Effects of Functional Partial Body Weight Support Treadmill Training on Mobility in Children with Ataxia: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Group Allocation
2.2. Intervention and Settings
2.3. Typical Therapy
2.4. Measurement Outcomes
- The Gross Motor Function Measure (GMFM-88) dimension D/E (GMFM-D /GMFM-E), were expressed as percentage scores (%) for the assessment of the motor performance and functional ability in standing, walking, running, and jumping [40].
- The Pediatric Balance Scale (PBS), with scores in a range of 0–56 (the higher score indicates better balance), was used for the assessment of functional balance skills [41].
- The Timed Up and Go (TUG) test for the assessment of dynamic balance control was expressed in seconds (s) [44].
- The 6-minute walk test (6 MWT) for the assessment of physical condition and endurance was expressed in meters (m) [45].
- The Scale for Assessment and Rating Ataxia (SARA), applied in the Greek version (SARAgr), was used for the assessment of ataxia features, with scores in a range of 0–40 (a higher score indicates more severe ataxia) [4].
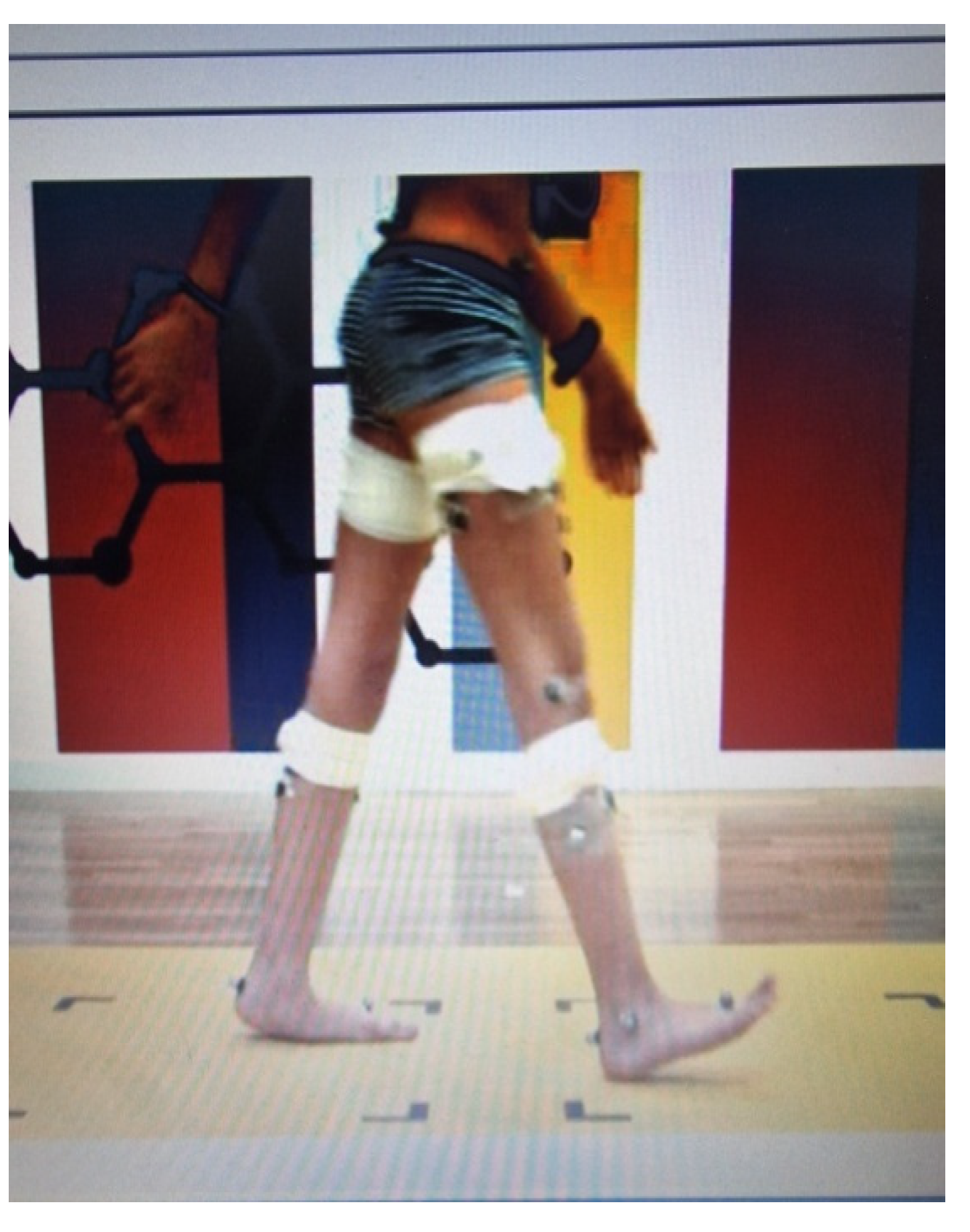
- Three-dimensional kinematic and kinetic analysis of lower limbs through motion and gait analysis was applied, with the electronic recording of kinematics elements for the pelvis, hip, knee, and foot of both lower limbs in the three planes of motion (sagittal, frontal, and transverse). Since laterality severity does not occur in ataxia, each participant’s dominant ankle power absorption and generation, expressed as Watt/kg, was collected for the kinetic variable. Similarly, the dominant lower limb’s mean gait deviations index (MGDI) during 1. pelvis movement in all three planes of motion; 2. hip, knee, and ankle movement in the sagittal plane; and 3. ankle movement in the transverse plane (foot progression) were collected and expressed as normally disturbed data, categorized as Normal Standard Deviations (NSDs) through Gait Deviation Index (GDI) analysis [46]. Data recording and collection were obtained by the Gait and Motion Analysis Lab of ELEPAP in Athens.
