Reversing Decline in Aging Muscles: Expected Trends, Impacts and Remedies
Abstract
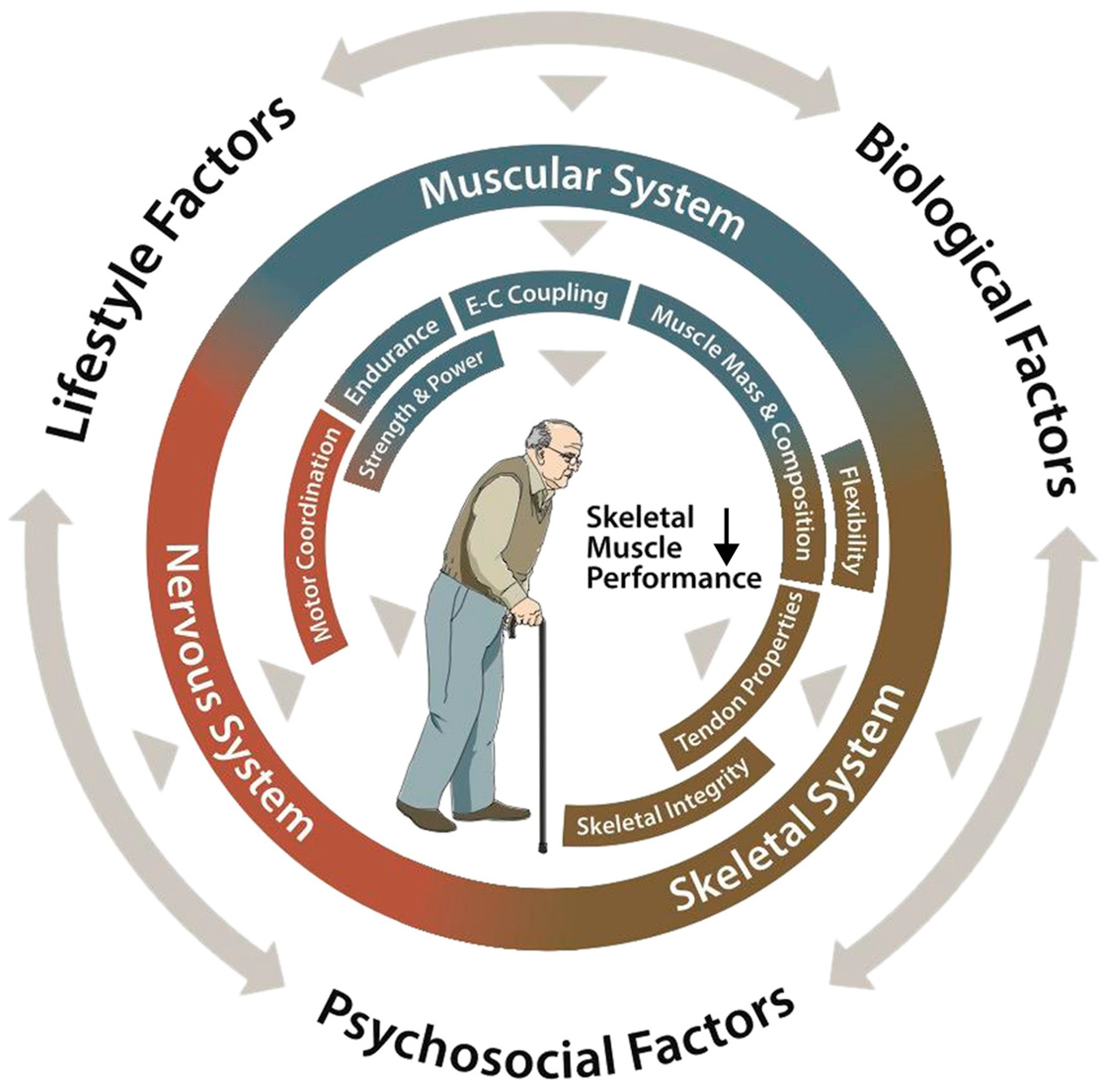
1. Introduction
2. The Effect of Aging on Physical Capacity
| System | Component | Changes | Rate/Magnitude | Association with Healthspan/QOL Measures | Intervention | Magnitude of Change | Mechanism of Adaptation |
|---|---|---|---|---|---|---|---|
| Muscular System | Muscle Mass | Overall muscle mass decline | Women: 0.37%/year Men: 0.47%/year Over 75 years: Women: 0.64–0.70%/year Men: 0.80–0.98%/year [6] | Correlation between thigh muscle area and telomere length [16] Association of appendicular lean mass normalized to body mass index and 10-year health-related quality of life [17] Muscle mass not associated with overall quality of life [18,19] Muscle mass correlated with physical vitality, emotional functioning, and physical functioning in older breast cancer survivors [20] | Strength training (2× per week for 24 weeks) | 3.8% [1.6%, 6.1%] increase in bone mineral-free lean tissue mass [21] | Activation of anabolic pathways [22] Satellite cell activation [23] Hormonal response [24] |
| Muscle Fibers | Type II (fast twitch) fiber reduction | 10–40% reduction in Type II fiber size [25] | In animal models, Type II muscle fibers help to regulate glucose metabolism [26] | Resistance training 3× per week for 12 weeks | 28% increase in area [27] | ||
| Type I (slow twitch) preservation | Decrease is much slower than for Type II [28] | Resistance training 3× per week for 12 weeks | Non-significant increase in Type I muscle fiber size [27] | ||||
| Muscle Function | Reduced force per unit area | Strong association between grip strength and mortality risk [29] Grip strength associated with level of independence in old age [30,31] | Leg press trained 3× per week for 12 weeks | 22% increase in leg press power [32] | |||
| Reduced motor unit firing | Maximum voluntary contraction (MVC) decreases by ~50 points or 9% per decade [33] Maximum firing rate 30–35% lower in older adults [34] | 6 weeks of resistance exercise | Maximal motor unit discharge rates were 49% higher for the older adults [35] | Increase in neural drive from CNS to activate muscle fibers [36] | |||
| 6 to 12 weeks of resistance training | Voluntary activation of knee extensors increased 1.8% following resistance training [37] | ||||||
| Impaired calcium handling | 33% reduction in calcium reuptake [38] | Unknown | Selenium supplementation and training | Improvement in calcium release in older mice [39] | Effect mediated through ryanodine receptor [39] and selenoprotein N [40] | ||
| Acute exercise training | Improvements in calcium release rate [41] | Modification of calcitropic hormone levels [42] | |||||
| 12 weeks of high-resistance strength training | Partial reversal of reduction in sarcoplasmic reticulum Ca 2+ uptake in skeletal muscle [38]. | ||||||
| Muscle Quality | Increased fat infiltration | Non-contractile area in leg anterior compartment approximately 2.5-fold larger in older subjects than in young subjects [43,44] | Arm fat mass index association with increased non-cardiovascular mortality [45] Low fat mass and high muscle mass associated with a 62% [32%,78%] decrease in total mortality [46] Skeletal muscle fat infiltration associated with higher all-cause and cardiovascular mortality [47] | 12-week resistance training program | 11% decrease in thigh intramuscular adipose tissue [48] | Increases fatty acid oxidation [49] Improves insulin sensitivity [50] Reduces adipogenic signaling [51] | |
| High-effort single-set exercise training 2×/week for 16 months | Fat infiltration stable in exercise group but increased in control group [52] | ||||||
| Greater fibrosis | Increase in tissue fibrosis observed in aging mouse model [53] Increase in muscle fibrosis biomarkers in humans [54] | Metformin | Lowered biomarkers of muscle fibrosis [55] | Inhibits TGF-β1 signaling, alters fate of myofibroblasts [56] | |||
| Nintedanib | Decreased expression of fibrotic genes [57] | Reduces proliferation and migration of fibroblasts [57], downregulation of extracellular matrix production [57], interference with profibrotic signaling [58] | |||||
| Resistance training and Dioscorea esculenta | Circulating levels of C1q, a biomarker associated with fibrosis, lower in experimental group [59] | Lowers C1q [59] | |||||
| Losartan | Decreases fibrosis and fibrotic biomarkers in animal models [60] | Interferes with TGF-β1 signaling [61] | |||||
| Suramin | Decreases fibrosis in animal models [60] | Reduces TGF-β signaling [62] | |||||
| Decorin | Decreases fibrosis in animal models [60] | Inhibits TGF-β [63] | |||||
| Halofuginone | Decreases fibrosis in animal models [60] | Inhibits collagen synthesis [64] | |||||
