Upper-Limb Muscle Fatigability in Para-Athletes Quantified as the Rate of Force Development in Rapid Contractions of Submaximal Amplitude
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment and Characteristics of Athletes
2.2. Anthropometric Measurements
2.3. Neuromuscular Function Evaluation
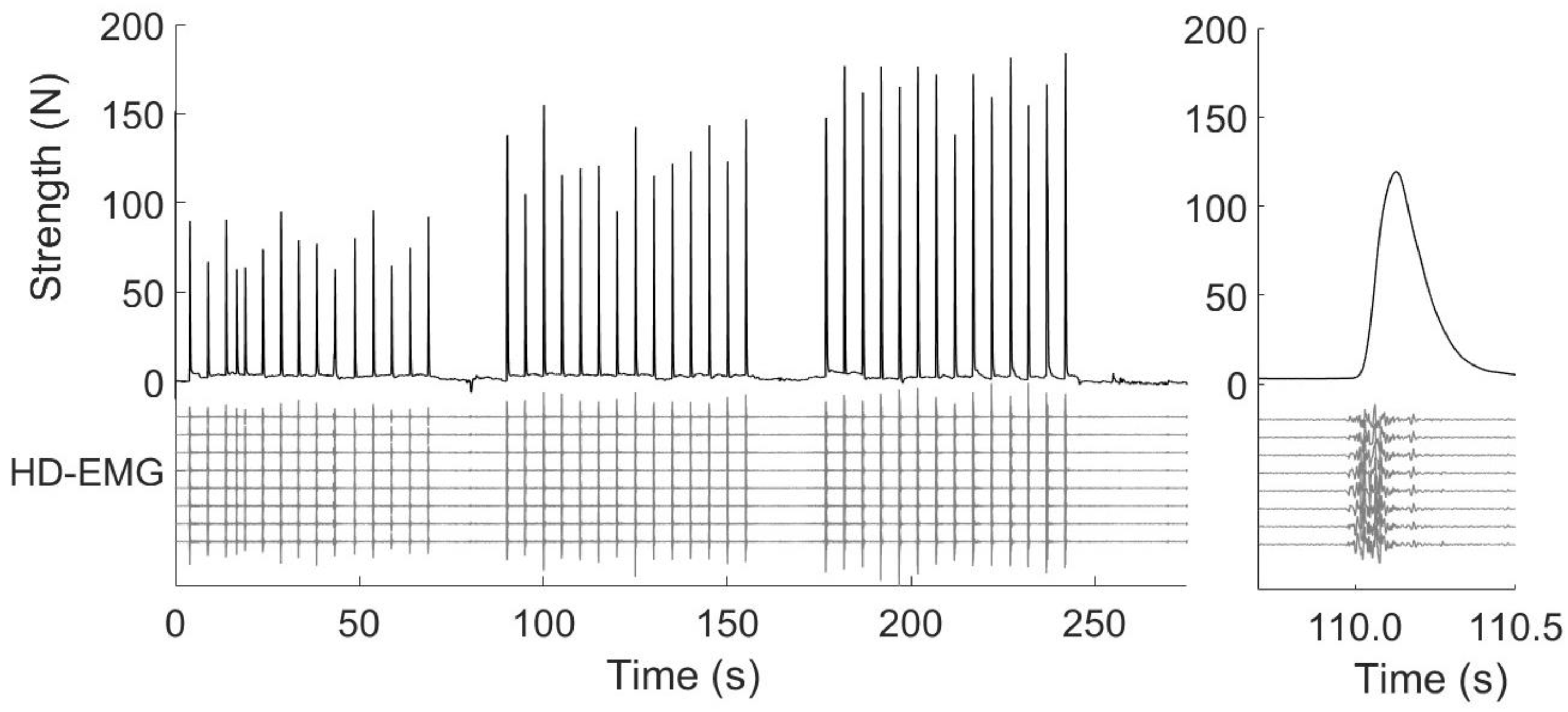
2.3.1. Setup
2.3.2. High-Density Surface Electromyography
2.3.3. Procedures
2.4. Incremental Arm-Cranking Graded Test
2.5. Signal Processing
2.5.1. Onset Determination and Time Windows
2.5.2. Force Signal
2.5.3. High-Density Surface Electromyography
2.6. Statistical Analysis
3. Results
3.1. Incremental Arm-Cranking Graded Test
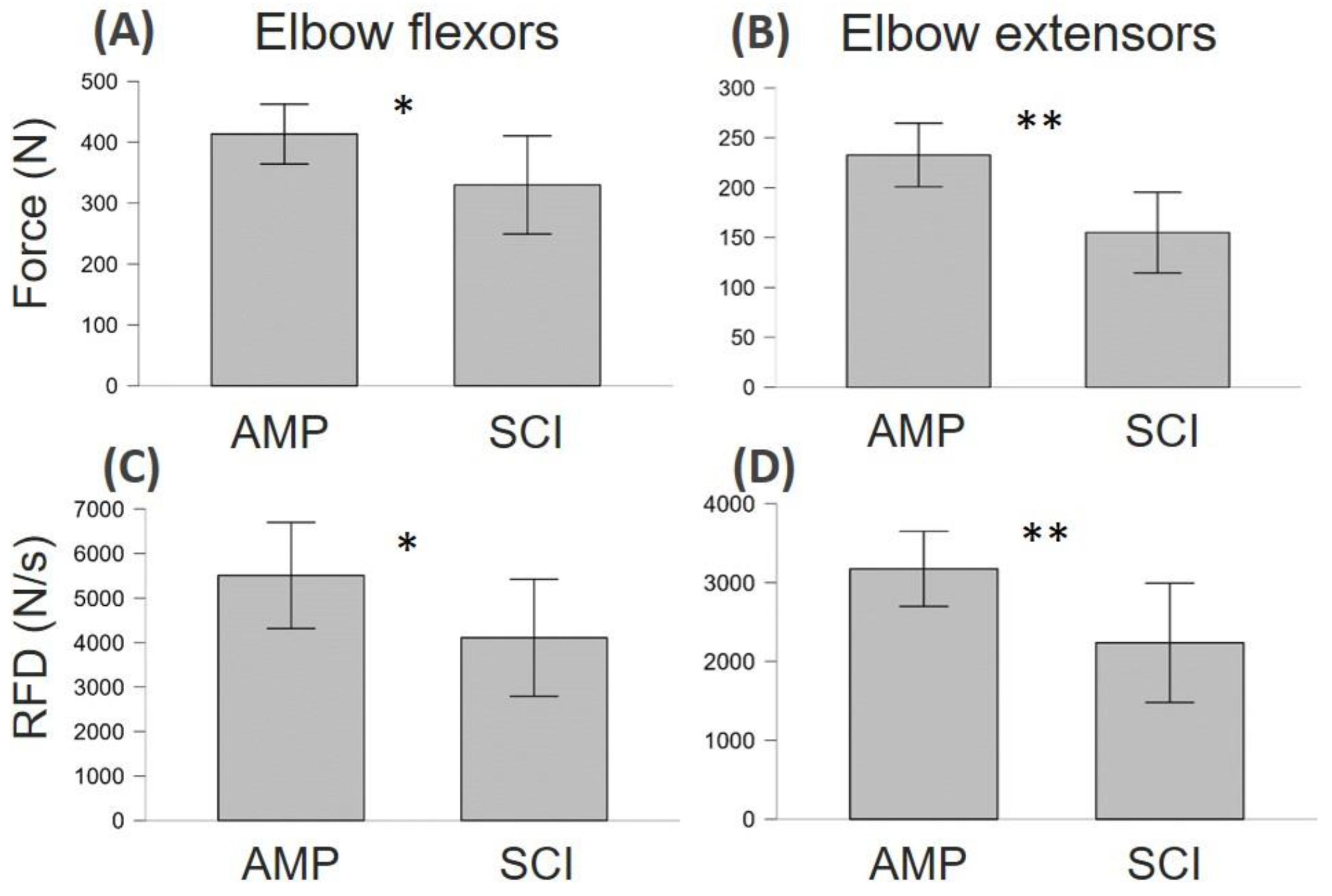
3.2. Neuromuscular Function Differences between AMP and SCI
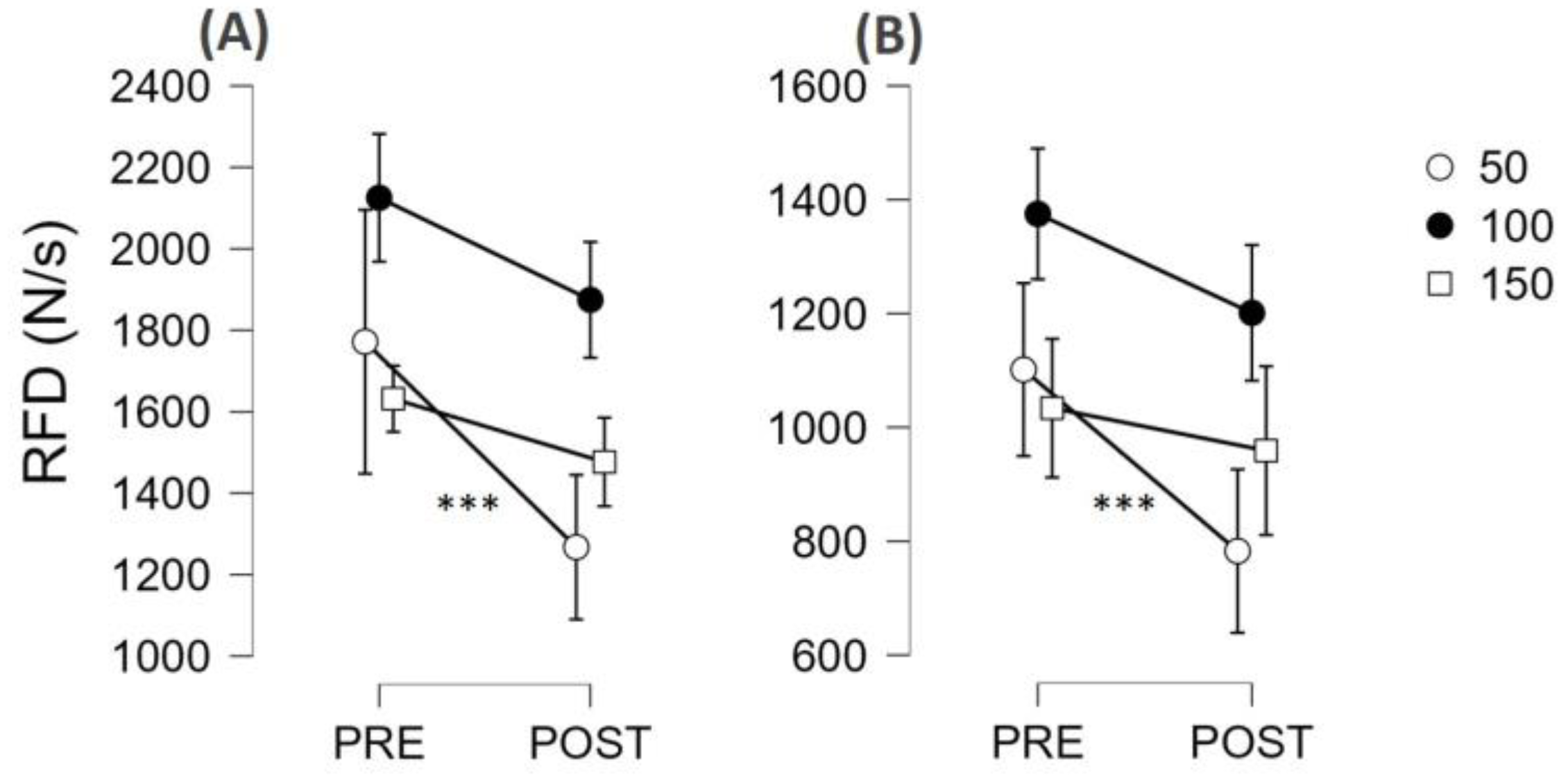
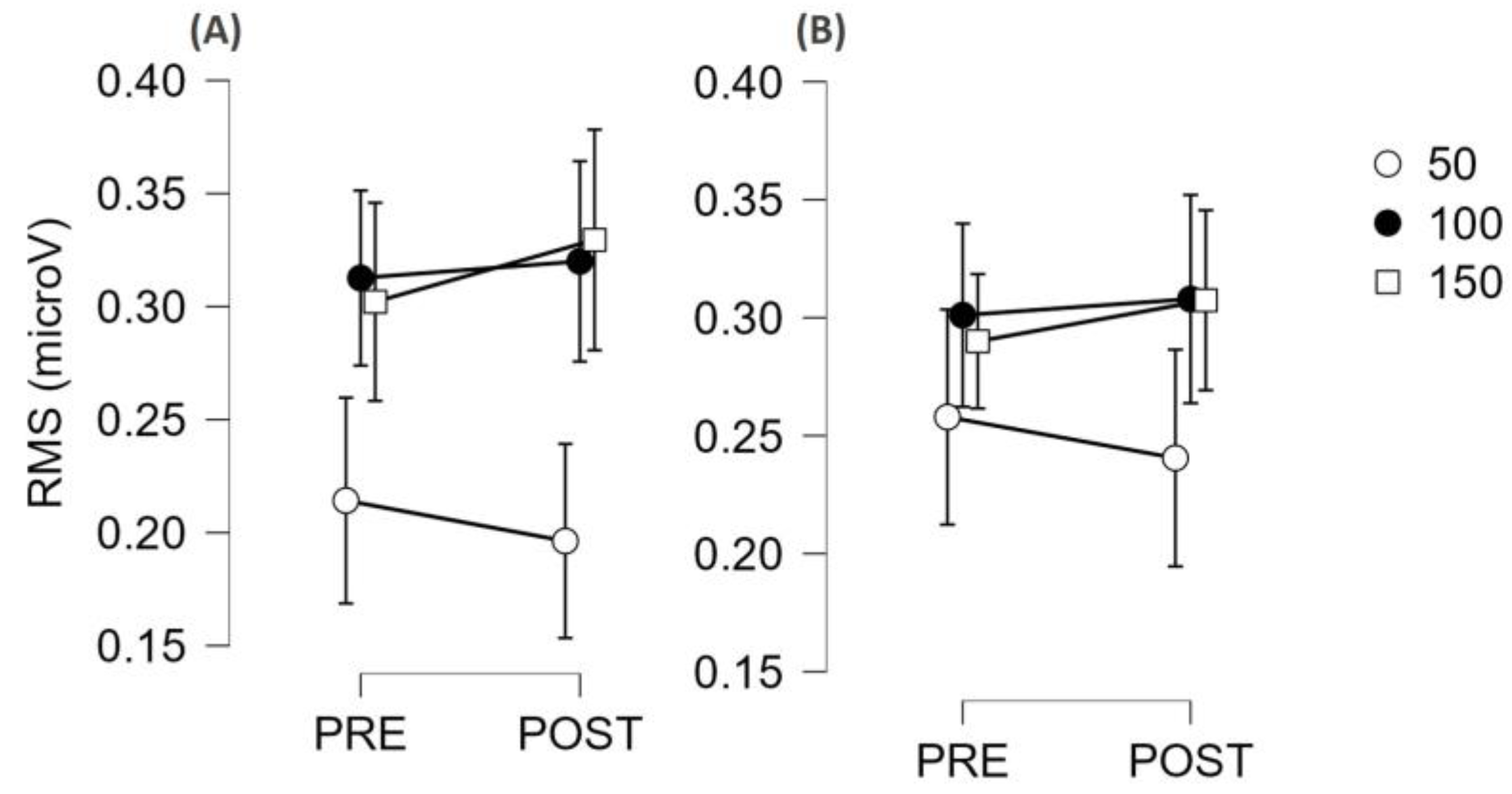
3.3. Fatigability Effect on Neuromuscular Function
4. Discussion
4.1. Differences and Similarities between SCI and AMP
4.2. Fatigability Expressed as Reduction of MVF or RFDpeak
4.3. Contraction Quickness in Ballistic Contractions of Submaximal Amplitude
4.4. Electromyographic Parameters
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, L.; Zhang, L.; Lin, X.-B.; Ferguson-Pell, M. Wheelchair propulsion fatigue thresholds in electromyographic and ventilatory testing. Spinal Cord 2020, 58, 1104–1111. [Google Scholar] [CrossRef]
- Van der Woude, L.H.; de Groot, S.; Janssen, T.W. Manual wheelchairs: Research and innovation in rehabilitation, sports, daily life and health. Med. Eng. Phys. 2006, 28, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.M.; McQuade, K.J.; Rasch, E.K.; Keyser, R.E.; Finley, M.A. Upper-limb fatigue-related joint power shifts in experienced wheelchair users and nonwheelchair users. J. Rehabil. Res. Dev. 2003, 40, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A.; Aagaard, P.; Blazevich, A.J.; Folland, J.; Tillin, N.; Duchateau, J. Rate of force development: Physiological and methodological considerations. Eur. J. Appl. Physiol. 