- Analysis of spatiotemporal gait features was performed through 2 min recordings on the treadmill with partial body weight support at a personalized slow walking speed (75% of each participant’s self-selected walking speed based on the 10 MWT over ground). GaitSens software (version 2.0), incorporated in the LiteGait equipment, was used for recording and collecting the gait parameters. The dominant lower limb’s step length, stride length, and width length were expressed in terms of meters (cm), while step and stride time were expressed in seconds (s).
2.5. Assessment Procedure and Raters
2.6. Statistical Analysis
- The percentage change from baseline at all time points, where we compared the percentage changes between the intervention groups using the independent samples t-test or the Mann–Whitney test if the data did not follow a normal distribution.
- The absolute change from baseline at all time points, where we compared the absolute change in the variables (dependent variable) between the intervention groups (factor) and the baseline assessment (covariate) using the Analysis of Covariance (ANCOVA) model.
3. Results
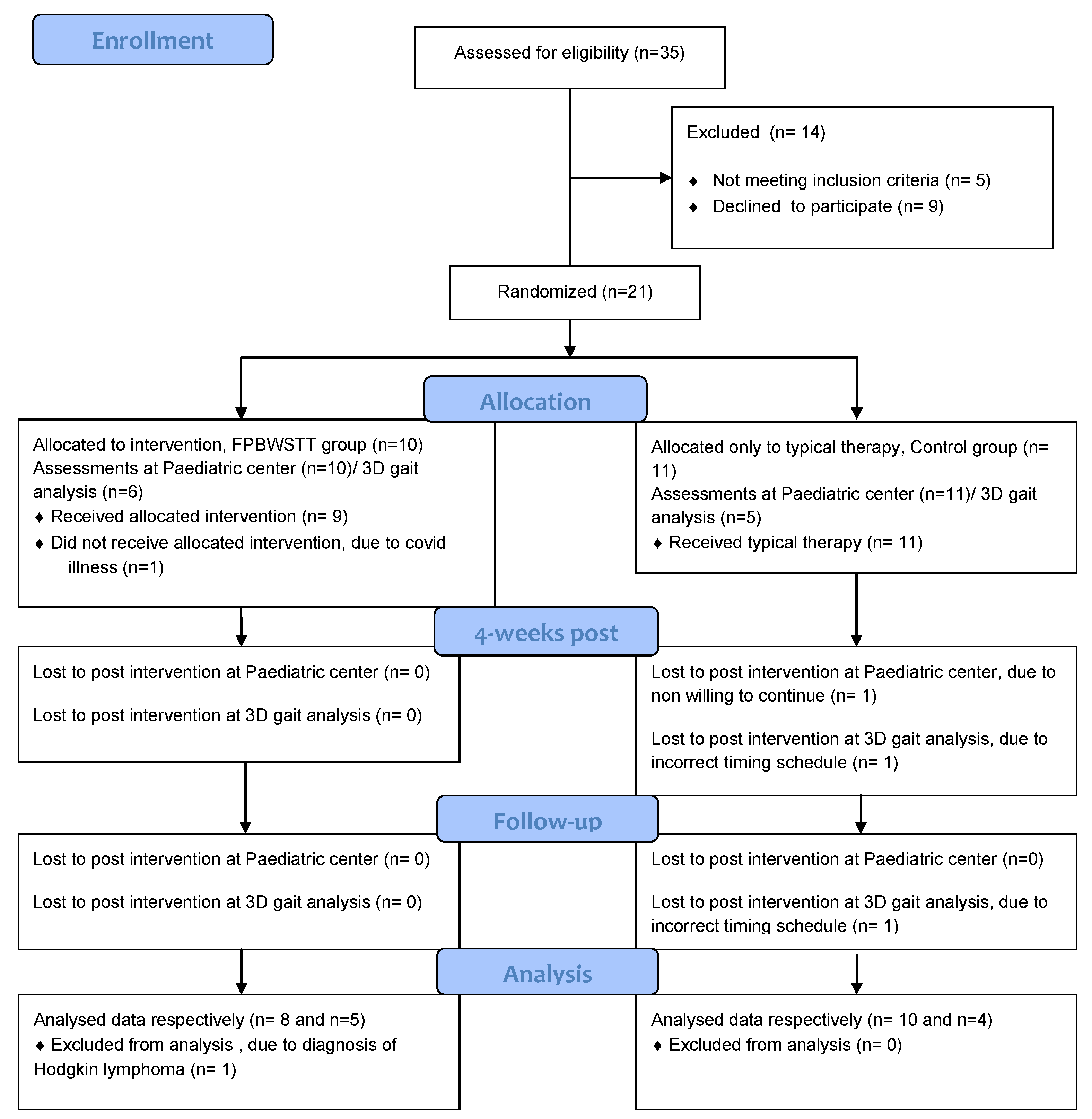
3.1. Participant Characteristics
3.2. Primary Outcomes: Domains D and E of the GMFM-88 (%)
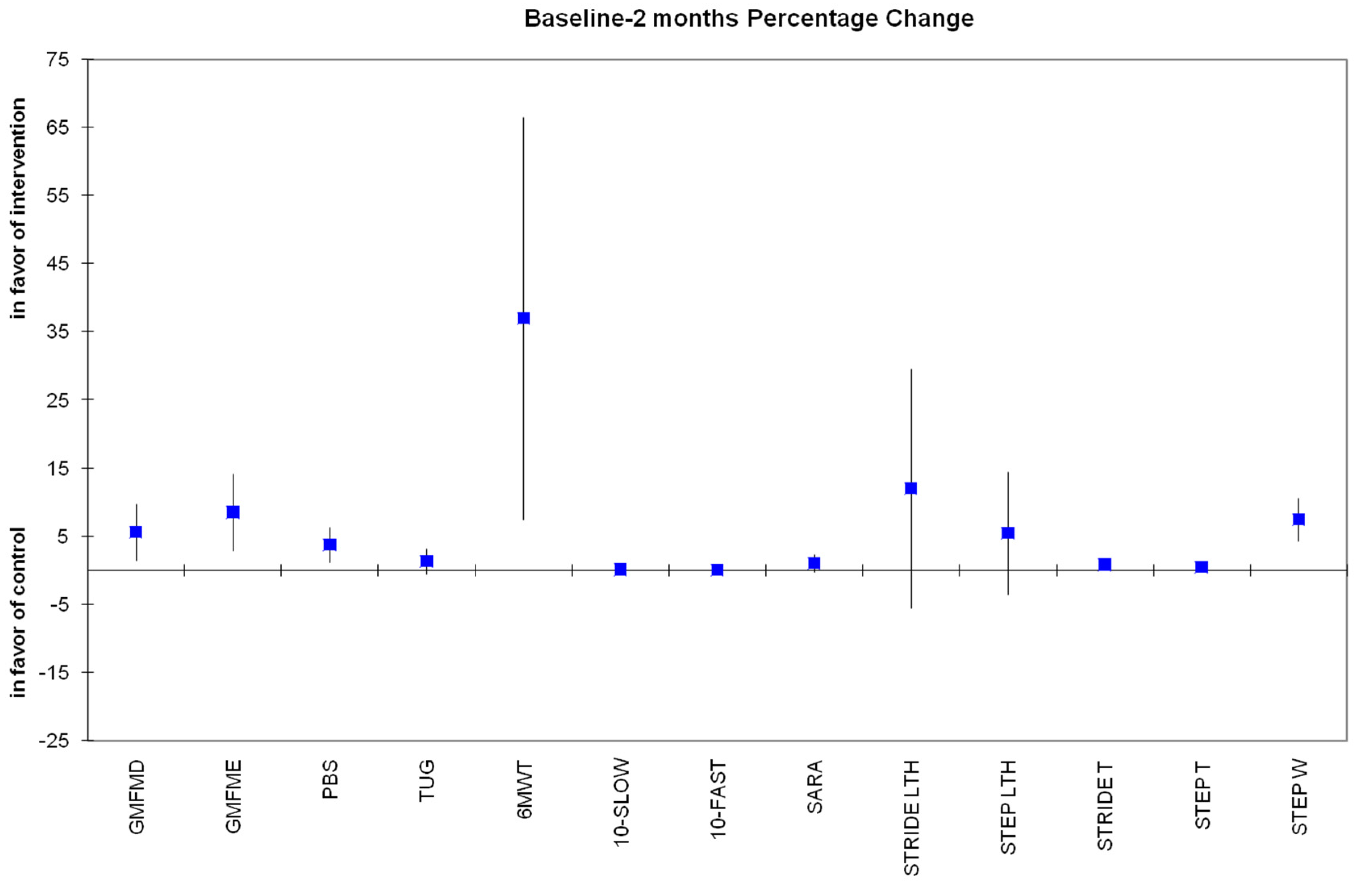
3.3. Secondary Outcomes
Secondary Variables from 3D Gait Analysis
4. Discussion
4.1. Kinematic Changes
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musselman, K.E.; Stoyanov, C.T.; Marasigan, R.; Jenkins, M.E.; Konczak, J.; Morton, S.M.; Bastian, A.J. Prevalence of ataxia in children: A systematic review. Neurology 2014, 82, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Hartley, H.; Cassidy, E.; Bunn, L.; Kumar, R.; Pizer, B.; Lane, S.; Carter, B. Exercise and Physical Therapy Interventions for Children with Ataxia: A Systematic Review. Cerebellum 2019, 18, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Pavone, P.; Praticò, A.D.; Pavone, V.; Lubrano, R.; Falsaperla, R.; Rizzo, R.; Ruggieri, M. Ataxia in children: Early recognition and clinical evaluation. Ital. J. Pediatr. 2017, 43, 6. [Google Scholar] [CrossRef] [PubMed]
- Lepoura, A.; Lampropoulou, S.; Galanos, A.; Papadopoulou, M.; Sakellari, V. Scale for Assessment and Rating Ataxia (SARA) in Children with Ataxia: Greek Cultural Adaptation and Psychometric Properties. Open Access J. Neurol. Neurosurg. 2023, 18, e555976. [Google Scholar]
- Schmitz-Hübsch, T.; du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C.; Giunti, P.; Globas, C.; Infante, J.; Kang, J.S.; et al. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 2006, 66, 1717–1720. [Google Scholar]
- Pinto, W.B.; Pedroso, J.L.; Souza, P.V.; Albuquerque, M.V.; Barsottini, O.G. Non-progressive cerebellar ataxia and previous undetermined acute cerebellar injury: A mysterious clinical condition. Arq. Neuropsiquiatr. 2015, 73, 823–827. [Google Scholar] [CrossRef]
- Panzeri, D.; Bettinelli, M.S.; Biffi, E.; Rossi, F.; Pellegrini, C.; Orsini, N.; Recchiuti, V.; Massimino, M.; Poggi, G. Application of the Scale for the Assessment and Rating of Ataxia (SARA) in pediatric oncology patients: A multicenter study. Pediatr. Hematol. Oncol. 2020, 37, 687–695. [Google Scholar] [CrossRef]
