Patellofemoral Pain Syndrome: Focused Vibrations Plus Kinesiotaping with Insights into Radiological Influences—An Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
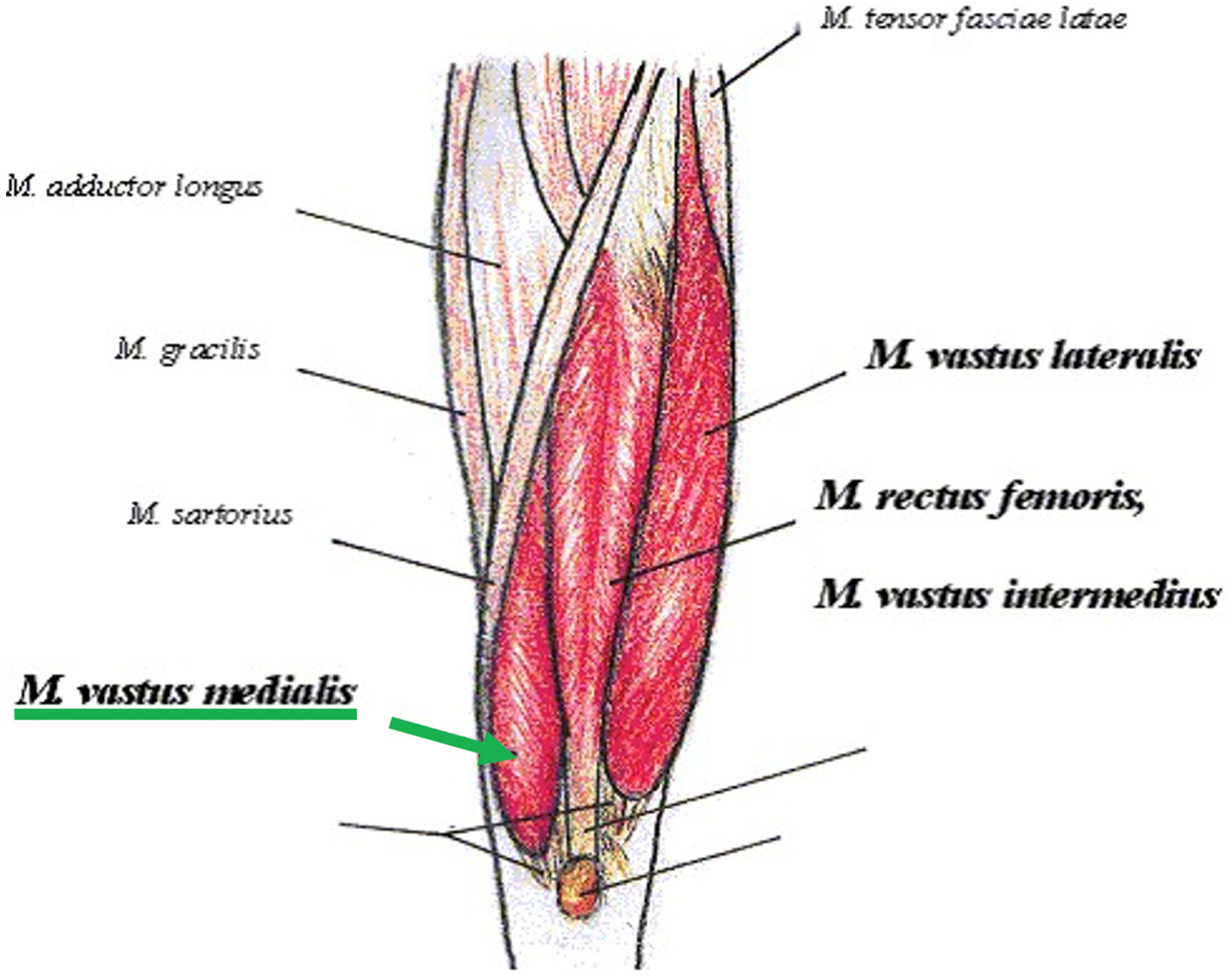
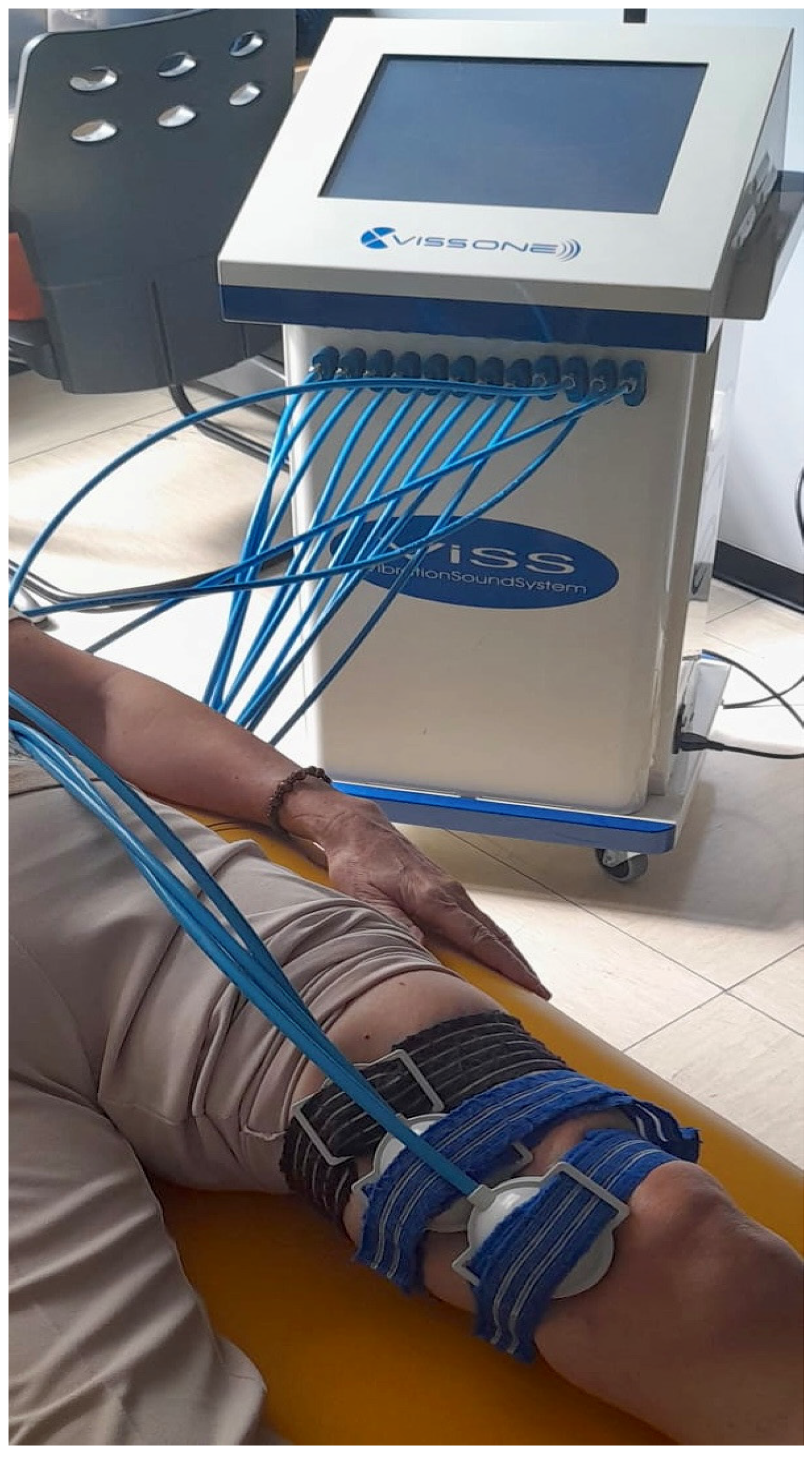
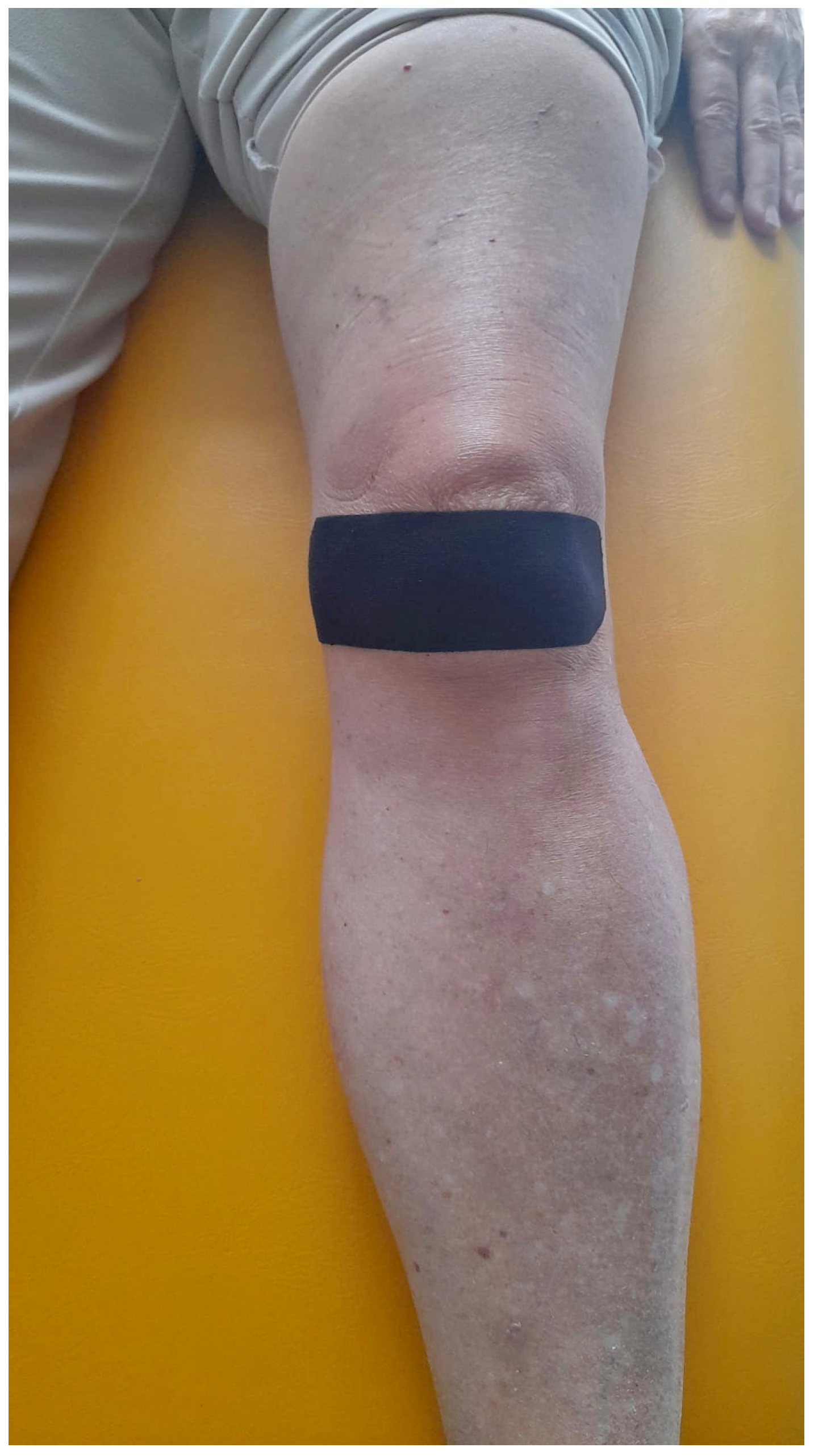
2.2. Intervention
Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Demographic and Clinical Characteristics
3.2. Safety
4. Discussion
5. The Strengths and Weaknesses of This Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crossley, K.M.; Stefanik, J.J.; Selfe, J.; Collins, N.J.; Davis, I.S.; Powers, C.M.; McConnell, J.; Vicenzino, B.; Bazett-Jones, D.M.; Esculier, J.-F.; et al. Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: Terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br. J. Sports Med. 2016, 50, 839–843. [Google Scholar] [PubMed]
- Crossley, K.M.; van Middelkoop, M.; Callaghan, M.J.; Collins, N.J.; Rathleff, M.S.; Barton, C.J. Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 2: Recommended physical interventions (exercise, taping, bracing, foot orthoses and combined interventions). Br. J. Sports Med. 2016, 50, 844–852. [Google Scholar] [CrossRef]
- Maclachlan, L.R.; Collins, N.J.; Matthews, M.L.G.; Hodges, P.W.; Vicenzino, B. The psychological features of patellofemoral pain: A systematic review. Br. J. Sports Med. 2017, 51, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, M.J.; Selfe, J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst. Rev. 2012, 18, CD006717. [Google Scholar] [CrossRef]
- Collins, N.; Vicenzino, B.; Macri, E.; Crossley, K. Prevalence and factors associated with radiographic PFJ OA in young to middle-aged adults with chronic patellofemoral pain. J. Sci. Med. Sport 2015, 19, e85. [Google Scholar] [CrossRef]
- Hinman, R.S.; Lentzos, J.; Vicenzino, B.; Crossley, K.M. Is patellofemoral osteoarthritis common in middle-aged people with chronic patellofemoral pain? Arthritis Care Res. 2014, 66, 1252–1257. [Google Scholar] [CrossRef]
- Macri, E.M.; Neogi, T.; Tolstykh, I.; Widjajahakim, R.; Lewis, C.E.; Torner, J.C.; Nevitt, M.C.; Roux, M.; Stefanik, J.J. Relation of patellofemoral joint alignment, morphology, and radiographic osteoarthritis to frequent anterior knee pain: TheMOST study. Arthritis Care Res. 2019, 72, 1066–1073. [Google Scholar] [CrossRef]
- Boling, M.; Padua, D.; Marshall, S.; Guskiewicz, K.; Pyne, S.; Beutler, A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand. J. Med. Sci. Sports 2010, 20, 725–730. [Google Scholar] [CrossRef]
- Lankhorst, N.E.; Bierma-Zeinstra, S.M.; van Middelkoop, M. Factors associated with patellofemoral pain syndrome: A systematic review. Br. J. Sports Med. 2013, 47, 193–206. [Google Scholar] [CrossRef]
- Grelsamer Ronald, P.; McConnell, J. La Rotula. Approccio d’Equipè; Albasini, A., Ed.; Galimberti, L., Translator; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Hrubes, M.; Nicola, T.L. Rehabilitation of the Patellofemoral Joint. Clin. Sports Med. 2014, 33, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Brotzman, B.S.; Manske, R.C. Clinical Orthopaedic Rehabilitation: An Evidence-Based Approach; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Rixe, J.A.; Glick, J.E.; Brady, J.; Olympia, R.P. A review of the management of patellofemoral pain syndrome. Phys. Sportsmed. 2013, 41, 19–28. [Google Scholar] [CrossRef]
- Kasitinon, D.; Li, W.X.; Wang, E.X.S.; Fredericson, M. Physical Examination and Patellofemoral Pain Syndrome: An Updated Review. Curr. Rev. Musculoskelet. Med. 2021, 14, 406–412. [Google Scholar] [CrossRef]
- Petersen, W.; Ellermann, A.; Gösele-Koppenburg, A.; Best, R.; Rembitzki, I.V.; Brüggemann, G.-P.; Liebau, C. Patellofemoral pain syndrome. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2264–2274. [Google Scholar] [CrossRef]
- Crossley, K.M.; Callaghan, M.J.; Linschoten, R.V. Patellofemoral pain. Br. J. Sports Med. 2016, 50, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Dutton, R.A.; Khadavi, M.J.; Fredericson, M. Update on Rehabilitation of Patellofemoral Pain. Curr. Sports Med. Rep. 2014, 13, 172–178. [Google Scholar] [CrossRef]
