Effects of Tactile Sensory Stimulation Training of the Trunk and Sole on Standing Balance Ability in Older Adults: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
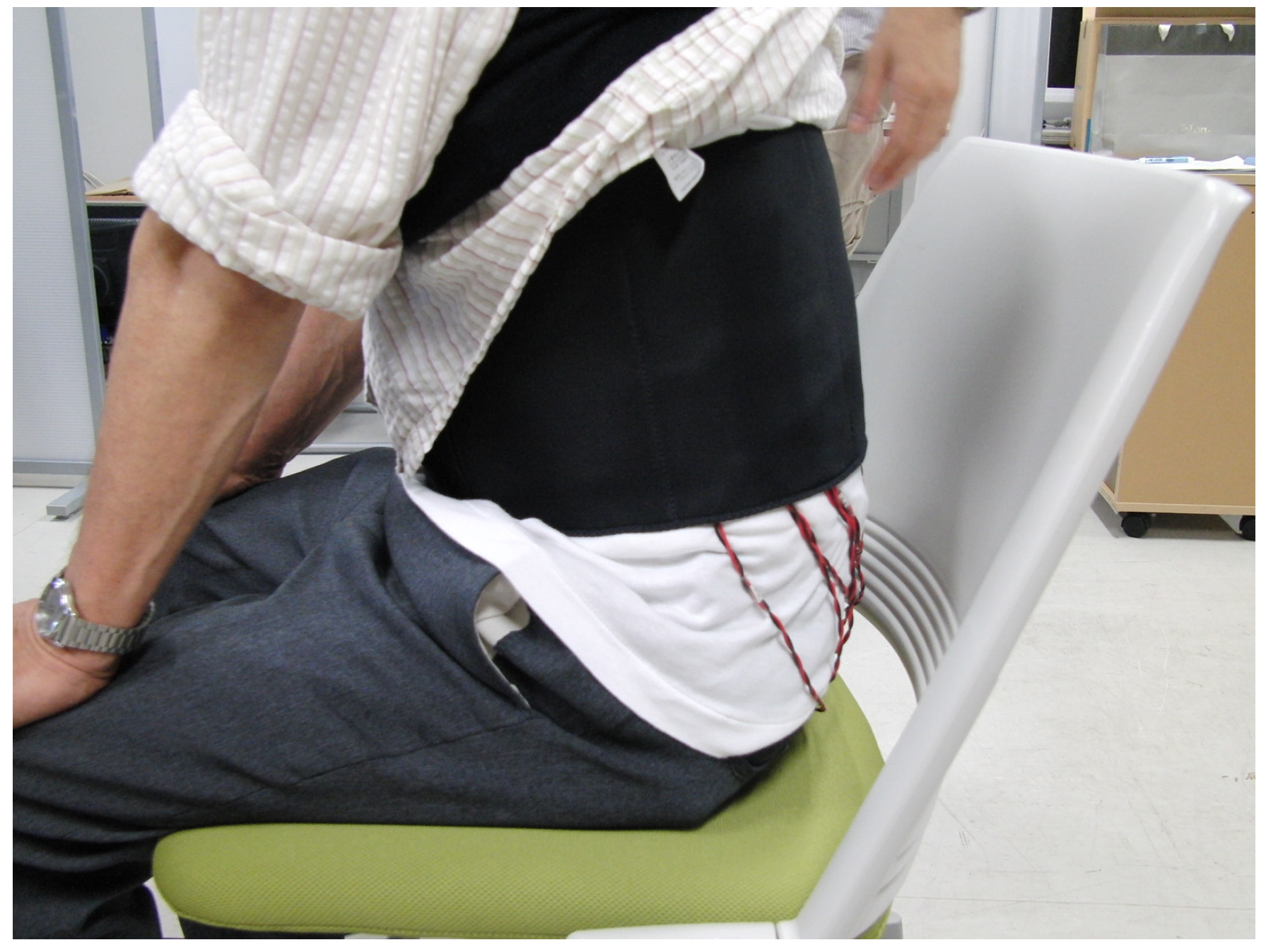
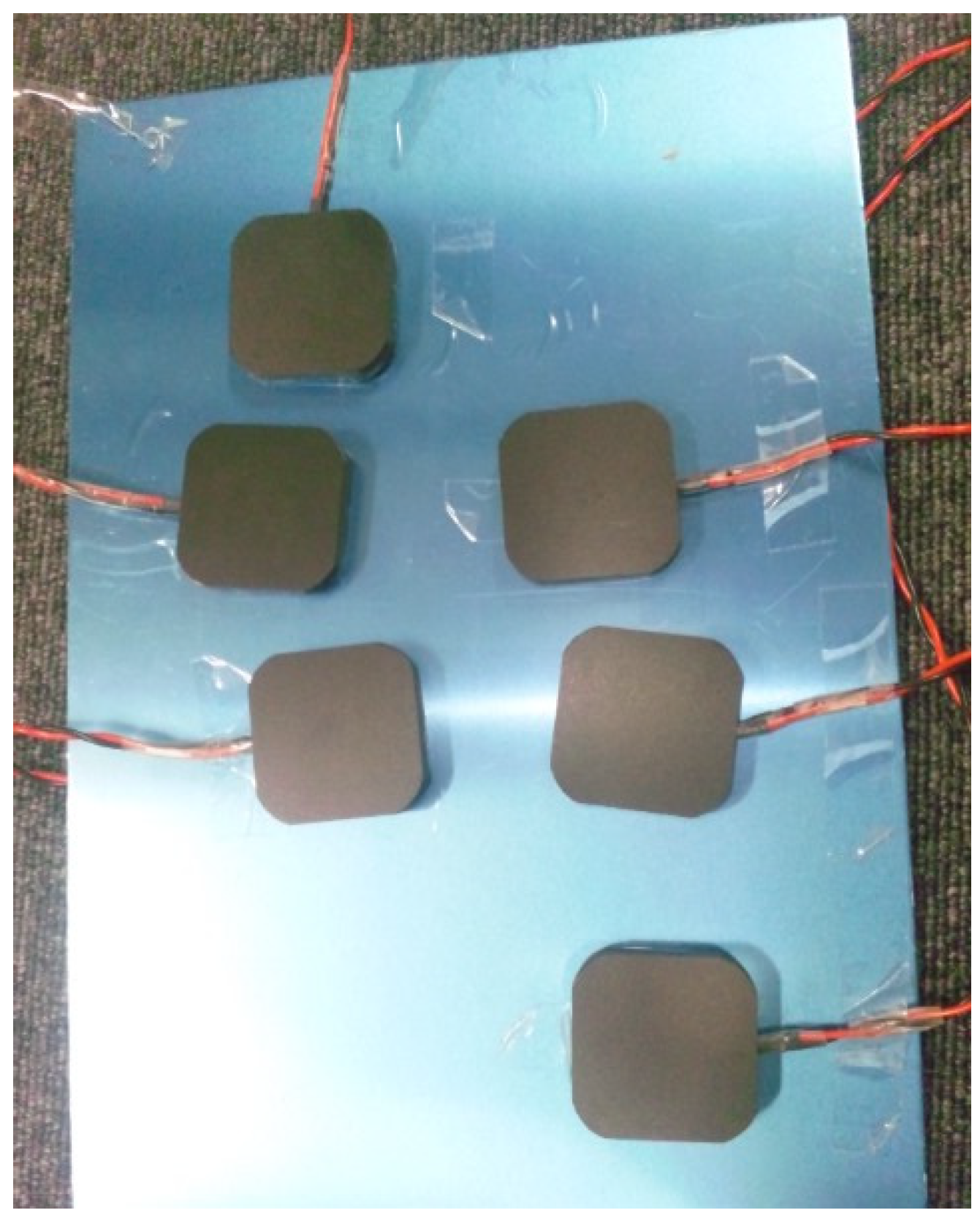
2.2. Details of the Trunk and Sole Training
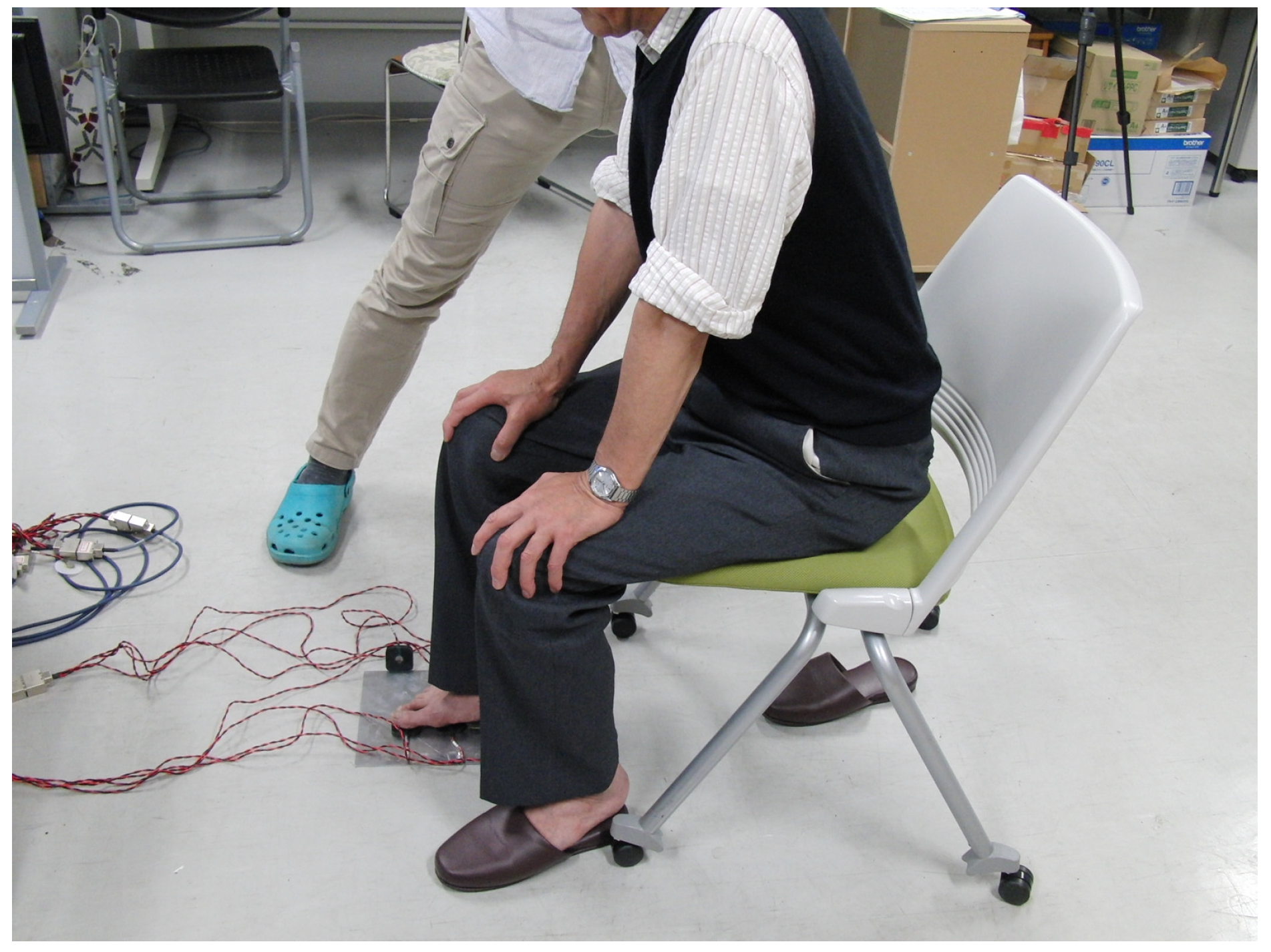
2.3. Evaluation of Trunk and Sole Region Vibratory Sensation
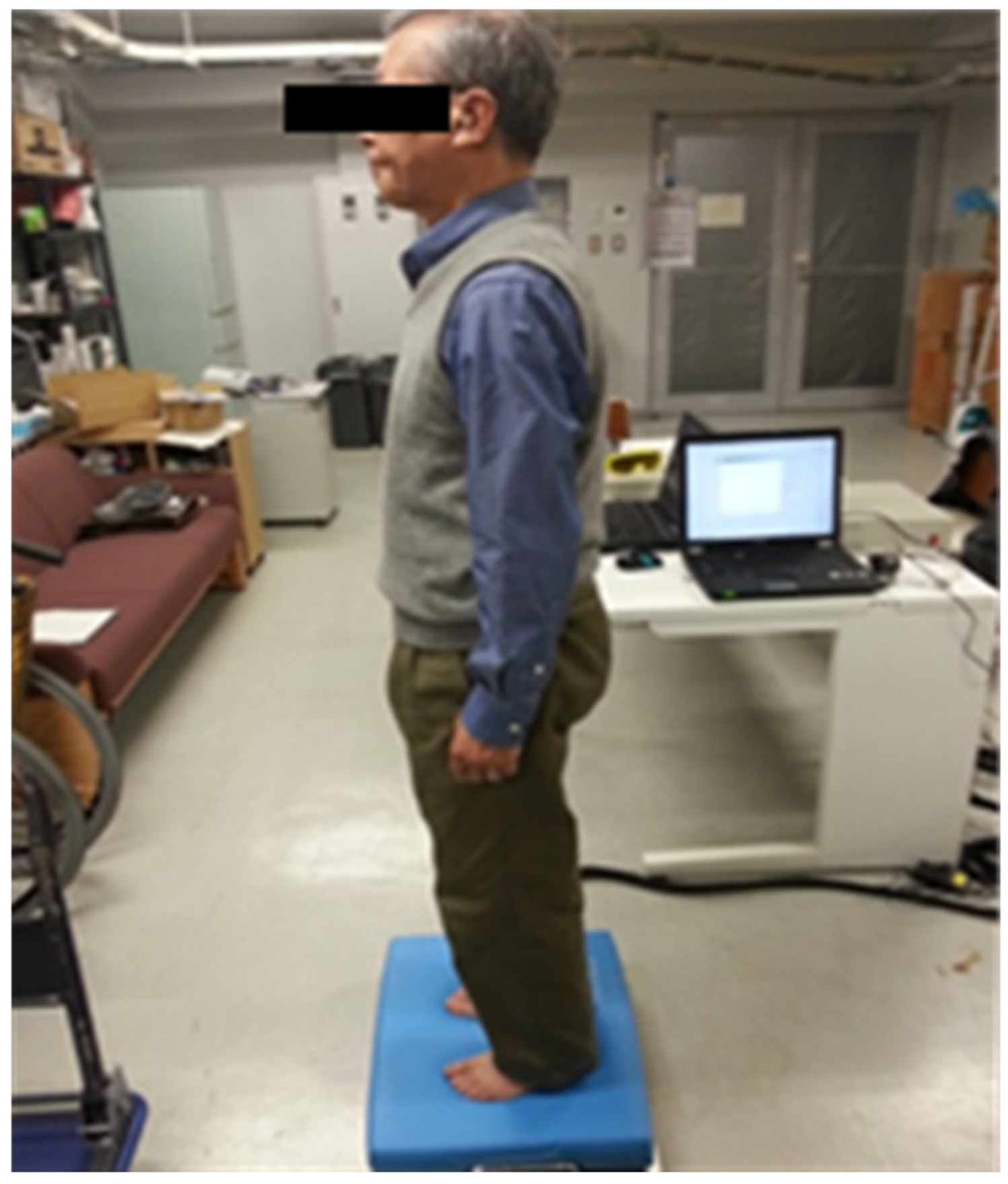
2.4. Evaluation of Standing Balance Using a Force Plate
2.5. Analysis
3. Results
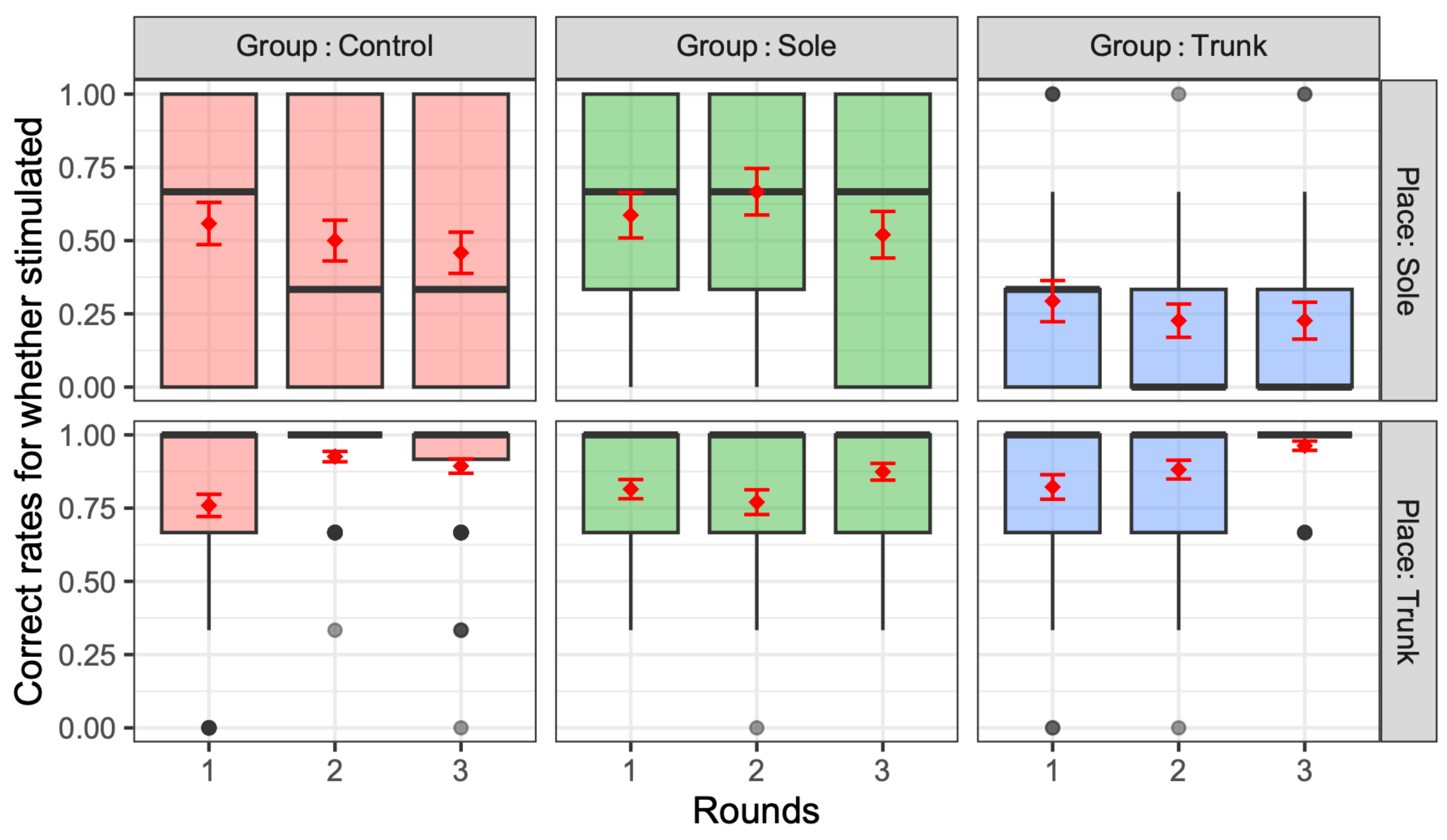
3.1. Vibratory Sensation Evaluation
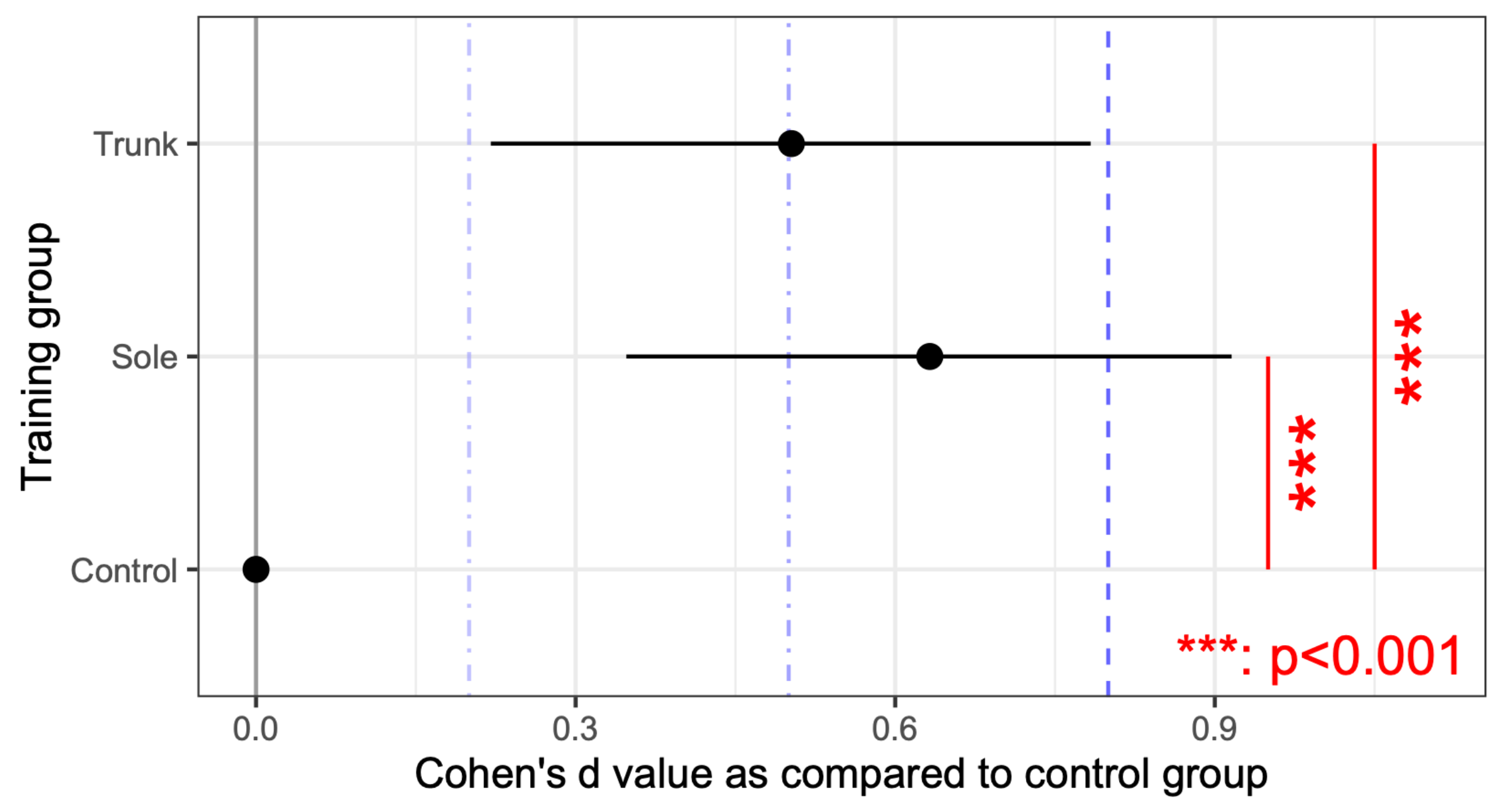
3.2. Evaluation of Standing Balance Performance with a Force Plate
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Geriatrics Society; British Geriatrics Society; American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J. Am. Geriatr. Soc. 2001, 49, 664–672. [Google Scholar] [CrossRef]
- Blake, A.J.; Morgan, K.; Bendall, M.J.; Dallosso, H.; Ebrahim, S.B.; Arie, T.H.; Fentem, P.H.; Bassey, E.J. Falls by elderly people at home: Prevalence and associated factors. Age Ageing 1988, 17, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hunkyung, K. Frequency of falls and their prevention. Clin. Calcium 2006, 16, 1444–1450. [Google Scholar] [PubMed]
- Birmingham, T. Test-retest reliability of lower extremity functional instability measures. Clin. J. Sport Med. 2000, 10, 264–268. [Google Scholar] [CrossRef]
