Post-Arthroplasty Spatiotemporal Gait Parameters in Patients with Hip Osteoarthritis or Developmental Dysplasia of the Hip: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
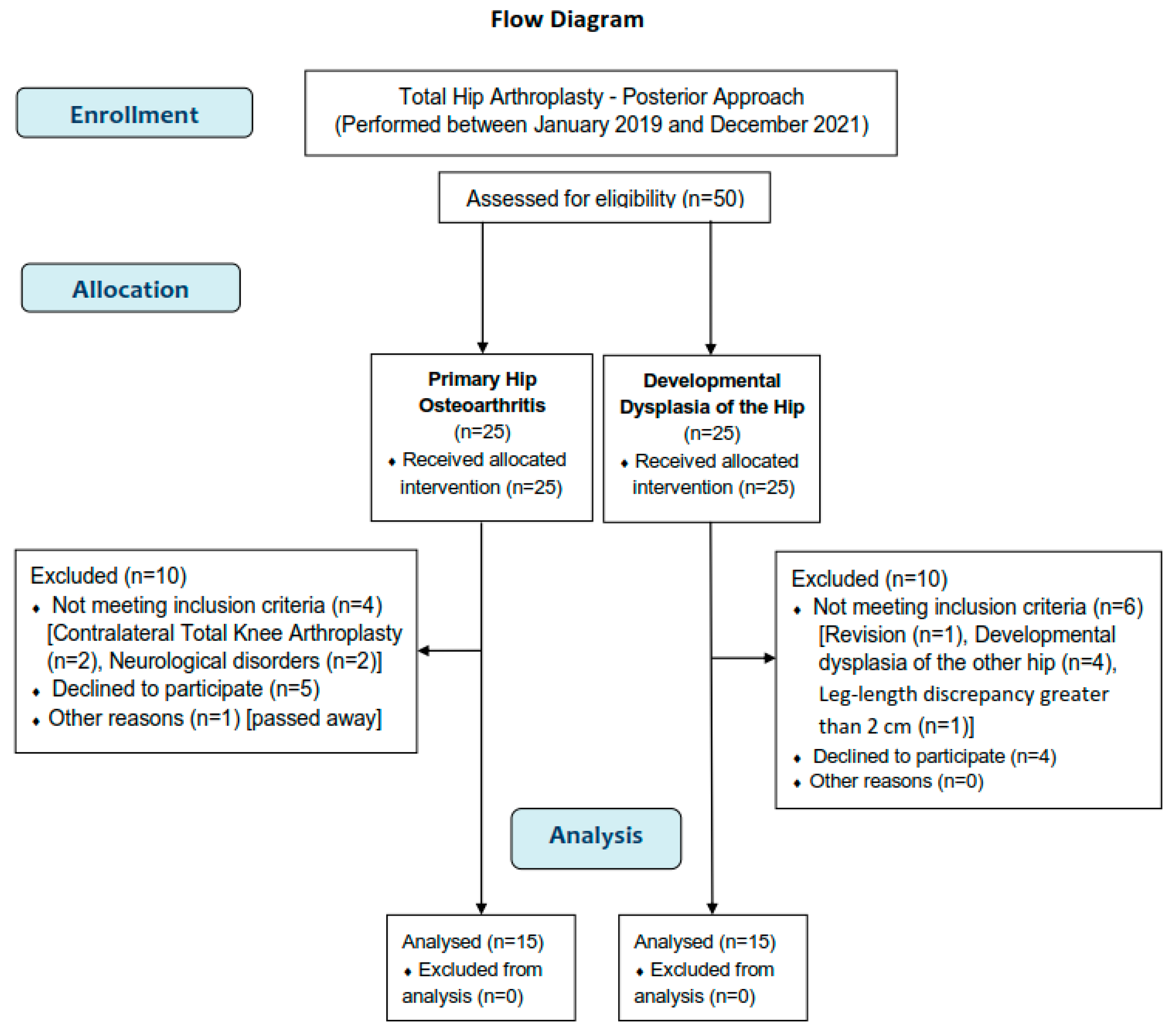
2.2. Participants
2.3. Outcomes
2.4. Instrumentation and Procedure
2.5. Modeling—Placement of Markers
2.6. Data Synthesis
2.7. Statistical Analysis
3. Results
3.1. Participants
3.2. Demographic and Clinical Characteristics
3.3. Correlation Analysis
3.4. Group Differences
3.5. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pivec, R.; Johnson, A.J.; Mears, S.C.; Mont, M.A. Hip arthroplasty. Lancet 2012, 380, 1768–1777. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Altman, R.D.; Hochberg, M.C.; Moskowitz, R.W.; Schnitzer, T.J. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000, 43, 1905–1915. [Google Scholar] [CrossRef]
- Moura, D.L.; Figueiredo, A. High congenital hip dislocation in adults—Arthroplasty and functional results. Rev. Bras. Ortop. 2018, 53, 226–235. [Google Scholar] [CrossRef]
- Hartofilakidis, G.; Stamos, K.; Ioannidis, T.T. Low friction arthroplasty for old untreated congenital dislocation of the hip. J. Bone Jt. Surg. Br. Vol. 1988, 70, 182–186. [Google Scholar] [CrossRef]
- Erdemli, B.; Yilmaz, C.; Atalar, H.; Güzel, B.; Cetin, I. Total hip arthroplasty in developmental high dislocation of the hip. J. Arthroplast. 2005, 20, 1021–1028. [Google Scholar] [CrossRef]
- Bennett, D.; Humphreys, L.; O’brien, S.; Kelly, C.; Orr, J.F.; Beverland, D.E. Gait kinematics of age-stratified hip replacement patients—A large scale, long-term follow-up study. Gait Posture 2008, 28, 194–200. [Google Scholar] [CrossRef]
- van den Akker-Scheek, I.; Stevens, M.; Bulstra, S.K.; Groothoff, J.W.; van Horn, J.R.; Zijlstra, W. Recovery of gait after short-stay total hip arthroplasty. Arch. Phys. Med. Rehabil. 2007, 88, 361–367. [Google Scholar] [CrossRef]
- Xu, C.; Wen, X.; Wei, W.; Huang, L.; Wang, J.; Yan, Y.; Lei, W. Gait parameters associated with untreated developmental dysplasia of the hip: A systematic review. Int. J. Clin. Exp. Med. 2017, 10, 13037–13047. [Google Scholar]
- Constantinou, M.; Barrett, R.; Brown, M.; Mills, P. Spatial-temporal gait characteristics in individuals with hip osteoarthritis: A systematic literature review and meta-analysis. J. Orthop. Sports Phys. Ther. 2014, 44, 291-B7. [Google Scholar] [CrossRef]