| Reduced sensitivity to anabolic stimuli | Decreased muscle protein synthesis after insulin infusion in older people compared to younger people [65] | N/A | Leucine | Leucine supplementation stimulates muscle protein synthesis [66] | Potent stimulator of mTORC1 and protein synthesis [67] | ||
| High protein intake | Doubling the recommended daily intake of protein increased muscle protein synthesis by 19% [68] | Increasing muscle protein synthesis [69] | |||||
| Pennation angle | Decrease in pennation angle | 4% decline per decade in vastus lateralis pennation angle [70] | No direct correlation, but pennation angle is correlated with age with r = −0.50 [70] | Leg press training for 10 weeks | 30% increase in pennation angle of vastus lateralis (VL) muscle [71] | Remodeling [72] | |
| Mitochondrial number | Decrease with age | Older participants (71+/−2 years) have 57% reduction in mitochondria compared to younger individuals (23+/−2 years) or 1.74% reduction per year. [73] | Association with VO2 max [74] | 30 to 60 min 3× per week for 4 months at moderate intensity (75% of maximum heartrate) | Increase in muscle mitochondrial density by 50.7% in exercise group [75] | Increases muscle GLUT−4 levels and insulin action [76,77] | |
| Energy expenditure at rest | Decreased basal metabolic rate | 4% decline per decade after 50 years of age [53,78] | Genetically predicted basal metabolic rate negatively associated with lifespan [79] | Fish oil with resistance training | Significant increase in resting metabolic rate [80] | Increases in the phosphorylation status of kinases related to the mTORC-1 signaling, increased muscle mass [81] | |
| Nutritional consultation and physical training | Increase in RMR [82] | Maximizes muscle protein synthesis (MPS) through the activation of mammalian target of rapamycin (mTOR) [83] | |||||
| Skeletal System | Bone Structure | Accelerated bone mineral density loss | Cortical zone in upper femoral neck declined by 6.4% per decade [84] | One standard deviation difference in bone mineral density is associated with a 1.39-fold increase in mortality [85] 1 SD increase in total hip BMD associated with a 0.77 [0.61,0.91] relative risk of overall mortality [86] | Physical exercise program | No decrease in bone mineral density of exercise group, decrease of 1.1% [0.1%,2.1%] in control group [87] | Activating mTORC1 and Mitogen-active protein kinases signaling for growth, repair, and adaptation [88] |
| Elastic modulus | 2.3% reduction per decade of life past age 35 [89] | Unknown | N/A | N/A | |||
| Bone strength | Reduction by 3.7% each decade after age 35 [89] | Unknown | Physical exercise | Reduction in fracture risk by 51% in the exercise group [90] | |||
| Fracture toughness | Kc: 4.1% reduction per decade after age 35 [89] | Unknown | |||||
| Work of fracture | 8.7% reduction per decade after age 35 [89] | Unknown | |||||
| Tendons | Reduced stiffness | Decreased maximum shortening velocity of tendons between older (75 years) and younger (20 years) adults by 16% [91] | Unknown | 14 weeks of high-load resistance training | Increase in tendon stiffness by 65% [92,93] | Tendon hypertrophy [94,95] | |
| Decreased shock absorption | F20 of occiput (inverse proxy for shock absorption capacity) roughly doubles between third and fifth decade of life, and between the fifth and seventh decade of life [96] | Decreased shock absorption capacity may contribute to greater risk from falls [97] | 14 weeks of high-load resistance training | Tendon Young’s modulus increased by 69% [92,93] | |||
| Function | Flexibility | 5–6 degree decline per decade in shoulder abduction [98] | Not associated with mortality [99]. | Dynamic and static stretching exercises for 12 weeks | Sit and reach test improvement by 23 +/−10% [100] | Stretching promotes the addition of sarcomeres in series, increasing the functional length of muscle fibers and improving the ability to generate an extended ROM [101], improved muscle–tendon unit (MTU) compliance [102,103] |
3. Interventions for Improving the Aging Musculoskeletal System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trombetti, A.; Reid, K.F.; Hars, M.; Herrmann, F.R.; Pasha, E.; Phillips, E.M.; Fielding, R.A. Age-Associated Declines in Muscle Mass, Strength, Power, and Physical Performance: Impact on Fear of Falling and Quality of Life. Osteoporos. Int. 2016, 27, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Lee, K. Population Aging and Fiscal Sustainability: Nonlinear Evidence from Europe. J. Int. Money Financ. 2022, 126, 102665. [Google Scholar] [CrossRef]
- Cristea, M.; Noja, G.G.; Stefea, P.; Sala, A.L. The Impact of Population Aging and Public Health Support on EU Labor Markets. Int. J. Environ. Res. Public Health 2020, 17, 1439. [Google Scholar] [CrossRef] [PubMed]