- Brouwer, B.; Culham, E.G.; Liston, R.A.; Grant, T. Normal variability of postural measures: Implications for the reliability of relative balance performance outcomes. Scand. J. Rehabil. Med. 1998, 30, 131–137. [Google Scholar] [CrossRef]
- Maeda, Y.; Tanaka, T.; Miyasaka, T.; Shimizu, K. A Preliminary Study of Static and Dynamic Standing Balance and Risk of Falling in an Independent Elderly Population with a Particular Focus on the Limit of Stability Test. J. Phys. Ther. Sci. 2011, 23, 803–806. [Google Scholar] [CrossRef]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional Reach: A New Clinical Measure of Balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef]
- Dibble, L.E.; Lopez-Lennon, C.; Lake, W.; Hoffmeister, C.; Gappmaier, E. Utility of disease-specific measures and clinical balance tests in prediction of falls in persons with multiple sclerosis. J. Neurol. Phys. Ther. 2013, 37, 99–104. [Google Scholar] [CrossRef]
- Poncumhak, P.; Srithawong, A.; Duangsanjun, W.; Amput, P. Comparison of the Ability of Static and Dynamic Balance Tests to Determine the Risk of Falls among Older Community-Dwelling Individuals. J. Funct. Morphol. Kinesiol. 2023, 8, 43. [Google Scholar] [CrossRef]
- Bronstein, A.M.; Brandt, T.; Woollacott, M.; Nutt, J.G. Clinical Disorders of Balance, Posture and Gait, 2nd ed.; A Hodder Arnold Publication: Cary, NC, USA, 2004. [Google Scholar]