- Drew, B.T.; Redmond, A.C.; Smith, T.O.; Penny, F.; Conaghan, P. Which patellofemoral joint imaging features are associated with patellofemoral pain? Systematic review and meta-analysis. Osteoarthr. Cartil. 2016, 24, 224–236. [Google Scholar] [CrossRef]
- Fischhoff, C.; Goorah, T.; Dowlut, S. Ultrasound measurements for patellofemoral pain syndrome: An inter-operator reliability study. Int. Musculoskelet. Med. 2015, 37, 59–67. [Google Scholar] [CrossRef]
- Pacini, P.; Martino, M.; Giuliani, L.; Santilli, G.; Agostini, F.; Del Gaudio, G.; Bernetti, A.; Mangone, M.; Paoloni, M.; Toscano, M.; et al. Patello-Femoral Pain Syndrome: Magnetic Resonance Imaging versus Ultrasound. Diagnostics 2023, 13, 1496. [Google Scholar] [CrossRef]
- Souza, R.B.; Draper, C.E.; Fredericson, M.; Powers, C.M. Femur rotation and patellofemoral joint kinematics: A weight-bearing magnetic resonance imaging analysis. J. Orthop. Sports Phys. Ther. 2010, 40, 277–285. [Google Scholar] [CrossRef]
- Garbagna, G.P.; Failoni, S.; Rampini, C.; Storti, L.; Gallazzi, M. L’imaging dell’articolazione femoro-rotulea. Arch. Ortop. Reumatol. 2008, 119, 5–6. [Google Scholar] [CrossRef]
- Logan, C.A.; Bhashyam, A.R.; Tisosky, A.J.; Haber, D.B.; Jorgensen, A.; Roy, A.; Provencher, M.T. Systematic Review of the Effect of Taping Techniques on Patellofemoral Pain Syndrome. Sports Health 2017, 9, 456–461. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; Curci, C.; Ferrara, M.; Losco, L.; Spalek, R.; Cisari, C.; Invernizzi, M.; Solaro, C. Efficacy of kinesio taping on hand functioning in patients with mild carpal tunnel syndrome. A double-blind randomized controlled trial. J. Hand Ther. 2021, 35, 605–612. [Google Scholar] [CrossRef]
- Chang, W.D.; Chen, F.C.; Lee, C.L.; Lin, H.Y.; Lai, P.T. Effects of Kinesio Taping versus McConnell Taping for Patellofemoral Pain Syndrome: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat Med. 2015, 2015, 471208. [Google Scholar] [CrossRef]
- Lan, T.Y.; Lin, W.P.; Jiang, C.C.; Chiang, H. Immediate effect and predictors of effectiveness of taping for patellofemoral pain syndrome: A prospective cohort study. Am. J. Sports Med. 2010, 38, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Lake, D.A.; Wofford, N.H. Effect of therapeutic modalities on patients with patellofemoral pain syndrome: A systematic review. Sports Health 2011, 3, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.O.; Drew, B.T.; Meek, T.H.; Clark, A.B. Knee orthoses for treating patellofemoral pain syndrome. Cochrane Database Syst. Rev. 2015, 2015, CD010513. [Google Scholar] [CrossRef]
- Lack, S.; Barton, C.; Sohan, O.; Crossley, K.; Morrissey, D. Proximal muscle rehabilitation is effective for patellofemoral pain: A systematic review with meta-analysis. Br. J. Sports Med. 2015, 49, 1365–1376. [Google Scholar] [CrossRef]
- Fischer, M.; Vialleron, T.; Laffaye, G.; Fourcade, P.; Hussein, T.; Chèze, L.; Deleu, P.-A.; Honeine, J.-L.; Yiou, E.; Delafontaine, A. Long-term effects of whole-body vibration on human gait: A systematic review and meta-analysis. Front. Neurol. 2019, 10, 627. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.P.; Mendonça, V.A.; Avelar, N.C.P.; da Fonseca, S.F.; Santos, J.M.; de Oliveira, A.C.C.; Tossige-Gomes, R.; Ribeiro, V.G.C.; Neves, C.D.C.; Balthazar, C.H.; et al. Whole body vibration training on muscle strength and brain-derived Neurotrophic factor levels in elderly woman with knee osteoarthritis: A randomized clinical trial study. Front. Physiol. 