- Žokalj, M. The Impact of Population Aging on Public Finance in the European Union. Financ. Theory Pract. 2016, 40, 383–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Gupta, A.; Nicholson, S.; Li, J. Elevated End-of-Life Spending: A New Measure of Potentially Wasteful Health Care Spending at the End of Life. Health Serv. Res. 2023, 58, 186–194. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, Dynapenia, and the Impact of Advancing Age on Human Skeletal Muscle Size and Strength; a Quantitative Review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- Martel, G.F.; Roth, S.M.; Ivey, F.M.; Lemmer, J.T.; Tracy, B.L.; Hurlbut, D.E.; Metter, E.J.; Hurley, B.F.; Rogers, M.A. Age and Sex Affect Human Muscle Fibre Adaptations to Heavy-Resistance Strength Training. Exp. Physiol. 2006, 91, 457–464. [Google Scholar] [CrossRef]
- Koopman, R.; van Loon, L.J.C. Aging, Exercise, and Muscle Protein Metabolism. J. Appl. Physiol. 2009, 106, 2040–2048. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal Muscle Performance and Ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- Miri, S.; Farhadi, B.; Takasi, P.; Ghorbani Vajargah, P.; Karkhah, S. Physical Independence and Related Factors among Older Adults: A Systematic Review and Meta-Analysis. Ann. Med. Surg. 2024, 86, 3400. [Google Scholar] [CrossRef]
- Raichandani, K.; Agarwal, S.; Jain, H.; Bharwani, N. Mortality Profile after 2 Years of Hip Fractures in Elderly Patients Treated with Early Surgery. J. Clin. Orthop. Trauma 2021, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Xi, I.L.; Ahn, J.; Bernstein, J. Median Survival Following Geriatric Hip Fracture among 17,868 Males from the Veterans Health Administration. Front. Surg. 2023, 10, 1090680. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Killington, M.; Cameron, I.D.; Li, R.; Kurrle, S.; Crotty, M. Life Expectancy of Older People Living in Aged Care Facilities after a Hip Fracture. Sci. Rep. 2021, 11, 20266. [Google Scholar] [CrossRef] [PubMed]
- Morri, M.; Ambrosi, E.; Chiari, P.; Orlandi Magli, A.; Gazineo, D.; D’ Alessandro, F.; Forni, C. One-Year Mortality after Hip Fracture Surgery and Prognostic Factors: A Prospective Cohort Study. Sci. Rep. 2019, 9, 18718. [Google Scholar] [CrossRef] [PubMed]
- Sadaqa, M.; Németh, Z.; Makai, A.; Prémusz, V.; Hock, M. Effectiveness of Exercise Interventions on Fall Prevention in Ambulatory Community-Dwelling Older Adults: A Systematic Review with Narrative Synthesis. Front. Public Health 2023, 11, 1209319. [Google Scholar] [CrossRef]
- Marques, A.; Peralta, M.; Marconcin, P.; Henriques-Neto, D.; Gouveia, É.R.; Ferrari, G.; Martins, J.; Sarmento, H.; Ihle, A. A Systematic Review of the Association Between Muscular Fitness and Telomere Length Across the Adult Lifespan. Front. Physiol. 2021, 12, 706189. [Google Scholar] [CrossRef]
- Balogun, S.; Winzenberg, T.; Wills, K.; Scott, D.; Jones, G.; Callisaya, M.L.; Aitken, D. Prospective Associations of Low Muscle Mass and Strength with Health-Related Quality of Life over 10-Year in Community-Dwelling Older Adults. Exp. Gerontol. 2019, 118, 65–71. [Google Scholar] [CrossRef]
- da Costa Pereira, J.P.; Freire, Y.A.; da Silva, A.M.B.; de Lucena Alves, C.P.; de Melo Silva, R.; Câmara, M.; Browne, R.A.V.; Costa, E.C.; Trussardi Fayh, A.P. Associations of Upper- and Lower-Limb Muscle Strength, Mass, and Quality with Health-Related Quality of Life in Community-Dwelling Older Adults. Geriatr. Gerontol. Int. 2024, 24, 683–692. [Google Scholar] [CrossRef]
- Haider, S.; Luger, E.; Kapan, A.; Titze, S.; Lackinger, C.; Schindler, K.E.; Dorner, T.E. Associations between Daily Physical Activity, Handgrip Strength, Muscle Mass, Physical Performance and Quality of Life in Prefrail and Frail Community-Dwelling Older Adults. Qual. Life Res. 2016, 25, 3129–3138. [Google Scholar] [CrossRef]
- Morishita, S.; Kasahara, R.; Yamamoto, Y.; Jinbo, R.; Takano, A.; Yasuda, M.; Tsubaki, A.; Aoki, O.; Fu, J.B.; Tsuji, T. Differences in the Relationships Between Muscle Strength, Muscle Mass, Balance Function, and Quality of Life for Middle-Aged and Older Breast Cancer Survivors. Integr. Cancer Ther. 2022, 21, 15347354221138574. [Google Scholar] [CrossRef]