- Tanaka, T.; Noriyasu, S.; Ino, S.; Ifukube, T.; Nakata, M. Objective method to determine the contribution of the great toe to standing balance and preliminary observations of age-related effects. IEEE Trans. Rehabil. Eng. 1996, 4, 84–90. [Google Scholar] [CrossRef]
- Tanaka, T.; Shirogane, S.; Izumi, T.; Ino, S.; Ifukube, T. The Effect of Brief Moving Vibratory Stimulation on the Feet for Postural Control in a Comparison Study. Phys. Occup. Ther. Geriatr. 2005, 24, 1–23. [Google Scholar] [CrossRef]
- Inglis, J.T.; Horak, F.B.; Shupert, C.L.; Jones-Rycewicz, C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Exp. Brain Res. 1994, 101, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kenshalo, D.R. Somesthetic sensitivity in young and elderly humans. J. Gerontol. 1986, 41, 732–742. [Google Scholar] [CrossRef]
- Ballardini, G.; Florio, V.; Canessa, A.; Carlini, G.; Morasso, P.; Casadio, M. Vibrotactile Feedback for Improving Standing Balance. Front. Bioeng. Biotechnol. 2020, 8, 94. [Google Scholar] [CrossRef]
- Xu, J.; Bao, T.; Lee, U.H.; Kinnaird, C.; Carender, W.; Huang, Y.; Sienko, K.H.; Shull, P.B. Configurable, wearable sensing and vibrotactile feedback system for real-time postural balance and gait training: Proof-of-concept. J. Neuroeng. Rehabil. 2017, 14, 102. [Google Scholar] [CrossRef]
- LeVeau, B.F. Williams and Lissner’s Biomechanics of Human Motion, 3rd ed.; WB Saunders: Philadelphia, PA, USA, 1992; pp. 12–14. [Google Scholar]
- Jijimol, G.; Fayaz, R.K.; Vijesh, P.V. Correlation of trunk impairment with balance in patients with chronic stroke. NeuroRehabilitation 2013, 32, 323–325. [Google Scholar] [CrossRef]