2019, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Khan, A.A.; Farooq, M. Effect of whole-body vibration on neuromuscular performance: A literature review. Work 2018, 59, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Anwer, S.; Alghadir, A.; Zafar, H.; Al-Eisa, E. Effect of whole body vibration training on quadriceps muscle strength in individuals with knee osteoarthritis: A systematic review and meta-analysis. Physiotherapy 2016, 102, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Alghadir, A.; Anwer, S.; Al-Eisa, E. Therapeutic effects of whole-body vibration training in knee osteoarthritis: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, X.; Yang, Y.; Yang, L.; Zhou, Y.; Liu, C.; Reinhardt, J.D.; He, C. Effects of whole body vibration on pain, stiffness and physical functions in patients with knee osteoarthritis: A systematic review and meta-analysis. Clin. Rehabil. 2015, 29, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.-Q.; Chen, B.-L.; Huang, L.-Y.; Liu, Y. Whole-body vibration exercise for knee osteoarthritis: A systematic review and meta-analysis. Evid. Based Complement. Alternat Med. 2015, 2015, 758147. [Google Scholar] [CrossRef]
- Corum, M.; Basoglu, C.; Yakal, S.; Sahinkaya, T.; Aksoy, C. Effects of whole body vibration training on isokinetic muscular performance, pain, function, and quality of life in female patients with patellofemoral pain: A randomized controlled trial. J. Musculoskelet. Neuronal Interact. 2018, 18, 473–484. [Google Scholar] [PubMed]
- Luo, J.; McNamara, B.P.; Moran, K. A portable vibrator for muscle performance enhancement by means of direct muscle-tendon stimulation. Med. Eng. Phys. 2005, 27, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Pamukoff, D.N.; Ryan, E.D.; Blackburn, J.T. The acute effects of local muscle vibration frequency on peak torque, rate of torque development, and EMG activity. J. Electromyogr. Kinesiol. 2014, 24, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Barati, K.; Esfandiari, E.; Kamyab, M.; Ebrahimi Takamjani, I.; Atlasi, R.; Parnianpour, M.; Yazdi, H.; Shahali, S.; Bidari, S. The effect of local muscle vibration on clinical and biomechanical parameters in people with knee osteoarthritis: A systematic review. Med. J. Islam. Repub. Iran 2021, 35, 124. [Google Scholar] [CrossRef]
- Gaitonde, D.Y.; Ericksen, A.; Robbins, R.C. Patellofemoral Pain Syndrome. Am. Fam. Physician 2019, 99, 88–94. [Google Scholar] [PubMed]
- Guerrero, P.; Li, X.; Patel, K.; Brown, M.; Busconi, B. Medial patellofemoral ligament injury patterns and associated pathology in lateral patella dislocation: An MRI study. BMC Sports Sci. Med. Rehabil. 2009, 1, 17. [Google Scholar] [CrossRef]
- Giurazza, G.; Caria, C.; Campi, S.; Franceschetti, E.; Papalia, G.F.; Basciani, S.; Zampoli, A.; Gregori, P.; Papalia, R.; Marinozzi, A. Femoral cartilage thickness measured on MRI varies among individuals: Time to deepen one of the principles of kinematic alignment in total knee arthroplasty. A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2024. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, E.; Fukuda, T.Y.; Sacramento, S.N.; Forgas, A.; Cohen, M.; Abdalla, R.J. A comparison of hip strength between sedentary females with and without patellofemoral pain syndrome. J. Orthop. Sports Phys. Ther. 2010, 40, 641–647. [Google Scholar] [CrossRef]
- Nijs, J.; Van Geel, C.; Van de Velde, B. Diagnostic value of five clinical tests in patellofemoral pain syndrome. Man. Ther. 2006, 11, 69–77. [Google Scholar] [CrossRef] [PubMed]
- van Linschoten, R.; van Middelkoop, M.; Berger, M.Y.; Heintjes, E.M.; Verhaar, J.A.; Willemsen, S.P.; Koes, B.W.; Bierma-Zeinstra, S.M. Supervised exercise therapy versus usual care for patellofemoral pain syndrome: An open label randomised controlled trial. BMJ 2009, 339, b4074. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of Digital Visual Analog Scale Pain Scoring with a Traditional Paper-based Visual Analog Scale in Adults. JAAOS: Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Lohmander, L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef]