- Taaffe, D.R.; Duret, C.; Wheeler, S.; Marcus, R. Once-Weekly Resistance Exercise Improves Muscle Strength and Neuromuscular Performance in Older Adults. J. Am. Geriatr. Soc. 1999, 47, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C.; Akimoto, T.; Blaauw, B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. J. Neuromuscul. Dis. 2021, 8, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Kadi, F.; Charifi, N.; Denis, C.; Lexell, J.; Andersen, J.L.; Schjerling, P.; Olsen, S.; Kjaer, M. The Behaviour of Satellite Cells in Response to Exercise: What Have We Learned from Human Studies? Pflug. Arch.-Eur. J. Physiol. 2005, 451, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength Cond. Res. 2010, 24, 2857. [Google Scholar] [CrossRef]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J.C. The Decline in Skeletal Muscle Mass with Aging Is Mainly Attributed to a Reduction in Type II Muscle Fiber Size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Castorena, C.M.; Arias, E.B.; Sharma, N.; Bogan, J.S.; Cartee, G.D. Fiber Type Effects on Contraction-Stimulated Glucose Uptake and GLUT4 Abundance in Single Fibers from Rat Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E223–E230. [Google Scholar] [CrossRef]
- Kryger, A.I.; Andersen, J.L. Resistance Training in the Oldest Old: Consequences for Muscle Strength, Fiber Types, Fiber Size, and MHC Isoforms. Scand. J. Med. Sci. Sports 2007, 17, 422–430. [Google Scholar] [CrossRef]
- Miljkovic, N.; Lim, J.-Y.; Miljkovic, I.; Frontera, W.R. Aging of Skeletal Muscle Fibers. Ann. Rehabil. Med. 2015, 39, 155–162. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic Value of Grip Strength: Findings from the Prospective Urban Rural Epidemiology (PURE) Study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Carson, R.G. Get a Grip: Individual Variations in Grip Strength Are a Marker of Brain Health. Neurobiol. Aging 2018, 71, 189–222. [Google Scholar] [CrossRef]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife Hand Grip Strength as a Predictor of Old Age Disability. JAMA 1999, 281, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Earles, D.R.; Judge, J.O.; Gunnarsson, O.T. Velocity Training Induces Power-Specific Adaptations in Highly Functioning Older Adults. Arch. Phys. Med. Rehabil. 2001, 82, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.M.; Conwit, R.A.; Ferrucci, L.; Metter, E.J. Age-Associated Changes in Motor Unit Physiology: Observations from the Baltimore Longitudinal Study of Aging. Arch. Phys. Med. Rehabil. 2009, 90, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Kamen, G.; Sison, S.V.; Du, C.C.; Patten, C. Motor Unit Discharge Behavior in Older Adults during Maximal-Effort Contractions. J. Appl. Physiol. 1995, 79, 1908–1913. [Google Scholar] [CrossRef]
- Kamen, G.; Knight, C.A. Training-Related Adaptations in Motor Unit Discharge Rate in Young and Older Adults. J. Gerontol. Ser. A 2004, 59, 1334–1338. [Google Scholar] [CrossRef]
- Gabriel, D.A.; Kamen, G.; Frost, G. Neural Adaptations to Resistive Exercise: Mechanisms and Recommendations for Training Practices. Sports Med. 2006, 36, 133–149. [Google Scholar] [CrossRef]
- Arnold, P.; Bautmans, I. The Influence of Strength Training on Muscle Activation in Elderly Persons: A Systematic Review and Meta-Analysis. Exp. Gerontol. 2014, 58, 58–68. [Google Scholar] [CrossRef]
- Hunter, S.K.; Thompson, M.W.; Ruell, P.A.; Harmer, A.R.; Thom, J.M.; Gwinn, T.H.; Adams, R.D. Human Skeletal Sarcoplasmic Reticulum Ca2+ Uptake and Muscle Function with Aging and Strength Training. J. Appl. Physiol. 1999, 86, 1858–1865. [Google Scholar] [CrossRef]
- Fodor, J.; Al-Gaadi, D.; Czirják, T.; Oláh, T.; Dienes, B.; Csernoch, L.; Szentesi, P. Improved Calcium Homeostasis and Force by Selenium Treatment and Training in Aged Mouse Skeletal Muscle. Sci. Rep. 2020, 10, 1707. [Google Scholar] [CrossRef]
- Wesolowski, L.T.; Semanchik, P.L.; White-Springer, S.H. Beyond Antioxidants: Selenium and Skeletal Muscle Mitochondria. Front. Vet. Sci. 2022, 9, 1011159. [Google Scholar] [CrossRef]
- Gejl, K.D.; Andersson, E.P.; Nielsen, J.; Holmberg, H.-C.; Ørtenblad, N. Effects of Acute Exercise and Training on the Sarcoplasmic Reticulum Ca2+ Release and Uptake Rates in Highly Trained Endurance Athletes. Front. Physiol. 2020, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Maïmoun, L.; Sultan, C. Effect of Physical Activity on Calcium Homeostasis and Calciotropic Hormones: A Review. Calcif. Tissue Int. 2009, 85, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kragstrup, T.W.; Kjaer, M.; Mackey, A.L. Structural, Biochemical, Cellular, and Functional Changes in Skeletal Muscle Extracellular Matrix with Aging. Scand. J. Med. Sci. Sports 2011, 21, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A.; Ng, A.V.; Young, K. Skeletal Muscle Contractile and Noncontractile Components in Young and Older Women and Men. J. Appl. Physiol. 2000, 88, 662–668. [Google Scholar] [CrossRef]
- Guo, J.; Wei, Y.; Heiland, E.G.; Marseglia, A. Differential Impacts of Fat and Muscle Mass on Cardiovascular and Non-Cardiovascular Mortality in Individuals with Type 2 Diabetes. J. Cachexia Sarcopenia Muscle 2024, 15, 1930–1941. [Google Scholar] [CrossRef]
- Srikanthan, P.; Horwich, T.B.; Tseng, C.H. Relation of Muscle Mass and Fat Mass to Cardiovascular Disease Mortality. Am. J. Cardiol. 2016, 117, 1355–1360. [Google Scholar] [CrossRef]