2016, 116, 1091–1116. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rosell, D.; Pareja-Blanco, F.; Aagaard, P.; Gonzalez-Badillo, J.J. Physiological and methodological aspects of rate of force development assessment in human skeletal muscle. Clin. Physiol. Funct. Imaging 2018, 38, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Tillin, N.A.; Jimenez-Reyes, P.; Pain, M.T.; Folland, J.P. Neuromuscular performance of explosive power athletes versus untrained individuals. Med. Sci. Sports. Exerc. 2010, 42, 781–790. [Google Scholar] [CrossRef]
- McLellan, C.P.; Lovell, D.I.; Gass, G.C. The role of rate of force development on vertical jump performance. J. Strength Cond. Res. 2011, 25, 379–385. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.D.; Maganaris, C.N.; Seynnes, O.R.; Rennie, M.J.; Narici, M.V. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J. Physiol. 2007, 583, 1079–1091. [Google Scholar] [CrossRef]
- Andersen, L.L.; Andersen, C.H.; Mortensen, O.S.; Poulsen, O.M.; Bjornlund, I.B.; Zebis, M.K. Muscle activation and perceived loading during rehabilitation exercises: Comparison of dumbbells and elastic resistance. Phys. Ther. 2010, 90, 538–549. [Google Scholar] [CrossRef]
- Angelozzi, M.; Madama, M.; Corsica, C.; Calvisi, V.; Properzi, G.; Mccaw, S.T.; Cacchio, A. Rate of Force Development as an Adjunctive Outcome Measure for Return-to-Sport Decisions After Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. 2012, 42, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Buckthorpe, M.; Pain, M.T.; Folland, J.P. Central fatigue contributes to the greater reductions in explosive than maximal strength with high-intensity fatigue. Exp. Physiol. 2014, 99, 964–973. [Google Scholar] [CrossRef]