- Ma, Y.T.; Dong, Y.L.; Wang, B.; Xie, W.P.; Huang, Q.M.; Zheng, Y.J. Dry needling on latent and active myofascial trigger points versus oral diclofenac in patients with knee osteoarthritis: A randomized controlled trial. BMC Musculoskelet. Disord. 2023, 24, 36. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Mancinelli, R.; Toniolo, L.; Cancellara, L.; Paoli, A.; Puglielli, C.; Iodice, P.; Doria, C.; Bosco, G.; D’Amelio, L.; et al. Effects of local vibrations on skeletal muscle trophism in elderly people: Mechanical, cellular, and molecular events. Int. J. Mol. Med. 2009, 24, 503–512. [Google Scholar] [CrossRef]
- Costantino, C.; Galuppo, L.; Romiti, D. Short-term effect of local muscle vibration treatment versus sham therapy on upper limb in chronic post-stroke patients: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Saggini, R.; Di Stefano, A.; Galati, V.; Panelli, E.; Valeri, M.; Di Pancrazio, L.; Iodice, P.; Bellomo, R. Long-Term Effectiveness of Combined Mechanotransduction Treatment in Jumper’s Knee. Eur. J. Inflamm. 2012, 10, 515–524. [Google Scholar] [CrossRef]
- McConnell, J.S. The management of chondromalacia patellae: A long term solution. Austr. J. Physiother. 1986, 32, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Thomeé, R.; Grimby, G.; Wright, B.D.; Linacre, J.M. Rasch analysis of Visual Analog Scale measurements before and after treatment of Patellofemoral Pain Syndrome in women. Scand. J. Rehabil. Med. 1995, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Botta, A.F.B.; Waiteman, M.C.; Ducatti, M.H.M.; Garcia, C.L.G.; Farinelli, L.A.L.B.; Bazett-Jones, D.M.; Briani, R.V.; de Azevedo, F.M. Patellofemoral pain over time: Protocol for a prospective, longitudinal study investigating physical and non-physical features. Front. Sports Act. Living 2023, 4, 1081943. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, J.; Wang, X.; Ren, Z. The effect of foot orthoses for patients with patellofemoral pain syndrome: A systematic review and meta-analysis. Heliyon 2022, 8, e09656. [Google Scholar] [CrossRef] [PubMed]
- Rathleff, M.S.; Roos, E.M.; Olesen, J.L.; Rasmussen, S.; Arendt-Nielsen, L. Lower mechanical pressure pain thresholds in female adolescents with patellofemoral pain syndrome. J. Orthop. Sports Phys. Ther. 2013, 43, 414–421. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Grassi, A.; Rotini, M.; Raggi, F.; di Sarsina, T.R. Nuovi principi di anatomia e biomeccanica della femoro-rotulea. Scalpello 2016, 30, 86–91. [Google Scholar] [CrossRef]
- Beaconsfield, T.; Pintore, E.; Maffulli, N.; Petri, G.J. Radiological measurements in patellofemoral disorders: A review. Clin. Orthop. 1994, 308, 18–28. [Google Scholar] [CrossRef]
- Ostermeier, S.; Holst, M.; Bohnsack, M.; Hurschler, C.; Stukenborg-Colsman, C.; Wirth, C.-J. In vitro measurement of patellar kinematics following reconstruction of the medial patellofemoral ligament. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Frings, J.; Dust, T.; Meyer, J.; Krause, M.; Frosch, K.-H.; Adam, G.; Henes, F.O.; Spink, C.; Maas, K.-J. The Influence of Surgical Realignment Procedures on Dynamic Patellar Tracking: A Dynamic Magnetic Resonance Imaging-Controlled Feasibility Study. Diagnostics 2022, 12, 2761. [Google Scholar] [CrossRef] [PubMed]
- Voleti, P.B.; Stephenson, J.W.; Lotke, P.A.; Lee, G.C. No Sex Differences Exist in Posterior Condylar Offsets of the Knee. Clin. Orthop. Relat. Res. 2015, 473, 1425–1431. [Google Scholar] [CrossRef]
- Krebs, C.; Tranovich, M.; Andrews, K.; Ebraheim, N. The medial patellofemoral ligament: Review of the literature. J. Orthop. 2018, 15, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Mangone, M.; Diko, A.; Giuliani, L.; Agostini, F.; Paoloni, M.; Bernetti, A.; Santilli, G.; Conti, M.; Savina, A.; Iudicelli, G.; et al. A Machine Learning Approach for Knee Injury Detection from Magnetic Resonance Imaging. Int. J. Environ. Res. Public Health 2023, 20, 6059. [Google Scholar] [CrossRef]