- Johansson, G.; Jarnlo, G. Balance training in 70-year-old women. Physiother. Theory Pract. 1991, 7, 121–125. [Google Scholar] [CrossRef]
- Dean, C.M.; Channon, E.F.; Hall, J.M. Sitting training early after stroke improves sitting ability and quality and carries over to standing up but not to walking: A randomized trial. Aust. J. Physiother. 2007, 53, 97–102. [Google Scholar] [CrossRef]
- Hopewell, S.; Adedire, O.; Copsey, B.J.; Boniface, G.J.; Sherrington, C.; Clemson, L.; Close, J.C.; Lamb, S.E. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2018, 23, 7. [Google Scholar] [CrossRef]
- Buchner, D.M.; Cress, M.E.; De Lateur, B.J.; Esselman, P.C.; Margherita, A.J.; Price, R.; Wagner, E.H. The effect of strength and endurance training on gait, balance, fall risk, and health services use in community-living older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1997, 52, M218–M224. [Google Scholar] [CrossRef]
- Lord, S.R.; Ward, J.A.; Williams, P.; Strudwick, M. The effect of a 12-month exercise trial on balance, strength, and falls in older women: A randomized controlled trial. J. Am. Geriatr. Soc. 1995, 43, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Liu, D.; Yu, D.Z.; Zhu, Y.T.; Xu, W.C.; Tian, E.; Guo, Z.Q.; Shi, H.B.; Yin, S.K.; Kong, W.J. Multisensory Exercise Improves Balance in People with Balance Disorders: A Systematic Review. Curr. Med. Sci. 