- Miljkovic, I.; Kuipers, A.L.; Cauley, J.A.; Prasad, T.; Lee, C.G.; Ensrud, K.E.; Cawthon, P.M.; Hoffman, A.R.; Dam, T.-T.; Gordon, C.L.; et al. Greater Skeletal Muscle Fat Infiltration Is Associated with Higher All-Cause and Cardiovascular Mortality in Older Men. J. Gerontol. Ser. A 2015, 70, 1133–1140. [Google Scholar] [CrossRef]
- Marcus, R.L.; Addison, O.; Kidde, J.P.; Dibble, L.E.; Lastayo, P.C. Skeletal Muscle Fat Infiltration: Impact of Age, Inactivity, and Exercise. J. Nutr. Health Aging 2010, 14, 362–366. [Google Scholar] [CrossRef]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Turcotte, L.P.; Fisher, J.S. Skeletal Muscle Insulin Resistance: Roles of Fatty Acid Metabolism and Exercise. Phys. Ther. 2008, 88, 1279–1296. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Ghasemikaram, M.; Chaudry, O.; Nagel, A.M.; Uder, M.; Jakob, F.; Kemmler, W.; Kohl, M.; Engelke, K. Effects of 16 Months of High Intensity Resistance Training on Thigh Muscle Fat Infiltration in Elderly Men with Osteosarcopenia. GeroScience 2021, 43, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt Signaling during Aging Alters Muscle Stem Cell Fate and Increases Fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.; Caldow, M.K.; Watts, R.; Levinger, P.; Cameron-Smith, D.; Levinger, I. Age and Sex Differences in Human Skeletal Muscle Fibrosis Markers and Transforming Growth Factor-β Signaling. Eur. J. Appl. Physiol. 2017, 117, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Petrocelli, J.J.; McKenzie, A.I.; de Hart, N.M.M.P.; Reidy, P.T.; Mahmassani, Z.S.; Keeble, A.R.; Kaput, K.L.; Wahl, M.P.; Rondina, M.T.; Marcus, R.L.; et al. Disuse-Induced Muscle Fibrosis, Cellular Senescence, and Senescence-Associated Secretory Phenotype in Older Adults Are Alleviated during Re-Ambulation with Metformin Pre-Treatment. Aging Cell 2023, 22, e13936. [Google Scholar] [CrossRef]
- Wu, M.; Xu, H.; Liu, J.; Tan, X.; Wan, S.; Guo, M.; Long, Y.; Xu, Y. Metformin and Fibrosis: A Review of Existing Evidence and Mechanisms. J. Diabetes Res. 2021, 2021, 6673525. [Google Scholar] [CrossRef]
- Piñol-Jurado, P.; Suárez-Calvet, X.; Fernández-Simón, E.; Gallardo, E.; de la Oliva, N.; Martínez-Muriana, A.; Gómez-Gálvez, P.; Escudero, L.M.; Pérez-Peiró, M.; Wollin, L.; et al. Nintedanib Decreases Muscle Fibrosis and Improves Muscle Function in a Murine Model of Dystrophinopathy. Cell Death Dis. 2018, 9, 776. [Google Scholar] [CrossRef]
- Rangarajan, S.; Kurundkar, A.; Kurundkar, D.; Bernard, K.; Sanders, Y.Y.; Ding, Q.; Antony, V.B.; Zhang, J.; Zmijewski, J.; Thannickal, V.J. Novel Mechanisms for the Antifibrotic Action of Nintedanib. Am. J. Respir. Cell Mol. Biol. 2016, 54, 51–59. [Google Scholar] [CrossRef]
- Iemitsu, K.; Fujie, S.; Uchida, M.; Inoue, K.; Shinohara, Y.; Iemitsu, M. Dioscorea Esculenta Intake with Resistance Training Improves Muscle Quantity and Quality in Healthy Middle-Aged and Older Adults: A Randomized Controlled Trial. Nutrients 2023, 15, 2438. [Google Scholar] [CrossRef]
- Garg, K.; Corona, B.T.; Walters, T.J. Therapeutic Strategies for Preventing Skeletal Muscle Fibrosis after Injury. Front. Pharmacol. 2015, 6, 87. [Google Scholar] [CrossRef]
- Miguel-Carrasco, J.L.; Beaumont, J.; San José, G.; Moreno, M.U.; López, B.; González, A.; Zalba, G.; Díez, J.; Fortuño, A.; Ravassa, S. Mechanisms Underlying the Cardiac Antifibrotic Effects of Losartan Metabolites. Sci. Rep. 2017, 7, 41865. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-S.; Li, Y.; Foster, W.; Fu, F.H.; Huard, J. The Use of Suramin, an Antifibrotic Agent, to Improve Muscle Recovery after Strain Injury. Am. J. Sports Med. 2005, 33, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Zhu, J.; Sun, B.; Branca, M.; Tang, Y.; Foster, W.; Xiao, X.; Huard, J. Decorin Gene Transfer Promotes Muscle Cell Differentiation and Muscle Regeneration. Mol. Ther. 2007, 15, 1616–1622. [Google Scholar] [CrossRef]
- Turgeman, T.; Hagai, Y.; Huebner, K.; Jassal, D.S.; Anderson, J.E.; Genin, O.; Nagler, A.; Halevy, O.; Pines, M. Prevention of Muscle Fibrosis and Improvement in Muscle Performance in the Mdx Mouse by Halofuginone. Neuromuscul. Disord. 2008, 18, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.M.; Volpi, E.; Rasmussen, B.B. Exercise and Nutrition to Target Protein Synthesis Impairments in Aging Skeletal Muscle. Exerc. Sport Sci. Rev. 2013, 41, 216–223. [Google Scholar] [CrossRef]
- Deane, C.S.; Cox, J.; Atherton, P.J. Critical Variables Regulating Age-Related Anabolic Responses to Protein Nutrition in Skeletal Muscle. Front. Nutr. 2024, 11, 1419229. [Google Scholar] [CrossRef]