- Sullivan, R.; Yau, W.Y.; O’Connor, E.; Houlden, H. Spinocerebellar ataxia: An update. J. Neurol. 2019, 266, 533–544. [Google Scholar]
- Steinlin, M. Non-progressive congenital ataxias. Brain Dev. 1998, 20, 199–208. [Google Scholar]
- Buckley, E.; Mazzà, C.; McNeill, A. A systematic review of the gait characteristics associated with Cerebellar Ataxia. Gait Posture 2018, 60, 154–163. [Google Scholar]
- Milne, S.C.; Hocking, D.R.; Georgiou-Karistianis, N.; Murphy, A.; Delatycki, M.B.; Corben, L.A. Sensitivity of spatiotemporal gait parameters in measuring disease severity in Friedreich ataxia. Cerebellum 2014, 13, 677–688. [Google Scholar] [CrossRef]
- Peri, E.; Panzeri, D.; Beretta, E.; Reni, G.; Strazzer, S.; Biffi, E. Motor Improvement in Adolescents Affected by Ataxia Secondary to Acquired Brain Injury: A Pilot Study. Biomed. Res. Int. 2019, 2019, 8967138. [Google Scholar] [CrossRef]
- Vasco, G.; Gazzellini, S.; Petrarca, M.; Lispi, M.L.; Pisano, A.; Zazza, M.; Della Bella, G.; Castelli, E.; Bertini, E. Functional and Gait Assessment in Children and Adolescents Affected by Friedreich’s Ataxia: A One-Year Longitudinal Study. PLoS ONE 2016, 11, e0162463. [Google Scholar] [CrossRef]
- Damiano, D.L.; DeJong, S.L. A systematic review of the effectiveness of treadmill training and body weight support in pediatric rehabilitation. J. Neurol. Phys. Ther. 2009, 33, 27–44. [Google Scholar] [CrossRef]
- Molina-Rueda, F.; Aguila-Maturana, A.M.; Molina-Rueda, M.J.; Miangolarra-Page, J.C. Pasarelarodante con o sin sistema de suspension del peso corporal enninos con paralisis cerebral infantil: Revision sistematica y metaanalisis [Treadmill training with or without partial body weight support in children with cerebral palsy: Systematic review and meta-analysis]. Rev. Neurol. 2010, 51, 135–145. [Google Scholar]
- Qian, G.; Cai, X.; Xu, K.; Tian, H.; Meng, Q.; Ossowski, Z.; Liang, J. Which gait training intervention can most effectively improve gait ability in patients with cerebral palsy? A systematic review and network meta-analysis. Front. Neurol. 2023, 13, 1005485. [Google Scholar] [CrossRef]
- Valentin-Gudiol, M.; Bagur-Calafat, C.; Girabent-Farrés, M.; Hadders-Algra, M.; Mattern-Baxter, K.; Angulo-Barroso, R. Treadmill interventions with partial body weight support in children under six years of age at risk of neuromotor delay: A report of a Cochrane systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2013, 49, 67–91. [Google Scholar]
- Bjornson, K.F.; Moreau, N.; Bodkin, A.W. Short-burst interval treadmill training walking capacity and performance in cerebral palsy: A pilot study. Dev. Neurorehabil. 2019, 22, 126–133. [Google Scholar] [CrossRef]
- Hamacher, D.; Herold, F.; Wiegel, P.; Hamacher, D.; Schega, L. Brain activity during walking: A systematic review. Neurosci. Biobehav. Rev. 2015, 57, 310–327. [Google Scholar] [CrossRef]