2021, 41, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Allison, L.K.; Kiemel, T.; Jeka, J.J. Sensory-Challenge Balance Exercises Improve Multisensory Reweighting in Fall-Prone Older Adults. J. Neurol. Phys. Ther. 2018, 42, 84–93. [Google Scholar] [CrossRef]
- Sienko, K.H.; Balkwill, M.D.; Oddsson, L.I.E.; Wall, C. Effects of multi-directional vibrotactile feedback on vestibular-deficient postural performance during continuous multi-directional support surface perturbations. J. Vestib. Res. 2013, 23, 33–43. [Google Scholar] [CrossRef]
- Ma, C.Z.; Wong, D.W.; Lam, W.K.; Wan, A.H.; Lee, W.C. Balance improvement effects of biofeedback systems with state-of-the-art wearable sensors: A systematic review. Sensors 2016, 16, 434. [Google Scholar] [CrossRef]
- Orr, R. The effect of whole body vibration exposure on balance and functional mobility in older adults: A systematic review and meta-analysis. Maturitas 2015, 80, 342–358. [Google Scholar] [CrossRef]
- Peters, R.M.; McKeown, M.D.; Carpenter, M.G.; Inglis, J.T. Losing touch: Age-related changes in plantar skin sensitivity, lower limb cutaneous reflex strength, and postural stability in older adults. J. Neurophysiol. 2016, 116, 1848–1858. [Google Scholar] [CrossRef]
- Allum, J.H.; Carpenter, M.G.; Honegger, F.; Adkin, A.L.; Bloem, B.R. Age-dependent variations in the directional sensitivity of balance corrections and compensatory arm movements in man. J. Physiol. 2002, 542, 643–663. [Google Scholar] [CrossRef]
