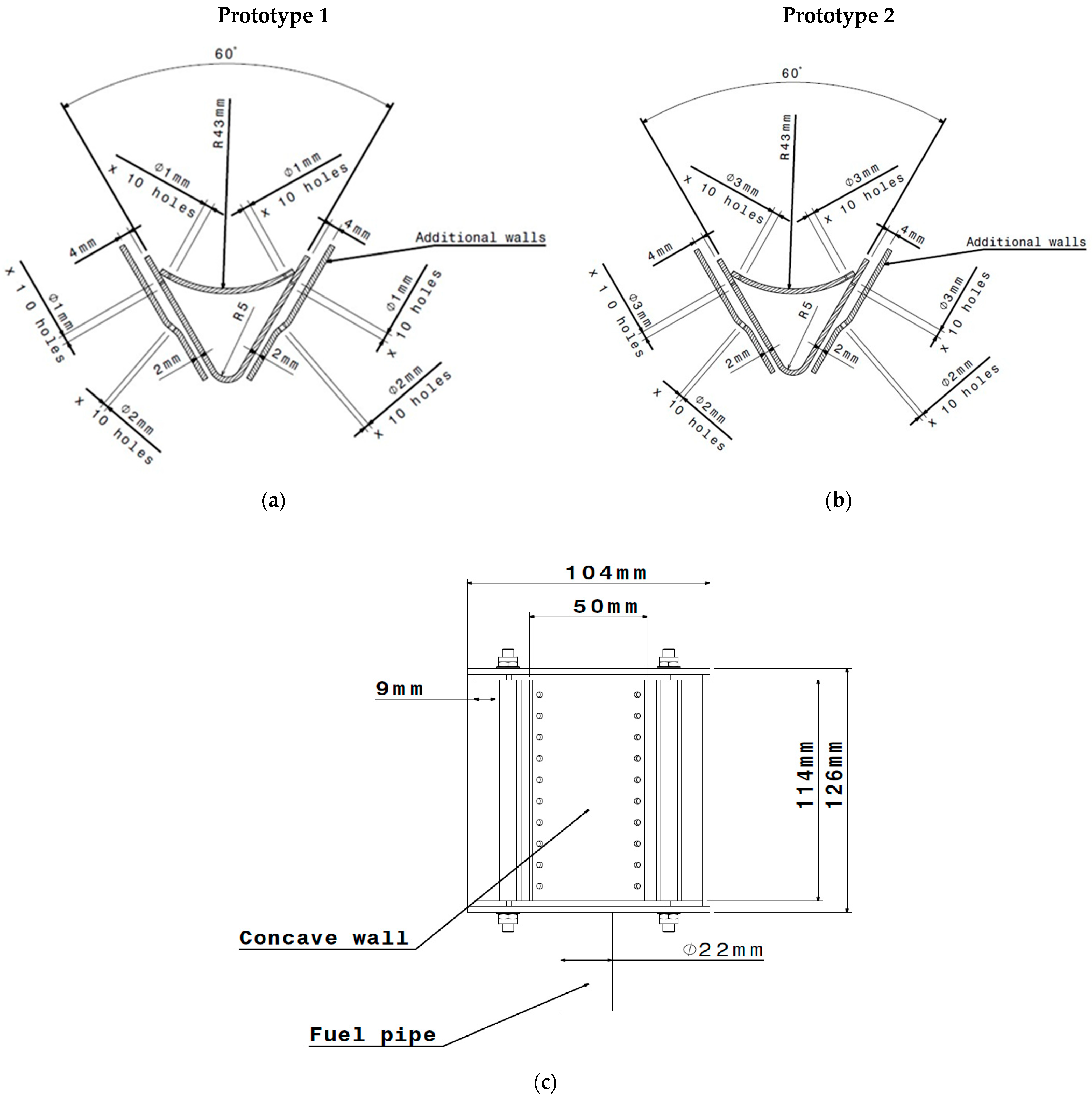
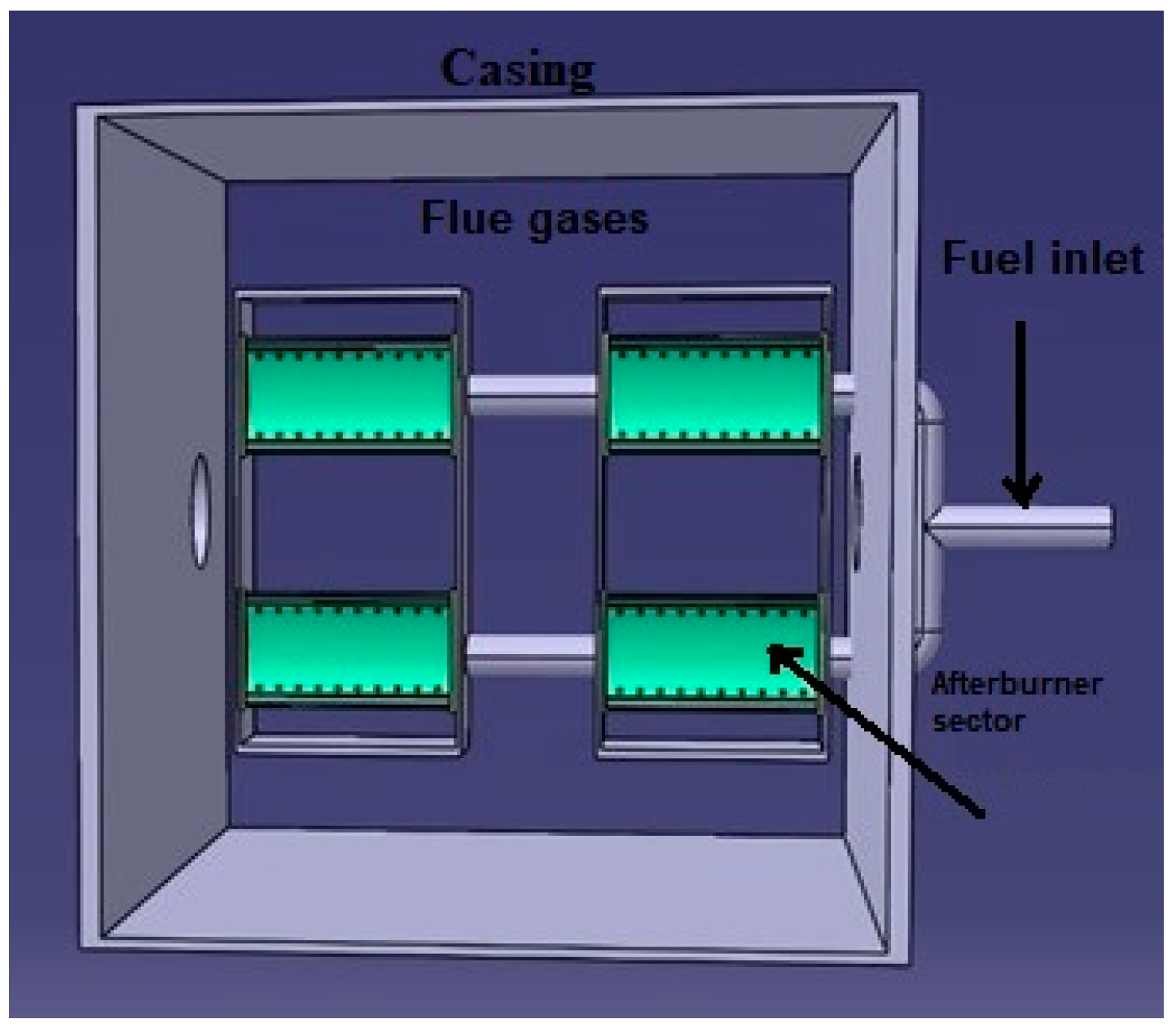
Test Rig Instrumentation