- Cichy, B.; Wilk, M. Gait analysis in osteoarthritis of the hip. Med. Sci. Monit. 2006, 12, CR507–CR513. [Google Scholar]
- Romano, C.L.; Frigo, C.; Randelli, G.; Pedotti, A. Analysis of the gait of adults who had residual of congenital dysplasia of the hip. J. Bone Jt. Surg. Am. 1996, 78, 1468–1479. [Google Scholar] [CrossRef]
- Jacobsen, J.S.; Nielsen, D.B.; Sorensen, H.; Soballe, K.; Mechlenburg, I. Changes in walking and running in patients with hip dysplasia. Acta Orthop. 2013, 84, 265–270. [Google Scholar] [CrossRef]
- Lai, K.A.; Lin, C.J.; Su, F.C. Gait analysis of adult patients with complete congenital dislocation of the hip. J. Formos. Med. Assoc. 1997, 96, 740–744. [Google Scholar]
- Cho, S.H.; Lee, S.H.; Kim, K.H.; Yu, J.Y. Gait analysis before and after total hip arthroplasty in hip dysplasia and osteonecrosis of the femoral head. J. Korean Orthop. Assoc. 2004, 39, 482–488. [Google Scholar] [CrossRef]
- Bennett, D.; Humphreys, L.; O’Brien, S.; Beverland, D.E. Temporospatial parameters of hip replacement patients ten years post-operatively. Int. Orthop. 2009, 33, 1203–1207. [Google Scholar] [CrossRef]
- Guedes, R.C.; Dias, J.M.; Dias, R.C.; Borges, V.S.; Lustosa, L.P.; Rosa, N.M. Total hip arthroplasty in the elderly: Impact on functional performance. Braz. J. Phys. Ther. 2011, 15, 123–130. [Google Scholar] [CrossRef]
- Lai, K.A.; Lin, C.J.; Jou, I.M.; Su, F.C. Gait analysis after total hip arthroplasty with leg-length equalization in women with unilateral congenital complete dislocation of the hip—Comparison with untreated patients. J. Orthop. Res. 2001, 19, 1147–1152. [Google Scholar] [CrossRef]
- Nie, Y.; Ning, N.; Pei, F.; Shen, B.; Zhou, Z.; Li, Z. Gait kinematic deviations in patients with developmental dysplasia of the hip treated with total hip arthroplasty. Orthopedics 2017, 40, e425–e431. [Google Scholar] [CrossRef]
- Marangoz, S.; Atilla, B.; Gök, H.; Yavuzer, G.; Ergin, S.; Tokgözoğlu, A.M.; Alpaslan, M. Gait analysis in adults with severe hip dysplasia before and after total hip arthroplasty. Hip Int. 2010, 20, 466–472. [Google Scholar] [CrossRef]
- John, S.; Esch, M.; Steinert, M.; Witte, K. Relationship between self-reported function, functional tests and biomechanical parameters in patients 12 months after total hip arthroplasty: A preliminary cross-sectional study. Indian J. Orthop. 2023, 57, 1032–1040. [Google Scholar] [CrossRef]