- San Martín Valenzuela, C.; Moscardó, L.D.; López-Pascual, J.; Serra-Añó, P.; Tomás, J.M. Effects of Dual-Task Group Training on Gait, Cognitive Executive Function, and Quality of Life in People With Parkinson Disease: Results of Randomized Controlled DUALGAIT Trial. Arch. Phys. Med. Rehabil. 2020, 101, 1849–1856.e1. [Google Scholar] [CrossRef]
- Yang, Y.R.; Chen, Y.C.; Lee, C.S.; Cheng, S.J.; Wang, R.Y. Dual-task-related gait changes in individuals with stroke. Gait Posture 2007, 25, 185–190. [Google Scholar] [CrossRef]
- Elhinidi, E.I.; Ismaeel, M.M.; El-Saeed, T.M. Effect of dual-task training on postural stability in children with infantile hemiparesis. J. Phys. Ther. Sci. 2016, 28, 875–880. [Google Scholar] [CrossRef]
- Lee, N.Y.; Lee, E.J.; Kwon, H.Y. The effects of dual-task training on balance and gross motor function in children with spastic diplegia. J. Exerc. Rehabil. 2021, 17, 21–27. [Google Scholar] [CrossRef]
- Koch, I.; Poljac, E.; Müller, H.; Kiesel, A. Cognitive structure, flexibility, and plasticity in human multitasking-An integrative review of dual-task and task-switching research. Psychol. Bull. 2018, 144, 557–583. [Google Scholar] [CrossRef]
- Lepoura, A.; Lampropoulou, S.; Galanos, A.; Papadopoulou, M.; Sakellari, V. Study protocol of a randomised controlled trial for the effectiveness of a functional partial body weight support treadmill training (FPBWSTT) on motor and functional skills of children with ataxia. BMJ Open 2022, 12, e056943. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, M.; Su, Z. The pursuit of balance: An overview of covariate-adaptive randomization techniques in clinical trials. Contemp. Clin. Trials. 2015, 45 Pt A, 21–25. [Google Scholar] [CrossRef]
- Beauchet, O.; Dubost, V.; Herrmann, F.; Rabilloud, M.; Gonthier, R.; Kressig, R.W. Relationship between dual-task related gait changes and intrinsic risk factors for falls among transitional frail older adults. Aging Clin. Exp. Res. 2005, 17, 270–275. [Google Scholar] [CrossRef]
- Iqbal, M.; Arsh, A.; Hammad, S.M.; Haq, I.U.; Darain, H. Comparison of dual task specific training and conventional physical therapy in ambulation of hemiplegic stroke patients: A randomized controlled trial. J. Pak. Med. Assoc. 2020, 70, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Yu, J.; Jung, J.; Kang, H.; Cho, K. Effects of motor dual task training on spatio-temporal gait parameters of post-stroke patients. J. Phys. Ther. Sci. 2012, 24, 845–848. [Google Scholar] [CrossRef]
- Bleyenheuft, Y.; Ebner-Karestinos, D.; Surana, B.; Paradis, J.; Sidiropoulos, A.; Renders, A.; Friel, K.M.; Brandao, M.; Rameckers, E.; Gordon, A.M. Intensive upper- and lower-extremity training for children with bilateral cerebral palsy: A quasi-randomized trial. Dev. Med. Child Neurol. 2017, 59, 625–633. [Google Scholar] [CrossRef]
- Salem, Y.; Godwin, E.M. Effects of task-oriented training on mobility function in children with cerebral palsy. NeuroRehabilitation 2009, 24, 307–313. [Google Scholar]
- Cano-de-la-Cuerda, R.; Molero-Sánchez, A.; Carratalá-Tejada, M.; Alguacil-Diego, I.M.; Molina-Rueda, F.; Miangolarra-Page, J.C.; Torricelli, D. Theories and control models and motor learning: Clinical applications in neuro-rehabilitation. Neurologia 2015, 30, 32–41. [Google Scholar]