and Afterburner Geometry
3. Results and Discussion
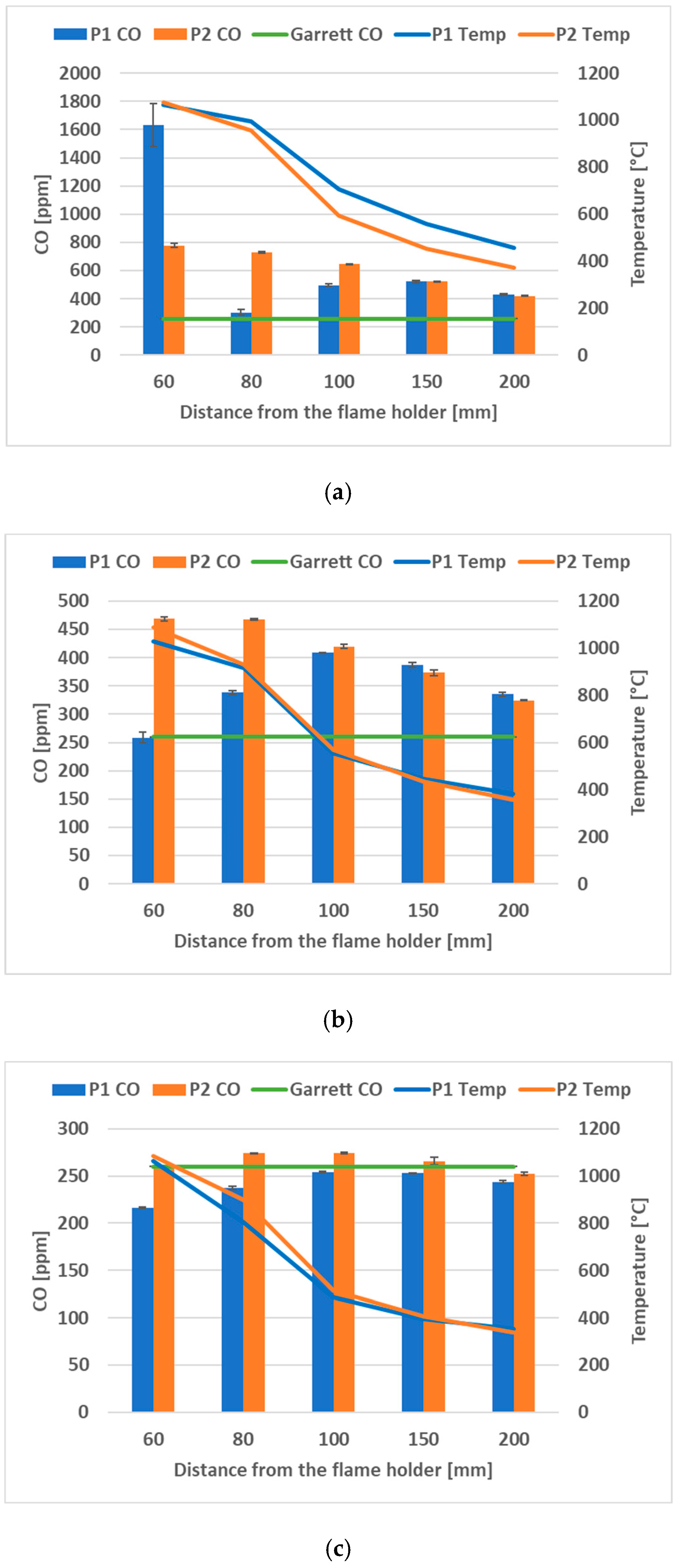