- Peñailillo, L.; Blazevich, A.; Numazawa, H.; Nosaka, K. Rate of force development as a measure of muscle damage. Scand. J. Med. Sci. Sports 2015, 25, 417–427. [Google Scholar] [CrossRef]
- Rice, D.A.; Mannion, J.; Lewis, G.N.; McNair, P.J.; Fort, L. Experimental knee pain impairs joint torque and rate of force development in isometric and isokinetic muscle activation. Eur. J. Appl. Physiol. 2019, 119, 2065–2073. [Google Scholar] [CrossRef]
- Del Vecchio, A. Neuromechanics of the Rate of Force Development. Exerc. Sport Sci. Rev. 2023, 51, 34–42. [Google Scholar] [CrossRef] [PubMed]
- D’Emanuele, S.; Maffiuletti, N.A.; Tarperi, C.; Rainoldi, A.; Schena, F.; Boccia, G. Rate of Force Development as an Indicator of Neuromuscular Fatigue: A Scoping Review. Front. Hum. Neurosci. 2021, 15, 701916. [Google Scholar] [CrossRef]
- Boccia, G.; D’Emanuele, S.; Brustio, P.R.; Rainoldi, A.; Schena, F.; Tarperi, C. Decreased neural drive affects the early rate of force development after repeated burst-like isometric contractions. Scand. J. Med. Sci. Sports 2024, 34, e14528. [Google Scholar] [CrossRef] [PubMed]
- D’Emanuele, S.; Tarperi, C.; Rainoldi, A.; Schena, F.; Boccia, G. Neural and contractile determinants of burst-like explosive isometric contractions of the knee extensors. Scand. J. Med. Sci. Sports 2023, 33, 127–135. [Google Scholar] [CrossRef]
- Cossich, V.; Maffiuletti, N. Early vs. late rate of torque development: Relation with maximal strength and influencing factors. J. Electromyogr. Kinesiol. 2020, 55, 102486. [Google Scholar] [CrossRef]