- Ham, D.J.; Caldow, M.K.; Lynch, G.S.; Koopman, R. Leucine as a Treatment for Muscle Wasting: A Critical Review. Clin. Nutr. 2014, 33, 937–945. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Schutzler, S.; Schrader, A.; Spencer, H.; Kortebein, P.; Deutz, N.E.P.; Wolfe, R.R.; Ferrando, A.A. Quantity of Dietary Protein Intake, but Not Pattern of Intake, Affects Net Protein Balance Primarily through Differences in Protein Synthesis in Older Adults. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E21–E28. [Google Scholar] [CrossRef]
- Garlick, P.J.; McNurlan, M.A.; Patlak, C.S. Adaptation of Protein Metabolism in Relation to Limits to High Dietary Protein Intake. Eur. J. Clin. Nutr. 1999, 53 (Suppl. S1), S34–S43. [Google Scholar] [CrossRef]
- Jacob, I.; Johnson, M.I.; Jones, G.; Jones, A.; Francis, P. Age-Related Differences of Vastus Lateralis Muscle Morphology, Contractile Properties, Upper Body Grip Strength and Lower Extremity Functional Capability in Healthy Adults Aged 18 to 70 Years. BMC Geriatr. 2022, 22, 538. [Google Scholar] [CrossRef]
- Franchi, M.V.; Atherton, P.J.; Reeves, N.D.; Flück, M.; Williams, J.; Mitchell, W.K.; Selby, A.; Beltran Valls, R.M.; Narici, M.V. Architectural, Functional and Molecular Responses to Concentric and Eccentric Loading in Human Skeletal Muscle. Acta Physiol. 2014, 210, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Blazevich, A.; Souza, N.; Celes, R.; Alex, S.; Tufano, J.J.; Bottaro, M. Acute Changes in Muscle Thickness and Pennation Angle in Response to Work-Matched Concentric and Eccentric Isokinetic Exercise. Appl. Physiol. Nutr. Metab. 2018, 43, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.D.; Devries, M.C.; Safdar, A.; Hamadeh, M.J.; Tarnopolsky, M.A. The Effect of Aging on Human Skeletal Muscle Mitochondrial and Intramyocellular Lipid Ultrastructure. J. Gerontol. Ser. A 2010, 65A, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.-C.; Saner, N.J.; Ferri, A.; García-Domínguez, E.; Broatch, J.R.; Bishop, D.J. Delineating the Contribution of Ageing and Physical Activity to Changes in Mitochondrial Characteristics across the Lifespan. Mol. Asp. Med. 2024, 97, 101272. [Google Scholar] [CrossRef] [PubMed]
- Broskey, N.T.; Greggio, C.; Boss, A.; Boutant, M.; Dwyer, A.; Schlueter, L.; Hans, D.; Gremion, G.; Kreis, R.; Boesch, C.; et al. Skeletal Muscle Mitochondria in the Elderly: Effects of Physical Fitness and Exercise Training. J. Clin. Endocrinol. Metab. 2014, 99, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zheng, J.; Liu, X.F.; Feng, Z.L.; Zhang, X.P.; Cao, L.L.; Zhou, Z.P. Exercise Improved Lipid Metabolism and Insulin Sensitivity in Rats Fed a High-Fat Diet by Regulating Glucose Transporter 4 (GLUT4) and Musclin Expression. Braz. J. Med. Biol. Res. 2016, 49, e5129. [Google Scholar] [CrossRef]
- Hughes, V.A.; Fiatarone, M.A.; Fielding, R.A.; Kahn, B.B.; Ferrara, C.M.; Shepherd, P.; Fisher, E.C.; Wolfe, R.R.; Elahi, D.; Evans, W.J. Exercise Increases Muscle GLUT-4 Levels and Insulin Action in Subjects with Impaired Glucose Tolerance. Am. J. Physiol.-Endocrinol. Metab. 1993, 264, E855–E862. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Ryu, J.Y.; Choi, H.Y.; Hong, H.C.; Yoo, H.J.; Kang, H.J.; Song, W.; Park, S.W.; Baik, S.H.; et al. Impact of Visceral Fat on Skeletal Muscle Mass and Vice Versa in a Prospective Cohort Study: The Korean Sarcopenic Obesity Study (KSOS). PLoS ONE 2014, 9, e115407. [Google Scholar] [CrossRef]
- Ng, J.C.M.; Schooling, C.M. Effect of Basal Metabolic Rate on Lifespan: A Sex-Specific Mendelian Randomization Study. Sci. Rep. 2023, 13, 7761. [Google Scholar] [CrossRef]
- Lee, S.-R.; Directo, D.; Khamoui, A.V. Fish Oil Administration Combined with Resistance Exercise Training Improves Strength, Resting Metabolic Rate, and Inflammation in Older Adults. Aging Clin. Exp. Res. 2022, 34, 3073–3081. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Lammes, E.; Rydwik, E.; Akner, G. Effects of Nutritional Intervention and Physical Training on Energy Intake, Resting Metabolic Rate and Body Composition in Frail Elderly. A Randomised, Controlled Pilot Study. J. Nutr. Health Aging 2012, 16, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Dreyer, H.C.; Drummond, M.J.; Glynn, E.L.; Cadenas, J.G.; Yoshizawa, F.; Volpi, E.; Rasmussen, B.B. Nutrient Signalling in the Regulation of Human Muscle Protein Synthesis. J. Physiol. 2007, 582, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, P.M.; Thomas, C.D.; Clement, J.G.; Loveridge, N.; Beck, T.J.; Bonfield, W.; Burgoyne, C.J.; Reeve, J. Relation between Age, Femoral Neck Cortical Stability, and Hip Fracture Risk. Lancet 2005, 366, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Black, D.; Johnell, O.; Odén, A.; Mellström, D. Bone Mineral Density Is a Predictor of Survival. Calcif. Tissue Int. 1998, 63, 190–196. [Google Scholar] [CrossRef]