3.1. Flue Gases Analysis
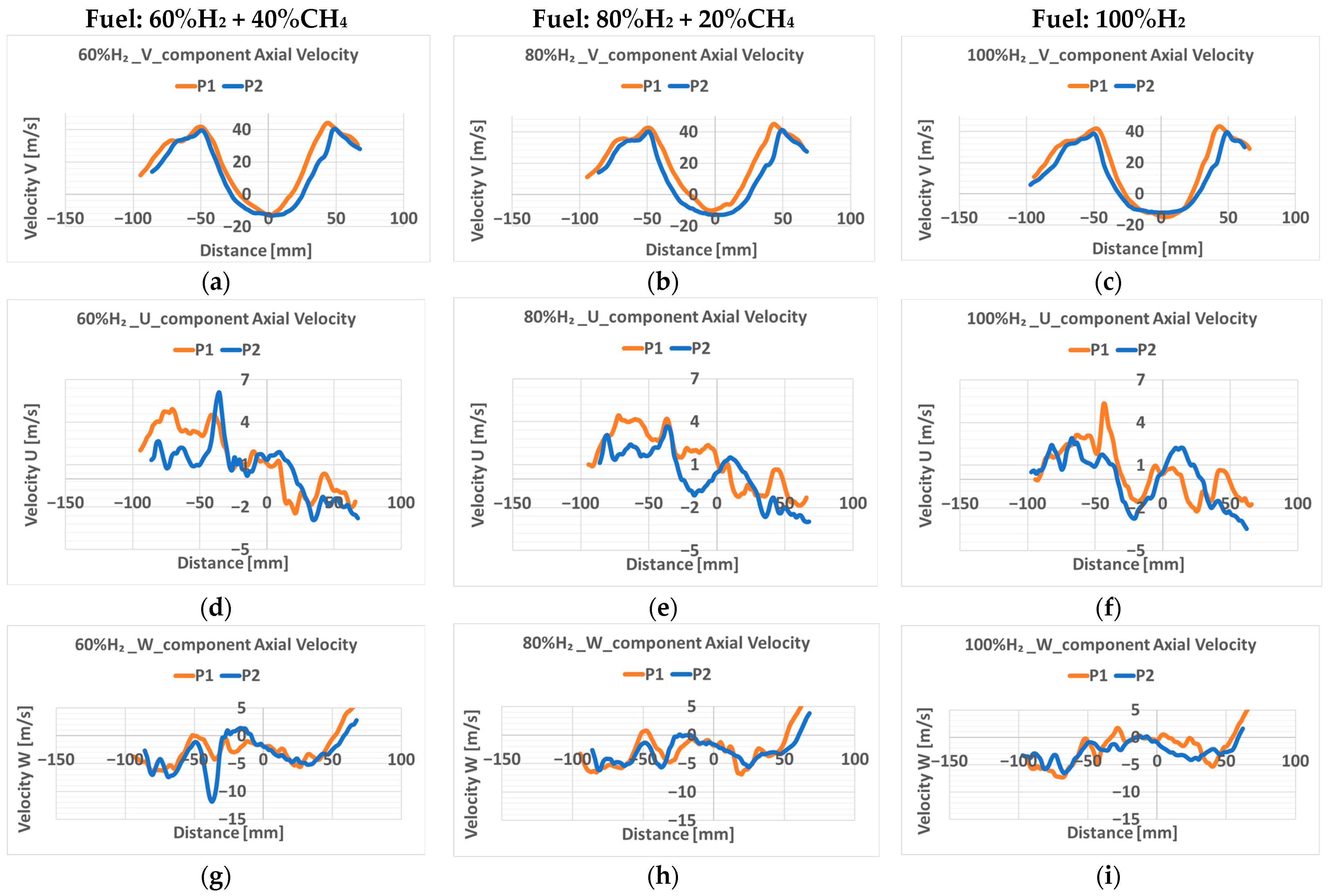
3.2. PIV Experiments
4. Conclusions
- A stable functioning of the afterburner assembly for all used fuels, in the case of both prototypes.