- Atan, T.; Özyemişci Taşkıran, Ö.; Bora Tokçaer, A.; Kaymak Karataş, G.; Karakuş Çalışkan, A.; Karaoğlan, B. Effects of different percentages of body weight-supported treadmill training in Parkinson’s disease: A double-blind randomized controlled trial. Turk. J. Med. Sci. 2019, 49, 999–1007. [Google Scholar]
- Hesse, S.; Helm, B.; Krajnik, J.; Gregoric, M.; Mauritz, K.H. Treadmill Training with Partial Body Weight Support: Influence of Body Weight Release on the Gait of Hemiparetic Patients. J. Neurol. Rehabil. 1997, 11, 15–20. [Google Scholar]
- Matsuno, V.M.; Camargo, M.R.; Palma, G.C.; Alveno, D.; Barela, A.M. Analysis of partial body weight support during treadmill and overground walking of children with cerebral palsy. Rev. Bras Fisioter. 2010, 14, 404–410. [Google Scholar]
- Arpino, C.; Vescio, M.F.; De Luca, A.; Curatolo, P. Efficacy of intensive versus nonintensive physiotherapy in children with cerebral palsy: A meta-analysis. Int. J. Rehabil. Res. 2010, 33, 165–171. [Google Scholar] [CrossRef]
- Tinderholt Myrhaug, H.; Østensjø, S.; Larun, L.; Odgaard-Jensen, J.; Jahnsen, R. Intensive training of motor function and functional skills among young children with cerebral palsy: A systematic review and meta-analysis. BMC Pediatr. 2014, 14, 292. [Google Scholar]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Landgraf, J.M. Child Health Questionnaire (CHQ). In Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Russell, D.J.; Rosenbaum, P.L.; Wright, M.; Avery, L.M. Gross Motor Function Measure (GMFM-66 & GMFM-88) User’s Manual; Mac Keith Press: London, UK, 2002. [Google Scholar]
- Franjoine, M.R.; Gunther, J.S.; Taylor, M.J. Pediatric Balance Scale: A modified version of the BBS for school-age child with mild to moderate motor impairment. Pediatr. Phys. Ther. 2003, 15, 114–128. [Google Scholar]
- Provost, B.; Dieruf, K.; Burtner, P.A.; Phillips, J.P.; Bernitsky-Beddingfield, A.; Sullivan, K.J.; Bowen, C.A.; Toser, L. Endurance and gait in children with cerebral palsy after intensive body weight-supported treadmill training. Pediatr. Phys. Ther. 2007, 19, 2–10. [Google Scholar]
- Wolf, S.L.; Catlin, P.A.; Gage, K.; Gurucharri, K.; Robertson, R.; Stephen, K. Establishing the reliability and validity of measurements of walking time using the Emory Functional Ambulation Profile. Phys Ther. 1999, 79, 1122–1133. [Google Scholar]
- Williams, E.N.; Carroll, S.G.; Reddihough, D.S.; Phillips, B.A.; Galea, M.P. Investigation of the timed “Up and Go” test in children. Dev. Med. Child. Neurol. 2005, 47, 518–524. [Google Scholar]
- Maher, C.A.; Williams, M.T.; Olds, T.S. The six-minute walk test for children with cerebral palsy. Int. J. Rehabil. Res. 2008, 31, 185–188. [Google Scholar]
- Baker, R.; McGinley, J.L.; Schwartz, M.H.; Beynon, S.; Rozumalski, A.; Graham, H.K.; Tirosh, O. The gait profile score and movement analysis profile. Gait Posture 2009, 30, 265–269. [Google Scholar] [CrossRef]
- Herson, J.; Wittes, J. The Use of Interim Analysis for Sample Size Adjustment. Drug Inf. J. 1993, 27, 753–760. [Google Scholar]