- Agostini, F.; de Sire, A.; Bernetti, A.; Damiani, C.; Santilli, G.; Alessio, G.; Ammendolia, A.; Paoloni, M.; Mangone, M. Effectiveness of Kinesiotaping and McConnell taping combined with physical exercise on gait biomechanics in patients with patellofemoral syndrome: Non-randomized clinical trial. Clin. Ter. 2023, 174, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Shenkin, S.D.; Harrison, J.K.; Wilkinson, T.; Dodds, R.M.; Ioannidis, J.P.A. Systematic reviews: Guidance relevant for studies of older people. Age Ageing 2017, 46, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Wageck, B.; Nunes, G.S.; Bohlen, N.B.; Santos, G.M.; de Noronha, M. Kinesio Taping does not improve the symptoms or function of older people with knee osteoarthritis: A randomised trial. J. Physiother. 2016, 62, 153–158. [Google Scholar] [CrossRef]
- Kauffmann, R.M.; Hamner, J.B.; Ituarte, P.H.G.; Yim, J.H. Age greater than 60 years portends a worse prognosis in patients with papillary thyroid cancer: Should there be three age categories for staging? BMC Cancer 2018, 18, 316. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Dunkerley, S.; Silver, D.; Redfern, A.; Talbot, N.; Sharpe, I.; Guyver, P. Extracorporeal shockwave therapy (ESWT) for refractory Achilles tendinopathy: A prospective audit with 2-year follow up. Foot 2016, 26, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.; Segal, N.A.; Sluka, K.A.; Torner, J.C.; Nevitt, M.C.; Felson, D.T.; Bradley, L.A.; Neogi, T.; Lewis, C.E.; Frey-Law, L.A. Examining sex differences in knee pain: The multicenter osteoarthritis study. Osteoarthr. Cartil. 2014, 22, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Buntin-Mushock, C.; Phillip, L.; Moriyama, K.; Palmer, P.P. Age-Dependent Opioid Escalation in Chronic Pain Patients. Anesth. Analg. 2005, 100, 1740–1745. [Google Scholar] [CrossRef]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef]
- Collins, N.J.; Misra, D.; Felson, D.T.; Crossley, K.M.; Roos, E.M. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res. 2011, 63, S208–S228. [Google Scholar] [CrossRef]
- Mizner, R.L.; Petterson, S.C.; Stevens, J.E.; Axe, M.J.; Snyder-Mackler, L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J. Rheumatol. 2005, 32, 1533–1539. [Google Scholar] [PubMed]
- Ho, K.Y.; Chen, Y.J.; Farrokhi, S.; Tsai, L.C.; Liao, T.C.; Haas, N.; Powers, C.M. Selective Atrophy of the Vastus Medialis: Does It Exist in Women with Nontraumatic Patellofemoral Pain? Am. J. Sports Med. 2021, 49, 700–705. [Google Scholar] [CrossRef]
- Pattyn, E.; Verdonk, P.; Steyaert, A.; Vanden Bossche, L.; Van den Broecke, W.; Thijs, Y.; Witvrouw, E. Vastus medialis obliquus atrophy: Does it exist in patellofemoral pain syndrome? Am. J. Sports Med. 2011, 39, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.M.; Martel, G.F.; Ivey, F.M.; Lemmer, J.T.; Tracy, B.L.; Hurlbut, D.E.; Metter, E.J.; Hurley, B.F.; Rogers, M.A. Ultrastructural muscle damage in young vs. older men after high-volume, heavy-resistance. strength training. J. Appl. Physiol. 1999, 86, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.M.; Martel, G.F.; Ivey, F.M.; Lemmer, J.T.; Metter, E.J.; Hurley, B.F.; Rogers, M.A.; Lovering, R.M.; Brooks, S.V.; Takada, S.; et al. High-volume, heavy-resistance strength training and muscle damage in young and older women. J. Appl. Physiol. 2000, 88, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Melotti, R.M.; Fanelli, A.; Sorella, M.C. Gender and Pain. In Health and Gender; Tarricone, I., Riecher-Rössler, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Mogil, J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef]
- Racine, M.; Tousignant-Laflamme, Y.; Kloda, L.A.; Dion, D.; Dupuis, G.; Choinière, M. A systematic literature review of 10 years of research on sex/gender and pain perception—Part 2: Do biopsychosocial factors alter pain sensitivity differently in women and men? Pain 2012, 153, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B. Sex, gender, and pain: Women and men really are different. Curr. Rev. Pain 2000, 4, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Medial Patellofemoral Ligament. Available online: https://jrfortho.org/images/prod-files/Medial_Patellofemoral_Ligament_%28MPFL%29.pdf (accessed on 30 November 2024).