- Bellumori, M.; Jaric, S.; Knight, C.A. The rate of force development scaling factor (RFD-SF): Protocol, reliability, and muscle comparisons. Exp. Brain Res. 2011, 212, 359–369. [Google Scholar] [CrossRef]
- Brustio, P.R.; Casale, R.; Buttacchio, G.; Calabrese, M.; Bruzzone, M.; Rainoldi, A.; Boccia, G. Relevance of evaluating the rate of torque development in ballistic contractions of submaximal amplitude. Physiol. Meas. 2019, 40, 025002. [Google Scholar] [CrossRef] [PubMed]
- Casartelli, N.C.; Lepers, R.; Maffiuletti, N.A. Assessment of the rate of force development scaling factor for the hip muscles. Muscle Nerve 2014, 50, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, D.; Uygur, M. Methodological considerations in the calculation of the rate of force development scaling factor. Physiol. Meas. 2017, 39, 015001. [Google Scholar] [CrossRef] [PubMed]
- Freund, H.J.; Budingen, H.J. The relationship between speed and amplitude of the fastest voluntary contractions of human arm muscles. Exp. Brain Res. 1978, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Klass, M.; Baudry, S.; Duchateau, J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J. Appl. Physiol. 2008, 104, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, M.M.; Wiegner, A.W.; Logigian, E.L.; Young, R.R. Abnormal most-rapid isometric contractions in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1991, 54, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. J. Neurophysiol. 2006, 95, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Gizzi, L.; Nielsen, J.F.; Felici, F.; Ivanenko, Y.P.; Farina, D. Impulses of activation but not motor modules are preserved in the locomotion of subacute stroke patients. J. Neurophysiol. 2011, 106, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Bellumori, M.; Jaric, S.; Knight, C.A. Age-related decline in the rate of force development scaling factor. Motor Control 2013, 17, 370–381. [Google Scholar] [CrossRef]