- Kwak, S.K.; Kim, J.H. Statistical data preparation: Management of missing values and outliers. Korean J. Anesthesiol. 2017, 70, 407–411. [Google Scholar]
- Küper, M.; Döring, K.; Spangenberg, C.; Konczak, J.; Gizewski, E.R.; Schoch, B.; Timmann, D. Location and restoration of function after cerebellar tumor removal-a longitudinal study of children and adolescents. Cerebellum 2013, 12, 48–58. [Google Scholar]
- Dodd, K.J.; Taylor, N.F.; Graham, H.K. A randomized clinical trial of strength training in young people with cerebral palsy. Dev. Med. Child Neurol. 2003, 45, 652–657. [Google Scholar]
- Mattern-Baxter, K.; McNeil, S.; Mansoor, J.K. Effects of home-based locomotor treadmill training on gross motor function in young children with cerebral palsy: A quasi-randomized controlled trial. Arch. Phys. Med. Rehabil. 2013, 94, 2061–2067. [Google Scholar] [CrossRef]
- Storm, F.A.; Petrarca, M.; Beretta, E.; Strazzer, S.; Piccinini, L.; Maghini, C.; Panzeri, D.; Corbetta, C.; Morganti, R.; Reni, G.; et al. Minimum Clinically Important Difference of Gross Motor Function and Gait Endurance in Children with Motor Impairment: A Comparison of Distribution-Based Approaches. Biomed. Res. Int. 2020, 2020, 2794036. [Google Scholar]
- Chen, C.L.; Shen, I.H.; Chen, C.Y.; Wu, C.Y.; Liu, W.Y.; Chung, C.Y. Validity, responsiveness, minimal detectable change, and minimal clinically important change of Pediatric Balance Scale in children with cerebral palsy. Res. Dev. Disabil. 2013, 34, 916–922. [Google Scholar] [PubMed]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang Hear Res. 2008, 51, S225–S239. [Google Scholar] [PubMed]
- Nichols-Larsen, D.S.; Kegelmeyer, D.A.; Buford, J.A.; Kloos, A.D.; Heathcock, J.C.; Michele Basso, D. Neurologic Rehabilitation: Neuroscience and Neuroplasticity in Physical Therapy Practice; McGraw-Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Schatton, C.; Synofzik, M.; Fleszar, Z.; Giese, M.A.; Schöls, L.; Ilg, W. Individualized exergame training improves postural control in advanced degenerative spinocerebellar ataxia: A rater-blinded, intra-individually controlled trial. Park. RelatDisord. 2017, 39, 80–84. [Google Scholar]
- Schmitz-Hübsch, T.; Fimmers, R.; Rakowicz, M.; Rola, R.; Zdzienicka, E.; Fancellu, R.; Mariotti, C.; Linnemann, C.; Schöls, L.; Timmann, D.; et al. Responsiveness of different rating instruments in spinocerebellar ataxia patients. Neurology 2010, 74, 678–684. [Google Scholar]
- Ilg, W.; Schatton, C.; Schicks, J.; Giese, M.A.; Schöls, L.; Synofzik, M. Video game-based coordinative training improves ataxia in children with degenerative ataxia. Neurology 2012, 79, 2056–2060. [Google Scholar]
- Verbecque, E.; Schepens, K.; Theré, J.; Schepens, B.; Klingels, K.; Hallemans, A. The Timed Up and Go Test in Children: Does Protocol Choice Matter? A Systematic Review. Pediatr. Phys. Ther. 2019, 31, 22–31. [Google Scholar]
- Nicolini-Panisson, R.D.; Donadio, M.V. Timed “Up & Go” test in children and adolescents. Rev. Paul. Pediatr. 2013, 31, 377–383. [Google Scholar]
- Fitzgerald, D.; Hickey, C.; Delahunt, E.; Walsh, M.; O’Brien, T. Six-Minute Walk Test in Children With Spastic Cerebral Palsy and Children Developing Typically. Pediatr. Phys. Ther. 2016, 28, 192–199. [Google Scholar]