- Luhmann, S.J.; Schoenecker, P.L.; Dobbs, M.B.; Eric Gordon, J. Adolescent patellofemoral pain: Implicating the medial patellofemoral ligament as the main pain generator. J. Child. Orthop. 2008, 2, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Liphardt, A.M.; Mündermann, A.; Koo, S.; Bäcker, N.; Andriacchi, T.P.; Zange, J.; Mester, J.; Heer, M. Vibration training intervention to maintain cartilage thickness and serum concentrations of cartilage oligometric matrix protein (COMP) during immobilization. Osteoarthr. Cartil. 2009, 17, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hosseini, A.; Li, J.S.; Gill, T.J.; Li, G. Quantitative magnetic resonance imaging (MRI) morphological analysis of knee cartilage in healthy and anterior cruciate ligament-injured knees. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1496–1502. [Google Scholar] [CrossRef]
- Ekim, A.A.; Hamarat, H.; Musmul, A. Relationship Between Q-Angle and Articular Cartilage in Female Patients with Symptomatic Knee Osteoarthritis: Ultrasonographic and Radiologic Evaluation. Arch. Rheumatol. 2017, 32, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Freijo, V.; Navarro, C.; Villalba, J. Gait, Quality of Life, and Knee Function in Advanced Knee Osteoarthritis: A Single-Center, Prospective, Observational Study. J. Clin. Med. 2024, 13, 5392. [Google Scholar] [CrossRef]
- Powers, C.M.; Witvrouw, E.; Davis, I.S.; Crossley, K.M. Evidence-based framework for a pathomechanical model of patellofemoral pain 2017 patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester, UK, part 3. Br. J. Sports Med. 2017, 51, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.O.; Bowyer, D.; Dixon, J.; Stephenson, R.; Chester, R.; Donell, S.T. Can vastus medialis oblique be preferentially activated? A systematic review of electromyographic studies. Physiother. Theory Pract. 2009, 25, 69–98. [Google Scholar] [CrossRef]
- Stephen, J.; Alva, A.; Lumpaopong, P.; Williams, A.; Amis, A.A. A cadaveric model to evaluate the effect of unloading the medial quadriceps on patellar tracking and patellofemoral joint pressure and stability. J. Exp. Orthop. 2018, 5, 34. [Google Scholar] [CrossRef]
- Souza, D.R.; Gross, M.T. Comparison of Vastus Medialis Obliquus:Vastus Lateralis Muscle Integrated Electromyographic Ratios Between Healthy Subjects and Patients with Patellofemoral Pain. Phys. Ther. 1991, 71, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Boccia, G.; Cavazzuti, L.; Magnani, E.; Mariani, E.; Rainoldi, A.; Casale, R. Localized muscle vibration reverses quadriceps muscle hypotrophy and improves physical function: A clinical and electrophysiological study. Int. J. Rehabil. Res. 2017, 40, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Iodice, P.; Bellomo, R.G.; Gialluca, G.; Fanò, G.; Saggini, R. Acute and cumulative effects of focused high-frequency vibrations on the endocrine system and muscle strength. Eur. J. Appl. Physiol. 2011, 111, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Lundeberg, T.; Nordemar, R.; Ottoson, D. Pain alleviation by vibratory stimulation. Pain 1984, 20, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Henry, J.L. Differential responses of nociceptive vs. non-nociceptive spinal dorsal horn neurones to cutaneously applied vibration in the cat. Pain 1990, 40, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Henry, J.L. Physiological characteristics of responses of wide dynamic range spinal neurones to cutaneously applied vibration in the cat. Brain Res. 1990, 507, 69–84. [Google Scholar] [CrossRef]
- Staud, R.; Robinson, M.E.; Goldman, C.T.; Price, D.D. Attenuation of experimental pain by vibro-tactile stimulation in patients with chronic local or widespread musculoskeletal pain. Eur. J. Pain 2011, 15, 836–842. [Google Scholar] [CrossRef]