- Bolink, S.A.; Lenguerrand, E.; Brunton, L.R.; Wylde, V.; Gooberman-Hill, R.; Heyligers, I.C.; Blom, A.W.; Grimm, B. Assessment of physical function following total hip arthroplasty: Inertial sensor based gait analysis is supplementary to patient-reported outcome measures. Clin. Biomech. 2016, 32, 171–179. [Google Scholar] [CrossRef]
- World Medical Association (WMA). Declaration of Helsinki—Ethical principles for medical research involving human subjects. 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 2 October 2021).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Prosser, G.H.; Yates, P.J.; Wood, D.J.; Graves, S.E.; de Steiger, R.N.; Miller, L.N. Outcome of primary resurfacing hip replacement: Evaluation of risk factors for early revision. Acta Orthop. 2010, 81, 66–71. [Google Scholar] [CrossRef]
- Huch, K.; Müller, K.A.C.; Stürmer, T.; Brenner, H.; Puhl, W.; Günther, K.-P. Sports activities 5 years after total knee or hip arthroplasty: The Ulm Osteoarthritis Study. Ann. Rheum. Dis. 2005, 64, 1715–1720. [Google Scholar] [CrossRef]
- Hoppenfeld, S.; DeBoer, P.; Buckley, R. Surgical Exposures in Orthopaedics: The Anatomic Approach, 5th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2017; pp. 800–820. [Google Scholar]
- Papachristou, G.C.; Pappa, E.; Chytas, D.; Masouros, P.T.; Nikolaou, V.S. Total Hip Replacement in Developmental Hip Dysplasia: A narrative review. Cureus 2021, 13, e14763. [Google Scholar] [CrossRef]
- Kellgren, J.H. Atlas of standard radiographs of arthritis. In The Epidemiology of Chronic Rheumatism; Ball, J.R., Jeffrey, M.R., Kellgren, J.H., Eds.; Blackwell: London, UK, 1963; Volume 2, pp. 22–23. [Google Scholar]
- Crowe, J.F.; Mani, V.J.; Ranawat, C.S. Total hip replacement in congenital dislocation and dysplasia of the hip. J. Bone Jt. Surg. Am. 1979, 61, 15–23. [Google Scholar] [CrossRef]
- Dawson, J.; Fitzpatrick, R.; Carr, A.; Murray, D. Questionnaire on the perceptions of patients about total hip replacement. J. Bone Jt. Surg. Br. 1996, 78, 185–190. [Google Scholar] [CrossRef]
- Murray, D.W.; Fitzpatrick, R.; Rogers, K.; Pandit, H.; Beard, D.J.; Carr, A.J.; Dawson, J. The use of the Oxford hip and knee scores. J. Bone Jt. Surg. Br. 2007, 89, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Vicon Motion Systems Ltd. Vicon Plug-in Gait Reference Guide. Available online: https://help.vicon.com/space/Nexus216/11607059/Plug-in+Gait+Reference+Guide (accessed on 6 May 2022).