- Corrêa, T.G.; Donato, S.V.; Lima, K.C.; Pereira, R.V.; Uygur, M.; de Freitas, P.B. Age-and Sex-Related Differences in the Maximum Muscle Performance and Rate of Force Development Scaling Factor of Precision Grip Muscles. Motor Control 2020, 24, 274–290. [Google Scholar] [CrossRef]
- Kim, J.J.; Delmas, S.; Choi, Y.J.; Hubbard, J.C.; Weintraub, M.; Arabatzi, F.; Yacoubi, B.; Christou, E.A. Unique Neural Mechanisms Underlying Speed Control of Low-Force Ballistic Contractions. J. Hum. Kinet. 2024, 90, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; Dardanello, D.; Brustio, P.R.; Tarperi, C.; Festa, L.; Zoppirolli, C.; Pellegrini, B.; Schena, F.; Rainoldi, A. Neuromuscular Fatigue Does Not Impair the Rate of Force Development in Ballistic Contractions of Submaximal Amplitudes. Front. Physiol. 2018, 9, 1503. [Google Scholar] [CrossRef] [PubMed]
- Toti, E.; Cavedon, V.; Raguzzini, A.; Fedullo, A.L.; Milanese, C.; Bernardi, E.; Bellito, S.; Bernardi, M.; Sciarra, T.; Peluso, I. Dietary intakes and food habits of wheelchair basketball athletes compared to gym attendees and individuals who do not practice sport activity. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2022, 22, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; D’Emanuele, S.; Brustio, P.R.; Beratto, L.; Tarperi, C.; Casale, R.; Sciarra, T.; Rainoldi, A. Strength Asymmetries Are Muscle-Specific and Metric-Dependent. Int. J. Environ. Res. Public Health 2022, 19, 8495. [Google Scholar] [CrossRef] [PubMed]
- Beretta Piccoli, M.; Rainoldi, A.; Heitz, C.; Wuthrich, M.; Boccia, G.; Tomasoni, E.; Spirolazzi, C.; Egloff, M.; Barbero, M. Innervation zone locations in 43 superficial muscles: Toward a standardization of electrode positioning. Muscle Nerve 2014, 49, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Muceli, S. Tutorial. Surface EMG detection in space and time: Best practices. J. Electromyogr. Kinesiol. 2019, 49, 102363. [Google Scholar] [CrossRef] [PubMed]