- Lehane, S.; O’Gorman, S.; Spink, K.; Agouris, I. Effects of Kinesiology tape (KT) on VMO activation and on proprioception at the knee in adults with anterior knee pain. Gait Posture 2017, 57, 306–307. [Google Scholar] [CrossRef]
- Maghbouli, N.; Khodadost, M.; Pourhassan, S. The effectiveness of vibration therapy for muscle peak torque and postural control in individuals with anterior cruciate ligament reconstruction: A systematic review and meta-analysis of clinical trials. J. Orthop. Traumatol. 2021, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Santilli, G.; Ioppolo, F.; Mangone, M.; Agostini, F.; Bernetti, A.; Forleo, S.; Cazzolla, S.; Mannino, A.C.; Fricano, A.; Franchitto, A.; et al. High Versus Low-Energy Extracorporeal Shockwave Therapy for Chronic Lateral Epicondylitis: A Retrospective Study. J. Funct. Morphol. Kinesiol. 2024, 9, 173. [Google Scholar] [CrossRef]
- Verstraelen, F.U.; In den Kleef, N.J.; Jansen, L.; Morrenhof, J.W. High-energy versus low-energy extracorporeal shock wave therapy for calcifying tendinitis of the shoulder: Which is superior? A meta-analysis. Clin. Orthop. Relat. Res. 2014, 472, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Bartel, L.; Mosabbir, A. Possible Mechanisms for the Effects of Sound Vibration on Human Health. Healthcare 2021, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.M.; Serrador, L.; da Silva, M.V.; Macedo, C.S.; Santos, C.P. Knee landmarks detection via deep learning for automatic imaging evaluation of trochlear dysplasia and patellar height. Eur. Radiol. 2024, 34, 5736–5747. [Google Scholar] [CrossRef] [PubMed]
- Santilli, G.; Mangone, M.; Agostini, F.; Paoloni, M.; Bernetti, A.; Diko, A.; Tognolo, L.; Coraci, D.; Vigevano, F.; Vetrano, M.; et al. Evaluation of Rehabilitation Outcomes in Patients with Chronic Neurological Health Conditions Using a Machine Learning Approach. J. Funct. Morphol. Kinesiol. 2024, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Karimzadehfini, A.; Mahdavinejad, R.; Zolaktaf, V.; Vahdatpour, B. Forecasting of rehabilitation treatment in sufferers from lateral displacement of patella using artificial intelligence. Sport Sci. Health 2018, 14, 37–45. [Google Scholar] [CrossRef]
- Santilli, G.; Vetrano, M.; Mangone, M.; Agostini, F.; Bernetti, A.; Coraci, D.; Paoloni, M.; de Sire, A.; Paolucci, T.; Latini, E.; et al. Predictive Prognostic Factors in Non-Calcific Supraspinatus Tendinopathy Treated with Focused Extracorporeal Shock Wave Therapy: An Artificial Neural Network Approach. Life 2024, 14, 681. [Google Scholar] [CrossRef]
- Ng, G.Y.; Fung, D.T. The combined treatment effects of therapeutic laser and exercise on tendon repair. Photomed. Laser Surg. 2008, 26, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Bernetti, A.; Santilli, G.; Paoloni, M.; Santilli, V.; Mangone, M. Laser and thermal therapy in athletes’ tennis elbow: An observational study. Med. Sport 2022, 75, 238–247. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Y. The therapeutic effect of extracorporeal shock wave therapy combined with Kinesio Tape on plantar fasciitis. J. Back. Musculoskelet. Rehabil. 2023, 36, 1203–1211. [Google Scholar] [CrossRef]
- Agostini, F.; Bernetti, A.; Santilli, G.; Damiani, C.; Santilli, V.; Paoloni, M.; Mangone, M. Efficacy of ultrasound therapy combined with cryotherapy in pain management and rehabilitation in patients with Achilles tendinopathy: A retrospective observational study. Clin. Ter. 2023, 174, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; St Louis, M.; Fournier, M. Vibration and pressure wave therapy for calf strains: A proposed treatment. Muscles Ligaments Tendons J. 2013, 3, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Delia, C.; Santilli, G.; Colonna, V.; Di Stasi, V.; Latini, E.; Ciccarelli, A.; Taurone, S.; Franchitto, A.; Santoboni, F.; Trischitta, D.; et al. Focal Versus Combined Focal Plus Radial Extracorporeal Shockwave Therapy in Lateral Elbow Tendinopathy: A Retrospective Study. J. Funct. Morphol. Kinesiol. 2024, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Goebel, R.; Haddad, M.; Kleinöder, H.; Yue, Z.; Heinen, T.; Mester, J. Does combined strength training and local vibration improve isometric maximum force? A pilot study. Muscles Ligaments Tendons J. 2017, 7, 186–191. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| Age (years) | 66 ± 12.2 |