- Cheng, D.K.; Nelson, M.; Brooks, D.; Salbach, N.M. Validation of stroke-specific protocols for the 10-meter walk test and 6-minute walk test conducted using 15-meter and 30-meter walkways. Top Stroke Rehabil. 2020, 27, 251–261. [Google Scholar] [CrossRef]
- Elder, G.C.; Kirk, J.; Stewart, G.; Cook, K.; Weir, D.; Marshall, A.; Leahey, L. Contributing factors to muscle weakness in children with cerebral palsy. Dev. Med. Child Neurol. 2003, 45, 542–550. [Google Scholar] [CrossRef]
- Darras, N.; Tziomaki, M.; Pasparakis, D. Motion Graph Deviation Index (MGDI): An index that enhances objectivity in clinical motion graph analysis. EEXOT 2015, 67, 53–60. [Google Scholar]
- Matsushima, A.; Yoshida, K.; Genno, H.; Murata, A.; Matsuzawa, S.; Nakamura, K.; Nakamura, A.; Ikeda, S. Clinical assessment of standing and gait in ataxic patients using a triaxial accelerometer. Cerebellum Ataxias 2015, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Chini, G.; Ranavolo, A.; Draicchio, F.; Casali, C.; Conte, C.; Martino, G.; Leonardi, L.; Padua, L.; Coppola, G.; Pierelli, F.; et al. Local Stability of the Trunk in Patients with Degenerative Cerebellar Ataxia During Walking. Cerebellum 2017, 16, 26–33. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | FPBWSTT Group | Control Group | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 14.69 ± 2.05 | 13.45 ± 2.73 | 0.304 |
| Sex, male/female | 7 (87.5%)/1 (12.5%) | 7 (70%)/3 (30%) | 0.588 |
| Weight (kg), mean ± SD | 41.56 ± 8.79 | 46.55 ± 14.53 | 0.407 |
| Height (cm), mean ± SD | 157.38 ± 11.17 | 151.90 ± 14.67 | 0.397 |
| BMI, mean ± SD | 16.86 ± 2.20 | 19.72 ± 3.56 | 0.058 |
| Type of ataxia, (non-P/P) | 7 (87.5%)/1 (12.5%) | 9 (90%)/1 (10%) | 1.000 |
| GMFCS, (II/III/IV) | 6 (75%)/1 (12.5%)/1 (12.5%) | 7 (70%)/2 (20%)/1 (10%) | 0.909 |
| Laterality, (right/left) | 8 (100%)/0 (0%) | 9 (90%)/1 (10%) | 1.000 |
| Quality of life Questionnaire | |||
| Friends/Family, mean ± SD | 7.18 ± 0.92 | 6.81 ± 0.82 | 0.375 |
| Participation, mean ± SD | 6.06 ± 2.31 | 5.65 ± 1.79 | 0.675 |
| Communication, mean ± SD | 6.58 ± 1.28 | 6.00 ± 1.50 | 0.395 |
| Use of limbs, mean ± SD | 5.99 ± 1.78 | 6.23 ± 1.62 | 0.683 |
| Self-care, median ± IR | 7.00 ± 0.0 | 7.00 ± 0.0 | 0.537 |
| Equipment, mean ± SD | 4.21 ± 1.38 | 4.21 ± 0.76 | 1.00 |
| Pain-discomfort, mean ± SD | 2.98 ± 0,77 | 2.98 ± 0.93 | 0.991 |
| Sense of happiness, median ± IR | 7.00 ± 1.0 | 7.00 ± 0.5 | 0.573 |
| Assistance with questionnaire completion, mean ± SD | 2.38 ± 1.06 | 2.40 ± 1.07 | 0.961 |
| Secondary Outcome | 4-Week Mean Difference (95% CI) | p-Value | 2-Month Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|
| PBS | 3.73 (1.13–6.33) | 0.008 | 3.85 (−0.14 to 7.71) | 0.05 |
| TUG (s) | 2.19 (0.7–3.67) | 0.007 | 1.29 (−0.53 to 3.10) | 0.151 |
| 10 MWT-SLOW (m/s) | 0.07 (0.04–0.18) | 0.193 | 0.05 (0.05–0.15) | 0.307 |
| 10 MWT-FAST (m/s) | 0.19 (0–0.38) | 0.046 | 0.04 (−0.12 to 0.21) | 0.620 |
| 6 MWT (m) | 56.09 (29.22–82.96) | <0.005 | 36.92 (7.34–66.50) | 0.018 |
| SARAgr | 1.22 (0.44–2.01) | 0.005 | 1.00 (−0.31 to 2.34) | 0.125 |