- The concentration of CO2 decreased as the proportion of H2 in the fuel mixture increased. The CO2 concentration monitored was lower in the case of prototype P2 for all the fuel mixtures tested. The most notable difference was observed at 60% H2, where the carbon content in the fuel was dominant in terms of mass participation; thus, the CO2 emissions were more distinguishable between P1 and P2 flame holders.
- The CO levels decreased as the percentage of H2 in the fuel increased. For the P1 prototype, the maximum value was only at the first measurement point, at a distance of 60 mm from the flame holder, in the case of using 60% H2 in the fuel mixture. The rest of the measurements indicate that the P2 prototype geometry obtains better behavior in terms of CO emissions.
- The NO emissions gradually decreased as the percentage of H2 in the fuel mixture increased. The NO concentration was significantly lower in the case of prototype P2 in comparison with prototype P1 for all the measurement points at all of the hydrogen concentrations in the fuel mixture.
- Increasing the V-gutter bluff body expansion angle extends the flame stability limits. The shortest recirculation zone was observed for P1, where the axial velocity reaches a negative peak of approximately 12 m/s at roughly 50 mm downstream of the edge of the flame holder, and the recirculation region spans about 90 mm. In comparison, the P2 prototype has a length of the recirculation region span of about 100 mm with a negative peak of approximately 14 m/s. The transversal velocity (U) components are minimal for both prototypes, reflecting the dominance of axial flow along the centerline. The slight fluctuations can be attributed to external wind currents in the experimental setup. The spanwise velocity (W) components exhibit small values for both prototypes. The data spread indicates variability, which is expected due to the calculation-based measurement method of PIV for spanwise velocities. Despite their similar design, P2 consistently performs better across all measured velocity components. This improvement can be attributed to the increased fuel injection holes, which enhance fuel–air mixing and combustion stability. Additionally, the presence of side walls directing the flow around the flame stabilizer further aids in maintaining a stable combustion process. The influence of ambient conditions, such as wind currents, is minimal but noticeable in the transversal velocity measurements. The spanwise velocity, calculated rather than directly measured, shows more variability but supports the conclusion that P2 and higher hydrogen concentrations result in improved flow characteristics.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stylianidis, N.; Azimov, U.; Birkett, M. Investigation of the Effect of Hydrogen and Methane on Combustion of Multicomponent Syngas Mixtures using a Constructed Reduced Chemical Kinetics Mechanism. Energies 2019, 12, 2442. [Google Scholar] [CrossRef]
- Pio, G.; Salzano, E. Laminar burning velocity of methane, hydrogen and their mixtures at extremely low-temperature conditions. Energy Fuels 2018, 32, 8830–8836. [Google Scholar] [CrossRef]
- Salzano, E.; Pio, G.; Ricca, A.; Palma, V. The effect of a hydrogen addition to the premixed flame structure of light alkanes. Fuel 2018, 234, 1064–1070. [Google Scholar] [CrossRef]