| VAS T0 | 6 ± 1.4 |
| VAS T1 | 2.8 ± 1.9 |
| KOOS T0 | 45.6% ± 9.6% |
| KOOS T1 | 72.7% ± 15.3% |
| Gender (Male/Female) | 14/38 |
| Patellar Alignment (Normal) | 15 (28.8%) |
| Patellar Alignment (Tilt/Hyperpressure) | 24 (46.2%) |
| Patellar Alignment (Subluxation/Dislocation) | 13 (25%) |
| Medial patellofemoral ligament thickness | 2 ± 0.7 |
| Cartilages Medial Thickness | 1.8 ± 0.4 |
| Cartilages Lateral Thickness | 2 ± 0.5 |
| Medial Meniscus Binay Value (healthy/injured) | 35/17 |
| Lateral Meniscus Binay Value (healthy/injured) | 41/11 |
| Age Group 1 < 60 | 13 |
| Age Group 2 ≥ 60 | 39 |
| Delta KOOS Group 1 < 59% | 11 |
| Delta KOOS Group 2 ≥ 60% | 41 |
| Age Group | VAS T0 (Mean ± SD) | VAS T1 (Mean ± SD) | p-Value (Time) | Interaction Effect (F, p, η2) |
|---|---|---|---|---|
| <60 years | 5.6 ± 1.3 | 1.8 ± 1.3 | <0.001 | F(1, 50) = 3.71, p < 0.05, η2 = 0.07 |
| ≥60 years | 6 ± 1.4 | 3.2 ± 2 | <0.01 |
| Delta KOOS Group | VAS T0 (Mean ± SD) | VAS T1 (Mean ± SD) | p-Value (Time) | Interaction Effect (F, p, η2) |
|---|---|---|---|---|
| Group 1 < 59% | 6.6 ± 1.1 | 2.7 ± 1.2 | <0.001 | F (1, 50) = 4.3, p < 0.05, η2 = 0.08 |
| Group 2 ≥ 60% | 5.7 ± 1.4 | 2.9 ± 2 | <0.01 |
| Patellar Alignment Group | VAS T0 (Mean ± SD) | VAS T1 (Mean ± SD) | p-Value (Time) | Interaction Effect (F, p, η2) | Post Hoc |
|---|---|---|---|---|---|
| Neutral (Group 0) | 5.3 ± 1.1 | 3.1 ± 2 | 0.01 | F(2, 49) = 3.83, p = 0.028, η2 = 0.135 | Group 2 vs. Group 0 (p < 0.05) |
| Hyperpressure (Group 1) | 6.1 ± 1.4 | 2.8 ± 2 | <0.001 | Group 2 vs. Group 1 (p < 0.05) | |
| Subluxation/Dislocation (Group 2) | 6.2 ± 1.5 | 2.5 ± 1.6 | 0.02 |
| Age Group | VAS T0 (Mean ± SD) | VAS T1 (Mean ± SD) | p-Value (Time) | Interaction Effect (F, p, η2) |
|---|---|---|---|---|
| <60 years | 5.7 ± 1.6 | 1.3 ± 1.2 | <0.001 | F(1, 36) = 4.9, p < 0.03, η2 = 0.12 |
| ≥60 years | 6.2 ± 1.5 | 3.2 ± 2.1 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santilli, G.; Martino, M.; Pacini, P.; Agostini, F.; Bernetti, A.; Giuliani, L.; Del Gaudio, G.; Mangone, M.; Colonna, V.; Vetrano, M.; et al. Patellofemoral Pain Syndrome: Focused Vibrations Plus Kinesiotaping with Insights into Radiological Influences—An Observational Study. J. Funct. Morphol. Kinesiol. 2025, 10, 2. https://doi.org/10.3390/jfmk10010002
Santilli G, Martino M, Pacini P, Agostini F, Bernetti A, Giuliani L, Del Gaudio G, Mangone M, Colonna V, Vetrano M, et al. Patellofemoral Pain Syndrome: Focused Vibrations Plus Kinesiotaping with Insights into Radiological Influences—An Observational Study. Journal of Functional Morphology and Kinesiology. 2025; 10(1):2. https://doi.org/10.3390/jfmk10010002
Chicago/Turabian StyleSantilli, Gabriele, Milvia Martino, Patrizia Pacini, Francesco Agostini, Andrea Bernetti, Luca Giuliani, Giovanni Del Gaudio, Massimiliano Mangone, Vincenzo Colonna, Mario Vetrano, and et al. 2025. "Patellofemoral Pain Syndrome: Focused Vibrations Plus Kinesiotaping with Insights into Radiological Influences—An Observational Study" Journal of Functional Morphology and Kinesiology 10, no. 1: 2. https://doi.org/10.3390/jfmk10010002
APA StyleSantilli, G., Martino, M., Pacini, P., Agostini, F., Bernetti, A., Giuliani, L., Del Gaudio, G., Mangone, M., Colonna, V., Vetrano, M., Vulpiani, M. C., Stella, G., Ciccarelli, A., Taurone, S., Franchitto, A., Ottonello, C., Cantisani, V., Paoloni, M., Fiore, P., & Gimigliano, F. (2025). Patellofemoral Pain Syndrome: Focused Vibrations Plus Kinesiotaping with Insights into Radiological Influences—An Observational Study. Journal of Functional Morphology and Kinesiology, 10(1), 2. https://doi.org/10.3390/jfmk10010002