- Trivedi, D.P.; Khaw, K.T. Bone Mineral Density at the Hip Predicts Mortality in Elderly Men. Osteoporos. Int. 2001, 12, 259–265. [Google Scholar] [CrossRef]
- Korpelainen, R.; Keinänen-Kiukaanniemi, S.; Heikkinen, J.; Väänänen, K.; Korpelainen, J. Effect of Impact Exercise on Bone Mineral Density in Elderly Women with Low BMD: A Population-Based Randomized Controlled 30-Month Intervention. Osteoporos. Int. 2006, 17, 109–118. [Google Scholar] [CrossRef]
- Thomas, A.C.Q.; Stead, C.A.; Burniston, J.G.; Phillips, S.M. Exercise-Specific Adaptations in Human Skeletal Muscle: Molecular Mechanisms of Making Muscles Fit and Mighty. Free Radic. Biol. Med. 2024, 223, 341–356. [Google Scholar] [CrossRef]
- Zioupos, P.; Currey, J.D. Changes in the Stiffness, Strength, and Toughness of Human Cortical Bone with Age. Bone 1998, 22, 57–66. [Google Scholar] [CrossRef]
- Kemmler, W.; Häberle, L.; von Stengel, S. Effects of Exercise on Fracture Reduction in Older Adults. Osteoporos. Int. 2013, 24, 1937–1950. [Google Scholar] [CrossRef]
- Narici, M.V.; Maffulli, N.; Maganaris, C.N. Ageing of Human Muscles and Tendons. Disabil. Rehabil. 2008, 30, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Grosset, J.-F.; Breen, L.; Stewart, C.E.; Burgess, K.E.; Onambélé, G.L. Influence of Exercise Intensity on Training-Induced Tendon Mechanical Properties Changes in Older Individuals. AGE 2014, 36, 9657. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.D.; Maganaris, C.N.; Narici, M.V. Effect of Strength Training on Human Patella Tendon Mechanical Properties of Older Individuals. J. Physiol. 2003, 548, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, G.; Bohm, S.; Peper, K.K.; Arampatzis, A.; Legerlotz, K. Evidence-Based High-Loading Tendon Exercise for 12 Weeks Leads to Increased Tendon Stiffness and Cross-Sectional Area in Achilles Tendinopathy: A Controlled Clinical Trial. Sports Med.-Open 2022, 8, 149. [Google Scholar] [CrossRef]
- Jerger, S.; Centner, C.; Lauber, B.; Seynnes, O.; Friedrich, T.; Lolli, D.; Gollhofer, A.; König, D. Specific Collagen Peptides Increase Adaptions of Patellar Tendon Morphology Following 14-Weeks of High-Load Resistance Training: A Randomized-Controlled Trial. Eur. J. Sport Sci. 2023, 23, 2329–2339. [Google Scholar] [CrossRef]
- Brzuszkiewicz-Kuźmicka, G.; Szczegielniak, J.; Bączkowicz, D. Age-Related Changes in Shock Absorption Capacity of the Human Spinal Column. Clin. Interv. Aging 2018, 13, 987–993. [Google Scholar] [CrossRef]
- Santello, M. Review of Motor Control Mechanisms Underlying Impact Absorption from Falls. Gait Posture 2005, 21, 85–94. [Google Scholar] [CrossRef]
- Stathokostas, L.; McDonald, M.W.; Little, R.M.D.; Paterson, D.H. Flexibility of Older Adults Aged 55–86 Years and the Influence of Physical Activity. J. Aging Res. 2013, 2013, e743843. [Google Scholar] [CrossRef]
- Perrone, F.; Jommi, C.; Di Maio, M.; Gimigliano, A.; Gridelli, C.; Pignata, S.; Ciardiello, F.; Nuzzo, F.; de Matteis, A.; Del Mastro, L.; et al. The Association of Financial Difficulties with Clinical Outcomes in Cancer Patients: Secondary Analysis of 16 Academic Prospective Clinical Trials Conducted in Italy†. Ann. Oncol. 2016, 27, 2224–2229. [Google Scholar] [CrossRef]
- Greca, S.L.; Rapali, M.; Ciaprini, G.; Russo, L.; Vinciguerra, M.G.; Giminiani, R.D. Acute and Chronic Effects of Supervised Flexibility Training in Older Adults: A Comparison of Two Different Conditioning Programs. Int. J. Environ. Res. Public Health 2022, 19, 16974. [Google Scholar] [CrossRef]
- Warneke, K.; Keiner, M.; Wohlann, T.; Lohmann, L.H.; Schmitt, T.; Hillebrecht, M.; Brinkmann, A.; Hein, A.; Wirth, K.; Schiemann, S. Influence of Long-Lasting Static Stretching Intervention on Functional and Morphological Parameters in the Plantar Flexors: A Randomized Controlled Trial. J. Strength Cond. Res. 2023, 37, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Panidi, I.; Bogdanis, G.C.; Terzis, G.; Donti, A.; Konrad, A.; Gaspari, V.; Donti, O. Muscle Architectural and Functional Adaptations Following 12-Weeks of Stretching in Adolescent Female Athletes. Front. Physiol. 2021, 12, 701338. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; Cè, E.; Bisconti, A.V.; Rampichini, S.; Doria, C.; Borrelli, M.; Limonta, E.; Coratella, G.; Esposito, F. The Effects of 12 Weeks of Static Stretch Training on the Functional, Mechanical, and Architectural Characteristics of the Triceps Surae Muscle–Tendon Complex. Eur. J. Appl. Physiol. 2021, 121, 1743–1758. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhang, S.; Liu, Y.; Gang, X.; Wang, G. Grip Strength and the Risk of Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis of Longitudinal Cohort Studies. Front. Aging Neurosci. 2021, 13, 625551. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jazwinski, S.M. Quantitative Measures of Healthy Aging and Biological Age. Healthy Aging Res. 2015, 4, 26. [Google Scholar] [CrossRef]