- Juras, G.; Słomka1, K.; Fredyk, A.; Sobota, G.; Bacik, B. Evaluation of the Limits of Stability (LOS) Balance Test. J. Hum. Kinet. 2008, 19, 39–52. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Horak, F.B. Assessing the influence of sensory interaction on balance. Phys. Ther. 1986, 66, 1548–1550. [Google Scholar] [CrossRef]
- Hu, M.H.; Woollacott, M.H. Multisensory training of standing balance in older adults: I. Postural stability and one-leg stance balance. J. Gerontol. 1994, 49, M52–M61. [Google Scholar] [CrossRef] [PubMed]
- Hirase, T.; Inokuchi, S.; Matsusaka, N.; Okita, M. Effects of a Balance Training Program Using a Foam Rubber Pad in Community-Based Older Adults. A Randomized Controlled Trial. J. Geriatr. Phys. Ther. 2015, 38, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The aligned rank transform was used for non-parametric factorial analyses using only anova. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Vancouver, BC, Canada, 7–12 May 2011; pp. 143–146. [Google Scholar]
- Elkin, L.A.; Kay, M.; Higgins, J.J.; Wobbrock, J.O. Rank transform procedure for multifactor contrast tests. In Proceedings of the 34th Annual ACM Symposium on User Interface Software and Technology, Virtual, 10–13 October 2021; pp. 754–768. [Google Scholar]
- Lenth, R.V. Least-squares means: R package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.10.7. 2018. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 13 March 2025).