- Hof, A.L. Scaling gait data to body size. Gait Posture 1996, 4, 222–223. [Google Scholar] [CrossRef]
- van Berkel, A.C.; Schiphof, D.; Waarsing, J.H.; Runhaar, J.; van Ochten, J.M.; Bindels, P.J.E.; Bierma-Zeinstra, S.M.A. 10-Year natural course of early hip osteoarthritis in middle-aged persons with hip pain: A CHECK study. Ann. Rheum. Dis. 2021, 80, 487–493. [Google Scholar] [CrossRef]
- Lespasio, M.J.; Sultan, A.A.; Piuzzi, N.S.; Khlopas, A.; Husni, M.E.; Muschler, G.F.; Mont, M.A. Hip osteoarthritis: A Primer. Perm. J. 2018, 22, 17–084. [Google Scholar] [CrossRef]
- Wang, Y. Current concepts in developmental dysplasia of the hip and Total hip arthroplasty. Arthroplasty 2019, 1, 2. [Google Scholar] [CrossRef]
- Murphy, L.B.; Helmick, C.G.; Schwartz, T.A.; Renner, J.B.; Tudor, G.; Koch, G.G.; Dragomir, A.D.; Kalsbeek, W.D.; Luta, G.; Jordan, J.M. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthr. Cartil. 2010, 18, 1372–1379. [Google Scholar] [CrossRef]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef]
- Mercieca-Bebber, R.; King, M.T.; Calvert, M.J.; Stockler, M.R.; Friedlander, M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat. Outcome Meas. 2018, 9, 353–367. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Papagiannis, G.; Nikolaou, V.S.; Papagelopoulos, P.J.; Babis, G.C. Similar biomechanical behavior in gait analysis between Ceramic-on-Ceramic and Ceramic-on-XLPE Total Hip Arthroplasties. Life 2021, 11, 1366. [Google Scholar] [CrossRef]

| Characteristics | OA Group (N = 15) | DDH Group (N = 15) | p-Value |
|---|---|---|---|
| Age (years) | 60.1 ± 3.82 | 46.13 ± 5.93 | <0.005 |
| Sex (Men/Women) [N (%)] | 5(33.3%)/10(66.7%) | 3(20%)/12(80%) | 0.409 |
| Height (cm) | 163.95 ± 3.6 | 164.42 ± 3.1 | 0.181 |
| Weight (kg) | 69.35 ± 5.6 | 68.41 ± 4.5 | 0.135 |
| Body Mass Index (kg/m2) | 25.78 ± 2.6 | 25.30 ± 2.07 | 0.289 |
| Years post-THA | 3.91 ± 0.52 | 3.69 ± 0.52 | 0.123 |
| Items | OA Group (N = 15) | DDH Group (N = 15) |
|---|---|---|
| 1. How would you describe the pain you usually have in your hip? | 3.40 ± 0.74 | 3.33 ± 0.72 |
| 2. Have you had any trouble with washing and drying yourself (all over) because of your hip? | 4.00 ± 0.00 | 3.93 ± 0.26 |
| 3. Have you had any trouble getting in and out of a car or using public transportation because of your hip? (whichever you tend to use) | 3.66 ± 0.5 | 3.60 ± 0.49 |
| 4. Have you been able to put on a pair of socks, stockings or tights? | 2.33 ± 0.47 | 2.47 ± 0.51 |
| 5. Could you do the household shopping on your own? | 4.00 ± 0.00 | 3.86 ± 0.26 |
| 6. For how long have you been able to walk before the pain in your hip becomes severe? (with or without a walking aid) | 3.60 ± 0.51 | 3.13 ± 0.77 |