- Pourali, M.; Esfahani, J.A.; Fanaee, S.A.; Bastiaans, R.J.; Kim, K.C. Effect of hydrogen addition on conjugate heat transfer in a planar micro-combustor with the detailed reaction mechanism: An analytical approach. Int. J. Hydrogen Energy 2020, 45, 15425–15440. [Google Scholar] [CrossRef]
- Chen, Z. Effects of hydrogen addition on the propagation of spherical methane/air flames: A computational study. Int. J. Hydrogen Energy 2009, 34, 6558–6567. [Google Scholar] [CrossRef]
- Huang, Z.; Peng, Q.; Shi, Z.; Fu, G.; Xiao, H.; Huang, C. Numerical and experimental investigation of H2/CH4/Air combustion characteristics and thermal performance in the combustor with multi-bluff-body. Fuel 2014, 367, 131435. [Google Scholar] [CrossRef]
- Leparoux, J.; Mercier, R.; Puggelli, S.; Cailler, M.; Moureau, V. Numerical Investigation of a Hydrogen–Air Flame for NOx Prediction. J. Eng. Gas Turbines Power. Sep. 2024, 146, 091015. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Mao, R.; Lin, W.; Lin, B.; Wu, Y.; Zhang, M.; Huang, Z. Experimental study of compact swirl flames with lean premixed CH4/H2/air mixtures at stable and near blow-off conditions. Exp. Therm. Fluid. Sci. 2021, 122, 110294. [Google Scholar] [CrossRef]
- Vance, F.H.; Shoshin, Y.; van Oijen, J.A.; de Goey, L.P.H. Effect of Lewis number on premixed laminar lean-limit flames stabilized on a bluff body. Proc. Combust. Inst. 2019, 37, 1663–1672. [Google Scholar] [CrossRef]
- Jiménez, C.; Michaels, D.; Ghoniem, A.F. Stabilization of ultra-lean hydrogen enriched inverted flames behind a bluff–body and the phenomenon of anomalous blow–off. Combust. Flame 2018, 191, 86–98. [Google Scholar] [CrossRef]
- Marragou, S.; Magnes, H.; Poinsot, T.; Selle, L.; Schuller, T. Stabilization regimes and pollutant emissions from a dual fuel CH4/H2 and dual swirl low NOx burner. Int. J. Hydrogen Energy 2022, 47, 19275–19288. [Google Scholar] [CrossRef]
- Kwak, S.; Choi, J.; Ahn, M.; Yoon, Y. Effects of hydrogen addition on the forced response of H2/CH4 flames in a dual-nozzle swirl-stabilized combustor. Int. J. Hydrogen Energy 2022, 47, 28139–28151. [Google Scholar] [CrossRef]
- Eckart, S.; Pio, G.; Krause, H.; Salzano, E. Chemical and thermal effects of trace components in hydrogen rich gases on combustion. Chem. Eng. Trans. 2022, 90, 361–366. [Google Scholar] [CrossRef]
- Konnov, A.A.; Mohammad, A.; Kishore, V.R.; Kim, N.I.; Prathap, C.; Kumar, S. A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel+air mixtures. Prog. Energy Combust. Sci. 2018, 68, 197–267. [Google Scholar] [CrossRef]
- Eckart, S.; Prieler, R.; Hochenauer, C.; Krause, H. Application and comparison of multiple machine learning techniques for the calculation of laminar burning velocity for hydrogen-methane mixtures. Therm. Sci. Eng. Prog. 2022, 32, 101306. [Google Scholar] [CrossRef]
- Xia, H.; Han, W.; Wei, X.; Zhang, M.; Wang, J.; Huang, Z.; Hasse, C. Numerical investigation of boundary layer flashback of CH4/H2/air swirl flames under different thermal boundary conditions in a bluff-body swirl burner. Proc. Combust. Inst. 2023, 39, 4541–4551. [Google Scholar] [CrossRef]
- Ebi, D.; Bombach, R.; Jansohn, P. Swirl flame boundary layer flashback at elevated pressure: Modes of propagation and effect of hydrogen addition. Proc. Combust. Inst. 2020, 38, 6345–6353. [Google Scholar] [CrossRef]