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35 (Suppl. S2), ii7–ii11. [Google Scholar] [CrossRef]
- Wright, W.G.; Ivanenko, Y.P.; Gurfinkel, V.S. Foot anatomy specialization for postural sensation and control. J. Neurophysiol. 2012, 107, 1513–1521. [Google Scholar] [CrossRef]
- Tsai, L.-C.; Yu, B.; Mercer, V.S.; Gross, M.T. Comparison of different structural foot types for measures of standing postural control. J. Orthop. Sports Phys. Ther. 2006, 36, 942–953. [Google Scholar] [CrossRef]
- Al Abdulwahab, S.S.; Kachanathu, S.J. The effect of various degrees of foot posture on standing balance in a healthy adult population. Somatosens. Mot. Res. 2015, 32, 172–176. [Google Scholar] [CrossRef]
- Anzai, E.; Nakajima, K.; Iwakami, Y.; Sato, M.; Ino, S.; Ifukube, T.; Yamashita, K.; Ohta, Y. Effects of foot arch structure on postural stability. Clin. Res. Foot Ankle 2014, 2, 132. [Google Scholar]
- Saghazadeh, M.; Tsunoda, K.; Tomohiro, O. Foot arch height and rigidity index associated with balance and postural sway in elderly women using a 3D foot scanner. Foot Ankle Online J. 2014, 7, 10-3827. [Google Scholar]
- Lee, S.W.; Cho, K.H.; Lee, W.H. Effect of a local vibration stimulus training programme on postural sway and gait in chronic stroke patients: A randomized controlled trial. Clin. Rehabil. 2013, 27, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Priplata, A.A.; Niemi, J.B.; Harry, J.D.; Lipsitz, L.A.; Collins, J.J. Vibrating insoles and balance control in elderly people. Lancet 2003, 362, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Khalifeloo, M.; Naghdi, S.; Ansari, N.N.; Akbari, M.; Jalaie, S.; Jannat, D.; Hasson, S. A study on the immediate effects of plantar vibration on balance dysfunction in patients with stroke. J. Exerc. Rehabil. 2018, 14, 259–266. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Time “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Shumway Cook, A.; Brauer, S.; Woolacott, M. Predicting the Probability for Falls in Community Dwelling Older Adults Using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Önala, B.; Sertelb, M.; Karacaca, G. Effect of plantar vibration on static and dynamic balance instroke patients: A randomised controlled study. Physiotherapy 2022, 116, 1–8. [Google Scholar] [CrossRef]
- Goldberg, A.; Hernandez, M.E.; Alexander, N.B. Trunk repositioning errors are increased in balance-impaired older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 1310–1314. [Google Scholar] [CrossRef]
- Sonasath, M.K.; Shah, D.S. Effect of Lumbar Position Sense on Lumbar Muscle Strength, Static versus Dynamic Balance and Functional Status in Children with Spastic Diplegic Cerebral Palsy: A Cross-Sectional Study. Int. J. Health Sci. Res. 2024, 14, 20–27. [Google Scholar] [CrossRef]
- Whanger, A.; Wang, H.S. Clinical correlates of the vibratory sense in elderly psychiatric patients. J. Gerontol. 1974, 29, 39–45. [Google Scholar] [CrossRef]
- Johansson, R.S.; Vallbo, A.B. Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci. 1983, 6, 27–32. [Google Scholar] [CrossRef]
- Ino, S.; Shimizu, S.; Hosoe, F.; Izumi, T.; Takahashi, M.; Ifukube, T. A Basic Study on Tactile Display for Tele-presence. In Proceedings of the IEEE International Workshop on Robot and Human Communication, RO-MAN’92, Tokyo, Japan, 1–3 September 1992; pp. 58–62. [Google Scholar]
- Franco, P.G.; Santos, K.B.; Rodacki, A.L. Joint positioning sense, perceived force level and two-point discrimination tests of young and active elderly adults. Braz. J. Phys. Ther. 2015, 19, 304–310. [Google Scholar] [CrossRef]
- Maurer, C.; Mergner, T.; Bolha, B.; Hlavacka, F. Human balance control during cutaneous stimulation of the plantar soles. Neurosci. Lett. 2001, 302, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.L.; Mansfield, A.; Inness, E.L.; Patterson, K.K. The relationship of plantar cutaneous sensation and standing balance post-stroke. Top. Stroke Rehabil. 2016, 23, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I.; Gayton, D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother. Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Wanderley, F.S.; Alburquerque-Sendin, F.; Parizotto, N.A.; Rebelatto, J.R. Effect of plantar vibration stimuli on the balance of older women: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2011, 92, 199–206. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; St George, R.; Fitzpatrick, R.C. Walking stability and sensorymotor function in older people with diabetic peripheral neuropathy. Arch. Phys. Med. Rehabil. 2004, 85, 245–252. [Google Scholar] [CrossRef]
- Lord, S.R.; Clark, R.D.; Webster, I.W. Postural stability and associated physiological factors in a population of aged persons. J. Gerontol. 1991, 46, M69–M76. [Google Scholar] [CrossRef]
- Kavounoudias, A.; Roll, R.; Roll, J.-P. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J. Physiol. 2001, 532, 869–878. [Google Scholar] [CrossRef]
- Dietz, V.; Trippel, M.; Horstmann, G.A. Significance of proprioceptive and vestibulo-spinal reflexes in the control of stance and gait. In Adaptability of Human Gait; Patla, A.E., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 17–52. [Google Scholar]
- Massion, J.; Wollacott, M. Normal balance and postural control. In Clinical Aspects of Balance and Gait Disorders; Bronstein, A.M., Brandit, T., Woollacott, M., Eds.; Edward Arnold: London, UK, 1996. [Google Scholar]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research into Clinical Practice, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 52–62. [Google Scholar]
- Lipsitz, L.A.; Lough, M.; Niemi, J.; Travison, T.; Howlett, H.; Manor, B. A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Arch. Phys. Med. Rehabil. 2015, 96, 432–439. [Google Scholar] [CrossRef]
- Hirosawa, M.; Takehara, I.; Moriyama, Y.; Amimoto, K. Effects of unilateral neck muscle vibration on standing postural orientation and spatial perception in healthy subjects based on stimulus duration and simultaneous stimulation of trunk muscles. PLoS ONE 2023, 18, e0281012. [Google Scholar] [CrossRef] [PubMed]
- Champely, S.; Ekstrom, C.; Dalgaard, P.; Gill, J.; Weibelzahl, S.; Anandkumar, A.; Ford, C.; Volcic, R.; De Rosario, H. pwr: Basic Functions for Power Analysis. 2017. Available online: https://cran.r-project.org/web/packages/pwr/ (accessed on 6 March 2025).