| 7. Have you been able to climb a flight of stairs? | 3.20 ± 0.40 | 3.07 ± 0.26 |
| 8. After a meal (sat at a table), how painful has it been for you to stand up from a chair because of your hip? | 3.36 ± 0.49 | 3.53 ± 0.52 |
| 9. Have you been limping when walking, because of your hip? | 3.13 ± 0.61 | 3.00 ± 0.55 |
| 10. Have you had any sudden, severe pain—“shooting”, “stabbing”, or “spasms”)—from your affected hip? | 3.40 ± 0.52 | 3.20 ± 0.91 |
| 11. How much has pain from your hip interfered with your usual work (including housework)? | 3.8 ± 0.47 | 3.53 ± 0.75 |
| 12. Have you been troubled by pain from your hip in bed at night? | 3.47 ± 0.72 | 3.06 ± 0.72 |
| Oxford Hip Score (total score) | 41.67 ± 2.19 | 39.73 ± 1.58 |
| Parameters | OA Group (N= 15) | DDH Group (N= 15) |
|---|---|---|
| Walking speed (cm/s) | 77.26 ± 4.83 | 74.75 ± 3.24 |
| Cadence (steps/min) | 94.69 ± 2.73 | 92.93 ± 3.17 |
| Double support time (% cycle) | 33.18 ± 2.13 | 31.78 ± 3.81 |
| Single support (% cycle) | 37.09 ± 3.8 | 35.12 ± 5.57 |
| Step time (s) | 0.66 ± 0.08 | 0.72 ± 0.11 |
| Step length (cm) | 48.62 ± 2.84 | 47.18 ± 2.87 |
| Stride time (s) | 1.23 ± 0.20 | 1.27 ± 0.11 |
| Stride length (cm) | 97.83 ± 5.45 | 95.45 ± 5.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasi, S.; Papagiannis, G.; Triantafyllou, A.; Papagelopoulos, P.; Koulouvaris, P. Post-Arthroplasty Spatiotemporal Gait Parameters in Patients with Hip Osteoarthritis or Developmental Dysplasia of the Hip: An Observational Study. J. Funct. Morphol. Kinesiol. 2024, 9, 110. https://doi.org/10.3390/jfmk9030110
Stasi S, Papagiannis G, Triantafyllou A, Papagelopoulos P, Koulouvaris P. Post-Arthroplasty Spatiotemporal Gait Parameters in Patients with Hip Osteoarthritis or Developmental Dysplasia of the Hip: An Observational Study. Journal of Functional Morphology and Kinesiology. 2024; 9(3):110. https://doi.org/10.3390/jfmk9030110
Chicago/Turabian StyleStasi, Sophia, Georgios Papagiannis, Athanasios Triantafyllou, Panayiotis Papagelopoulos, and Panagiotis Koulouvaris. 2024. "Post-Arthroplasty Spatiotemporal Gait Parameters in Patients with Hip Osteoarthritis or Developmental Dysplasia of the Hip: An Observational Study" Journal of Functional Morphology and Kinesiology 9, no. 3: 110. https://doi.org/10.3390/jfmk9030110
APA StyleStasi, S., Papagiannis, G., Triantafyllou, A., Papagelopoulos, P., & Koulouvaris, P. (2024). Post-Arthroplasty Spatiotemporal Gait Parameters in Patients with Hip Osteoarthritis or Developmental Dysplasia of the Hip: An Observational Study. Journal of Functional Morphology and Kinesiology, 9(3), 110. https://doi.org/10.3390/jfmk9030110