- Hirase, T.; Okubo, Y.; Delbaere, K.; Menant, J.C.; Lord, S.R.; Sturnieks, D.L. Risk Factors for Falls and Fall-Related Fractures in Community-Living Older People with Pain: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 6040. [Google Scholar] [CrossRef]
- Runge, M.; Hunter, G. Determinants of Musculoskeletal Frailty and the Risk of Falls in Old Age. J. Musculoskelet. Neuronal Interact. 2006, 6, 167–173. [Google Scholar]
- Zech, A.; Hübscher, M.; Vogt, L.; Banzer, W.; Hänsel, F.; Pfeifer, K. Balance Training for Neuromuscular Control and Performance Enhancement: A Systematic Review. J. Athl. Train. 2010, 45, 392–403. [Google Scholar] [CrossRef]
- Araújo, C.G.S.D. Flexibility Assessment: Normative Values for Flexitest from 5 to 91 Years of Age. Arq. Bras. Cardiol. 2008, 90, 280–287. [Google Scholar] [CrossRef]
- Polsgrove, M.J.; Eggleston, B.M.; Lockyer, R.J. Impact of 10-Weeks of Yoga Practice on Flexibility and Balance of College Athletes. Int. J. Yoga 2016, 9, 27. [Google Scholar] [CrossRef]
- Duizer, D. Cardiorespiratory Fitness Assessment and Treatment for Health Span and Lifespan. CAND J. 2020, 27, 22–25. [Google Scholar] [CrossRef]
- Costa, J.N.A.; Ribeiro, A.L.A.; Ribeiro, D.B.G.; Neri, S.G.R.; Barbosa, D.F.; Avelar, B.P.; Safons, M.P. Balance Exercise Circuit for Fall Prevention in Older Adults: A Randomized Controlled Crossover Trial. J. Frailty Sarcopenia Falls 2022, 7, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Stathokostas, L.; Little, R.M.D.; Vandervoort, A.A.; Paterson, D.H. Flexibility Training and Functional Ability in Older Adults: A Systematic Review. J. Aging Res. 2012, 2012, 306818. [Google Scholar] [CrossRef] [PubMed]
- Ghanvatkar, S.; Kankanhalli, A.; Rajan, V. User Models for Personalized Physical Activity Interventions: Scoping Review. JMIR mHealth uHealth 2019, 7, e11098. [Google Scholar] [CrossRef] [PubMed]
- Davergne, T.; Meidinger, P.; Dechartres, A.; Gossec, L. The Effectiveness of Digital Apps Providing Personalized Exercise Videos: Systematic Review with Meta-Analysis. J. Med. Internet Res. 2023, 25, e45207. [Google Scholar] [CrossRef]
- Monroe, C.M.; Cai, B.; Edney, S.; Jake-Schoffman, D.E.; Brazendale, K.; Bucko, A.; Armstrong, B.; Yang, C.-H.; Turner-McGrievy, G. Harnessing Technology and Gamification to Increase Adult Physical Activity: A Cluster Randomized Controlled Trial of the Columbia Moves Pilot. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 129. [Google Scholar] [CrossRef]
- Halma, M.; Marik, P. Preventing Functional Decline with Age: Biomarkers and Best Practices-[V1]. Preprints 2024. [Google Scholar] [CrossRef]
| Training Type | Trend (Absent Training) | System | Associated Tests | Training | Adaptations |
|---|---|---|---|---|---|
| Strength Training | Sarcopenia, muscle loss, bone loss | Musculoskeletal | Grip strength [104] | Weightlifting | Increase in muscle mass and bone density |
| Endurance training | Lower VO2 max | Metabolic, cardiopulmonary | Resting metabolic rate, creatine phosphokinase [105] | Running, swimming, walking, cycling, cross-country skiing, hiking, etc. | Increased mitochondrial size, greater ability to metabolize fat, increased (heart) stroke volume |
| Balance training | Poorer coordination | Musculoskeletal, nervous | Self-selected gait velocity [106], chair rise test (timed 5 chair rises), tandem standing and walking, timed up and go test, clinical gait analysis with special focus on regularity, mechanography [107] | Yoga | Neuromuscular control [108] |
| Flexibility | Decrease in joint flexion [98,109] | Musculoskeletal, tendons, fascia | Flexibility tests: Flexindex [109] | Yoga, pilates | Improved flexibility and stability [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halma, M.; Marik, P.; Varon, J.; Tuszynski, J. Reversing Decline in Aging Muscles: Expected Trends, Impacts and Remedies. J. Funct. Morphol. Kinesiol. 2025, 10, 29. https://doi.org/10.3390/jfmk10010029
Halma M, Marik P, Varon J, Tuszynski J. Reversing Decline in Aging Muscles: Expected Trends, Impacts and Remedies. Journal of Functional Morphology and Kinesiology. 2025; 10(1):29. https://doi.org/10.3390/jfmk10010029
Chicago/Turabian StyleHalma, Matthew, Paul Marik, Joseph Varon, and Jack Tuszynski. 2025. "Reversing Decline in Aging Muscles: Expected Trends, Impacts and Remedies" Journal of Functional Morphology and Kinesiology 10, no. 1: 29. https://doi.org/10.3390/jfmk10010029
APA StyleHalma, M., Marik, P., Varon, J., & Tuszynski, J. (2025). Reversing Decline in Aging Muscles: Expected Trends, Impacts and Remedies. Journal of Functional Morphology and Kinesiology, 10(1), 29. https://doi.org/10.3390/jfmk10010029