- Bulus, M. Pwrss: Statistical Power and Sample Size Calculation Tools. Available online: https://CRAN.R-project.org/package=pwrss (accessed on 6 March 2025).
- Lakens, D.; Caldwell, A.R. Simulation-based power analysis for factorial analysis of variance designs. Adv. Methods Pract. Psychol. Sci. 2021, 4, 2515245920951503. [Google Scholar] [CrossRef]
- Pohlert, T. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended. R Package Version 1.9.10. 2023. Available online: https://CRAN.R-project.org/package=PMCMRplus (accessed on 6 March 2025).







| Condition | Vibrators in the Trunk Position | ||
|---|---|---|---|
 | One stimulus | 1, 2, 3, 4, 5, 6, 7, 8, 9 | |
| Two stimuli | (Vertical) | 1–2, 4–5, 7–8, 2–3, 5–6, 8–9 | |
| (Horizontal) | 1–4, 4–7, 2–5, 5–8, 3–6, 6–9 | ||
| (Diagonal) | 1–5, 5–9, 7–5, 5–3 | ||
| No stimulus | N/A | ||
| Condition | Vibrators | Plantar Position(s) | (Abbreviation) | |
|---|---|---|---|---|
 | One stimulus | a | Hallux | Hallux |
| b | Thenar eminence | TE | ||
| c | Hypothenar eminence | HE | ||
| d | Proximal first metatarsal | P1M | ||
| f | Heel | Heel | ||
| Two stimuli | a–b | Hallux–Thenar eminence | Hallux–TE | |
| a–d | Hallux–Proximal first metatarsal | Hallux–P1M | ||
| b–d | Thenar eminence–Proximal first metatarsal | TE–P5M | ||
| b–f | Thenar eminence–Heel | TE–Heel | ||
| c–e | Hypothenar eminence–Proximal fifth metatarsal | HE–P5M | ||
| c–f | Hypothenar eminence–Heel | HE–Heel | ||
| e–f | Proximal fifth metatarsal–Heel | P5M–Heel | ||
| No stimulus | N/A | No vibration | No vibration |
| Anterior Tilt | Posterior Tilt | Right Tilt | Left Tilt | Total Cross Test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | p | p | p | p | ||||||
| Rounds | <0.001 | *** | <0.001 | *** | 0.130 | 0.415 | 0.029 | * | ||
| Mattress usage | <0.001 | *** | <0.001 | *** | 0.008 | ** | 0.169 | <0.001 | *** | |
| Correct rates (sole) | 0.626 | 0.324 | 0.896 | 0.599 | 0.550 | |||||
| Correct rates (trunk) | 0.024 | * | 0.003 | ** | 0.004 | ** | 0.005 | ** | 0.015 | ** |
| Training group (sole) | <0.001 | *** | <0.001 | *** | 0.007 | ** | 0.007 | *** | <0.001 | *** |
| Training group (trunk) | <0.001 | *** | <0.001 | *** | 0.001 | ** | 0.001 | *** | <0.001 | *** |
| Sole | Trunk | |||||
|---|---|---|---|---|---|---|
| 0 Vibration | 1 Vibration | 2 Vibrations | 0 Vibration | 1 Vibration | 2 Vibrations | |
| Anterior tilt | 0.10 | −0.06 | 0.19 | 0.16 | −0.02 | 0.41 |
| Posterior tilt | 0.08 | −0.29 | 0.16 | 0.08 | 0.05 | 0.43 |
| Right tilt | 0.10 | −0.19 | 0.27 | 0.01 | 0.03 | 0.45 |
| Left tilt | 0.07 | −0.20 | 0.28 | 0.08 | −0.02 | 0.48 |
| Total tilt | 0.10 | −0.21 | 0.25 | 0.09 | 0.01 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Maeda, Y.; Miura, T. Effects of Tactile Sensory Stimulation Training of the Trunk and Sole on Standing Balance Ability in Older Adults: A Randomized Controlled Trial. J. Funct. Morphol. Kinesiol. 2025, 10, 96. https://doi.org/10.3390/jfmk10010096
Tanaka T, Maeda Y, Miura T. Effects of Tactile Sensory Stimulation Training of the Trunk and Sole on Standing Balance Ability in Older Adults: A Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology. 2025; 10(1):96. https://doi.org/10.3390/jfmk10010096
Chicago/Turabian StyleTanaka, Toshiaki, Yusuke Maeda, and Takahiro Miura. 2025. "Effects of Tactile Sensory Stimulation Training of the Trunk and Sole on Standing Balance Ability in Older Adults: A Randomized Controlled Trial" Journal of Functional Morphology and Kinesiology 10, no. 1: 96. https://doi.org/10.3390/jfmk10010096
APA StyleTanaka, T., Maeda, Y., & Miura, T. (2025). Effects of Tactile Sensory Stimulation Training of the Trunk and Sole on Standing Balance Ability in Older Adults: A Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology, 10(1), 96. https://doi.org/10.3390/jfmk10010096