- Smajla, D.; Žitnik, J.; Šarabon, N. Advancements in the Protocol for Rate of Force Development/Relaxation Scaling Factor Evaluation. Front. Hum. Neurosci. 2021, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Crotty, E.D.; Furlong, L.M.; Hayes, K.; Harrison, A.J. Onset detection in surface electromyographic signals across isometric explosive and ramped contractions: A comparison of computer-based methods. Physiol. Meas. 2021, 42, 035010. [Google Scholar] [CrossRef] [PubMed]
- Haff, G.G.; Ruben, R.P.; Lider, J.; Twine, C.; Cormie, P. A Comparison of Methods for Determining the Rate of Force Development During Isometric Midthigh Clean Pulls. J. Strength Cond. Res. 2015, 29, 386–395. [Google Scholar] [CrossRef]
- Ditroilo, M.; Forte, R.; Benelli, P.; Gambarara, D.; De Vito, G. Effects of age and limb dominance on upper and lower limb muscle function in healthy males and females aged 40–80 years. J. Sport Sci. 2010, 28, 667–677. [Google Scholar] [CrossRef]
- Cook, R.D. Detection of influential observation in linear regression. Technometrics 1977, 19, 15–18. [Google Scholar]
- Balshaw, T.G.; Fry, A.; Maden-Wilkinson, T.M.; Kong, P.W.; Folland, J.P. Reliability of quadriceps surface electromyography measurements is improved by two vs. single site recordings. Eur. J. Appl. Physiol. 2017, 117, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; Dardanello, D.; Tarperi, C.; Festa, L.; La Torre, A.; Pellegrini, B.; Schena, F.; Rainoldi, A. Fatigue-induced dissociation between rate of force development and maximal force across repeated rapid contractions. Hum. Mov. Sci. 2017, 54, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; Dardanello, D.; Zoppirolli, C.; Bortolan, L.; Cescon, C.; Schneebeli, A.; Vernillo, G.; Schena, F.; Rainoldi, A.; Pellegrini, B. Central and peripheral fatigue in knee and elbow extensor muscles after a long-distance cross-country ski race. Scand. J. Med. Sci. Sports 2016, 27, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Negro, F.; Holobar, A.; Casolo, A.; Folland, J.P.; Felici, F.; Farina, D. You are as fast as your motor neurons: Speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J. Physiol. 2019, 597, 2445–2456. [Google Scholar] [CrossRef] [PubMed]
- Kozinc, Z.; Smajla, D.; Sarabon, N. The rate of force development scaling factor: A review of underlying factors, assessment methods and potential for practical applications. Eur. J. Appl. Physiol. 2022, 122, 861–873. [Google Scholar] [CrossRef]
- Kozinc, Ž.; Šarabon, N. Inter-Limb Asymmetries in Volleyball Players: Differences between Testing Approaches and Association with Performance. J. Sports Sci. Med. 2020, 19, 745–752. [Google Scholar] [PubMed]
- Smajla, D.; Žitnik, J.; Sarabon, N. Quantification of inter-limb symmetries with rate of force development and relaxation scaling factor. Front. Physiol. 2021, 12, 871. [Google Scholar] [CrossRef]
- Bellumori, M.; Uygur, M.; Knight, C.A. High-Speed Cycling Intervention Improves Rate-Dependent Mobility in Older Adults. Med. Sci. Sports Exerc. 2017, 49, 106–114. [Google Scholar] [CrossRef]
- Uygur, M.; Barone, D.A.; Dankel, S.J.; DeStefano, N. Isometric tests to evaluate upper and lower extremity functioning in people with multiple sclerosis: Reliability and validity. Mult. Scler. Relat. Disord. 2022, 63, 103817. [Google Scholar] [CrossRef]



| AMP | SCI | |
|---|---|---|
| Age, years | 43.3 ± 2.8 | 44.6 ± 3.4 |