- Guo, S.; Wang, J.; Zhang, W.; Zhang, M.; Huang, Z. Effect of hydrogen enrichment on swirl/bluff-body lean premixed flame stabilization. Int. J. Hydrogen Energy 2020, 45, 10906–10919. [Google Scholar] [CrossRef]
- Mao, R.; Wang, J.; Zhang, W.; An, Z.; Lin, W.; Zhang, M.; Huang, Z. Effect of high hydrogen enrichment on the outer-shear-layer flame of confined lean premixed CH4/H2/air swirl flames. Int. J. Hydrogen Energy 2021, 46, 17969–17981. [Google Scholar] [CrossRef]
- Emadi, M.; Karkow, D.; Salameh, T.; Gohil, A.; Ratner, A. Flame structure changes resulting from hydrogen-enrichment and pressurization for low-swirl premixed methane–air flames. Int. J. Hydrogen Energy 2012, 37, 10397–10404. [Google Scholar] [CrossRef]
- Halter, F.; Chauveau, C.; Gökalp, I. Characterization of the effects of hydrogen addition in premixed methane/air flames. Int. J. Hydrogen Energy 2007, 32, 2585–2592. [Google Scholar] [CrossRef]
- Nakaya, S.; Omi, K.; Okamoto, T.; Ikeda, Y.; Zhao, C.; Tsue, M.; Taguchi, H. Instability and mode transition analysis of a hydrogen-rich combustion in a model afterburner. Proc. Combust. Inst. 2020, 38, 5933–5942. [Google Scholar] [CrossRef]
- Shoshin, Y.; Bastiaans, R.J.M.; de Goey, L.P.H. Anomalous blow-off behavior of laminar inverted flames of ultra-lean hydrogen–methane–air mixtures. Combust. Flame 2013, 160, 565–576. [Google Scholar] [CrossRef]
- Florean, F.G.; Mangra, A.; Enache, M.; Deaconu, M.; Ciobanu, R.; Carlanescu, R. Experimental Research on an Afterburner System Fueled with Hydrogen–Methane Mixtures. Inventions 2024, 9, 46. [Google Scholar] [CrossRef]
- Available online: https://www.findlight.net/lasers/solid-state-lasers/lamp-pumped/litron-nano-l-200-15-piv (accessed on 15 May 2024).
- La Vision Product Manual for Davis 10.2, Flow Master, Item-Number (s): 1105011-4, Produced by LaVision GmbH, Göttingen, Printed in Germany, Göttingen, 2 November 2022. Available online: https://www.lavision.de/en/news/2021/4701/ (accessed on 1 July 2024).
- Porumbel, I. LES of Bluff Body Stabilized Premixed and Partially Premixed Combustion; VDM Verlag Dr. Muller: Saarbrucken, Germany, 2007; ISBN 978-3-639-43666-2. [Google Scholar]



| Mass Flow Kerosene/Mass Flow H2/CH4 | Flue Gases GT (Garrett) | ||||||
|---|---|---|---|---|---|---|---|
| 60%H2 + 40%CH4 | 80%H2 + 40%CH4 | 100%H2 | NO [ppm] | CO [ppm] | CO2 [%] | O2 [%] | |
| P1 | 6.20 | 6.77 | 11.58 | 0.8 | 260 | 1.25 | 19 |
| P2 | 6.88 | 8.15 | 13.33 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florean, F.G.; Mangra, A.; Enache, M.; Carlanescu, R.; Taranu, A.; Botu, M. Analysis of a Newly Developed Afterburner System Employing Hydrogen–Methane Blends. Inventions 2024, 9, 74. https://doi.org/10.3390/inventions9040074
Florean FG, Mangra A, Enache M, Carlanescu R, Taranu A, Botu M. Analysis of a Newly Developed Afterburner System Employing Hydrogen–Methane Blends. Inventions. 2024; 9(4):74. https://doi.org/10.3390/inventions9040074
Chicago/Turabian StyleFlorean, Florin Gabriel, Andreea Mangra, Marius Enache, Razvan Carlanescu, Alexandra Taranu, and Madalina Botu. 2024. "Analysis of a Newly Developed Afterburner System Employing Hydrogen–Methane Blends" Inventions 9, no. 4: 74. https://doi.org/10.3390/inventions9040074
APA StyleFlorean, F. G., Mangra, A., Enache, M., Carlanescu, R., Taranu, A., & Botu, M. (2024). Analysis of a Newly Developed Afterburner System Employing Hydrogen–Methane Blends. Inventions, 9(4), 74. https://doi.org/10.3390/inventions9040074