| Step Length (cm) | 8.84 (1.67–16.01) | 0.019 | 5.36 (−3.64 to 14.35) | 0.224 |
| Stride Length (cm) | 8.29 (−3.98 to 20.57) | 0.170 | 12 (−5.52 to 29.54) | 0.165 |
| Step Time (s) | 0.38 (0.01–0.74) | 0.043 | 0.45 (0.01–0.90) | 0.048 |
| Stride Time (s) | 0.45 (0.16–0.75) | 0.005 | 0.77 (0.05–1.49) | 0.038 |
| Step Width (cm) | 6.74 (4.20–9.29) | <0.005 | 7.41 (4.18–10.64) | <0.005 |
| Groups | Step Length (cm) | Stride Length (cm) | ||||
|---|---|---|---|---|---|---|
| Baseline | 4-Week | 2 Months | Baseline | 4-Week | 2 Months | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| FPBWSTT | 22.85 ± 7.56 | 28.19 ± 8.69 * | 27.49 ± 8.65 | 47.72 ± 12.74 | 54.79 ± 14.69 | 58.16 ± 18.66 |
| Control | 38.01 ± 7.04 | 35.99 ± 9.63 | 32.88 ± 7.00 | 72.29 ± 17.77 | 68.51 ± 18.27 | 64.57 ± 16.63 |
| Comparison between groups by time | p < 0.005 | p = 0.094 | p = 0.163 | p = 0.005 | p = 0.104 | p = 0.453 |
| Interaction between group and time | F(2.32) = 6.94, p < 0.005 | F(2.32) = 6.34, p = 0.005 | ||||
| Kinematic Variables | 4-Week Mean Difference (95% CI) | p-Value | 2-Month Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Pelvis Frontal Plane (NSDs) | −0.11 (−0.76 to 0.74) | 0.972 | 0.57 (−0.42 to 1.57) | 0.210 |
| Pelvis Transverse Plane (NSDs) | −0.08 (−1.45 to 1.29) | 0.888 | −0.22 (−1.41 to 0.97) | 0.671 |
| Hip Sagittal Plane (NSDs) | −0.21 (−0.98 to 0.54) | 0.512 | −0.54 (−0.45 to 1.53) | 0.232 |
| Knee Sagittal Plane (NSDs) | −0.42 (−1.68 to 0.85) | 0.450 | −0.06 (−1.41 to 1.29) | 0.919 |
| Ankle Sagittal Plane (NSDs) | −0.21 (−0.70 to 0.28) | 0.337 | 0.18 (−0.42 to 0.78) | 0.482 |
| Ankle Transverse Plane (NSDs) | −0.09 (−01.05 to 0.88) | 0.829 | −0.42 (−1.69 to 0.86) | 0.452 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepoura, A.; Lampropoulou, S.; Galanos, A.; Papadopoulou, M.; Gkrimas, G.; Tziomaki, M.; Sakellari, V. Effects of Functional Partial Body Weight Support Treadmill Training on Mobility in Children with Ataxia: A Randomized Controlled Trial. J. Funct. Morphol. Kinesiol. 2025, 10, 123. https://doi.org/10.3390/jfmk10020123
Lepoura A, Lampropoulou S, Galanos A, Papadopoulou M, Gkrimas G, Tziomaki M, Sakellari V. Effects of Functional Partial Body Weight Support Treadmill Training on Mobility in Children with Ataxia: A Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology. 2025; 10(2):123. https://doi.org/10.3390/jfmk10020123
Chicago/Turabian StyleLepoura, Alexandra, Sofia Lampropoulou, Antonis Galanos, Marianna Papadopoulou, Georgios Gkrimas, Magda Tziomaki, and Vasiliki Sakellari. 2025. "Effects of Functional Partial Body Weight Support Treadmill Training on Mobility in Children with Ataxia: A Randomized Controlled Trial" Journal of Functional Morphology and Kinesiology 10, no. 2: 123. https://doi.org/10.3390/jfmk10020123
APA StyleLepoura, A., Lampropoulou, S., Galanos, A., Papadopoulou, M., Gkrimas, G., Tziomaki, M., & Sakellari, V. (2025). Effects of Functional Partial Body Weight Support Treadmill Training on Mobility in Children with Ataxia: A Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology, 10(2), 123. https://doi.org/10.3390/jfmk10020123