| Lesion level (incomplete for SCI, prosthesis for AMP) | Above knee, n = 3 Below knee, n = 6 Forearm, n = 1 Wheelchair users (2/10) | Tetraplegia (C6-C7), n = 3 Paraplegia (T7-T12), n = 7 Wheelchair users (10/10) |
| Sport practiced | Athletics, Basketball, Cycling, Sitting volleyball, Swimming, Tennis | Athletics, Archery, Hand-bike, Sitting Volleyball, Swimming, Power Soccer |
| Training/h week | 5.3 ± 0.9 | 5.2 ± 1.0 |
| Television/h week | 12.0 ± 3.6 | 11.8 ± 2.6 |
| Screening/h week | 27.9 ± 7.7 | 22.4 ± 4.1 |
| Sleeping/h day | 7.1 ± 0.3 | 7.6 ± 0.2 |
| Neurogenic bowel, % | - | 40% |
| Neurogenic bladder, % | - | 40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccia, G.; Brustio, P.R.; Beratto, L.; Peluso, I.; Ferrara, R.; Munzi, D.; Toti, E.; Raguzzini, A.; Sciarra, T.; Rainoldi, A. Upper-Limb Muscle Fatigability in Para-Athletes Quantified as the Rate of Force Development in Rapid Contractions of Submaximal Amplitude. J. Funct. Morphol. Kinesiol. 2024, 9, 108. https://doi.org/10.3390/jfmk9020108
Boccia G, Brustio PR, Beratto L, Peluso I, Ferrara R, Munzi D, Toti E, Raguzzini A, Sciarra T, Rainoldi A. Upper-Limb Muscle Fatigability in Para-Athletes Quantified as the Rate of Force Development in Rapid Contractions of Submaximal Amplitude. Journal of Functional Morphology and Kinesiology. 2024; 9(2):108. https://doi.org/10.3390/jfmk9020108
Chicago/Turabian StyleBoccia, Gennaro, Paolo Riccardo Brustio, Luca Beratto, Ilaria Peluso, Roberto Ferrara, Diego Munzi, Elisabetta Toti, Anna Raguzzini, Tommaso Sciarra, and Alberto Rainoldi. 2024. "Upper-Limb Muscle Fatigability in Para-Athletes Quantified as the Rate of Force Development in Rapid Contractions of Submaximal Amplitude" Journal of Functional Morphology and Kinesiology 9, no. 2: 108. https://doi.org/10.3390/jfmk9020108






